Abstract
Background & Aims
Among the reprogrammed metabolic pathways described in cancer stem cells, aberrant lipid metabolism has recently drawn increasing attention. Our study explored the contribution of fatty acids (FA) in the regulation of stem-like features in intrahepatic cholangiocarcinoma (iCCA).
Methods
We previously identified a functional stem-like subset in human iCCA by using a three-dimensional sphere (SPH) model in comparison to parental cells grown as monolayers (MON). In this study, quantification of intracellular free FA and lipidomic analysis (triacylglycerol [TAG] composition, de novo synthesis products) was performed by Liquid chromatography–mass spectrometry (LC–MS); quadrupole time-of-flight liquid chromatography/mass spectrometry (Q-TOF LC/MS), respectively, in both SPH and MON cultures.
Results
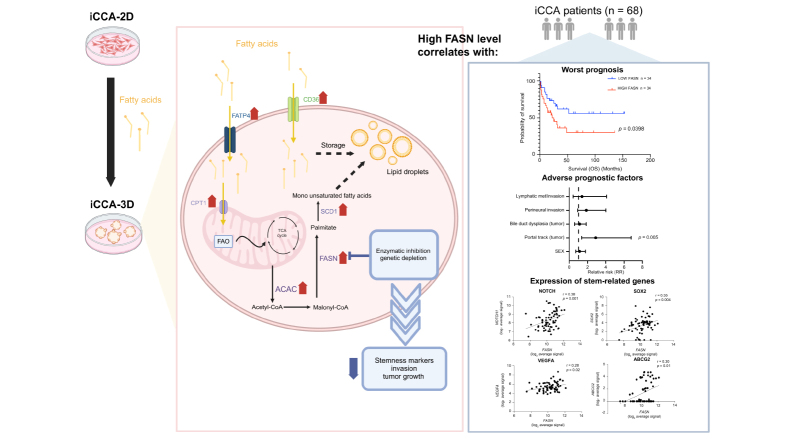
Stem-like SPH showed a superior content of free FA (citric, palmitic, stearic, and oleic acids) and unsaturated TAG. Molecularly, SPH showed upregulation of key metabolic enzymes involved in de novo FA biosynthesis (AceCS1, ACLY, ACAC, FASN, ACSL1) and the mTOR signalling pathway. In patients with iCCA (n = 68), tissue expression of FASN, a key gene involved in FA synthesis, correlated with 5-year overall survival. Interference with FASN activity in SPH cells through both specific gene silencing (siRNA) or pharmacological inhibition (orlistat) decreased sphere-forming ability and expression of stem-like markers. In a murine xenograft model obtained by injection of iCCA-SPH cells, FASN inhibition by orlistat or injection of FASN-silenced cells significantly reduced tumour growth and expression of stem-like genes.
Conclusion
Altered FA metabolism contributes to the maintenance of a stem-like phenotype in iCCA. FASN inhibition may represent a new approach to interfere with the progression of this deadly disease.
Impact and implications
Recent evidence indicates that metabolic disorders correlate with an increased susceptibility to intrahepatic cholangiocarcinoma (iCCA). Our investigation emphasises the pivotal involvement of lipid metabolism in the tumour stem cell biology of iCCA, facilitated by the upregulation of crucial enzymes and the mTOR signalling pathway. From a clinical perspective, this underscores the dual role of FASN as both a prognostic indicator and a therapeutic target, suggesting that FASN inhibitors could enhance patient outcomes by diminishing stemness and tumour aggressiveness. These findings pave the way for novel therapeutic strategies for iCCA and shed light on its relationship with metabolic disorders such as diabetes, obesity, metabolic syndrome, and metabolic dysfunction-associated steatotic liver disease.
Keywords: Hepatobiliary tumours, Cancer stem cells, Lipid metabolism, Oleic acid, Palmitoleic acid, triglycerides, Lipid droplets
Graphical abstract
Highlights
-
•
Stem cells in iCCA exhibit disrupted fatty acid metabolism and increased production of specific fatty acids.
-
•
Exposure of iCCA cells to fatty acids results in a notable increase in tumour stem cells.
-
•
FASN is upregulated in iCCA SPH and its expression correlates with poor prognosis in patients with CCA.
-
•
Depletion of FASN or its inhibition reduces the aggressive characteristics of the iCCA cancer stem cell subset.
Introduction
Stem cell programs in cancer initiation, progression, and therapy resistance are considered the centrepiece of tumour biology. The identification of cancer stem cells (CSC) in many solid tumours, including hepatic cancer,[1], [2], [3] has provided novel insights into the mechanisms of carcinogenesis. In cholangiocarcinoma (CCA), the presence of stem-like cells has been correlated with more aggressive tumour characteristics and poorer patient prognosis.4 Investigation on CSC has been favoured by the development of in vitro systems enriched in stem-like cells. In this regard, we and others have developed and adopted three-dimensional (3D) tumour sphere (SPH) formation as an efficient in vitro tool to enrich stem-like cells.[1], [2], [3]
Although studies on CSC biology have identified relevant signals sustaining tumour stemness, the exploration of metabolic reprogramming in the control of the stem state is in its infancy. Novel lines of evidence are shedding light on the dependence of CSC on lipid metabolism and particularly on fatty acids (FA).[5], [6], [7], [8] This emerging concept linking lipid metabolism and stem cell fate mostly derives from studies assigning a CSC-promoting function to the excess of monounsaturated FA.9,10
Recently, new experimental evidence, although divergent, has highlighted a role for lipid metabolism in the pathogenesis of CCA. In line with the histological and molecular heterogeneity of this neoplasm, highly proliferative CCA cells are strongly lipid-dependent, as shown by their upregulated lipid and lipoprotein uptake and catabolism. Conversely, more advanced stages of disease or CCAs associated with Clonorchis sinensis infection exhibit elevated expression of FASN. Furthermore, in lymph node metastases, CCA colonisation appears to be driven by PPARγ-regulated lipid metabolic reprogramming.[11], [12], [13], [14], [15]
Despite these recent lines of evidence, little is known about the role of lipids and lipid metabolism in iCCA-stemness. Here we show that altered FA metabolism is a distinctive feature of tumour stem-like cells in iCCA.
Materials and methods
Extraction of metabolites
For the monolayer (MON) cell culture, cells cultured in a six well-plate were quickly rinsed with NaCl 0.9% and quenched with 500 μl ice-cold 70:30 acetonitrile:water. Plates were placed at -80 °C for 10 min, then cells were collected by scraping and sonicated 5 s for five pulses at 70% power twice. For the SPH cell culture, 48 spheroids were collected from each well of a 96-well plate and centrifuged at 600 rpm for 5 min at 4 °C. Pellets were washed with 1 ml of NaCl 0.9%, centrifuged as above, and resuspended in 500 μl ice-cold 70:30 acetonitrile:water. Samples were placed at -80 °C for 10 min and then sonicated 5 s for five pulses at 70% power twice. At this point, for both MON and SPH experiments, samples were centrifuged at 12,000 × g for 10 min and supernatants were collected using a glass insert and dried in a centrifugal vacuum concentrator (Concentrator plus/Vacufuge plus, Eppendorf) at 30 °C for about 2.5 h. Samples were then resuspended with 150 μl H2O prior to analyses.
Liquid chromatography-mass spectrometry analysis
Liquid chromatography (LC) separation was performed using an Agilent 1290 Infinity UHPLC system and an InfintyLab Poroshell 120 PFP column (2.1 × 100 mm, 2.7 μm; Agilent Technologies). Mobile phase A was water with 0.1% formic acid. Mobile phase B was acetonitrile with 0.1% formic acid. The injection volume was 10 μl and LC gradient conditions were: 0 min: 100% A; 2 min: 100% A; 4 min: 99% A; 10 min: 98% A; 11 min: 70% A; 15 min: 70% A; and 16 min: 100% A with 2 min of post-run. The flow rate was 0.2 ml/min and the column temperature was 35 °C. MS detection was performed using an Agilent 6550 iFunnel Q-TOF mass spectrometer with Dual JetStream source operating in negative ionisation mode. MS parameters were: gas temperature: 285 °C; gas flow: 14 L/min; nebuliser pressure: 45 psig; sheath gas temperature: 330 °C; sheath gas flow: 12 L/min; VCap: 3,700 V; Fragmentor: 175 V; Skimmer: 65 V; and Octopole RF: 750 V. Active reference mass correction was done through a second nebuliser using masses with m/z: 112.9855 and 1,033.9881. Data were acquired from m/z 60–1,050. Data analysis and isotopic natural abundance correction were performed using MassHunter Profinder (version 10.0.2). Data preprocessing was performed using the Batch Targeted Feature Extraction algorithm and Agile 2 algorithm. This software assigned identities to metabolites by searching against an in-house compound database built with Agilent PCDL Manager (version B.08.00) based on the metabolite formula and its corresponding retention time with a score >75. Peak areas obtained were normalised for protein content for each sample. LC-MS analysis was performed by the Metabolomics Unit of JRU ISBE-SYSBIO Center and Elixir European Infrastructure in Milan (Milan, Italy).
In vivo experiments
Animal experiments were performed in accordance with national guidelines and approved by the ethical committee of the Animal Welfare Office of Italian Health Ministry. All procedures conformed to the legal mandates and the Italian guidelines for the care and maintenance of laboratory animals. All animals received humane care, and the study protocols complied with the institutional guidelines. Studies involving animal experiments conformed to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (http://www.nc3rs.org.uk/arriveguidelines), developed by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) to improve the standards and reporting of animal research. Male 6-week-old NOD/SCID mice (n = 6 per group) (Charles River Laboratories International, Wilmington, Massachusetts, MA, US) were subcutaneous injected with 3 × 106 CCLP1 SPH cells. When tumours became palpable, mice were randomly divided into two experimental groups to receive intraperitoneal injection of solution (10% DMSO, 40% PEG300, 5% TWEEN80, and 45% saline) or 240 mg/kg orlistat (three times a week). Animals were monitored daily. In parallel, we performed subcutaneous xenografts using injected CCLP1 cells (3 × 106) silenced for FASN (SPH-T shFASN) expression or cells transfected with shCTR (SPH-T shCTR) (male 6-week-old NOD/SCID mice (n = 6 per group) (Charles River Laboratories International, Wilmington, Massachusetts, MA, US).
Statistical analysis
Statistical tests were performed using GraphPad Prism v.9 software, Boston, Massachusetts, MA, US and R version 4.1.0. software Indianapolis, Indiana, IN, US. For each experiment, statistical details can be found in the figure legends, including the statistical tests applied and the sample sizes. All in vitro experiments were confirmed by independent biological replicates. Data is represented as mean ± SD or ± SEM. Group comparison was performed using the nonparametric Mann–Whitney U test. Clinical data were examined using Fisher’s exact test. The Kaplan–Meier method was employed to determine survival rates, with significance assessed using the Log-rank test. Pearson’s correlation was utilised to assess the relationship between gene expression.
Detailed information is provided in the Supporting Information and in the Supplementary CTAT Table.
Results
Fatty acids enhance the stemness features of iCCA cells
To assess the relevance of FA in the biology of iCCA stem cells, CCLP1 and HUCCT1 cells were first exposed to increasing concentrations of unsaturated (oleic acid [OA], palmitoleic acid (POA], linoleic acid) or saturated (palmitic acid) FA for different time periods (Figs. S1 and S2, Tables S1 and S2). In line with previous studies,14 all tested FA promoted cell growth (Figs. S1 and S2, Tables S1 and S2). Thus, based on the highest proliferative effect (Figs. S1 and S3), we focused our study on the role of OA and POA in the modulation of iCCA stemness.
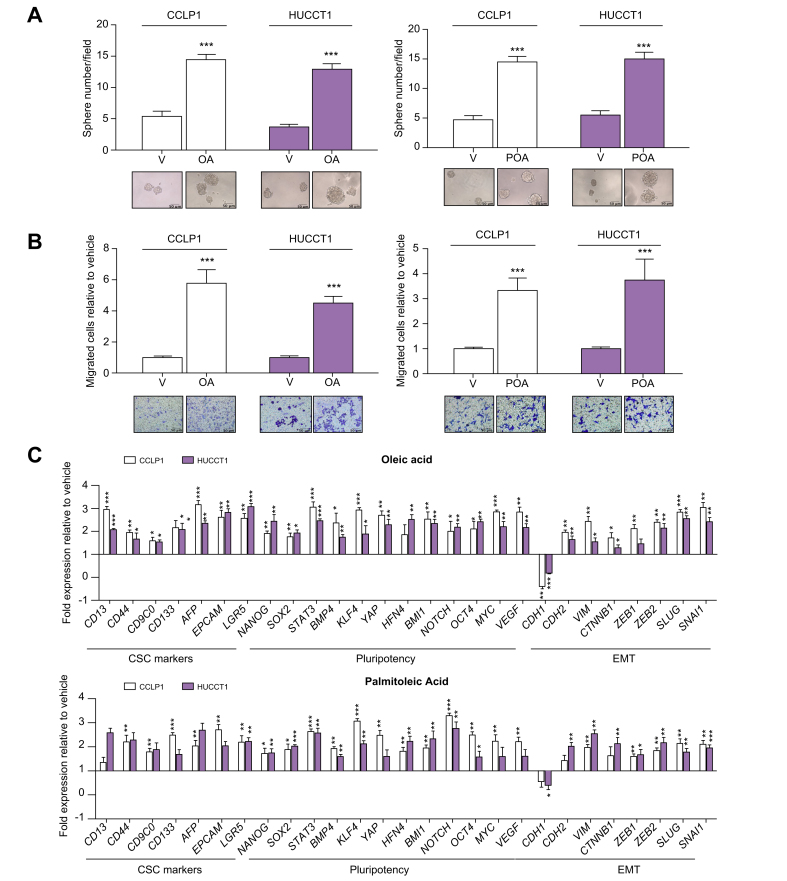
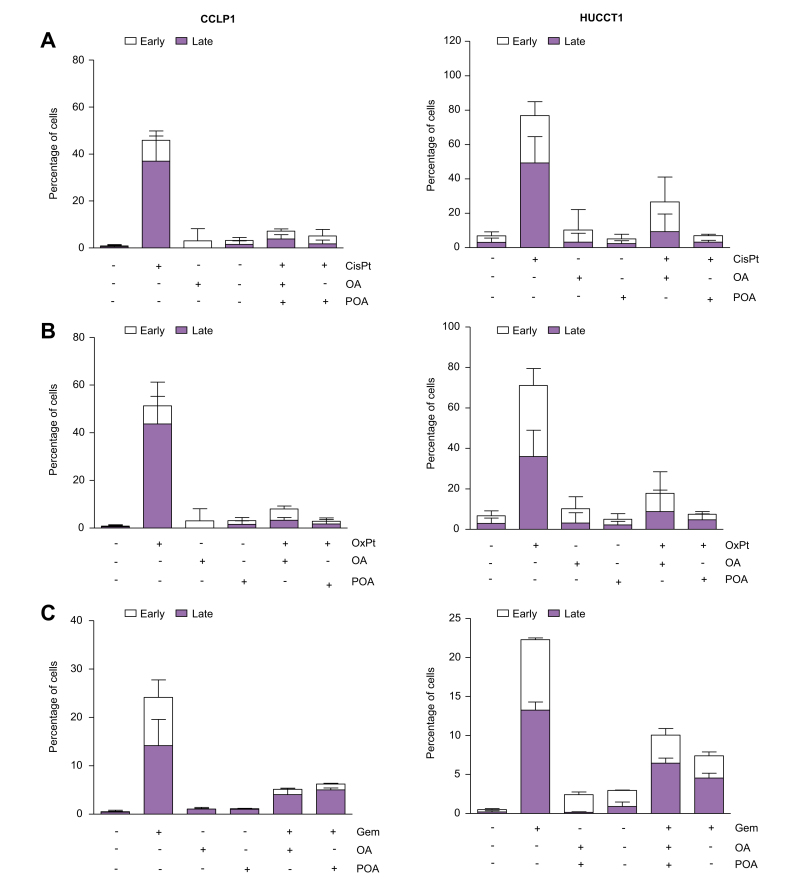
Since CSC are key players in tumorigenesis including tumour initiation and metastasis, we next evaluated whether FA are able to modulate these aspects in vitro by reprogramming parental cells grown in MON cultures. Indeed, treatment of MON cells (HUCCT1 or CCLP1) with OA or POA markedly enhanced their sphere-forming ability (i.e. sphere number, volume, and growth over time) as an indication of self-renewal potential (Fig. 1A, Fig. S4A and B), significantly increased aldehyde dehydrogenase activity, serving as a functional marker of tumour stemness (Fig. S4C) and the notably improved the capacity to invade a basement-like membrane (Fig. 1B, Fig. S5). Accordingly, the expression of multiple genes implicated in the maintenance of stemness, self-renewal, and regulation of epithelial-to-mesenchymal transition (EMT) were modulated in a pro-malignant fashion (Fig. 1C), thus further supporting the in vitro functional importance of FA in acquiring a stem-like traits. Conversely, due to their drug-resistance, cancer stem cells can escape cytotoxicity and survive chemotherapy and radiotherapy. Therefore, we assessed the influence of monounsaturated FA on iCCA resistance to chemotherapeutic agents. Pre-treatment with OA or POA before exposure to cisplatin, oxaliplatin, or gemcitabine, commonly used drugs for iCCA therapy,1 led to a significant rescue of cell viability under pharmacological treatment as shown by analysis of apoptosis (Fig. 2, Fig. S6, Table S3).
Fig. 1.
Effects of monounsaturated FAs on stem-like proprieties in iCCA cells.
(A) iCCA cells were grown as spheres for 7 days, in presence or absence of FAs. Then SPH were counted, and their volume was measured. Mean ± SEM (n = 3, ∗∗∗p ≤0.001). Representative images of iCCA SPH are reported below the graphs (original magnification 40×). (B) Migration of iCCA cells was measured in modified Boyden chambers, after 48 h treatment. Mean ± SEM (n = 3, ∗∗∗p ≤0.001). Representative images of the filters are shown below the barograms (original magnification 40×, scale bar 10 μM). (C) Expression of different genes involved in EMT, drug-resistance and stem-like acquisition in CCLP1 and HUCCT1 cells were treated with OA or POA for 48 h, reported as fold changes normalised to mean expression of vehicle-treated cells. Mean ± SEM (n = 3, ∗p ≤0.05, ∗∗p ≤0.01, ∗∗∗p ≤0.001; Mann–Whitney U test). EMT, epithelial to mesenchymal transition; FA, fatty acids; iCCA, intrahepatic cholangiocarcinoma; SPH, sphere cultures; OA, oleaic acid; POA, palmitoleic acid; V, vehicle.
Fig. 2.
Monounsaturated FAs pretreatment protects iCCA cells from antiblastic toxic effects.
CCLP1 and HUCCT1 cells were pretreated for 24 h with oleic acid (OA) or palmitoleic acid (POA) in starvation medium, then with (A) cisplatin, (B) oxaliplatin, or (C) gemcitabine for a further 24 h. Apoptosis was measured with Annexin V/PI staining. Data are presented as mean ± SEM (n = 3). Complete statistical data are shown in Table S3; FA, fatty acids; iCCA, intrahepatic cholangiocarcinoma; OA, oleic acid; POA, palmitoleic acid; CisPt, cisplatin; OxPt, oxaliplatin; Gem, gemcitabine.
The fatty acid metabolic machinery was enhanced in CCA stem-like cells
Based on the observed enrichment of stem cell properties in vitro in MON cells treated with exogenous OA or POA, we further investigated the metabolic apparatus of FA in the SPH culture system. This system functions as a model of tumour stemness in CCA, as previously described by our group1 and confirmed at the molecular level by RNA-sequencing profiling of SPH cells (Fig. S7, Table S4). Utilising Enrichr analysis, we observed a significant enrichment of stem-related molecules among the differentially expressed genes in SPH compared to MON in both cell lines. The acquisition of stemness was further supported by the predominant activation of stem cell signalling pathways, including PI3K/Akt-, Hippo-, and TGF-beta-dependent pathways. Additionally, there was an upregulation of significant networks associated with the EMT process, TNF-alpha signalling via NF-kB, the p53 pathway, as well as factors related to pluripotency, stemness, and EMT, such as POU5F1, STAT3, EZH2, ZEB1, MYC, SOX2, and ZEB2 genes (Fig. S7, Table S4).
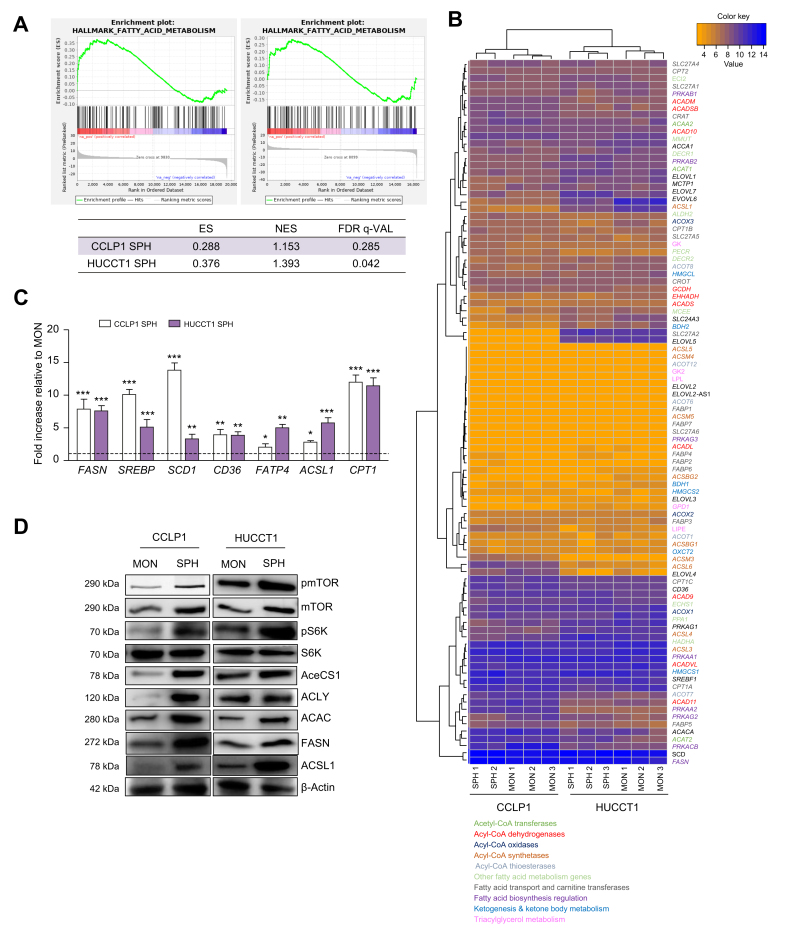
Gene set enrichment analysis (GSEA) of SPH RNA sequencing data indicated a marked increase in the expression of several genes participating in FA metabolism, including both anabolic and catabolic pathways (Fig. 3A). Accordingly, an unsupervised clustering heatmap indicated a primary divergence in the expression pattern of differentially expressed genes involved in FA metabolism comparing SPH with MON culture systems for both cell lines (Fig. 3B). Furthermore, overexpression of key enzymes involved in FA synthesis (FASN, SREB, ACSL1, SCD1), desaturation (SCD1), and exogenous uptake (CD36, FATP4) was further confirmed at the mRNA level in SPH cells compared to MON cells (Fig. 3C). Of note, protein members of the mTOR signalling pathway and key metabolic enzymes involved in de novo fatty acid biosynthesis (cytoplasmic acetyl-CoA synthetase [AceCS1], ATP citrate lyase [ACLY], Acetyl-CoA carboxylase [ACAC] the rate-limiting step in FA synthesis, FASN, Acyl-CoA synthetase long chain family member 1 (ACSL1) were upregulated in SPH with respect to MON cultures of both CCLP1 and HUCCT1 cells lines, as shown by Western blotting analysis and the relative densitometric analysis (Fig. 3D, Fig. S8). Notably, it is well known that mTOR stimulates de novo lipogenesis via a SREB-dependent pathway through S6K phosphorylation.16
Fig. 3.
Molecular aspects behind altered FA pathways in iCCA stem-like cells.
(A) Results of a GSEA Pre-ranked analysis on the fatty acids metabolism gene set of the Hallmark MSigDB collection. (B) Heatmap plot of differentially expressed genes of the genes involved in fatty acid metabolism in SPH and MON, using Euclidean distance as similarity metrics and complete linkage as linkage method. Modified Z-scores of the individual genes, as median-centred log2 intensity values divided by standard deviation, are shown by a blue-to-red gradient variation. Different colours specified different FA metabolic pathways. (C) CCLP1 and HUCCT1 cells were grown as MON) or as SPH, then RNA was extracted. Expression of different genes involved in FA metabolism is reported as fold changes normalised to mean expression of MON. Mean ± SEM (n = 3, ∗p ≤0.05, ∗∗ p ≤0.01, ∗∗∗ p ≤0.001; Mann–Whitney U test). (D) Representative immunoblot of mTOR, phospho-mTOR, S6K, phosphoS6K, AceCS1, ACLY, ACAC, FASN, ACSL1 protein levels in CCLP1 and HUCCT1 cells grown as MON and SPH. β-Actin immunoblot was performed to ensure equal loading. ES, enrichment score; FA, Fatty Acids; FDR, false discovery rate; GSEA, gene set enrichment analysis; MFI, mean fluorescent intensity; NES, normalised enrichment score; iCCA, intrahepatic cholangiocarcinoma; MON, monolayer; SPH, sphere cultures.
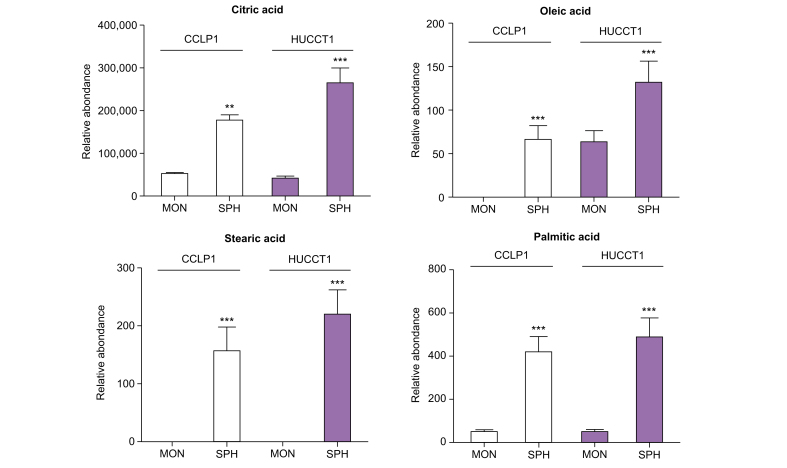
Based on this evidence, the content of selected intracellular free FAs in iCCA stem-like cells was quantified by LC-MS. Notably, an abundance of citric acid, a precursor of FA as well as POA, stearic acid, and OA was identified in SPH cells compared to the respective MON in both cell lines (Fig. 4) in accordance with molecular data (Fig. 3) showing differences in the expression of key enzymes of FA biosynthesis. Coherently, BODIPY staining showed that iCCA-SPH had a higher amount of lipid droplets, which are dynamic organelles constituted mainly by triglycerides containing FA with different degrees of saturation or size (i.e. number of carbons due to elongation) (Fig. 5A and B).
Fig. 4.
Content of free FA in iCCA stem-like compartment.
Relative metabolite abundance of intracellular free citric, stearic, oleic, and palmitic acid in CCLP1 or HUCCT1 cell lines, grown in MON or as SPH, identified by LC-MS analysis. Peak areas obtained were normalised for protein content for each sample. Mean ± SEM (n = 9, ∗∗p ≤0.01, ∗∗∗p ≤0.001; Mann–Whitney U test). FA, fatty acids; iCCA, intrahepatic cholangiocarcinoma; MON, monolayer; SPH, sphere cultures.
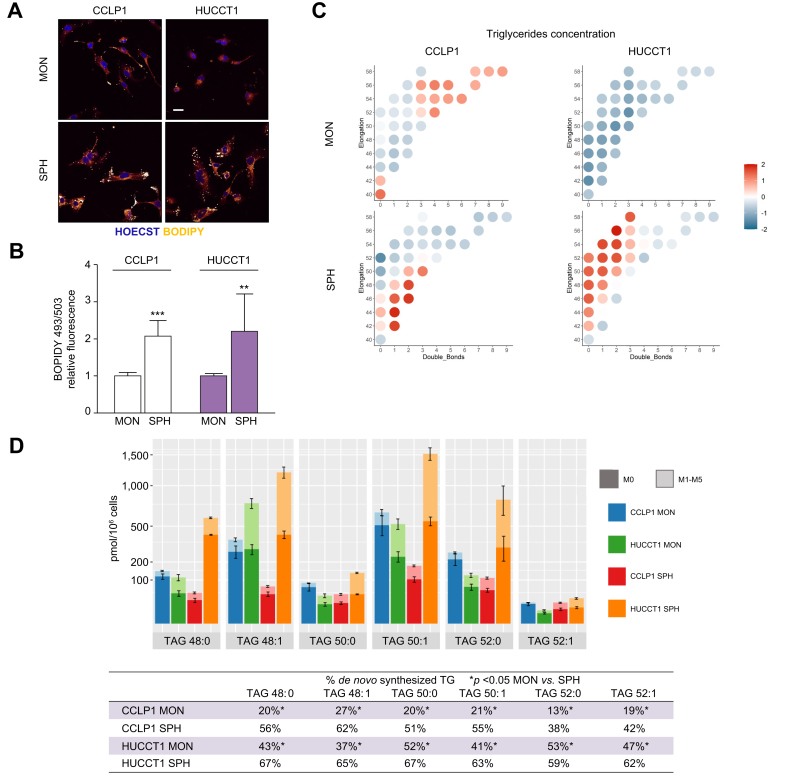
Fig. 5.
Triglyceride composition in iCCA cells grown as monolayers or spheres.
(A) Intrahepatic CCA cells were grown as MON or SPH, then cells were stained with Bodipy 493/503. Confocal microscopy analysis of lipid droplets in MON and SPH. (light yellow: Bodipy 493/503; blue: Hoechst; scale bar = 5 μm). (B) Lipid droplets quantification by cytofluorimetric analysis. Histograms represent the MFI of the Bodipy probe normalised to mean MFI of MON. Results are mean ± SEM (n = 3, ∗∗p ≤0.01, ∗∗∗p ≤0.001; Mann–Whitney U test). (C) Representation of triglycerides concentration in CCLP1 and HUCCT1 grown as MON or SPH, respectively. Single triglycerides were identified according to their degree of saturation (x axis) i.e., the number of double bonds, and elongation number (y axis) i.e., the number of carbons. Triglycerides concentrations were scaled to zero mean and unit variance and reported as median for each cell line grown as MON or SPH. (D) Concentration of the main saturated TAG and their desaturated counterparts in CCLP1 MON (blue), SPH (green), HUCCT1 MON (red), SPH (orange) cultured cells, respectively. The measured concentrations are composed by two parts: the de novo synthesised component, characterised by deuterium incorporation levels ranging from 1 to 5 (M1-M5) and displayed in lighter shades, and the pre-existing component that was not newly synthesised i.e., without any incorporation of deuterium (M0), and is depicted in darker tones. In the table, molar percents of de novo synthesised TAG, measured as deuterium enrichment after D2O incubation, were reported. ∗p <0.05; non-parametric test of the Mann Whitney ranks. FA, fatty acids; iCCA, intrahepatic cholangiocarcinoma; MON, monolayer; SPH, sphere cultures; TAG, triglycerides; MFI, Mean Fluorescence Intensity.
Therefore, an untargeted lipidomic analysis was performed using high-resolution MS and targeted quantification of the most relevant lipids. By comparing the amount and composition of triglycerides in SPH cultures and the respective parental cells grown as monolayers, we observed that iCCA-SPH were characterised by a higher TAG composition including saturated and monounsaturated fatty acids (i.e. TAG with 1–2 double bonds) with respect to MON (data not shown). This was particularly evident in HUCCT1 cells, although both SPH cultures contained TAG of lower length than MON cultures (i.e. TAG with less than 52 carbons). Incubation with deuterated water was used to quantify the contribution of FA from de novo lipogenesis (DNL) of single triglycerides species. Palmitic acid (C16:0) is the main product of DNL and can either be desaturated to POA (C16:1) or elongated to stearic acid (C18:0) that in turn can be desaturated to OA (C18:1). The iCCA-SPH culture exhibited a larger percentage of de novo synthesised triglycerides (Fig. 5C), as assessed through the measurement of incorporation of deuterium. Within TAGs with lower length, SPH contained more TAG with monounsaturated triglycerides than MON, i.e. TAGs with POA and OA FA (48:1, 50:1, 52:1, than TAG incorporating only saturated FA like palmitic and stearic acid, (48:0, 50:0, 52:0), as shown in Fig. 5C-D. Accordingly, the desaturation index, i.e. the ratio between triglycerides with one double bond in the FA chain and triglycerides without double bonds, indicated a larger amount of monounsaturated FA in SPH (Fig. S9).
We next evaluated the pathways responsible for FA metabolism. SPH cells were characterised by elevated activity of intracellular FA beta-oxidation (FAO) compared to MON cultures (Fig. S10). This observation was supported by the increased content in lipid droplets evident in CCA-SPH cultures (Fig. 5A and B). Collectively, these findings identify a distinct alteration in mitochondrial function within SPH compared to MON cultured cells, corroborated by a previous study from our group.2 To provide a more comprehensive perspective, we explored the classification of patients with CCA based on stemness and FA metabolic pathways using publicly available datasets (GSE32879 and GSE89749). Gene expression profiles for GSE89749 and GSE32879 were obtained from the Gene Expression Omnibus. A curated list of stemness and chemoresistance genes, as well as Gene Ontology FAO and KEGG Biosynthesis of unsaturated fatty acids (DNL) terms were used in the analysis. Within each dataset, samples underwent z-scoring and hierarchical clustering based on the expression of stemness genes. Next, GSEA was used to establish the association of clusters with chemoresistance, DNL, and FAO. Hierarchical clustering allowed a clear definition of two clusters (A and B), based on the expression of 139 resistance and stemness genes (resistance&stemness signature). In each dataset, Cluster B was significantly enriched in the expression of stemness and resistance genes that was validated with GSEA (Figs. S11 and S12). In both GSE32879 and GSE89749 datasets, Cluster B was enriched in both FAO and DNL, suggesting positive association of stemness and FAO and stemness and DNL. Taken together, these data suggest that the stem-like subset has an amplified machinery for FA metabolism in iCCA.
Higher expression of FASN was associated with a more aggressive course in patients with iCCA
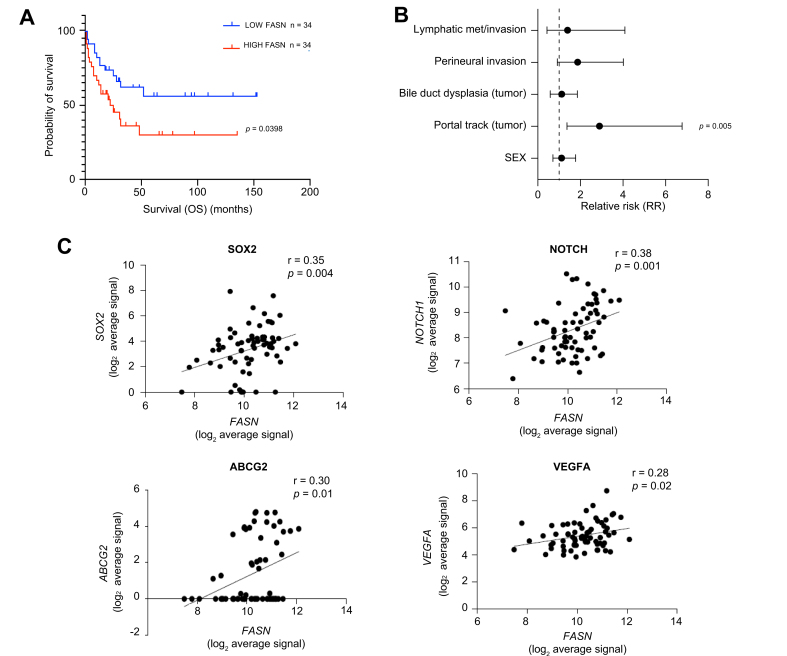
To explore the possible relevance of our in vitro data with the clinical setting, we analysed transcriptomic data from a published cohort of patients with iCCA to search for possible correlations between genes implicated in FA metabolism and clinical outcomes.17 In particular, we focused our attention on the FASN gene, the master regulator of FA synthesis, which was upregulated in SPH compared to MON iCCA cultured cells. Clustering data based on FASN expression, Kaplan–Meyer curves demonstrated that higher FASN was strongly associated with significantly lower survival (Fig. 6A), in accordance with recently published data.13 Notably FASN levels correlated with available clinical pathological parameters (Table S5) in particular with portal trunk invasion, a significant adverse prognostic factor in iCCA (Fig. 6B). In addition, FASN expression significantly correlated with a number of stemness-related genes including SOX2, NOTCH1 and ABCG2 in the same iCCA dataset (n = 68) (Fig. 6C).
Fig. 6.
Expression of Fatty acid synthase is associated with poor survival in iCCA patients.
(A) Kaplan–Meyer plot showing overall survival in iCCA patients (n = 68) stratified according to good or bad prognosis. (B) Univariate association of FASN expression with clinical pathological parameters. (C) Scatterplot representing the correlation between FASN and SOX2, NOTCH, ABCG2 and VEGF expression in a public dataset.17 FASN, Fatty acid synthase; iCCA, intrahepatic cholangiocarcinoma; OS, overall survival.
FASN promotes the growth of experimental iCCA and confers stem cell features to the tumour
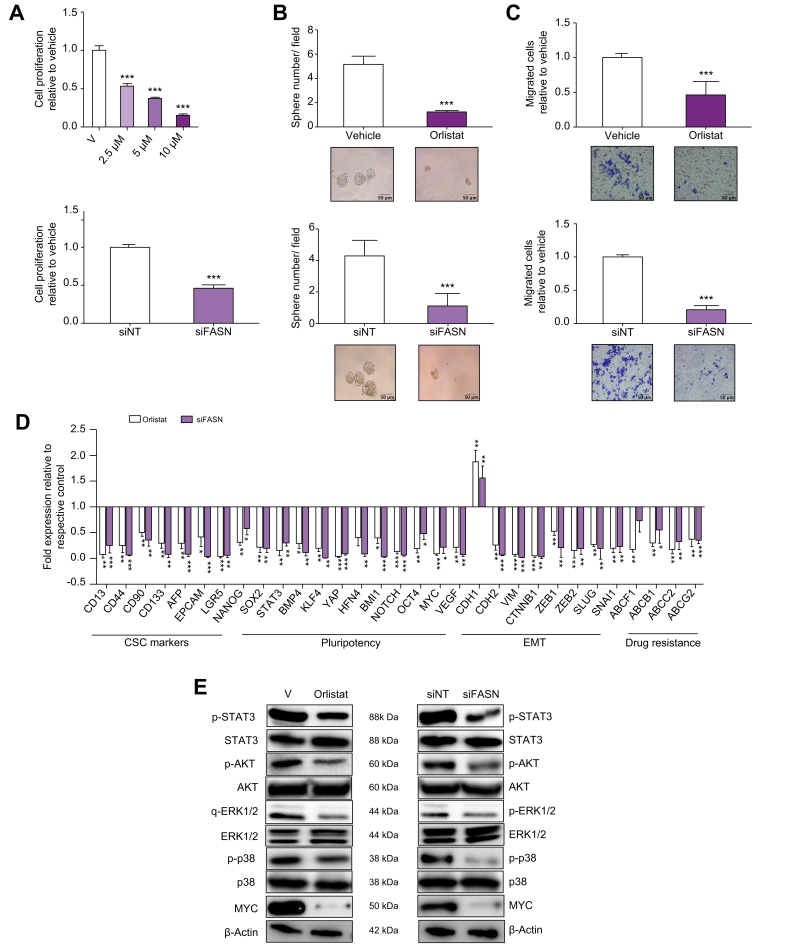
Based on the results of transcriptomic analysis in patients, we next investigated the effect of genetic (siRNA) or pharmacological inhibition of FASN in SPH cells. Drug inhibition was performed using orlistat, an anti-obesity drug that irreversibly inhibits the enzymatic activity of FASN.[18], [19], [20], [21], [22] Both specific genetic depletion (siRNA) (Fig. S13) or enzymatic inhibition (orlistat) of FASN in SPH cells significantly reduced in vitro functionally properties such as proliferation, sphere-forming ability, invasion, as well drug-resistance.13 (Fig. 7A–C; Figs. S13 and 14). At the molecular level, FASN reduction impaired the expression of several key genes implicated in pluripotency (i.e. NANOG, SOX2, KLF4, OCT4, BMI1), drug resistance (ABC transporters) and EMT (CHD2, VIM, ZEB1, ZEB2, SNAI1), and impact the phosphorylation of pro-survival proteins (i.e. AKT, p38 and ERK1/2), as well as expression of MYC oncoprotein (Fig. 7D and E) (Tables S6, S7).
Fig. 7.
Effects of FASN depletion in CCLP1 SPH.
(A) Effect of FASN depletion on proliferation by BrdU incorporation 48 h after orlistat treatment (upper panel) or gene silencing (lower panel). Mean ± SEM (n = 3, ∗∗∗p ≤0.001). (B) Effects of orlistat (upper panel) or FASN gene silencing (lower panel) on iCCA sphere forming efficiency. Mean ± SEM (n = 3, ∗∗∗p ≤0.001; Mann–Whitney U test). Representative images of iCCA SPH are shown below the graphs (original magnification 40×). (C) Migration of orlistat treated SPH (upper panel) or FASN silenced SPH (lower panel) was measured in modified Boyden chambers. The number of migrated cells is represented as fold increase with respect to vehicle. Mean ± SEM (n = 3, ∗∗∗p ≤0.001; Mann–Whitney U test). Representative images of filters are shown below the barograms (original magnification 40×). (D) Expression of different genes involved in CSCs pathways, EMT and drug resistance expressed as fold changes normalised to mean expression of the respective vehicle. Mean ± SEM (n = 3, ∗p ≤0.05, ∗∗ p ≤0.01, ∗∗∗ p ≤0.001; Mann–Whitney U test). (E) Immunoblot of several proteins and phosphoproteins involved in cell proliferation and survival, following orlistat treatment or FASN silencing. β-Actin immunoblot was performed to ensure equal loading. EMT, epithelial to mesenchymal transition; FASN, Fatty acid synthase; iCCA, intrahepatic cholangiocarcinoma; V, vehicle; CSC, cancer stem cells.
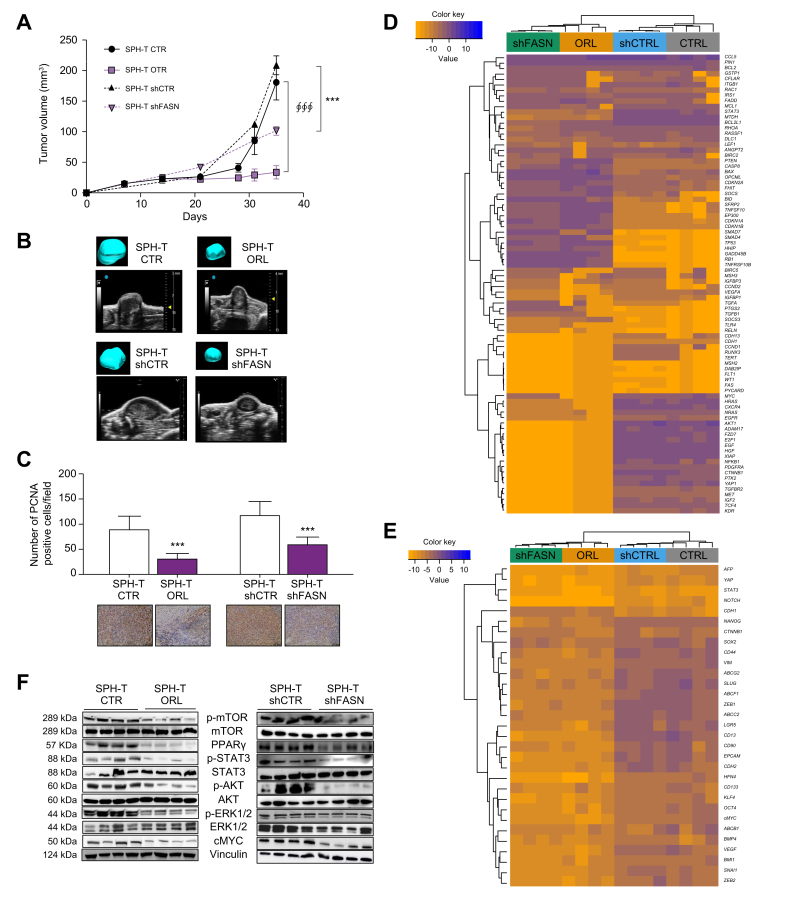
Based on the strong in vitro data indicating the involvement of FASN in the features of iCCA stem compartment relevant for tumour progression, we evaluated the possible impact of the inhibition of this protein in an in vivo model of CCA. To this aim, the effect of both orlistat pharmacological treatment and genetic FASN inhibition (shRNA) was investigated in a mouse xenograft model in which dissociated CCLP1 SPH cells were injected subcutaneously into the flank(s) of NOD/SCID mice.2
After confirming that treatment with orlistat at a dose of 240 mg/kg/day was not toxic (Fig. S15A) and in accordance with previously reported studies,18,21,22 mice were randomised once the tumour was palpable and treated with orlistat via i.p. injection (SPH-T ORL) or vehicle (SPH-T CTR) for 4 weeks. In parallel, we performed subcutaneous xenografts using CCLP1 cells silenced for FASN (SPH-T shFASN), or cells transfected with shCTR (SPH-T shCTR) after confirming FASN depletion at the protein level (Fig. S15B). Tumour growth was monitored with a dedicated in vivo imaging system. Tumour volume and weight in the ORL-treated and shFASN groups were significantly reduced compared with SPH-T CTR and SPH-T shCTR, respectively (Fig. 8A and B, Fig. S15C), while no differences in liver/body weight or in lung/body weight ratios were observed (Fig. S15D and E). At the end of treatment, in vivo cell proliferation was evaluated by PCNA staining (Fig. 8C). Both SPH-T ORL and SPH-T shFASN showed a lower number of cycling cells, in agreement with the observed impact on tumour progression. Moreover, comparison of the molecular characteristics of the xenografts (Fig. 8D and E) revealed in both SPH-T ORL and SPH-T shFASN tissues a downregulation of genes involved in proliferation (i.e. STAT3, MTDH, BCL2L1, XIAP, AKT, EGF, EGFR, E2F1, HGF, HRAS, IGF2, NRAS, PDGFRA, FZD7, PTK2, c-MYC), invasion (i.e. ZEB1, SLUG, N-cadherin, beta-catenin, ADAM17, CXCR4, FDZ7, MET, PTK2), drug-resistance (ABCG2, ABCF1, ABCC2) and pluripotency (NANOG, NOTCH, HNF4, KLF4), cancer stem cell markers (CD44, CD13, EpCAM) together with upregulation of tumour-suppressor (i.e. OPCML, TP53, HHIP, SMAD4, RB1, GADD45B, PTEN, SFRP2, SOCS3) and proapoptotic genes (i.e. FADD, CASP8, BAX,BUD, TNFRSF10B) (Fig. 8E).
Fig. 8.
Effects of orlistat treatment in iCCA xenografts mouse model.
(A) Analysis of tumour volume by Vevo LAZR-X photoacoustic imaging. Tumours (SPH-T) were obtained by subcutaneous injection of SPH cells in NOD/SCID mice. Mice were treated with vehicle (SPH-T CTR) or orlistat (240 mg/kg/day) (SPH-T ORL) (n = 6 per group) or injected with FASN silenced SPH cells (SPH-T shFASN) or cells transfected with shCTR (SPH-T shCTR) (∮∮∮p ≤0.001 SPH-T ORL vs. SPH-T CTR; SPH-T shFASN vs. SPH-T shCTR ∗∗∗p ≤0.001; Mann–Whitney U test). (B) Ultrasound images of representative subcutaneous tumour masses derived from injection of CCLP1 SPH generated tumours in different conditions. Three-dimensional rendering of the tumour mass is shown in the inset. (C) PCNA and haematoxylin eosin co-staining by immunohistochemical analysis. Representative staining is shown below the histogram. Ki67 positive nuclei are in brown colour (∗∗∗p ≤0.001; Mann–Whitney U test). (D and E) Heatmap of different tumour samples based on qRT-PCR of arrays of genes focused on liver cancer pathways (84 genes) or cancer stem-like markers (31 genes). Gene expression levels are expressed as log2 transformed values in colour code from blue (low) to orange (high) according to the colour key scale bar. Hierarchical clustering was based on complete linkage on Euclidean distances between genes (rows) or samples (columns). (F) Immunoblot of several proteins involved in proliferation and survival in tumour samples. Vinculin immunoblot was performed to ensure equal loading. FASN, Fatty acid synthase; iCCA, intrahepatic cholangiocarcinoma; SPH-T, tumors obtained by subcutaneous injection of SPH cells; CTR, control; ORL, orlistat; SPH-T shFASN, tumor derived by FASN silenced SPH cells; SPH-T shCTR, tumor derived by control silenced SPH cells.
Accordingly, at the protein level, FASN inhibition and depletion were associated with a lower activation/expression of proteins involved in proliferation or survival (i.e. STAT3, AKT, ERK1/2, c-MYC) and anabolic pathways (mTOR and PPARɣ) (Fig. 8F), confirming the key role of FASN and FA metabolism in tumour growth.
Discussion
Metabolic rewiring is a new hallmark of cancer, and in recent years has changed the view of tumour biology. Among several tumour metabolic alterations, lipid metabolism is receiving increasing attention, and several studies have demonstrated its central role in the process of carcinogenesis.23 As major components of lipids, FA provide energy, membrane building blocks, signalling molecules and post-translational modifications of proteins that are determinants for cancer cell survival.
The present study provides several lines of evidence supporting the role of increased FA metabolism in conferring a stem-like phenotype in iCCA. Notably, exposure to monounsaturated FA increased stem-like features at both functional (resistance to antineoplastic drugs, spherogenicity) and molecular (expression of stemness-associated genes) levels. Taking advantage of a 3D SPH culture system as a model for cancer stem cells1,2 and comparing these cultures with more differentiated parental cells grown in monolayers, we provide compelling evidence for the importance of FA in the regulation of the stemness features of iCCA. Several key enzymes involved in FA synthesis and transport were significantly overexpressed in the iCCA stem-compartment, indicating that a CSC phenotype is strictly associated with altered FA metabolism. Additionally, lipidomic analysis demonstrated that iCCA-SPH cultured cells exhibit higher de novo synthesis and desaturated FA that are then esterified to triglycerides, which then results in enrichment of monosaturated FA. This finding may suggest that de novo synthesis and desaturation of FA may have an essential role in fuelling the activation of pluripotency and self-renewal associated signalling networks contributing either reprogramming iCCA cells towards a less differentiated stem-like phenotype or favouring the expansion of the stem subpopulation by supporting their metabolism. In line with our results, a recently published study14 highlighted the pathogenic role of FA, showing that an exogenous supply of FA modulated the growth of CCA cells. Unlike more differentiated tumour cells, enhanced FA biosynthetic apparatus of iCCA stem-like cells probably render them more independent from the external source.
Additionally, the increased expression of FASN, the key enzyme in FA synthesis, was correlated with shorter survival in a dataset of 68 patients with iCCA thus suggesting that tumours with a worse prognosis are characterised by an increased content of intracellular FA. This might be potentially interesting considering our in vitro evidence of increased drug-resistance after exposure to FA. However, a detailed analysis of the underlying FA-driven molecular mechanism(s) is needed to provide the underlying mechanisms involved in the effective acquisition of a pharmacologically-resistant iCCA phenotype.
Nevertheless, we went further to demonstrate the functional relevance of FASN in stem-like iCCA cells both in the in vitro and in vivo settings. In vitro, FASN inhibition with both orlistat and specific gene silencing induced a significant decrease in pluripotency and stemness markers, as well as in the expression of genes involved in EMT and drug-resistance. These effects were replicated in a preclinical model of iCCA in vivo, where FASN inhibition caused a significant reduction in tumour growth after injection of resuspended iCCA-SPH. This was associated with dramatic changes in the expression of genes related to stemness and aggressiveness of iCCA. These data, together with those obtained in patients with iCCA, collectively indicate that tumour stem-like cells have distinct rearrangements of FA metabolism compared to more differentiated cells, and metabolic rewiring contributes to self-renewal and stemness maintenance with a major impact on the aggressiveness of iCCA cells.
Although the development and progression of cancer are frequently associated with increased de novo production of FA in several tumour types, the role of FASN in CCA is still controversial.[12], [13], [14], [15] Indeed, several studies,12 have shown that cholangiocarcinogenesis can be insensitive to FASN deprivation since CCA cells displayed enhanced FA uptake-related machinery. Differences observed in various studies may be ascribed to variances in patient cohorts, including different underlying aetiological factors (e.g. higher prevalence of MASLD, metabolic syndrome, or diabetes), and the intricate dynamics of the tumour microenvironment, especially the immune milieu. Furthermore, the diversity among liver tumour populations may result in variable reliance on FASN across cellular subsets. An additional explanation for these apparently divergent results can be that enhanced de novo lipogenesis and increased FASN expression are strictly related to the stemness component rather than to the more differentiated iCCA cells. Moreover, the abnormally activated de novo synthesis of FA may contribute to satisfy the energy needs for the maintenance of a stemness state in iCCA, as also suggested by our previous study.2 Thus, the results of the present study identify a new player in the regulation of lipid metabolism in iCCA with particular relevance to the biology of the stem cell compartment, and which could represent a target for treatment to be explored in dedicated studies. Of note, new FASN inhibitors are being developed, including TVB-2640, which is already being evaluated in clinical trials for the treatment of MASLD.24
An important aspect that will deserve additional research is related to the possible interaction between altered FA metabolism and metabolic disorders, such as MASLD, which are associated with an elevated risk of iCCA.[25], [26], [27], [28]
Recently, it has been shown that metabolic disorders are associated with an elevated risk of intrahepatic CCA.[25], [26], [27], [28] These metabolic risk factors encompass diabetes, obesity, metabolic syndrome and MASLD, which are frequently associated with dyslipidaemia. Unfortunately, the current datasets including clinical data do not specifically report the presence of MASLD, metabolic syndrome, or diabetes. However, it is imperative to investigate the potential presence and role of increased stemness in iCCA patients with these comorbidities. Further exploration and additional clinical data encompassing these specific aspects are warranted.
In conclusion, this study provides evidence that an altered FA metabolism contributes to the maintenance of stem-like phenotype in iCCA. These data allow to better understand the biology of CSCs in iCCA and suggest that inhibition of FASN may be a new potential target to interfere with tumour initiation of this deadly disease.
Abbreviations
ACAC, Acetyl-CoA carboxylase; AceCS1, cytoplasmic acetyl-CoA synthetase; ACLY, ATP citrate lyase; ACSL1, Acyl-CoA Synthetase Long Chain Family Member 1; CCA, cholangiocarcinoma; CSC, cancer stem cells; DNL, de novo lipogenesis; EMT, epithelial to mesenchymal transition; FA, fatty acids; FAO, Fatty acids beta-oxidation; FASN, Fatty acid synthase; GSEA, gene set-enrichment analysis; iCCA, intrahepatic cholangiocarcinoma; LC-MS, Liquid chromatography-mass spectrometry; MASLD, metabolic dysfunction-associated steatotic liver disease; MON, monolayer; OA, Oleic Acid; POA, Palmitoleic Acid; SPH, sphere cultures; TAGs, triacylglycerols.
Financial support
Funding for this work was provided by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) (IG23117) to CR, (IG27094) to MLT, Cassa di Risparmio di Pistoia e Pescia, and the University of Florence to FM. JBA and CR are members of the European Network for the Study of Cholangiocarcinoma (ENSCCA) and participate in the initiative COST Action EURO-CHOLANGIO-NET granted by the COST Association (CA18122).
Conflicts of interest
The authors declare no competing interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Designed the study and wrote the manuscript: CR, FM. Provided materials, performed the experiments, collected the data, and analysed the results: GL, MP, NN, BP, RB, ER, IT, ML, JBA, TL, AA, MLT, EP, CM, CA, SM, ES, SR, AT, GA, MR, PO, CPN, MP, FC, SS, AG. Supervised the project and critically revised the manuscript: CR and MF. All authors read and approved the final manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Acknowledgements
The authors thank Dr AJ Demetris (University of Pittsburgh, Pittsburgh, PA, USA) for cholangiocarcinoma cell lines (HUCCT1 and CCLP1).
Footnotes
Author names in bold designated shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101182.
Contributor Information
Fabio Marra, Email: fabio.marra@unifi.it.
Chiara Raggi, Email: chiara.raggi@unifi.it.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Raggi C., Correnti M., Sica A., et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raggi C., Taddei M.L., Sacco E., et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J Hepatol. 2021;74:1373–1385. doi: 10.1016/j.jhep.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Raggi C., Factor V.M., Seo D., et al. Epigenetic reprogramming modulates malignant properties of human liver cancer. Hepatology. 2014;59:2251–2262. doi: 10.1002/hep.27026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banales J.M., Marin J.J.G., Lamarca A., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo C.Y., Ann D.K. When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun (Lond) 2018;38:47. doi: 10.1186/s40880-018-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini R., Noto A., Pisanu M.E., et al. Metabolic features of cancer stem cells: the emerging role of lipid metabolism. Oncogene. 2018;37:2367–2378. doi: 10.1038/s41388-018-0141-3. [DOI] [PubMed] [Google Scholar]
- 7.Yi M., Li J., Chen S., et al. Emerging role of lipid metabolism alterations in Cancer stem cells. J Exp Clin Cancer Res. 2018;37:118. doi: 10.1186/s13046-018-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begicevic R.R., Arfuso F., Falasca M. Bioactive lipids in cancer stem cells. World J Stem Cells. 2019;11:693–704. doi: 10.4252/wjsc.v11.i9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noto A., De Vitis C., Pisanu M.E., et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene. 2017;36:4573–4584. doi: 10.1038/onc.2017.75. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Condello S., Thomes-Pepin J., et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20:303–314.e5. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Zhu K., Zhang R., et al. Oleic acid-PPARγ-FABP4 loop fuels cholangiocarcinoma colonization in lymph node metastases microenvironment. Hepatology. 2024;80:69–86. doi: 10.1097/HEP.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 12.Li L., Che L., Tharp K.M., et al. Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology. 2016;63:1900–1913. doi: 10.1002/hep.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomacha J., Dokduang H., Padthaisong S., et al. Targeting fatty acid synthase modulates metabolic pathways and inhibits cholangiocarcinoma cell progression. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.696961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz de Gauna M., Biancaniello F., González-Romero F., et al. Cholangiocarcinoma progression depends on the uptake and metabolization of extracellular lipids. Hepatology. 2022;76:1617–1633. doi: 10.1002/hep.32344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L., Zhang Y., Lin Z., et al. FASN-mediated fatty acid biosynthesis remodels immune environment in Clonorchis sinensis infection-related intrahepatic cholangiocarcinoma. J Hepatol. 2024;81:265–277. doi: 10.1016/j.jhep.2024.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Peterson T.R., Sen Gupta S.S., Harris T.E., et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen J.B., Spee B., Blechacz B.R., et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031.e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kridel S.J., Axelrod F., Rozenkrantz N., et al. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 19.Peng H., Wang Q., Qi X., et al. Orlistat induces apoptosis and protective autophagy in ovarian cancer cells: involvement of Akt-mTOR-mediated signaling pathway. Arch Gynecol Obstet. 2018;298:597–605. doi: 10.1007/s00404-018-4841-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Zhou Y., Feng X., Gao Y., et al. Low-dose orlistat promotes the therapeutic effect of oxaliplatin in colorectal cancer. Biomed Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113426. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C., Sheng L., Yuan M., et al. Orlistat delays hepatocarcinogenesis in mice with hepatic co-activation of AKT and c-Met. Toxicol Appl Pharmacol. 2020;392 doi: 10.1016/j.taap.2020.114918. [DOI] [PubMed] [Google Scholar]
- 22.Seguin F., Carvalho M.A., Bastos D.C., et al. The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br J Cancer. 2012;107:977–987. doi: 10.1038/bjc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baenke F., Peck B., Miess H., et al. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loomba R., Mohseni R., Lucas K.J., et al. TVB-2640 (FASN Inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterology. 2021;161:1475–1486. doi: 10.1053/j.gastro.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Da Fonseca L.G., Hashizume P.H., de Oliveira I.S., et al. Association between metabolic disorders and cholangiocarcinoma: impact of a postulated risk factor with rising incidence. Cancers (Basel) 2022;14 doi: 10.3390/cancers14143483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J.H., Hong J.Y., Park Y.S., et al. Persistent status of metabolic syndrome and risk of cholangiocarcinoma: a Korean nationwide population-based cohort study. Eur J Cancer. 2021;155:97–105. doi: 10.1016/j.ejca.2021.06.052. [DOI] [PubMed] [Google Scholar]
- 27.Yugawa K., Itoh S., Iseda N., et al. Obesity is a risk factor for intrahepatic cholangiocarcinoma progression associated with alterations of metabolic activity and immune status. Sci Rep. 2021;11:5845. doi: 10.1038/s41598-021-85186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadamuro M., Sarcognato S., Camerotto R., et al. Intrahepatic cholangiocarcinoma developing in patients with metabolic syndrome is characterized by osteopontin overexpression in the tumor stroma. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24054748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.