Abstract
Riboflavin (vitamin B2) is an essential water-soluble vitamin that serves as a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). FMN and FAD are coenzymes involved in key enzymatic reactions in energy metabolism, biosynthesis, detoxification and electron scavenging pathways. Riboflavin deficiency is prevalent worldwide and impacts women’s health due to riboflavin demands linked to urogenital and reproductive health, hormonal fluctuations during the menstrual cycle, pregnancy, and breastfeeding. Innovative functional foods and nutraceuticals are increasingly developed to meet women’s riboflavin needs to supplement dietary sources. An emerging and particularly promising strategy is the administration of riboflavin-producing lactic acid bacteria, combining the health benefits of riboflavin with those of probiotics and in situ riboflavin production. Specific taxa of lactobacilli are of particular interest for women, because of the crucial role of Lactobacillus species in the vagina and the documented health effects of other Lactobacillaceae taxa in the gut and on the skin. In this narrative review, we synthesize the underlying molecular mechanisms and clinical benefits of riboflavin intake for women’s health, and evaluate the synergistic potential of riboflavin-producing lactobacilli and other microbiota.
Subject terms: Microbiome, Anatomy
Introduction
Micronutrient status and dietary habits are crucial for human health and quality of life1–3, especially for women of reproductive age and their children4. Riboflavin is an essential water-soluble vitamin that cannot be synthesized in humans and thus requires regular intake5. The need for riboflavin is prominent in women, due to their increased riboflavin demands in a variety of life stages and physiological processes6,7. For example, riboflavin intake should be increased during pregnancy and lactation, since it is taken up by the placenta and fetus to support growth and prevent birth defects6 and lost via breast milk to meet the infant’s nutritional needs and immune development7. To this end, the recommended dietary allowance (RDA) is 1.6 mg/day for adults (both sexes), 1.9 mg/day for pregnant women and 2.0 mg/day for lactating women in Europe8. These values are higher than RDA values set for healthy U.S. and Canadian populations, namely 1.1 mg/day for adult women, 1.4 mg/day during pregnancy, and 1.6 mg/day for lactation9, which can be attributed to corresponding regulatory institutions that define criteria for nutritional adequacy in different ways depending on age, sex, and physiological status10. Documented dietary sources of riboflavin include milk and other dairy products, dark-green vegetables, cereals, fatty fish, and organ meat11. Yet, between 31%12 and 92%13 of women worldwide are reported to have a biochemical riboflavin deficiency. Although the majority of deficiencies occur in developing countries such as in Africa (e.g., Côte d’Ivoire14) and Asia (e.g., India15 and Cambodia13), also populations in developed countries can suffer from riboflavin deficiency due to inadequate intake via diet as a result of veganism16,17, lactose intolerance18, aging19, anorexia nervosa20, or alcoholism21. Consequently, both developing and developed countries call for an acquisition of the necessary riboflavin levels by readily available and cost-efficient supplementary means22.
Dietary intake of riboflavin is associated with health claims defined and evaluated for causality and level of evidence for the general population by the European Food Safety Authority (EFSA)23 (Fig. 1, indicated in bold). Apart from these, additional health benefits have been suggested by observational and intervention studies (Fig. 1, indicated in light), although the level of evidence is often still limited. Yet, riboflavin supplementation is increasingly explored in specific clinical settings, for example in case of preeclampsia24, anemia during pregnancy25, Parkinson’s disease and migraine26, and female27, and postpartum depression28, as further discussed below.
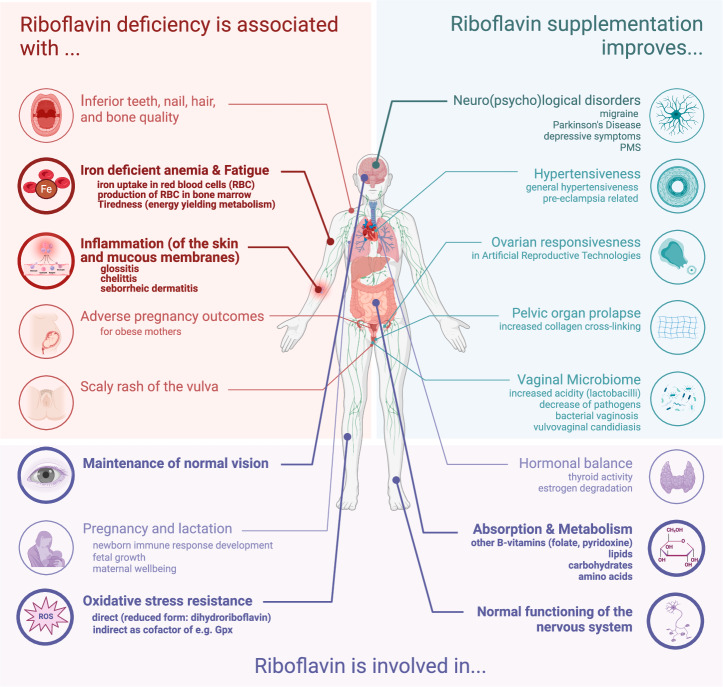
Fig. 1. Impact of riboflavin deficiency on various aspects of women’s health.
The different body sites where riboflavin is crucial are indicated. The evidence is categorized based on the available documentation on associations between (1) riboflavin deficiency and specific health problems, (2) riboflavin supplementation and how it improves specific health outcomes and (3) how normal riboflavin levels support and maintain physiological processes. EFSA-approved health claims are indicated in bold. This figure was created with Biorender.com and based on the following key refs. 23–28,42,44,78,87,90,91,96,128,129,215.
Apart from dietary sources and supplements, riboflavin synthesized by microbiota members in the human body forms an additional source that can contribute to the overall riboflavin homeostasis29. Bacteria such as Lactobacillaceae and Bifidobacteriaceae species have been reported to produce riboflavin that can be used by colonic epithelial cells, but the magnitude of their in situ contribution in the gut is not yet clear30,31. Whereas the riboflavin biosynthesis pathway is most extensively studied in Bacillus subtilis32 and Escherichia coli33, which can reside in the gut at different levels, one systematic genome assessment has found that each of the eight B vitamins (B1, B2, B3, B5, B6, B7, B9, and B12) can theoretically be produced by 40–65% of the 256 studied human gut strains34. The strains predicted to produce these vitamins belong to dominant gut microbiota genera such as Bacteroides, Prevotella, Clostridium, Faecalibacterium and Fusobacterium, and less dominant but prevalent gut taxa such as lactobacilli from the Limosilactobacillus reuteri, Limosilactobacillus fermentum and Lactiplantibacillus plantarum species. Riboflavin (B2) and niacin (B9) were predicted to be synthesized by more than half of the gut microbiota members studied34. In addition, the authors validated the genome predictions with experimental data from sixteen human gut microorganisms, published elsewhere, showing that 88% of the predictions matched34. Of interest, patients with metabolic diseases, such as obesity and type 2 diabetes35 have been reported to have a reduction of bacterial riboflavin synthesis genes in their gut microbiome, suggesting that microbially-produced riboflavin could play a role in these diseases, although the causal association remains to be established. Similarly, members of the microbiota at other body sites such as Lactobacillaceae in the vagina, have been reported to produce B vitamins in laboratory conditions36, but—to the best of our knowledge—so far without reference to potential physiological functions for the host. In infants, it is currently common practice to promote vitamin K supplementation to prevent uncontrolled bleeding until the microbiome is sufficiently matured37. A similar practice to counterbalance certain microbiome imbalances or deficiencies in infants and/or adults is not yet implemented for B vitamins but could be of interest. In this review, we present several arguments why this is of interest to explore, by providing a mechanistic overview of riboflavin’s involvement in women’s health based on dedicated molecular studies and clinical trials and associations. We also summarize the current knowledge on riboflavin production by microbiota members and exogenously added probiotics.
Riboflavin’s modes of action for women’s health
Riboflavin’s general properties: antioxidation and anti-inflammation
Riboflavin’s health benefits are generally based on the capacity of its active forms flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) to function as coenzymes for ca. 70 human proteins (cfr. the flavoproteome)38,39 in biochemical reactions related to energy production, macro- and micronutrient metabolism, cell respiration, cell growth and immune responses (Fig. 2)40,41. By now, it is common knowledge that riboflavin possesses considerable antioxidative and anti-inflammatory properties42,43. The antioxidant effects result from the (in)direct capacity to deactivate reactive oxygen species (ROS), such as O2- and H2O2, either by riboflavin in its reduced form, as dihydroriboflavin, or as a cofactor of antioxidative enzymes, such as glutathione peroxidase, superoxide dismutase, glutathione reductase, and catalase (as reviewed in refs. 42,44). In this regard, the erythrocyte glutathione reductase activity coefficient (EGRac), defined as the ratio of reductase activity in red blood cells (RBCs) after FAD addition to the activity before addition, is a functional indicator of riboflavin status in the blood and therefore used as golden standard to clinically monitor riboflavin deficiency (EGRac > 1.40)22. In general, the higher the EGRac, the less endogenous FAD is available, thus the poorer the blood riboflavin levels22.
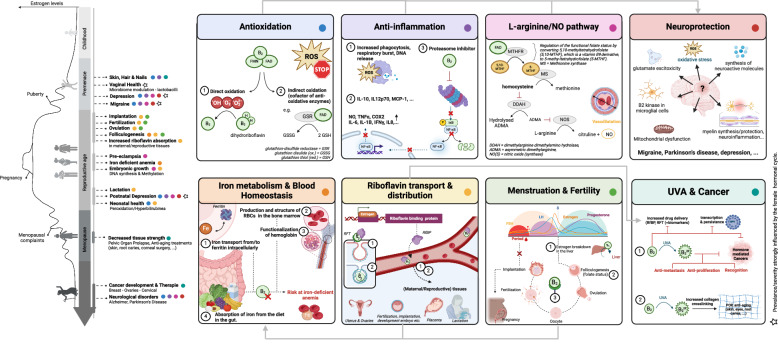
Fig. 2. Riboflavin mechanisms of action with a focus on women’s health (overview).
On the left part of the figure, a female’s life trajectory is depicted according to five different stages based on associated estrogen levels. Riboflavin’s interference in these life stages is indicated, and the involved modes of action of riboflavin (right) are highlighted via colored dots. Starred items are strongly influenced by the female hormonal cycle. Mechanisms affecting each other/acting together are connected with gray arrows. FMN flavin mononucleotide, FAD flavin adenine dinucleotide, IL interleukin, IFN-γ interferon gamma, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, NO nitric oxide, TNFα tumor necrosis factor alpha, COX2 cyclooxygenase 2, ROS reactive oxygen species, RfBP riboflavin binding protein, RFT riboflavin transporter, FSH follicle stimulating hormone, LH luteinizing hormone. This figure was created with Biorender.com and based on the following key refs. 22,26,43–45,72–76,88,93,97,101,103,104,118,128,130,140.
Riboflavin’s anti-inflammatory properties have mainly been studied using cell and murine models43,45, but are not yet well understood. Interference of riboflavin with the generally pro-inflammatory nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor signaling seems to play a major role45. Riboflavin seems to function as a proteasome inhibitor43,45, resulting in the decreased degradation of the NF-κB inhibitor, phosphorylated-inhibitor kappa (P-IκB), thus preventing nuclear translocation of NF-κB and subsequent pro-inflammatory gene activation46. This has been shown to result in decreased production of pro-inflammatory cytokines such as Tumor Necrosis Factor alpha (TNF-α), interleukin 6 (IL-6), interleukin 8 (IL-8), and interferon gamma (IFN-γ), and inflammation markers such as cyclooxygenase 2 (COX2)26,45. In similar study setups, riboflavin has also been shown to prevent mitochondrial ROS production and DNA release47. In addition to inhibiting NF-kB signaling, riboflavin can also inhibit critical components of the non-canonical inflammasomes, such as caspase-1 activity47, either via the AIM2 cytosolic innate immune receptor, which recognizes double-stranded DNA (dsDNA) released during cellular perturbation, or via the Nod-like receptor NLRC4, which recognizes bacterial ligands such as flagellin and the type three secretion system (T3SS)47. This appears to also result in reduction of pro-inflammatory cytokines such as IL-1β and IL-18 in macrophages and mice48. Moreover, riboflavin has been shown to directly stimulate the release of anti-inflammatory cytokines (e.g., IL-10, IL-12p70) and immune modulators (i.e., MCP-1, HMGB1, Hsp72, Hsp25)49,50. Riboflavin has also been reported to have an indirect role in anti-inflammatory mechanisms via its involvement in vitamin D biosynthesis as a cofactor for flavin-dependent monooxygenases and oxidoreductases (as reviewed in ref. 51).
The antioxidant and anti-inflammatory properties of riboflavin have also been documented in clinical trials. For example, in a 3-week prospective intervention with a high dose of 100 mg riboflavin/day with 70 patients with Crohn’s Disease, a reduction in systemic oxidative stress (measured by higher free thiol plasma levels), inflammatory markers (IL-2 and C-reactive protein (CRP)) and clinical symptoms of Crohn’s disease (i.e., a reduction in clinical disease activity index and quality of life improvement) were observed52. Contrarily, in a shorter placebo-controlled study with healthy individuals, daily supplementation of 50 mg (n = 32) or 100 mg (n = 33) riboflavin showed no reduction in free thiols compared to placebo (n = 34)53. In a prospective study with pregnant women and their children (n = 2797 with singleton births), riboflavin levels in the blood were inversely associated with inflammation markers in the blood54.
Indirect clinical validation of antioxidant and anti-inflammatory effects is linked to roles for riboflavin in the maintenance of healthy skin, nails, hair55, and good eyesight56. However, when this role of riboflavin and associated health claims were evaluated by the EFSA8 for the general population, the expert panel concluded that a cause-and-effect relationship has only been established for dietary intake of riboflavin in the case of contribution to normal skin and mucous membranes and maintenance of normal vision. For the other indications, the panel concluded that the mechanistic documentation available was too limited. For example, riboflavin has been shown to be involved in the conversion of tryptophan to niacin (vitamin B3)57, which stimulates the production of collagen (type I, III, and V), elastin and fibrillin (1 and 2), but—to the best of our knowledge—this has only been substantiated ex vivo in dermal fibroblasts58.
Riboflavin and neurological disorders
Several large observational studies in human cohorts have also associated B vitamins intake (e.g., vitamin B2, B9, and B12) and other dietary habits with mental health, particularly in adolescent girls (Japanese cross-sectional study, n = 3450)59, and women who suffer from postpartum depression (cross-sectional study, n = 344)28 or premenstrual syndrome (PMS) (case-control study, n = 3025)60. More specifically, the latter nested case-control study (1057 cases and 1968 controls) showed that when women consumed more riboflavin (from fortified cereals, cow milk and/or green vegetables), the risk for PMS was lowered with 35%60. In the Japanese cross-sectional study on adolescents (aged 12–15), higher riboflavin in blood were also associated with less depressive symptoms in girls (n = 3450), but not boys (n = 3067)59. Another peculiar sex difference was reported in an Iranian observational study (n = 3362) where lower dietary riboflavin levels were associated with anxiety and depression in middle-aged men (n = 1403), and psychological distress in middle-aged women (n = 1959)61. However, a recent systematic review of 20 randomized controlled trials (RCTs) (n = 2256)62 did not substantiate the potential of riboflavin as adjuvant for depressive symptom alleviation. Riboflavin supplementation is also discussed for neurodegenerative diseases such as Parkinson’s disease, where women have higher mortality rates and faster disease progression compared to men63,64 but the cause of the improved motor capacity of the patients was not solely attributed to riboflavin (n = 19)65.
More clinical evidence exists for the use of riboflavin as prophylaxis against migraine headaches66. Women suffer from a three-fold increased risk for migraine symptoms compared to men67. This can possibly be explained by a hormonally lowered neuro-excitability threshold for these headache attacks68. Strikingly, in a large cross-sectional study (n = 5725 females and n = 1061 males) menstruation was found as the main trigger factor for migraine episodes in female patients (78% of women)69, while postmenopausal women appear to have more similar migraine triggers to male migraine patients than fertile female patients concerning hormonal levels69. High-dose supplementation (400 mg/day) of riboflavin over a time course of three months was found to significantly reduce the frequency, duration and pain score of migraine attacks in both men and women in a meta-analysis of eight RCTs and one intervention study66. Consequently, riboflavin is also included as additional prophylactic therapy in the treatment guidelines of conditions and disorders with co-morbid migraine headaches70. For example, the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Association recommend riboflavin for cyclic vomiting, a gastrointestinal and psychological condition characterized by sudden episodes of nausea and vomiting70.
The neurological mode of action of riboflavin and its active forms FMN and FAD appears to be complex and multifactorial, involving its antioxidant and anti-inflammatory properties, as well as the homocysteine/L-arginine/NO pathway. For example, in a mouse model of lipopolysaccharide-induced neuro-inflammation and Alzheimer’s disease, FMN supplementation with specific nanoparticles targeting riboflavin metabolism in the microglia ameliorated cognitive dysfunction, synaptic plasticity, and inflammation71. These FMN particles appeared to lower riboflavin kinase expression in the microglia, preventing TNFR1/NF-κB signaling and pro-inflammatory cytokine release71. A recent integrative systematic review of 21 (pre)clinical studies (including 8 studies in mice, 12 clinical and one translational) indicated the potential of riboflavin treatment to improve brain damage following oxygen deprivation in children, adults, and elderly people72. Apart from these modes of action, riboflavin has also been shown to activate specific neuroactive molecules such as vitamin B6 (which confers neuroprotection based on a role in serotonin production)73, homocysteine (which has a double-edged neurological role)74 and kynurenine involved in glutamate excitotoxicity (i.e., excess of glutamate in neural synapse, resulting in death of neural cells)75. Moreover, the synthesis and protection of myelin, which insulates nerve cell axons and increases their electrical pulse rate, require riboflavin76. In line with this, riboflavin has been shown to prevent myelin degeneration in murine models of multiple sclerosis by supporting the levels of the protective brain-derived neurotrophic factor77. Clinical substantiation remains to be provided in suitable safety and efficacy trials.
Riboflavin and vaginal health
The above reviewed mechanistic and clinical studies with riboflavin have mainly focused on systemic outcomes, with some also highly relevant for women’s health. In addition to systemic benefits, as early as 1940, it was observed that riboflavin could also have local benefits: vaginal administration of riboflavin in ten women via a lactose tablet was shown to result in an increased acidity and a favorable impact on vaginal bacteria through increased growth of acid-forming bacilli and decrease of pathogens78. Considering the current knowledge on the vaginal microbiome79,80, these observations suggest an increased activity of lactic acid production by vaginal lactobacilli. To the best of our knowledge, local vaginal applications of riboflavin have not been further explored, so that direct measurements linking vaginal riboflavin supplementation to increased growth of lactobacilli are currently lacking. In contrast, oral supplementation has been explored. In one RCT (n = 158), an oral vitamin B complex, including riboflavin sodium phosphate, showed efficacy as adjuvant therapy to fluconazole for women with complicated vulvovaginal candidiasis (VVC)81. The B vitamin complex appeared to significantly increase the anti-Candida effect, with more women testing negative for hyphae and spore formation81. This finding was mechanistically supported by an enhanced antifungal effect of fluconazole in a VVC vaginal epithelial cell model and mouse model when administered together with vitamin B complex injection81. In other research, lactobacilli have also been shown to reduce the growth and hyphae formation of Candida in vitro82–84 and in patients with acute VVC85. Unfortunately, no data on the impact of riboflavin supplementation on the vaginal microbiome composition or endogenous vaginal lactobacilli have been reported, making it difficult to assess whether an increase in lactobacilli by riboflavin could play a role in these observed enhanced antifungal effects81.
Riboflavin and reproductive health
Modulation of hormonal fluctuations and impact on fertility
Apart from its role in systemic and vaginal health, riboflavin also seems to impact reproductive health, in part through its reciprocal interaction with estradiol, the most prominent female sex hormone essential for fertility and pregnancy68,86. For example, in a longitudinal prospective cohort study with 259 premenopausal women, a secondary analysis showed that higher dietary intake of riboflavin, assessed via a 24h dietary recall, was associated with lower serum levels of estradiol and homocysteine, which are signals required for folliculogenesis68. Similar inverse associations between riboflavin intake and blood estradiol levels were found by the Nurses’ Health Study II, which is one of the longest running investigations of factors influencing women’s health (n = 116,469) by combining food-frequency questionnaires (FFQs), health surveys, and biological samples (urine, blood and cheek cell samples)87. Likewise, higher intake of riboflavin, vitamin B6 and B12 was also associated with lower incidence of ovulatory infertility87. On the one hand, these observations might be explained by the fact that an estrogen drop is required for the onset of ovulation88, and several flavoproteins (proteins using FMN and FAD as coenzyme) assist estrogen degradation by cytochrome P45051,89 as they balance electrons from these reactions. On the other hand, the inverse association of riboflavin with plasma homocysteine levels can also be attributed to riboflavin’s interaction with methyltetrahydrofolate reductase (MTHFR), another peculiar flavoprotein involved in follicular activity, embryo quality and pregnancy success90–92. In short, MTHFR initiates the conversion of folate (vitamin B9) to its functional form 5-methyltetrahydrofolate93,94, which on its turn acts as a coenzyme of methionine synthase for the formation of methionine from homocysteine93,94. Remarkably, elevated homocysteine levels have frequently been associated with poor oocyte maturity, reduced fertilization and poor in vitro embryo quality95, though direct clinical evidence linking riboflavin, homocysteine and anovulation is sparse. Nevertheless, riboflavin and other B vitamins might assist future subfertility treatments and fertility preservation methods. For example, in a subset of 100 women relying on assisted reproductive technologies (ART), higher preconceptional vitamin B9 and B12 levels in blood were associated with higher live birth rates96.
Adding to these positive effects of vitamin B9 and B12, higher serum riboflavin levels were associated with increased probabilities of high-quality embryos as well as clinical pregnancy after embryo transfer in a prospective Chinese follow-up study (n = 216, age <35)97. Moreover, in a mechanistic study in pre-puberty mice, riboflavin, together with vitamin B1 and B6, seemed to stimulate in vitro maturation of follicles through granulocyte proliferation and upregulation of oocyte-specific genes, including genes encoding bone morphogenetic protein 15 (BMP15), growth differentiation factor 9 (GDF9), zona pellucida glycoprotein 3 (ZP3) and estrogen receptor alpha (ESR1) and beta (ESR2)98. This follicle maturation activity is of interest to substantiate in human mechanistic and efficacy studies because peri-menopausal women and female cancer survivors relying on fertility preservation methods have typically smaller number of follicles with a reduced probability to mature98.
Compared to limited evidence for the aforementioned effect of riboflavin on estrogen levels and menstrual cycle, estrogen seems to have a clearer impact on plasma riboflavin levels, as well as its distribution throughout the female body68. In general, riboflavin and FMN are transported across the body by binding to non-specific carrier proteins in the plasma, such as albumin and immunoglobulins (IgA, IgG, IgM), through hydrogen bond formation99. As a result of the varying affinity between the flavins and their carrier, the flavins are deposited across the body. Besides these non-specific carriers, there also exist specific riboflavin-binding proteins (RfBPs) in mature females, which are mainly synthesized by the liver under influence of an estrogen-sensitive promoter when becoming sexually mature or when treated with estrogen100–102. These RfBPs, highly conserved across mammals and avian species, scavenge riboflavin in blood and transfer the vitamin to specific tissues through receptor-mediated endocytosis by riboflavin transporters (RFTs)86,100.
Many reproductive and maternal tissues, such as the ovaries, placenta, and mammary glands, make use of estrogen-sensitive RfBPs and RFTs for highly sophisticated riboflavin transport to support fertilization and/or offspring’s growth101,102. For instance, in rodents and subhuman primates, immunoneutralization of RfBPs has been shown to result in female infertility, peri-implantation embryonic loss and pregnancy termination103,104. Evidently, it is more difficult to explore and substantiate such role in humans, but specific intervention studies with riboflavin in relation to female fertility seem worth investigation. The same holds true for the role of other micronutrients, such as folate, vitamin D, and iron, which all depend on the riboflavin status for their activation and which have been positively associated with female fertility, as reviewed in ref. 105. Moreover, it seems of interest to explore the role of riboflavin in male fertility in more detail. For instance, riboflavin seems to be involved in sperm motility and energy generation as well as fertilization (cfr. acrosome reaction) and oxidative stress management, but this has so far only been shown in animal models106.
We hypothesize that this hormonally induced scavenging of riboflavin might also explain why women taking high-dosed oral contraceptives containing estrogen and progesterone have been reported to be at risk for riboflavin deficiency as measured by the EGRac107–111. Similarly, it could clarify that migraine attacks, as side effects of the older generation oral contraceptives, are possibly linked to a reduction in plasma riboflavin67. Nevertheless, these mechanisms remain to be substantiated.
Riboflavin, pregnancy, and child’s development
Riboflavin is also crucial for the health and well-being of infants during pregnancy and after birth112. During pregnancy, estradiol levels are heightened, and riboflavin consumption strongly increases due to a particularly high demand by fetal tissues. In one study of 44 women and their infants, a maternal-fetal riboflavin plasma ratio of 1:4.7 was measured6. This high riboflavin demand could be explained by its involvement in DNA synthesis and methylation during embryonic growth8,105, as well as neural tube formation (cfr. regulation of the functional folate status as mentioned before). The transplacental transport of riboflavin is associated with high RfBP and RFT expression by placental trophoblast cells and appears to be the most intense during the third trimester6. To reduce perinatal mortality113, recommended riboflavin intake is therefore increased for pregnant women to 1.9 mg/day in Europe8 and 1.4 mg/day in U.S. and Canada9.
Riboflavin remains essential around birth, with FAD functioning as a crucial cofactor of glutathione, to oppose peroxidation reactions that arise during the rapid change in oxygen concentration in the baby during delivery. After birth, active riboflavin transport by maternal tissues using RfBPs and RFTs and other proteins appears to remain essential, for instance to pump riboflavin into the breast milk and support the child’s nutritional needs as shown in mice and humans101,114. Therefore, as mentioned before, the recommended daily intake for riboflavin is also increased for breastfeeding women to 2.0 and 1.6 mg/day in Europe (EFSA)8 and the U.S. and Canada (RDA)9, respectively.
Riboflavin and iron-deficient anemia: during pregnancy & beyond
Riboflavin is also important for the health of mother during pregnancy and this stems in part from its involvement in iron metabolism. Iron-deficient anemia remains one of the most prevalent medical concerns during pregnancy115. Women are specifically at risk for anemia during the first four months of gestation, since hemoglobin levels naturally decrease due to elevated iron demands to support fetal growth and a disproportionate rise of blood plasma volume to RBCs97. Iron-deficient anemia has been systematically associated with extreme fatigue and severe pregnancy complications such as postpartum hemorrhage, preterm delivery, stillbirth and reduced offspring birthweight25. Besides insufficient iron intake, low riboflavin status, which is more common among women of reproductive age than generally recognized25,116, appears to be also involved in the development of iron-deficient anemia22. For instance, in a large randomized controlled intervention study with 2153 healthy pregnant women in Ireland, 68% of the cohort measured low or deficient blood riboflavin levels25. This riboflavin status was found to be a significant determinant of hemoglobin levels25 and predictor of anemia development at the 12th gestational week25.
The underlying mechanism of riboflavin in anemia appears multifaceted: flavin-dependent enzymes are involved in the absorption of iron from the diet117, in the mobilization of iron from/to ferritin (the main intracellular iron storage protein in cells)118, and the uptake of iron in RBCs116. In addition, flavin-dependent enzymes are needed for the functionalization of hemoproteins (cfr. reduction of insoluble Fe3+ to soluble Fe2+), as observed for the conversion of inactive methemoglobin into hemoglobin required for oxygen transport22,119. Apart from RBC physiology, riboflavin is also involved in RBC structure, by preventing hemolysis through oxidative stress management120, and RBC generation in the bone marrow, mediated through its interference with corticosteroid metabolism121, with health implications far beyond pregnancy induced anemia.
Indeed, in an observational study of non-pregnant Malaysian (n = 210) and Canadian (n = 206) women, it was also shown that riboflavin deficiency (EGRac > 1.40) was a weak, but significant predictive biomarker of hemoglobin and anemia22. Altogether, these findings indicate that pregnant women could benefit from preventative riboflavin supplementation as it reduces the risk of iron-deficient anemia112,122, however it is not yet in treatment guidelines. Other cardiovascular concerns during pregnancy and the postpartum period, such as maternal systemic endothelial dysregulation, intravascular inflammation, and preeclampsia have also been associated with riboflavin deficiency123. Especially the latter is of interest, as it affects 3 to 13% of pregnant women, with incidence up to 20% among high-risk women according to the World Health Organization (WHO)124. Preeclampsia is a dangerous condition of persistent hypertension, associated with high urinary protein levels or decreased blood platelet development, failure of kidneys, liver or lungs, and neurological complications123. In recent years, riboflavin supplementation is increasingly explored to prevent preeclampsia. It is postulated that riboflavin induces NO-mediated vasodilatation, resulting in hypertension relief, through stimulation of the conversion of homocysteine and its subsequent impact on the L-arginine/NO pathway125 (Fig. 2). In a prospective, randomized, double-blind trial in Tanzania and Venezuela with 455 women, taking riboflavin (15 mg/day) from the 20th week of pregnancy appeared to be associated with prevention of severe cases of preeclampsia24. However, compared to anemia, the evidence for riboflavin supplementation to treat preeclampsia is limited, as recently reviewed112.
Riboflavin, aging and cancer
Pelvic organ prolapse and related issues
One of the most important aging-related conditions for women where riboflavin plays a role is pelvic organ prolapse (POP). POP is a condition with a worldwide prevalence of 9%126, in which a woman’s pelvic muscles and tissues weaken, resulting in bulging of the pelvic organs (uterus, bladder, rectum) into the vagina127. Besides vaginal birth and being heavily overweight, one of the causes for POP is diminished vaginal tissue stiffness through the reduction of collagen with age and hormonal changes128,129. This loss of collagen and epithelial stiffness is also suggested to be a cause of other diseases and aging-related complications such as corneal130, skin131 and teeth tissue132 deteroriation. While the latter two have been substantiated with in vitro work133,134, clinical efficacy has been documented for the treatment of corneal disorders with UVA-activated riboflavin, resulting in its incorporation in routine ophthalmologic procedures135. During the exposure of riboflavin to UVA, singlet oxygen molecules are generated, which induces covalent bonding between amino groups of collagen fibrils, and thus strengthens tissue stiffness136. Consequently, UVA-activated riboflavin was proposed as potential therapy for POP as well, especially due to its ability to attenuate UVA damage and inhibit necrosis in vaginal cells128,129. Until now, this hypothesis has only been substantiated by ex vivo experiments where vaginal tissue strips from POP cases were exposed to riboflavin and subsequent UVA photoactivation cells128,129. Dedicated clinical studies are required to substantiate the hypothesized benefits of local and systemic riboflavin application on vaginal health outcomes, including a potential beneficial impact on the vaginal microbiome as a key read-out.
Cancer
For several decades, poor riboflavin intake has been associated with increased risk of cervical cancer in epidemiological studies137,138. In addition, a case-control study (n = 257 cases, 133 controls) in 1993 reported that lower riboflavin intake, assessed via 24 h dietary recall, was associated with increased risk of cervical intraepithelial dysplasia, an early stage proceeding invasive cervical cancer139. More recently, a Chinese observational study (n = 146) showed that not only plasma, but also tissue riboflavin levels were inversely associated with high-risk human papillomavirus type 16 (HR-HPV16) and HPV18 infection140. Moreover, compared to healthy control specimens, plasma and tissue riboflavin levels were decreased in patients with cervical squamous epithelial cancer and cervical intraepithelial dysplasia, respectively. These findings suggest a role for riboflavin in HPV-induced cervical cancer development and progression140, which is potentially mediated through riboflavin transporter C20orf54140, and riboflavin’s antioxidant properties (see above). Although direct mechanical evidence for riboflavin is lacking, other antioxidants have been reported to reduce HPV transcription and expression in vitro through redox regulation141,142. Understanding what drives the progression from precancerous lesions to invasive cervical cancer, which especially impacts HPV-infected women, remains a necessary topic for further research.
Besides cervical cancer, insufficient riboflavin intake has also been associated with breast carcinogenesis143. A systematic review and meta-analysis of 21 prospective cohorts and 6 nested case-control studies (n = 49,707 cases and 1,274,060 individuals) indicated that a higher dietary intake of riboflavin, together with folate and vitamin B6, might be associated with a decreased risk of estrogen and progesterone receptor-positive breast cancers143. However, other studies report more complicated associations between B vitamins and cancer (as also reviewed in ref. 144). For instance, pharmacokinetic vitamin-drug interaction studies have indicated a decreased uptake of the anticancer drug antifolate methotrexate in cancer cells, and the complexation of methotrexate and doxorubicin C into inactive adducts by riboflavin, thereby reducing the efficacy of these drugs145. Care should thus be taken when implementing riboflavin in clinical practice for cancer patients in different disease stages.
Riboflavin-producing microbiota and women’s health
The microbiota at different body sites (gut, skin, vagina) also plays an important role in women’s health throughout all life stages, although the level of evidence and mechanistic substantiation for its role is fragmented. In the vagina, Lactobacillus species are generally dominant in healthy, complaint-free women, such as shown in a pioneering study in the US (n = 396)146 and a large-scale citizen science cohort study in Belgium (n = 3345)79. Nevertheless, more diverse vaginal microbiomes have been observed globally across different healthy populations (North America146, Scandinavia147, South Africa148, and Kenya149), but are usually associated with adverse sexual and reproductive outcomes150. Systematic reviews have now established that a vaginal composition dominated by Lactobacillaceae taxa such as Lactobacillus crispatus is linked to protection against conditions such as preterm birth151, bacterial vaginosis152 and progression of an HPV infection into cervical cancer153. The protective mode of action of lactobacilli in the vagina appears to be mainly due to their capacity to produce lactic acid as antimicrobial factor80, while a metabolic role for metabolites such as riboflavin is largely underexplored. Research on the gut microbiome in patients (male and female) with metabolic diseases, such as obesity154 and type 2 diabetes35,155, has reported a reduction of riboflavin synthesis genes in their gut metagenome. Of interest, in a female-specific gut metagenome study of women with type 2 diabetes (n = 53), impaired glucose tolerance (n = 49), and normal glucose tolerance (n = 43), riboflavin synthesis genes were also more abundant in the normal group156.
Main documentation is based on in vitro and preclinical data
A variety of microorganisms including bacteria (e.g., Clostridium difficile157), archaea (e.g., Methanococcus jannaschii158), fungi (e.g., Eremothecium ashbyii159, Saccharomyces cerevisiae160) have a documented capacity to produce riboflavin at different levels40,161, although in general, under physiological conditions the microbial riboflavin production is very low (few µg/L, or less, in culture media). Therefore, some of these microorganisms, such as Bacillus subtilis, Ashbya gossypii, and Candida famata, have even been genetically, metabolically and/or chemically optimized for industrial-scale riboflavin production160,162. For most commercially available dietary supplements with riboflavin, these microorganisms produce riboflavin industrially in bioreactors and the vitamin is extracted to be formulated in supplements163. Many of the producing microorganisms do not have an assigned safety status such as ‘Generally Recognized as Safe’ (GRAS)164 in the United States and/or ‘Qualified Presumption of Safety’ (QPS)165 as evaluated by EFSA for Europe, nor do they comply with the scientific definition of probiotic166. This definition states that probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”166. Consequently, many of the known riboflavin-producing taxa cannot be used live in food products for human consumption167.
Compared to these traditional industrial-scale producing microorganisms that cannot be consumed, riboflavin-producing Lactobacillaceae and Bifidobacteriaceae taxa with a GRAS/QPS or related status are of interest, because they can be safely consumed and allow the combination of the benefits of riboflavin with some of the probiotic health benefits for several strains of these taxa (Fig. 3). Probiotic benefits for Lactobacillaceae and Bifidobacteriaceae evaluated in systemic reviews and meta-analyses include improved gastrointestinal health and prevention of antibiotic-associated diarrhea168, prevention of relapse of bacterial vaginosis169, and prevention of respiratory tract infections170. It is not yet well-understood how these bacteria can exert these benefits, but documented mechanisms of action include antimicrobial activity against major gastrointestinal171 and urogenital pathogens172, barrier-promoting effects at the gut epithelium173 and other mucosa174, immunomodulatory effects by stimulating host antimicrobial compounds such as α-defensins175 and modulating the secretion of cytokines such as IL-10, IL-6, IL-1b, IL-2, TNF-α, and impacting different cell types such as intestinal epithelial cells, dendritic cells, macrophages and regulatory T cells176–178. These probiotic mechanisms can be postulated to have direct and indirect physiological effects on women and their health80 (Fig. 3), but require further validation. A role for (B-)vitamin production in probiotic modes of action is also not well-studied. Yet, when administered, Lactobacillaceae and Bifidobacteriaceae taxa that have the genetic and biochemical potential34 might produce riboflavin in situ as a (temporary) part of the gut (and other) microbiota, resulting in altered epithelial morphology as shown in a murine in vivo model179.
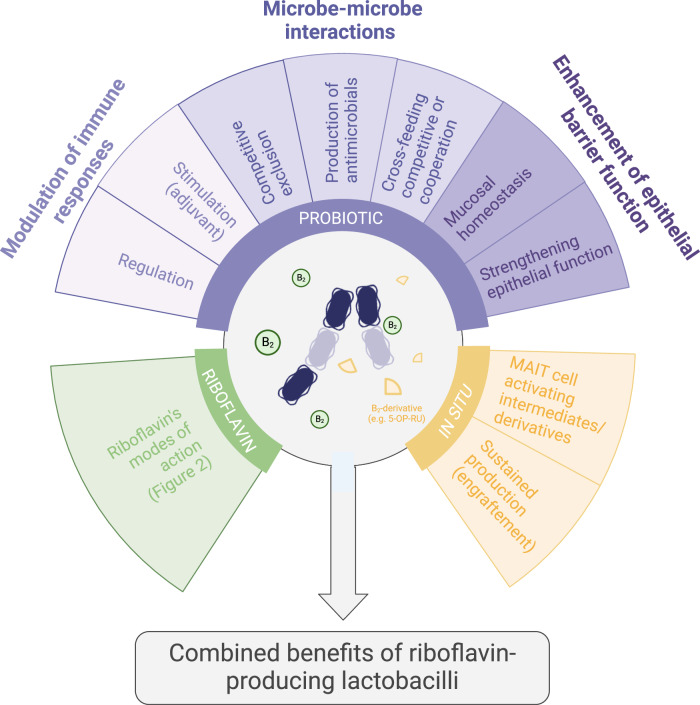
Fig. 3. The postulated synergistic effects of riboflavin-producing microbiota members such as probiotic lactobacilli and riboflavin.
The beneficial properties consist of riboflavin-mediated effects (as previously described in Fig. 2), probiotic and microbiome-promoting effects such as modulation of immune responses, microbe-microbe interactions and enhancement of the epithelial barrier function178 and effects mediated by unstable riboflavin intermediates/derivatives leading to MR1-dependent activation of Mucosal Associated Invariant T cells (MR1 = Major Histocompatibility complex class 1 related protein)187. This figure was created with Biorender.com.
Many Lactobacillaceae and Bifidobacteriaceae species have such capacity to produce riboflavin, at variable concentrations36. For example, Lactiplantibacillus plantarum strains M5MA1-B2180,181 and HY7715182 have been reported to produce 3–5 μg/mL riboflavin under laboratory conditions. Strain HY7715, isolated from food, could even reach up to 34.5 µg/mL when cultivated in optimized growth media182. However, such exceptionally high and industrially relevant riboflavin levels are usually not encountered amongst spontaneous riboflavin-producing lactobacilli183. Therefore, a well-established directed evolution method, using the toxic riboflavin analogue roseoflavin, can be applied to enhance riboflavin production in promising probiotic lactobacilli whilst remaining food-grade184. Most roseoflavin-resistant strains studied until now carry mutations in the regulatory region upstream of the rib operon (more specifically, in the aptamer of the riboswitch), disrupting the negative feedback mechanism, resulting in significantly higher expression184,185. Similar nucleotide replacements and deletions are also observed in spontaneous overproducing isolates, including the vaginal isolate Limosilactobacillus reuteri AMBV339, that can reach high riboflavin levels of approximately 18.36 μg/mL in laboratory conditions and food matrices36. Such high-producing levels are of interest as this could theoretically be sufficient to meet daily needs of 1.6 mg riboflavin with one fermented beverage consumption of 100 mL, as validated by Spacova et al.36. However, to the best of our knowledge, no clinical studies in humans have been performed to validate health effects of in situ riboflavin production after administration of riboflavin-producing probiotic strains. Of note, strain L. reuteri AMBV339 is currently in clinical evaluation for its impact on the gut and vaginal microbiome and metabolome upon administration as an oral dietary supplement (ClinicalTrials.gov ID NCT06425081)36.
Colonic epithelial cells are capable of transporting riboflavin basolaterally186. They could thus—theoretically—take up microbially produced riboflavin and benefit the physiology of the host. Moreover, riboflavin pathway derivatives, such as 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU), 5-(2-oxoethylideneamino)-6-D-ribitylaminouracil (5-OE-RU) and 6,7-Dimethyl-8-(1-D-ribityl)lumazine (RL-6,7-diME), function as evolutionarily conserved non-peptidic antigens to a subpopulation of innate-like T cells, Mucosal Associated Invariant T (MAIT) cells, particularly enriched at mucosal surfaces of mammals, such as the gut, bronchea, skin and uterus187–189. These MAIT cells have a semi-invariant T-cell receptor TCRα(TRAV1-2–TRAJ33)β(TRBV20-TRBV6) and can be activated upon recognition of the riboflavin derivatives 5-OP-RU, 5-OE-RU, or RL-6,7-diMe bound to Major Histocompatibility complex class 1 related (MR1) protein, which is ubiquitously expressed on the cell surface of epithelial cells and immune cells187. While researchers traditionally focused on the anti-pathogenic response of MAIT cells, such as their direct cytotoxic activity and/or indirect antimicrobial activity in bacterial190, fungal191, and viral infections192, their protective role in epithelial barrier enforcement was only recently discovered in the context of the immune response to SARS-CoV-2 infections193–196. In particular, MAIT cells have been shown to establish commensal-driven tissue homeostasis—tissue maintenance, tissue repair, and wound healing—crucial processes both in absence and presence of infections, as reviewed in refs. 197,198. For instance, the riboflavin derivate 5-OP-RU has been shown to activate MAIT cells and this stimulated the healing of a punch-biopsy induced skin wound in mice199. Of interest, early-life exposure to riboflavin-producing commensals has been shown to promote the correct development of the gut and skin MAIT cell response in germ-free murine neonates primed with a synthetic early-life gut microbial community consisting of two Lactobacillaceae, two Enterobacteriaceae and Enterococcus faecalis and the skin commensal Staphylococcus epidermidis, respectively200,201. In mice, riboflavin-overproducing strains L. plantarum ACTT8014202 and L. plantarum CRL2130203 have also been shown to significantly attenuate pathological changes of chemotherapy-induced mucositis during cancer treatments compared to the non-overproducing L. plantarum CRL725 and commercial riboflavin203. The same riboflavin-overproducing strain also showed antioxidant and anti-inflammatory mechanisms by attenuating motor deficits and prevented dopaminergic neuronal death in murine models of Parkinson’s disease204. Yet—to the best of our knowledge—no clinical trial has been initiated or performed linking riboflavin-producing probiotics and impact on host health.
Need for in vivo documentations in humans
The above mentioned studies are mainly based on in vitro and preclinical data, while the field would largely benefit from a more solid documentation of the capacity of riboflavin-producing organisms to increase riboflavin in vivo in humans by for example metagenomic/proteomic/metabolomic studies of the gut and vaginal microbiomes. For example, riboflavin could—theoretically—also support the mutualism between microbiota members, a concept that is increasingly evaluated as (gut) microbiome resilience, also by EFSA205. Within microbial communities, riboflavin is produced by prototrophic species (i.e., species that are equipped with a complete and functional pathway for de novo biosynthesis of certain micronutrients) to cross-feed auxotrophic species (i.e., species that lack the corresponding biosynthesis pathways) in exchange for other metabolites, mostly nutrients or energy206. For instance, in vitro co-cultures and synthetic gut microbiome communities have shown that riboflavin promotes cross-feeding networks involving butyrate production pathways207,208. In addition, riboflavin can stimulate flavin-based extracellular electron transfer (FLEET) by Lactobacillaceae, as recently learned from Lactiplantibacillus plantarum and vegetable fermentations209. In general, these fermentations function as valuable models for studying fundamental microbial interactions, while excluding the host as complicating factor. It is now increasingly understood that FLEET allows respirofermentation in LAB, a hybrid metabolism form that integrates some aspects of (anaerobic) respiration, such as EET, in fermentation (substrate level phosphorylation)209. It is suggested that the ability to transfer electrons outside the cell, and thus maintain redox balance during rapid growth, results in a vast fitness advantage, and seemingly more resilient microbial population. However, the role of FLEET in mammalian physiology is still unclear. Yet, FLEET by cecal microbiota has already been observed in mice, rats, and guinea pigs using cyclic voltammetry, while it appears absent in germ-free mice210. These findings are in line with the population dynamics hypothesis that cooperation (e.g., cross-feeding) enhances the resilience of microbial communities during ecological disturbances211,212. Conformingly, in the gut, where diet is the predominant ecological driver, short-term dietary changes and nutritional shortages do not, according to a systematic review213, significantly alter the microbiome composition. Conversely, microbiome function is impacted by the diet, for example the production of microbial riboflavin is influenced by a fiber-rich diet214. This highlights the potential of modulating the human microbiome in the gut and other body niches through addition of riboflavin-producing microorganisms with multifactorial ecosystem-wide modes of action. This is also one of the core objectives in previous clinical trials such as the observational studies on the association between riboflavin synthesis genes and type 2 diabetes using metagenomics156 and the ongoing clinical trial with the riboflavin-overproducing strain L. reuteri AMBV339 (ClinicalTrials.gov ID NCT06425081). The field would largely benefit from such substantiations of the association between riboflavin-producing probiotics and various aspects of health.
Concluding remarks and future perspectives
In conclusion, riboflavin deficiency could have a large impact on women’s health in both developed and developing countries. Due to specific riboflavin demands of women linked to pregnancy, iron deficiency, hormonal homeostasis, contraceptive use, and other physiological and lifestyle aspects, it is crucial to ensure an adequate riboflavin status. Considering that a large proportion of women lack sufficient riboflavin intake, alternative riboflavin sources such as functional foods and nutraceuticals enriched with riboflavin, as well as probiotics, should be considered. Particularly for lactic acid bacteria-based probiotics, women could benefit from the synergistic effect of riboflavin and beneficial bacteria such as lactobacilli with great potential for women’s health. However, more research is needed on the underlying mechanisms of the way micronutrient production can shape the female microbiome; the ability to share produced vitamins in microbe-microbe interactions; the beneficial effect of bacterial vitamin production on human and more specifically women’s health. Furthermore, formulation, dosage and safety aspects of microbial supplementation should be considered for efficient application of riboflavin-producing bacteria in clinical settings. Ultimately, this research should include large-scale clinical intervention studies in humans with an integrated approach that combines microbiome, multi-omics, metabolomics and immunological readouts, and compares administration formulations and routes. Overall, leveraging riboflavin and riboflavin-producing lactobacilli is a promising avenue with a wide range of potential benefits for women’s health.
Competing interests
This review was written in the framework of the PhD research projects of C.D., I.E., and S.A. However, for transparency, the authors want to declare relationships and interests that could be perceived as a potential conflict of interest. This involves the patent application EP20210606.8 that has been submitted by the host institution (University of Antwerp) on findings related to microbially produced riboflavin in strain Limosilactobacillus reuteri AMBV339. S.L., I.S., and S.A. are listed as inventors. Moreover, the authors declare that they have received funding from different probiotic and food supplement companies to perform mechanistic and clinical research related to aspects discussed in this review.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Sarah Ahannach, Sarah Lebeer.
References
- 1.Bartley, K. A., Underwood, B. A. & Deckelbaum, R. J. A life cycle micronutrient perspective for women’s health. Am. J. Clin. Nutr.81, 1188–1193 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Han, S. J. & Kim, H. W. Nutritional status and women’s Health in university students. Information20, 1795–1803 (2017).
- 3.Torheim, L. E., Ferguson, E. L., Penrose, K. & Arimond, M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J. Nutr.140, 2051–2058 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan, S., Thomas, T. & Kurpad, A. V. B-vitamin interventions for women and children in low-income populations. Curr. Opin. Clin. Nutr. Metab. Care18, 295–306 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Suwannasom, N., Kao, I., Pruß, A., Georgieva, R. & Bäumler, H. Riboflavin: the health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 21, 950–972 (2020). [DOI] [PMC free article] [PubMed]
- 6.Zempleni, J., Link, G. & Bitsch, I. Intrauterine Vitamin B, Uptake of Preterm and Full-Term Infants Vol. 38 (International Pediatric Research Foundation, Inc, 1995). [DOI] [PubMed]
- 7.Mosegaard, S. et al. Riboflavin deficiency—Implications for general human health and inborn errors of metabolism. Int. J. Mol. Sci.21, 3847 (2020). [DOI] [PMC free article] [PubMed]
- 8.Turck, D. et al. Dietary reference values for riboflavin. EFSA J.15, 4919–4984 (2017). [DOI] [PMC free article] [PubMed]
- 9.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, O. B. V. and C. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline 592 (National Academies Press, 1998). [PubMed]
- 10.The United Nations University. Recommended dietary intakes and allowances around the world- an introduction. Food Nutr. Bull.4, 1–14 (1982). [Google Scholar]
- 11.Powers, H. J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr.77, 1352–1360 (2003). [DOI] [PubMed]
- 12.Ter Borg, S. et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br. J. Nutr.113, 1195–1206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitfield, K. C. et al. Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. J. Nutr.145, 628–633 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Rohner, F., Zimmermann, M. B., Wegmueller, R., Tschannen, A. B. & Hurrell, R. F. Mild riboflavin deficiency is highly prevalent in school-age children but does not increase risk for anaemia in Côte d’Ivoire. Br. J. Nutr.97, 970–976 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Potdar, R. D. et al. Improving women’s diet quality preconceptionally and during gestation: Effects on birth weight and prevalence of low birth weight—A randomized controlled efficacy trial in India (Mumbai maternal nutrition project). Am. J. Clin. Nutr.100, 1257–1268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drewnowski, A., Henry, C. J. & Dwyer, J. T. Proposed nutrient standards for plant-based beverages intended as milk alternatives. Front. Nutr.8, 761442 (2021). [DOI] [PMC free article] [PubMed]
- 17.Park, Y. W. The impact of plant-based non-dairy alternative milk on the dairy industry. Food Sci. Anim. Resour.41, 8–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghimeray, P. S. et al. Riboflavin bioavailability varies with milk type and is altered in self-reported dairy intolerance states (P24-012-19). Curr. Dev. Nutr.3, nzz044.P24–012-19 (2019). [Google Scholar]
- 19.McKinley, M. C., McNulty, H., McPartlin, J., Strain, J. J. & Scott, J. M. Effect of riboflavin supplementation on plasma homocysteine in elderly people with low riboflavin status. Eur. J. Clin. Nutr.56, 850–856 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Capo-chichi, C. D. et al. Riboflavin and riboflavin-derived cofactors in adolescent girls with anorexia nervosa. Am. J. Clin. Nutr.69, 672–678 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Langohr, H. D., Petruch, F. & Schroth, G. Vitamin B 1, B 2 and B 6 deficiency in neurological disorders. J. Neurol.225, 95–108 (1981). [DOI] [PubMed]
- 22.Aljaadi, A. M. et al. Suboptimal biochemical riboflavin status is associated with lower hemoglobin and higher rates of anemia in a sample of Canadian and Malaysian women of reproductive age. J. Nutr.149, 1952–1959 (2019). [DOI] [PubMed] [Google Scholar]
- 23.European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to riboflavin (vitamin B2). EFSA J.8, 1814 (2010). [Google Scholar]
- 24.Elsen, C. et al. Vitamins E, A and B2 as possible risk factors for preeclampsia under consideration of the PROPER study (‘prevention of preeclampsia by high-dose riboflavin supplementation’). Geburtshilfe Frauenheilkd.72, 846–852 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy, B. et al. Impact of riboflavin status on haemoglobin and risk of anaemia in pregnancy. Proc. Nutr. Soc.80, (2021).
- 26.Marashly, E. T. & Bohlega, S. A. Riboflavin has neuroprotective potential: focus on Parkinson’s disease and migraine. Front. Neurol.10.3389/fneur.2017.00333 (2017). [DOI] [PMC free article] [PubMed]
- 27.Murakami, K., Miyake, Y., Sasaki, S., Tanaka, K. & Arakawa, M. Dietary folate, riboflavin, vitamin B6, and vitamin B12 and depressive symptoms in early adolescence: the Ryukyu child health study. Psychosom. Med. 72, 763–768 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Lin, Y. H. et al. Association between postpartum nutritional status and postpartum depression symptoms. Nutrients11, 1204–1217 (2019). [DOI] [PMC free article] [PubMed]
- 29.Hill, M. J. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 6, s41–s43 (1997). [DOI] [PubMed]
- 30.LeBlanc, J. G. et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol.24, 160–168 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Thakur, K., Tomar, S. K. & De, S. Lactic acid bacteria as a cell factory for riboflavin production. Microbial. Biotechnol. 9, 441–451 (2016). [DOI] [PMC free article] [PubMed]
- 32.Chu, R., Li, R., Wang, C. & Ban, R. Production of vitamin B2 (riboflavin) by Bacillus subtilis. J. Chem. Technol. Biotechnol. 97, 1941–1949 (2022).
- 33.Liu, B. et al. Escherichia coli O157:H7 senses microbiota-produced riboflavin to increase its virulence in the gut. Proc. Natl Acad. Sci. USA119, e2212436119 (2022). [DOI] [PMC free article] [PubMed]
- 34.Magnúsdóttir, S., Ravcheev, D., De Crécy-Lagard, V. & Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests cooperation among gut microbes. Front. Genet.6, 148 (2015). [DOI] [PMC free article] [PubMed]
- 35.Toubal, A. & Lehuen, A. Role of MAIT cells in metabolic diseases. Mol. Immunol.130, 142–147 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Spacova, I. et al. Spontaneous riboflavin-overproducing Limosilactobacillus reuteri for biofortification of fermented foods. Front. Nutr.9, (2022). [DOI] [PMC free article] [PubMed]
- 37.Fiesack, S. et al. Belgian consensus recommendations to prevent vitamin K deficiency bleeding in the term and preterm infant. Nutrients10.3390/nu13114109 (2021). [DOI] [PMC free article] [PubMed]
- 38.Lienhart, W. D., Gudipati, V. & Macheroux, P. The human flavoproteome. Arch. Biochem. Biophys.535, 150–162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross, N. S. & Hansen, T. P. Riboflavin deficiency is associated with selective preservation of critical flavoenzyme-dependent metabolic pathways. BioFactors3, 185–190 (1992). [PubMed] [Google Scholar]
- 40.Fischer, M. & Bacher, A. Biosynthesis of flavocoenzymes. Nat. Prod. Rep.22, 324–350 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Anaya, A. & Mayersohn, M. Quantification of riboflavin, riboflavin 5′-phosphate and flavin adenine dinucleotide in plasma and urine by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 423, 105–113 (1987). [DOI] [PubMed] [Google Scholar]
- 42.Ashoori, M. & Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: a review. Brit. J. Nutr. 111, 1985–1991 (2014). [DOI] [PubMed]
- 43.Dey, S. & Bishayi, B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J. Inflamm.13, 1–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olfat, N., Ashoori, M. & Saedisomeolia, A. Riboflavin is an antioxidant: a review update. Br. J. Nutr.128, 1887–1895 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Qureshi, A. A. et al. Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models. Lipids Health Dis.10, (2011). [DOI] [PMC free article] [PubMed]
- 46.Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther.2, 17023 (2017). [DOI] [PMC free article] [PubMed]
- 47.Ahn, H. & Lee, G. S. Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity. Sci. Rep.10, 19091 (2020). [DOI] [PMC free article] [PubMed]
- 48.Menezes, R. R., Godin, A. M., Rodrigues, F. F. & Coura, G. M. E. Thiamine and riboflavin inhibit production of cytokines and increase the anti-inflammatory activity of a corticosteroid in a chronic model of inflammation induced by complete Freund’s adjuvant. Pharmacol. Rep.69, 1036–1043 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Shih, C. K. et al. Riboflavin protects mice against liposaccharide-induced shock through expression of heat shock protein 25. Food Chem. Toxicol.48, 1913–1918 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Toyosawa, T., Suzuki, M., Kodama, K. & Araki, S. Potentiation by amino acid of the therapeutic effect of highly purified vitamin B2 in mice with lipopolysaccharide-induced shock. Eur. J. Pharm.493, 177–182 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Pinto, J. T. & Cooper, A. J. L. From cholesterogenesis to steroidogenesis: role of riboflavin and flavoenzymes in the biosynthesis of vitamin D. Adv. Nutr.5, 144–163 (2014). [DOI] [PMC free article] [PubMed]
- 52.Von Martels, J. Z. H. et al. Riboflavin supplementation in patients with Crohn’s disease [the RISE-UP study]. J. Crohns Colitis14, 595–607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourgonje, A. R. et al. The effect of riboflavin supplementation on the systemic redox status in healthy volunteers: a post-hoc analysis of the RIBOGUT trial. Free Radic. Biol. Med.190, 169–178 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Bjørke-Monsen, A. L. et al. Impact of pre-pregnancy BMI on B vitamin and inflammatory status in early pregnancy: an observational cohort study. Nutrients8, 776 (2016). [DOI] [PMC free article] [PubMed]
- 55.DiBaise, M. & Tarleton, S. M. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr. Clin. Pract.34, 490–503 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Struble, M. B. Nutrition Guide for Physicians (2010).
- 57.Henderson, L. M., Koski, R. E. & D’angelis, F. The role of riboflavin and vitamin B6 in tryptophan metabolism. J. Biol. Chem. 215, 369–376 (1954). [PubMed]
- 58.Philips, N., Chalensouk-Khaosaat, J. & Gonzalez, S. Simulation of the elastin and fibrillin in non-irradiated or UVA radiated fibroblasts, and direct inhibition of elastase or matrix metalloptoteinases activity by nicotinamide or its derivatives. J. Cosmet. Sci.69, 47–56 (2018). [PubMed] [Google Scholar]
- 59.Murakami, K., Miyake, Y., Sasaki, S., Tanaka, K. & Arakawa, M. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus child health study. Psychosom. Med. 72, 763–768 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Chocano-Bedoya, P. O. et al. Dietary B vitamin intake and incident premenstrual syndrome. Am. J. Clin. Nutr.93, 1080–1086 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rouhani, P. et al. Dietary riboflavin intake in relation to psychological disorders in Iranian adults: an observational study. Sci. Rep.13, (2023). [DOI] [PMC free article] [PubMed]
- 62.Borges-Vieira, J. G. & Cardoso, C. K. S. Efficacy of B-vitamins and vitamin D therapy in improving depressive and anxiety disorders: a systematic review of randomized controlled trials. Nutr. Neurosci.26, 187–207 (2022). [DOI] [PubMed] [Google Scholar]
- 63.Cerri, S., Mus, L. & Blandini, F. Parkinson’s disease in women and men: what’s the difference? J. Parkinson’s Dis. 9, 501–515 (2019). [DOI] [PMC free article] [PubMed]
- 64.Solla, P. et al. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson’s disease. J. Neurol. Sci.323, 33–39 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Coimbra, C. G. & Junqueira, V. B. C. High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson’s disease patients. Braz. J. Med. Biol. Res.36, 1409–1417 (2003). [DOI] [PubMed]
- 66.Chen, Y. S. et al. Effect of vitamin B2 supplementation on migraine prophylaxis: a systematic review and meta-analysis. Nutr. Neurosci.25, 1801–1812 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Vetvik, K. G. & MacGregor, E. A. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol.16, 76 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Kim, K. et al. Dietary intakes of vitamin B2 (riboflavin), vitamin B6, and vitamin B12 and ovarian cycle function among premenopausal women. J. Acad. Nutr. Diet.120, 885–892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Casteren, D. S., Verhagen, I. E., Onderwater, G. L. J., MaassenVanDenBrink, A. & Terwindt, G. M. Sex differences in prevalence of migraine trigger factors: a cross-sectional study. Cephalalgia41, 643–648 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venkatesan, T. et al. Guidelines on management of cyclic vomiting syndrome in adults by the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association. Neurogastroenterol. Motil. 10.1111/nmo.13604 (2019). [DOI] [PMC free article] [PubMed]
- 71.Zhang, M. et al. Biomimetic remodeling of microglial riboflavin metabolism ameliorates cognitive impairment by modulating neuroinflammation. Adv. Sci.10, e2300180 (2023). [DOI] [PMC free article] [PubMed]
- 72.Silva-Araújo, E. R. D. et al. Effects of riboflavin in the treatment of brain damage caused by oxygen deprivation: an integrative systematic review. Nutr. Neurosci.27, 989–1007 (2024). [DOI] [PubMed]
- 73.Stipanuk, M. H. & Caudill M. A. In Biochemical, Physiological, and Molecular Aspects of Human Nutrition (Elsevier Health Sciences, 2006).
- 74.Moretti, R. & Caruso, P. The controversial role of homocysteine in neurology: From labs to clinical practice. Int. J. Mol. Sci.10.3390/ijms20010231 (2019). [DOI] [PMC free article] [PubMed]
- 75.Martami, F. & Holton, K. F. Targeting glutamate neurotoxicity through dietary manipulation: potential treatment for migraine. Nutrients10.3390/nu15183952 (2023). [DOI] [PMC free article] [PubMed]
- 76.Ogunleye, A. J. & Odutuga, A. A. The effect of riboflavin deficiency on cerebrum and cerebellum of developing rat brain. J. Nutr. Sci. Vitaminol. 35, 193–197 (1989). [DOI] [PubMed]
- 77.Naghashpour, M. et al. Brain-derived neurotrophic and immunologic factors: beneficial effects of riboflavin on motor disability in murine model of multiple sclerosis. Iran. J. Basic Med. Sci.19, 439–448 (2016). [PMC free article] [PubMed] [Google Scholar]
- 78.Stahler, E. The effect of lactoflavin, vitamin B2, on biology of the vagina. Z. Vitam. Forsch.10, 26–31 (1940). [Google Scholar]
- 79.Lebeer, S. et al. A citizen-science-enabled catalogue of the vaginal microbiome and associated factors. Nat. Microbiol.8, 2183–2195 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petrova, M. I., Lievens, E., Malik, S., Imholz, N. & Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 10.3389/fphys.2015.00081 (2015). [DOI] [PMC free article] [PubMed]
- 81.Sun, M. G., Huang, Y., Xu, Y. H. & Cao, Y. X. Efficacy of vitamin B complex as an adjuvant therapy for the treatment of complicated vulvovaginal candidiasis: an in vivo and in vitro study. Biomed. Pharmacother.88, 770–777 (2017). [DOI] [PubMed]
- 82.Allonsius, C. N. et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Micro. Biotechnol.10, 1753–1763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allonsius, C. N. et al. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci. Rep.9, 2900–2912 (2019). [DOI] [PMC free article] [PubMed]
- 84.Spacova, I. et al. Multifactorial inhibition of Candida albicans by combinations of lactobacilli and probiotic Saccharomyces cerevisiae CNCM I-3856. Sci. Rep.14, 9365–9376 (2024). [DOI] [PMC free article] [PubMed]
- 85.Oerlemans, E. F. M. et al. Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci. Rep.10, 7976–7986 (2020). [DOI] [PMC free article] [PubMed]
- 86.Foraker, A. B., Khantwal, C. M. & Swaan, P. W. Current perspectives on the cellular uptake and trafficking of riboflavin. Adv. Drug Deliv. Rev.55, 1467–1483 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Chavarro, J. E., Rich-Edwards, J. W., Rosner, B. A. & Willett, W. C. Use of multivitamins, intake of B vitamins and risk of ovulatory infertility. Fertil. Steril. 89, 668–676 (2008). [DOI] [PMC free article] [PubMed]
- 88.Maman, E., Adashi, E. Y., Baum, M. & Hourvitz, A. Prediction of ovulation: new insight into an old challenge. Sci. Rep.13, 20003–20013 (2023). [DOI] [PMC free article] [PubMed]
- 89.Miller, W. L. Minireview: Regulation of steroidogenesis by electron transfer. Endocrinology146, 2544–2550 (2005). [DOI] [PubMed]
- 90.Hecht, S. et al. Common 677C→T mutation of the 5,10-methylenetetrahydrofolate reductase gene affects follicular estradiol synthesis. Fertil. Steril.91, 56–61 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Thaler, C. J., Budiman, H., Ruebsamen, H., Nagel, D. & Lohse, P. Effects of the common 677C>T mutation of the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene on ovarian responsiveness to recombinant follicle-stimulating hormone. Am. J. Reprod. Immunol.55, 251–258 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Rosen, M. P. et al. Methylenetetrahydrofolate reductase (MTHFR) is associated with ovarian follicular activity. Fertil. Steril.88, 632–638 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Stühlinger, M. C. et al. Homocysteine impairs the nitric oxide synthase pathway role of asymmetric dimethylarginine. Basic Sci. Rep.104, 2569–2575 (2001). [DOI] [PubMed] [Google Scholar]
- 94.Bajic, Z. et al. Homocysteine, vitamins B6 and folic acid in experimental models of myocardial infarction and heart failure—How strong is that link? Biomolecule10.3390/biom12040536 (2022). [DOI] [PMC free article] [PubMed]
- 95.Altmäe, S., Laanpere, M., Campoy, C. & Salumets, A. In Handbook of Diet and Nutrition in the Menstrual Cycle, Periconception and Fertility 431–448 (Brill | Wageningen Academic, 2014).
- 96.Gaskins, A. J. et al. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am. J. Clin. Nutr.102, 943–950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang, L. et al. Status of maternal serum B vitamins and pregnancy outcomes: new insights from in vitro fertilization and embryo transfer (IVF-ET) treatment. Front. Nutr. 9, 962212 (2022). [DOI] [PMC free article] [PubMed]
- 98.Kim, Y. Y. et al. Modulatory effects of single and complex vitamins on the in vitro growth of murine ovarian follicles. Tissue Eng. Regen. Med.16, 275–283 (2019). [DOI] [PMC free article] [PubMed]
- 99.Innis, W. S., McCormick, D. B. & Merrill, A. H. Variations in riboflavin binding by human plasma: identification of immunoglobulins as the major proteins responsible. Biochem Med.34, 151–165 (1985). [DOI] [PubMed] [Google Scholar]
- 100.Combs, G. F. & McClung, J. P. The Vitamins 315–329 (2017).
- 101.van Herwaarden, A. E. et al. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell Biol.27, 1247–1253 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hampel, D. et al. Thiamin and riboflavin in human milk: effects of lipid-based nutrient supplementation and stage of lactation on vitamer secretion and contributions to total vitamin content. PLoS ONE11, e0149479 (2016). [DOI] [PMC free article] [PubMed]
- 103.Subramanian, S. et al. Immunocontraceptive efficacy of synthetic peptides corresponding to major antigenic determinants of chicken riboflavin carrier protein in the female rats. Am. J. Reprod. Immunol.44, 184–191 (2000). [DOI] [PubMed] [Google Scholar]
- 104.Rao, J., Seshagiri, P. B., Shetty, G., Ramesh, G. & Adiga, P. R. Active immunization against riboflavin carrier protein results in peri-implantation embryonic loss leading to pregnancy termination in rats: use of alternate adjuvants. Indian J. Exp. Biol.38, 863–872 (2000). [PubMed] [Google Scholar]
- 105.Kohil, A. et al. Female infertility and diet, is there a role for a personalized nutritional approach in assisted reproductive technologies? A Narrative Review. Front Nutr.9, 927972 (2022). [DOI] [PMC free article] [PubMed]
- 106.Kuang, W. et al. SLC22A14 is a mitochondrial riboflavin transporter required for sperm oxidative phosphorylation and male fertility. Cell Rep.35, 109025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Briggs, M. & Briggs, M. Oral contraceptives and vitamin nutrition. Lancet303, 1234–1235 (1974). [DOI] [PubMed] [Google Scholar]
- 108.Newman, L. J., Lopez, R., Cole, H. S., Boria, M. C. & Cooperman, J. M. Riboflavin deficiency in women taking oral contraceptive agents. Am. J. Clin. Nutr.31, 247–249 (1978). [DOI] [PubMed] [Google Scholar]
- 109.Basciani, S. & Porcaro, G. Counteracting side effects of combined oral contraceptives through the administration of specific micronutrients. Eur Rev Med Pharmacol Sci.26, 4846–4862 (2022). [DOI] [PubMed]
- 110.Palmery, M., Saraceno, A., Vaiarelli, A. & Carlomagno, G. Oral contraceptives and changes in nutritional requirements. Eur. Rev. Med Pharm. Sci.17, 1804–1813 (2013). [PubMed] [Google Scholar]
- 111.Ahmed, F. & Bamji, M. S. Biochemical basis for the “riboflavin defect” associated with the use of oral contraceptives, a study in female rats. Contraception14, 297–307 (1976). [DOI] [PubMed] [Google Scholar]
- 112.Adams, J. B., Kirby, J. K., Sorensen, J. C., Pollard, E. L. & Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: vitamins and related nutrients. Matern. Health Neonatol. Perinatol. 8, 4–41 (2022). [DOI] [PMC free article] [PubMed]
- 113.Torreggiani, C. et al. Premature farrowing and stillbirths in two organic sow farms due to riboflavin deficiency. Porcine Health Manag. 9, 12–20 (2023). [DOI] [PMC free article] [PubMed]
- 114.Wynn, P. C., Morgan, A. J. & Sheehy, P. A. In Encyclopedia of Dairy Sciences:Second Edition 795–800 (2011).
- 115.O’Toole, F., Sheane, R., Reynaud, N., McAuliffe, F. M. & Walsh, J. M. Screening and treatment of iron deficiency anemia in pregnancy: A review and appraisal of current international guidelines. Int. J. Gynecol. Obstetr.10.1002/ijgo.15270 (2023). [DOI] [PubMed]
- 116.Powers, H. J. et al. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am. J. Clin. Nutr.93, 1274–1284 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Aljaadi, A. M., Devlin, A. M. & Green, T. J. Riboflavin intake and status and relationship to anemia. Nutr. Rev.81, 114–132 (2023). [DOI] [PubMed] [Google Scholar]
- 118.Ulvik, R. J. Reduction of exogenous flavins and mobilization of iron from ferritin by isolated mitochondria. J. Bioenerg. Biomembr.15, 151–160 (1983). [DOI] [PubMed]
- 119.Crossley, R. A. et al. Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Appl. Environ. Microbiol.73, 7819–7825 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orrico, F. et al. Oxidative stress in healthy and pathological red blood cells. Biomolecules10.3390/biom13081262 (2023). [DOI] [PMC free article] [PubMed]
- 121.Foy, H., Kondi, A. & Verjee, Z. H. Relation of riboflavin deficiency to corticosteroid metabolism and red cell hypoplasia in baboons. J. Nutr.102, 571–582 (1972). [DOI] [PubMed] [Google Scholar]
- 122.Suprapto, B., Widardo & Suhanantyo. Effect of low-dosage vitamin A and riboflavin on iron-folate supplementation in anaemic pregnant women. Asia Pac. J. Clin. Nutr.11, 263–267 (2002). [DOI] [PubMed] [Google Scholar]
- 123.Magee, L. A., Nicolaides, K. H., & von Dadelszen, P. Preeclampsia. N. Engl. J. Med.389, 1817–1832 (2022). [DOI] [PubMed] [Google Scholar]
- 124.Molina, L. et al. PROPER: a pilot study of the Role of Riboflavin Supplementation for the Prevention of Preeclampsia. Int. J. Biol. Biomed. Eng.6, 43–50 (2012). [Google Scholar]
- 125.Mcnulty, H., Strain, J. J., Hughes, C. F., Pentieva, K. & Ward, M. Evidence of a role for one-carbon metabolism in blood pressure: can B vitamin intervention address the genetic risk of hypertension owing to a common folate polymorphism? Curr. Dev. Nutr.4, nzz102 (2019). [DOI] [PMC free article] [PubMed]
- 126.Masenga, G. G., Shayo, B. C. & Rasch, V. Prevalence and risk factors for pelvic organ prolapse in Kilimanjaro, Tanzania: a population based study in Tanzanian rural community. PLoS ONE13, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elneil, S. Complex pelvic floor failure and associated problems. Best. Pr. Res. Clin. Gastroenterol.23, 555–573 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Schultz, K. J. et al. UVA-photoactivated riboflavin treatment of vaginal cells derived from pelvic organ prolapse cases. Gynecol. Obstet. Invest.77, 100–103 (2014). [DOI] [PubMed] [Google Scholar]
- 129.McMillan, K. S., Siddighi, S., Hardesty, J. S., Yune, J. J. & Chan, P. J. UVA-photoactivated riboflavin effect on isolated vaginal tissues derived from pelvic organ prolapse cases. Int Urol. Nephrol.47, 75–79 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Gogola, A. et al. Spatial patterns and age-related changes of the collagen crimp in the human cornea and sclera. Invest Ophthalmol. Vis. Sci.59, 2987–2998 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yyorimoto, Y. Age-related changes in the elastic properties and thickness of human facial skin. Brit. J. Dermatol.131, 641–648 (1994). [DOI] [PubMed]
- 132.Schuh, C. M. A. P. et al. Nanomechanical and molecular characterization of aging in dentinal collagen. J. Dent. Res.101, 840–847 (2022). [DOI] [PubMed] [Google Scholar]
- 133.Kang, Y., Kim, J. H., Kim, S. Y., Koh, W. G. & Lee, H. J. Blue light-activated riboflavin phosphate promotes collagen crosslinking to modify the properties of connective tissues. Materials14, 5788–5799 (2021). [DOI] [PMC free article] [PubMed]
- 134.Uemura, R. et al. UVA-activated riboflavin promotes collagen crosslinking to prevent root caries. Sci. Rep. 9, 1252–1263 (2019). [DOI] [PMC free article] [PubMed]
- 135.Wu, D. et al. Corneal cross-linking: the evolution of treatment for corneal diseases. Front. Pharmacol. 10.3389/fphar.2021.686630 (2021). [DOI] [PMC free article] [PubMed]
- 136.Ibusuki, S. et al. Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering. Tissue Eng.13, 1995–2001 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Brock, K. E. et al. Nutrients in diet and plasma and risk of in situ cervical cancer. J. Natl Cancer Inst. 80, 580–585 (1988). [DOI] [PubMed]
- 138.Hernandez, B. Y. et al. Diet and premalignant lesions of the cervix: evidence of a protective role for folate, riboflavin, thiamin, and vitamin B12. Cancer Causes Control14, 859–B870 (2003). [DOI] [PubMed] [Google Scholar]
- 139.Liu, T. et al. A case control study of nutritional factors and cervical dysplasia. Cancer Epidemiol., Biomark. Prev.2, 525–530 (1993). [PubMed] [Google Scholar]
- 140.Aili, A. et al. Association of the plasma and tissue riboflavin levels with C20orf54 expression in cervical lesions and its relationship to HPV16 infection. PLoS ONE8, e79937 (2013). [DOI] [PMC free article] [PubMed]
- 141.Prusty, B. K. & Das, B. C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer113, 951–960 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Rösl, F. et al. Antioxidant-induced changes of the AP-1 transcription complex are paralleled by a selective suppression of human papillomavirus transcription. J. Virol.71, 362–370 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeng, J. et al. Association between one-carbon metabolism-related vitamins and risk of breast cancer: a systematic review and meta-analysis of prospective studies. Clin. Breast Cancer20, e469–e480 (2020). [DOI] [PubMed] [Google Scholar]
- 144.Van de Roovaart, H., Stevens, M. & Goodridge, A. Safety and efficacy of vitamin B in cancer treatments: a systematic review. J. Oncol. Pharm. Pract.30, 451–463 (2024). [DOI] [PubMed] [Google Scholar]
- 145.Berginc, K. Pharmacokinetic Interactions between Drugs and Dietary Supplements: Carbohydrate, Protein, Vitamin and Mineral Supplements. Dietary Supplements: Safety, Efficacy and Quality (Woodhead Publishing Limited, 2015).
- 146.Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA108, 4680–4687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Drell, T. et al. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE8, (2013). [DOI] [PMC free article] [PubMed]
- 148.Lennard, K. et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect. Immun.86, e00410–17 (2018). [DOI] [PMC free article] [PubMed]
- 149.Mehta, S. D. et al. Host genetic factors associated with vaginal microbiome composition in Kenyan women. mSystems5, e00502-20 (2020). [DOI] [PMC free article] [PubMed]
- 150.Symul, L. et al. Sub-communities of the vaginal microbiota in pregnant and non-pregnant women. Proc. R. Soc. B: Biol. Sci.290, 20231461 (2023). [DOI] [PMC free article] [PubMed]
- 151.Gudnadottir, U. et al. The vaginal microbiome and the risk of preterm birth: a systematic review and network meta-analysis. Sci. Rep.12, 7926–7934 (2022). [DOI] [PMC free article] [PubMed]
- 152.van de Wijgert, J. & Verwijs, M. C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG127, 287–299 (2020). [DOI] [PubMed] [Google Scholar]
- 153.Norenhag, J. et al. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG127, 171–180 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Turnbaugh, P., Ley, R. & Mahowald, M. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature444, 1027–1031 (2006). [DOI] [PubMed]
- 155.Wang, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature490, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature498, 99–103 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Yoshii, K., Hosomi, K., Sawane, K. & Kunisawa, J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr.6, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haase, I., Mörtl, S., Köhler, P., Bacher, A. & Fischer, M. Biosynthesis of riboflavin in Archaea 6,7-dimethyl-8-ribityllumazine synthase of Methanococcus jannaschii. Eur. J. Biochem.270, 1025–1032 (2003). [DOI] [PubMed] [Google Scholar]
- 159.Kalingan, A. E. The kinetics of riboflavin secretion by Eremothecium ashbyii nrrl 1363. Bioprocess Eng.18, 445–449 (1998). [Google Scholar]
- 160.Averianova, L. A., Balabanova, L. A., Son, O. M., Podvolotskaya, A. B. & Tekutyeva, L. A. Production of vitamin B2 (Riboflavin) by microorganisms: an overview. Front. Bioeng. Biotechnol.8, 570828 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bacher, A., Eberhardt, S., Fischer, M., Kis, K. & Richter, G. Biosynthesis of vitamin B2 (riboflavin). Annu. Rev. Nutr.20, 153–167 (2000). [DOI] [PubMed] [Google Scholar]
- 162.Stahmann, K. P., Revuelta, J. L. & Seulberger, H. Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl. Microbiol. Biotechnol.53, 509–516 (2000). [DOI] [PubMed] [Google Scholar]
- 163.Rychen, G. et al. Safety of vitamin B2 (80%) as riboflavin produced by Bacillus subtilis KCCM-10445 for all animal species. EFSA J.16, e05223 (2018). [DOI] [PMC free article] [PubMed]
- 164.Burdock, G. A. & Carabin, I. G. Generally recognized as safe (GRAS): history and description. Toxicol. Lett.150, 3–18 (2004). [DOI] [PubMed] [Google Scholar]
- 165.Leuschner, R. G. K. et al. Qualified presumption of safety (QPS): a generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA). Trend. Food Sci. Technol. 21, 425–435 (2010).
- 166.Hill, C. et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol.11, 506–514 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Averianova, L. A., Balabanova, L. A., Son, O. M., Podvolotskaya, A. B. & Tekutyeva, L. A. Production of vitamin B2 (riboflavin) by microorganisms: an overview. Front. Bioeng. Biotechnol.10.3389/fbioe.2020.570828 (2020). [DOI] [PMC free article] [PubMed]
- 168.Guo, Q., Goldenberg, J. Z., Humphrey, C., El Dib, R. & Johnston, B. C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev.10.1002/14651858.cd004827.pub5 (2019). [DOI] [PMC free article] [PubMed]
- 169.Parma, M., Stella Vanni, V., Bertini, M. & Candiani, M. Probiotics in the prevention of recurrences of bacterial vaginosis. Alter. Ther. Health Med.20, 52–57 (2014). [PubMed] [Google Scholar]
- 170.Zhao, Y., Dong, B. R. & Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev.10.1002/14651858.CD006895.pub4 (2022). [DOI] [PMC free article] [PubMed]
- 171.Johnston, B. C. et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann. Intern. Med.157, 878–888 (2012). [DOI] [PubMed] [Google Scholar]
- 172.Abad, C. L. & Safdar, N. The role of Lactobacillus probiotics in the treatment or prevention of urogenital infections—a systematic review. J. Chemother.21, 243–252 (2009). [DOI] [PubMed] [Google Scholar]
- 173.Sharif, S., Meader, N., Oddie, S. J., Rojas-Reyes, M. X. & McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev.10.1002/14651858.CD005496.pub5 (2020). [DOI] [PMC free article] [PubMed]
- 174.He, Y. et al. Evaluation of the inhibitory effects of lactobacillus gasseri and lactobacillus crispatus on the adhesion of seven common lower genital tract infection-causing pathogens to vaginal epithelial cells. Front. Med.7, 284 (2020). [DOI] [PMC free article] [PubMed]
- 175.Menendez, A. et al. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal paneth cell α-defensins. J. Innate Immun.5, 39–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hao, H. et al. Effect of extracellular vesicles derived from Lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front. Immunol.12, 777147 (2021). [DOI] [PMC free article] [PubMed]
- 177.Happel, A. U. et al. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog.16, e1008559 (2020). [DOI] [PMC free article] [PubMed]
- 178.Lebeer, S., Vanderleyden, J. & De Keersmaecker, S. C. J. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev.72, 728–764 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hernández-Alcántara, A. M. et al. The Ability of Riboflavin-Overproducing Lactiplantibacillus plantarum Strains to Survive Under Gastrointestinal Conditions. Front. Microbiol. 11, 591945 (2020). [DOI] [PMC free article] [PubMed]
- 180.Mohedano, M. L. et al. Real-time detection of riboflavin production by Lactobacillus plantarum strains and tracking of their gastrointestinal survival and functionality in vitro and in vivo using mCherry labeling. Front. Microbiol.10, 1748 (2019). [DOI] [PMC free article] [PubMed]
- 181.Yépez, A. et al. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 77, 61–68 (2019). [DOI] [PubMed]
- 182.Kim, J. Y. et al. Probiotic potential of a novel vitamin B2-overproducing lactobacillus Plantarum strain, HY7715, isolated from Kimchi. Appl. Sci.11, 5765 (2021).
- 183.Thakur, K. & Tomar, S. K. Exploring indigenous Lactobacillus species from diverse niches for riboflavin production. J. Young Pharm.7, 126–131 (2015). [Google Scholar]
- 184.Burgess, C. M., Smid, E. J., Rutten, G. & van Sinderen, D. A general method for selection of riboflavin-overproducing food grade micro-organisms. Microb. Cell Fact5, 24 (2006). [DOI] [PMC free article] [PubMed]
- 185.Ripa, I. et al. A single change in the aptamer of the Lactiplantibacillus plantarum rib operon riboswitch severely impairs its regulatory activity and leads to a vitamin B2- overproducing phenotype. Micro. Biotechnol.15, 1253–1269 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Subramanian, V. S. et al. Differential expression of human riboflavin transporters -1, -2, and -3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim. Biophys. Acta Biomembr.1808, 3016–3021 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nel, I., Bertrand, L., Toubal, A. & Lehuen, A. MAIT cells, guardians of skin and mucosa? Mucosal Immunol.14, 803–814 (2021). [DOI] [PMC free article] [PubMed]
- 188.Hinks, T. S. C. et al. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep.28, 3249–3262.e5 (2019). [DOI] [PMC free article] [PubMed]
- 189.Wang, H. et al. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat. Commun.9, 3350 (2018). [DOI] [PMC free article] [PubMed]
- 190.Vorkas, C. K. et al. Mucosal-associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight3, e121899 (2018). [DOI] [PMC free article] [PubMed]
- 191.Oh, S. F., Jung, D.-J. & Choi, E. Gut microbiota-derived unconventional T cell ligands: contribution to host immune modulation. Immunohorizons6, 476–487 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Van Wilgenburg, B. et al. MAIT cells are activated during human viral infections. Nat. Commun.7, 11653 (2016). [DOI] [PMC free article] [PubMed]
- 193.Jouan, Y. et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J. Exp. Med.217, e20200872 (2020). [DOI] [PMC free article] [PubMed]
- 194.Parrot, T. et al. MAIT cell activation and dynamics associated with COVID-19 disease severity. Sci. Immunol. 5, eabe1670 (2020). [DOI] [PMC free article] [PubMed]
- 195.Kuri-Cervantes, L. et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 5, eabd7114 (2020). [DOI] [PMC free article] [PubMed]
- 196.Flament, H. et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol.22, 322–335 (2021). [DOI] [PubMed] [Google Scholar]
- 197.Godfrey, D. I., Koay, H. F. & McCluskey, J. The biology and functional importance of MAIT cells. Nat. Immunol.20, 1110–1128 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Klenerman, P., Hinks, T. S. C. & Ussher, J. E. Biological functions of MAIT cells in tissues. Mol. Immunol.130, 154–158 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.du Halgouet, A. et al. Role of MR1-driven signals and amphiregulin on the recruitment and repair function of MAIT cells during skin wound healing. Immunity56, 78–92.e6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science366, eaax6624 (2019). [DOI] [PMC free article] [PubMed]
- 201.Legoux, F. et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science366, 494–499 (2019). [DOI] [PubMed]
- 202.Ciobanu, L. et al. Effect of Lactobacillus plantarum ACTT 8014 on 5-fluorouracil induced intestinal mucositis in Wistar rats. Exp. Ther. Med.20, 1–1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Levit, R., Savoy de Giori, G., de Moreno de LeBlanc, A. & LeBlanc, J. G. Protective effect of the riboflavin-overproducing strain Lactobacillus plantarum CRL2130 on intestinal mucositis in mice. Nutrition54, 165–172 (2018). [DOI] [PubMed] [Google Scholar]
- 204.Perez Visñuk, D. et al. Neuroprotective effect of riboflavin producing lactic acid bacteria in parkinsonian models. Neurochem. Res.47, 1269–1279 (2022). [DOI] [PubMed] [Google Scholar]
- 205.Hardy, A. et al. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA J.15, e04970 (2017). [DOI] [PMC free article] [PubMed]
- 206.Sharma, V. et al. B-vitamin sharing promotes stability of gut microbial communities. Front. Microbiol. 10, 1485 (2019). [DOI] [PMC free article] [PubMed]
- 207.Soto-Martin, E. C. et al. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. mBio. 11, 1–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Buckel, W. & Thauer, R. K. Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chem. Rev.118, 3862–3886 (2018). [DOI] [PubMed] [Google Scholar]
- 209.Tejedor-Sanz, S. et al. Extracellular electron transfer increases fermentation in lactic acid bacteria via a hybrid metabolism. Elife11, e70684 (2022). [DOI] [PMC free article] [PubMed]
- 210.Wang, W. et al. Bacterial extracellular electron transfer occurs in mammalian gut. Anal. Chem.91, 12138–12141 (2019). [DOI] [PubMed] [Google Scholar]
- 211.Oña, L. & Kost, C. Cooperation increases robustness to ecological disturbance in microbial cross-feeding networks. Ecol. Lett.25, 1410–1420 (2022). [DOI] [PubMed] [Google Scholar]
- 212.Canon, F., Nidelet, T., Guédon, E., Thierry, A. & Gagnaire, V. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Front. Microbiol.11, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fassarella, M. et al. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut70, 595–605 (2021). [DOI] [PubMed] [Google Scholar]
- 214.Bourgonje, A. R. Microbial riboflavin biosynthesis associates with response to dietary fiber consumption: time to personalize adjunct therapy in patients with inflammatory bowel disease? Gastroenterology165, 515–516 (2023). [DOI] [PubMed]
- 215.Haggarty, P. et al. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet367, 1513–1519 (2006). [DOI] [PubMed]