Summary
During the COVID-19 pandemic, people with multiple sclerosis (MS) and their healthcare providers have faced unique challenges related to the interaction between SARS-CoV-2, underlying neurological disease and the use of disease-modifying treatments (DMTs). Key concerns arose, primarily related to the possibility that SARS-CoV-2 infection could trigger the initial demyelinating event or exacerbate disease activity. Another major concern was the safety and efficacy of the COVID-19 vaccines, especially for patients undergoing specific treatments that could weaken their antibody responses. In the post-infection phase, identifying long COVID in patients with MS has been complicated due to the large overlap between post-infection sequelae and MS symptoms. In addition, disruptions in health and rehabilitation services have made it difficult for MS patients to access care. This Series article explores current evidence on the interaction between MS and SARS-CoV-2, identifies the challenges posed by the COVID-19 pandemic in the care of patients with MS, and discusses the significant adoption of digital health solutions, including telemedicine and new technology-based rehabilitation approaches. Based on lessons learned, recommendations and future directions are offered for managing patients with MS, rethinking healthcare systems and improving health outcomes in the post-COVID-19 pandemic era.
Keywords: Multiple sclerosis, COVID-19, SARS-CoV-2
Key messages.
-
1.
The risk of SARS-CoV-2 infection in people with MS does not differ from that in the general population
-
2.
An increased risk of severe COVID-19 outcomes (hospitalization, admission to intensive care unit, death) was observed in patients with higher disability level, progressive disease course and under specific treatments (i.e. some anti-CD20 agents)
-
3.
The immune response to both SARS-CoV-2 infection and vaccination might be blunted in patients under specific treatments (i.e. some anti-CD20 agents and unselective sphingosine-1-phosphate receptor modulators)
-
4.
The anti-SARS-CoV-2 vaccination showed a good safety and tolerability profile in MS, including a negligible risk of disease reactivation, being therefore recommended
-
5.
Symptoms of long COVID in people with MS might overlap with disabling features of the disease
-
6.
The disruption of healthcare and rehabilitation services during the COVID-19 pandemic represented an opportunity to re-think healthcare system and trigger a digital revolution towards new rehabilitation approaches and implementation of telemedicine
Search strategy and selection criteria.
References for this Series paper were identified through searches of PubMed with the search terms “multiple sclerosis”, “COVID-19”, “SARS-CoV-2” from March 1, 2020 until December 31, 2023. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Introduction
One of the most pressing matters regarding the outbreak of COVID-19 pandemic was determining its impact on underlying neurological conditions, such as multiple sclerosis (MS), in terms of morbidity and mortality risk. The interplay between MS-related disability, disease-modifying treatments (DMTs) and infection by SARS-CoV-2 represented also a matter of concern.1 The availability of a large-scale vaccination against SARS-CoV-2, even though representing the single most effective public health measure, raised issues regarding its efficacy and safety in patients with MS, such as the potential risk of both disease reactivation triggered by vaccines2 and ineffective immunization due to impaired immune response in individuals under treatment with specific DMTs.3 While the pandemic presented significant obstacles for MS care and rehabilitation, it also highlighted the importance of adaptable healthcare systems and the resilience of MS patients and their support networks.1 The aim of this Series paper is to outline the challenges that were faced by individuals with MS and their healthcare providers during the COVID-19 pandemic. Clinically relevant recommendations on how to manage MS after the COVID-19 era and future perspective to improve health outcomes are also discussed.
According to the Oxford Centre for Evidence-Based Medicine, studies collected in our synthesis are, at best, on a type 2a level of evidence, given the lack of randomized clinical trials (RCTs), the availability of few high-quality multicentre case–control and cohort data, expert opinions, and meta-analysis with relevant heterogeneity across included articles. Therefore, syntheses and recommendations in this review can be graded as moderate to low.
Course of COVID-19 in people with MS
The risk of SARS-CoV-2 infection in patients with MS does not differ from that in the general population.4 However, despite the similar infection incidence, several studies documented an increased risk of severe events by COVID-19 (hospitalization, admission to intensive care unit, death), as well as a longer time to recover from COVID-19, in patients with MS than in the general population5, 6, 7 According to a meta-analysis pooling data from 18 studies on people with MS conducted in the earlier pandemic phases, the crude death rate was about 2% (ranging from 0 to 10%), yielding to a 24%-increased risk of death after indirect age-standardization using case-fatality rates obtained from the detailed surveillance data available at the World Health Organization (WHO) website.8 Another study found an approximately two-fold increased risk of worse outcomes, including a more symptomatic COVID-19 course, in patients with MS as compared to the sex- and age-matched Italian population.9 This excess of severe events is attributable not only to risk factors in common with the general population (older age, male sex, and concomitant comorbidities), but also to MS-specific features, such as higher disability level and progressive disease course.5,6,10,11 Notably, earlier reports suggested no increased risk of death by COVID-19 in the presence of MS, with a mortality rate comparable to that of general population at approximately 2–3%.12 However, several methodological considerations should be raised when interpreting such discrepancies: (i) the demographic characteristics of patients with MS, who tend to be on average younger and more female than the average general population; (ii) differences across countries in terms of societal and public health issues, measures taken to face with pandemic, variation in data transmission and in case definition over time; (iii) the possibility of sampling or referral bias as patients with more severe outcomes were more likely to be reported, especially in the earlier outbreak phases due to the disruption of healthcare systems; (iv) the largest amount of data was collected in the first pandemic wave, dominated by the ancestral SARS-CoV-2 variants (more virulent and less contagious than the newer variants) and when vaccines were not available yet. The risk of worse outcomes due to COVID-19 in patients with MS is indeed declining thanks to anti-SARS-CoV-2 mass vaccination, together with shift towards less deadly SARS-CoV-2 variants, increased testing capacity allowing for detection of milder cases, and the improved standard of care measures, supportive care and management over time.3
Finally, children with MS, pregnant and post-partum women with MS do not seem to carry a higher risk of poor COVID-19 outcomes.13,14 However, the risk of preterm birth should be considered as in general population; notably, this risk began to wane after mass vaccination.15
Synthesis: the available evidence is inconclusive to support an increased risk of worse outcomes by COVID-19 in people with MS, although it is reasonable that older patients with comorbidities and worse disability levels might experience a greater risk of hospitalization and death than the general population.
Effect of DMTs on COVID-19 outcomes
Currently available DMTs for MS act on the autoimmune cascade leading to lesion formation, via an either immunomodulatory or immunosuppressive effect for the most effective treatments. In the first few months after the start of the SARS-CoV-2 pandemic, anti-CD20 agents, specifically rituximab and ocrelizumab, were found to be associated with an increased risk of severe COVID-19, hospitalisation with oxygen therapy, hospitalisation in intensive care or death. This increased risk was identified in the Italian MUSC-19 registry,16 then confirmed in a joint meta-analysis between the Italian MUSC-19 and French COVISEP registries,17 in the North American COVIMS registry,18 in the Swedish registry,19 and in the international MSIF registry.11 In a population-based study in Sweden among patients on rituximab, trends for differences in risk of hospitalization due to COVID-19 remained in the model adjusted by demographics, socio-economic status, comorbidity, and disease severity.20 Other DMTs, in particular sphingosine-1-phosphate receptor (S1P-R) modulators, were not associated with an increased risk of severe forms of COVID-19, despite the chronic lymphopenia usually associated with these treatments, but whose mechanism of action is linked to lymphocyte sequestration. On the other hand, a potentially beneficial effect of interferon β formulations has been suggested,10,17 which could be linked to the anti-viral properties of this type of molecule, and which would be consistent with the identification of a signalling deficit linked to type I interferon in certain patients with a severe form of COVID-19.21 Interestingly, there is an interaction between demographic or neurological risk factors on the one hand, and risk factors linked to DMTs on the other (Panel 1). Indeed, an Italian study on the MUSC-19 registry showed that the increase in COVID-19 severity linked to anti-CD20 agents in patients with MS compared with the general population mainly concerned patients without comorbidity and with an EDSS ≤3.0 (odds ratio = 3.0)6; similarly, a study on the French COVISEP registry also showed that the increased risk associated with anti-CD20 agents mainly concerned patients with relapsing-remitting (RR) MS (odds ratio = 5.2).22 In this latter study, patients with progressive MS, whether treated with anti-CD20 or not, had a higher risk of developing severe COVID-19 (∼19%) than patients with RRMS (∼9%). The increased risk of severe forms of COVID-19 associated with anti-CD20 therapy was also found in the paediatric population, with an odds ratio of 15.3.23 However, on the basis of the currently available knowledge, and considering the mild course of COVID-19 and the protective effect of mass vaccination, all DMTs can be started and sequenced of in a similar way to that in the pre-pandemic era, with special attention (but not overt contraindication) for anti-CD20 agents.
Panel 1. Summary of risk factors for severe COVID-19 outcomes in patients with MS.
In common with general population
-
1.
Older Age
-
2.
Male Sex
-
3.
More comorbidities
-
4.
Lymphopenia
-
5.
Recent exposure to steroids
Specific for multiple sclerosis
-
1.
Higher disability levels
-
2.
Primary and secondary progressive phenotypes
-
3.
Ongoing anti-CD20 treatments
Recommendation: there is no evidence for altering the initiation and sequencing of the currently available DMTs; however, patients starting or under treatment with some anti-CD20 agents should be informed about the possibility of an increased risk of worse outcomes by COVID-19.
Effect of COVID-19 on clinical course of MS
In the early pandemic phases, there was great concern that SARS-CoV-2 infection (like other viruses) could contribute either to trigger the initial demyelinating event or to increase disease activity in people with MS or other autoimmune conditions of the central nervous system (CNS). As such, COVID-19 has been associated with an increased risk of triggering several autoimmune disorders, including MS (adjusted hazard ratio = 2.7 versus non-COVID-19 individuals)24 and neuromyelitis optica spectrum disorder (NMOSD).25 Experimental data on animal models of MS-like demyelinating diseases, together with the neuro-invasive potential of SARS-CoV-2, corroborate the indirect evidence of an increased incidence of MS due to COVID-19.26
By contrast, the link between disease activity and COVID-19 is more controversial. The occurrence of pseudo-exacerbations, i.e. a transient clinical worsening triggered by the undercurrent infection by SARS-CoV-2, is an expected phenomenon. There were also a number of anecdotal reports of either new demyelinating lesions or relapses following getting infected by SARS-CoV-2,27,28 and one retrospective study that found an accelerated disability worsening in patients with MS after severe COVID-19.29 However, larger studies suggest neither increased risk of clinic-radiological disease activity nor motor and cognitive worsening in patients with MS in the short- and long-term period.30, 31, 32, 33 The possibility of under-reporting of clinical exacerbations and missed detection of radiological activity due to difficult to access routine care and unscheduled assessments during the pandemic should be taken into account.34
Synthesis: available data did not support the evidence that COVID-19 increases MS activity and progression, but the possibility of an increased risk of triggering several autoimmune disorders, including MS, should be considered after the SARS-CoV-2 infection.
Long COVID: implications for people with MS
The definition of long COVID is otherwise unexplained and persisting symptoms for more than 3 months, in individuals with a history of probable or confirmed SARS-CoV-2 infection.35 Among neurological and neuropsychiatric symptoms fatigue, cognitive impairment (‘brain fog’), depression, and anxiety prevail; these symptoms can be both fluctuating and persistent and significantly overlap with disabling MS features. Many viral, bacterial, and parasitic infections are known to potentially lead to neurocognitive impairment in post-acute infection syndromes, with a subset of patients developing myalgic encephalomyelitis/chronic fatigue syndrome, a condition characterized by persistent fatigue that is not relieved by sleep or rest.36 Chronic inflammation, viral reactivation, immune dysregulation and autoimmunity have been proposed as putative mechanisms for long COVID37,38 as well as direct brain infection.36,39 One study showed indeed persistence of SARS-CoV-2 RNA in the brain at autopsy long after the onset of symptoms39 and elevated levels of neurofilaments have been found in the acute phase of the disease, indicating neuroinflammation.40 Moreover, long COVID patients with neurological symptoms exhibited higher levels of neurofilament light chain and glial fibrillary acidic protein.41 Reactivation of latent herpesviruses by SARS-CoV-2 virus has also been hypothesized among long COVID causes.42 Among different viruses, infection by SARS-CoV-2 has been found associated with EBV reactivation37; interestingly, a deep multi-omic, longitudinal investigation demonstrated that EBV viremia at the time of initial COVID-19 diagnosis predicts long COVID.43 Such evidence is of particular interest in the context of MS, since epidemiological studies recently confirmed the strong link between the disease and EBV infection.44 Up-regulated interleukin 6, C-reactive protein, and tumor necrosis factor alpha (TNF-α) have been suggested as potential diagnostic biomarkers for long COVID.41 Like MS, long COVID is associated with adaptive and innate immune systems dysregulation and evidence exists that cerebrospinal fluid levels of pro-inflammatory proteins (tumor necrosis factor receptor superfamily member-9 [TNFRSM9], interferon γ) and lacking anti-inflammatory mediators (TNF-α-related apoptosis-inducing ligand [TRANCE], receptor activator of nuclear factor kappa-Β ligand [RANKL], tumor necrosis factor-related apoptosis-inducing ligand [TRAIL]) are predictive for long COVID.45 Recently, multimodal proteomics studies with machine learning approaches demonstrated that active long COVID is characterized by dysregulated activation of the complement system, increased antibody titers against several herpesviruses and with the presence of thrombo-inflammatory proteins,46 elements known to be associated with MS pathogenesis.
The frequency of long COVID in the general population varies with the definition used and the observed cohorts, but it is higher in hospitalized compared to more benign cases and in unvaccinated compared to vaccinated subjects.38 The difference between females and males are minor and the majority of symptoms resolves within one year.47 A prospective and longitudinal cohort study reported that 29.7% of MS patients experiencing COVID-19 had long-standing symptoms for ≥4 weeks and 12.4% for ≥12 weeks. Recovery from COVID-19 was less likely in people with web-EDSS scores ≥7, probable anxiety and/or depression before COVID-19 onset, and in women.7 Interestingly, a recent study demonstrated that people with MS are more likely to experience new weakness, mobility difficulties, and cognitive dysfunction than controls, even after controlling for the presence of these symptoms prior to their infection and other risk factors.48 Long COVID symptoms, such as cognitive impairment, fatigue and psychiatric manifestations, greatly overlap with common disturbances of MS, and it may be difficult to assign the symptoms to either MS or long COVID. A study on memory deficits in patients with long COVID recently showed the presence of a significantly reduced overnight consolidation and a non-significant trend to reduced learning rates.49 Notably, cognitive and psychiatric manifestations of MS have been proposed to rely on a disrupted neuro–immune interaction at the synaptic level due to glial cells activation,50,51 a condition thought to occur during COVID-19.36 Unfortunately studies on long COVID pathogenesis and its clinical manifestations in MS, including newly onset symptoms attributable to long COVID, are still largely lacking. Likewise, differences across the variants of SARS-CoV-2 have not been investigated yet. Understanding the mechanistic bases of long COVID in MS might be of utmost importance since it could provide unifying hypotheses for the pathobiology of the symptoms shared by both the conditions. In the meantime it remains important to bear in mind the possibility of long COVID when evaluating people with MS, thus avoiding unnecessary escalation of treatment and instead prompt information, counselling and appropriate rehabilitation.
Recommendation: in patients with MS and a history of probable or confirmed SARS-CoV-2 infection, the possibility of long COVID should be considered as differential diagnosis of relapses and/or MS progression and in the management of disabling MS symptoms.
COVID-19 vaccines and MS: tolerability and safety issues
The widespread use of COVID-19 vaccines has engendered concerns about their safety, especially regarding their potential to elicit an immunological response capable of inducing a disease reactivation.52 Very few cases of CNS demyelination (transverse myelitis, new onset MS, NMOSD, optic neuritis), not exceeding the expected rate, have been observed following all types of approved COVID-19 vaccines.53 These heterogeneous demyelinating events occurred approximately within one months from vaccine administration, mainly after the first dose; booster vaccination schedules were not associated with recurrent adverse events (AEs).54
Several case-reports and case-series documented the occurrence of MS relapses after SARS-CoV-2 vaccination,55 prompting the design of larger studies to address these concerns. A seminal observational study evaluated the risk of immediate relapses following mRNA-based vaccination on a cohort of 555 patients with MS receiving the first dose and 435 undergoing the second dose. The rate of patients with acute relapse was 2.1% and 1.6%, respectively. No increased risk of relapse activity was estimated in comparison with a cohort of non-vaccinated patients during a corresponding pre-pandemic period.56 Thereafter, numerous observational studies explored the occurrence of MS relapses after COVID-19 vaccines. For instance, a multi-centric Italian work involving 324 patients with MS demonstrated no increased relapse rate in the two months after the initial mRNA-based vaccine administration when compared with the prior two months.57 Even following repeated immune system stimuli, such as a third booster dose, there was no observable heightened risk of disease reactivation.58 A systemic review and meta-analysis including data from 14,755 patients who received a cumulative number of 23,088 doses of any SARS-CoV-2 vaccine (i.e. mRNA-based, adenovector-based, inactivated virus) documented a similar pooled proportion (1.9%) of patients experiencing relapses at an average time interval of 20 days post-vaccination.2 Overall, the prevailing body of evidence seems reassuring in terms of short-term relapse risk after COVID-19 vaccination in MS. The benefits of COVID-19 vaccination in preventing severe illness and hospitalization along with disease reactivation induced by the infection itself, appear to outweigh the potential associated risks.3,6 Nevertheless, a comprehensive evaluation of individual medical history remains crucial, as well as the need to overcome the methodological limits of current observational studies to provide further evidence. Sequential SARS-CoV-2 vaccinations to patients suffering from immune-mediated inflammatory diseases like MS, often treated with immunosuppressive agents, have been offered on a large scale world-wide during the pandemic. Even though various AEs were reported in healthy individuals after consecutive vaccinations, large studies on AEs after repeated vaccinations in patients with MS under DMTs are scarce.59,60 The occurrence of short-term AEs after 5454 documented vaccinations was recorded in the Netherlands,59 including first, second and third vaccinations in patients with autoimmune diseases like MS (n = 343), Crohn's disease (n = 302) and rheumatoid arthritis (n = 266). Clinically relevant AEs were observed in 57.3% of participants after the first, 61.5% after the second and 58.0% after the third vaccination. Patients were rarely admitted to the hospital and only sporadic allergic reactions were reported. Patients with immune-mediated inflammatory diseases had a modestly increased risk of clinically relevant AEs after vaccination when compared to controls.60 The large majority of AEs resolved within one week. Data obtained from a large cohort of 6142 patients with MS from Germany and United Kingdom who took at least one dose of anti-SARS-CoV-2 vaccine showed up to 65.4% who reported vaccination reactions.61 The most common reported AEs were pain at the site of inoculation, followed by fatigue and headache, occurring more often in women than men. Despite the absence of a control population of healthy individuals, patients with MS did not seem to experience a higher occurrence of vaccination reactions with respect to the general population.62 Two smaller studies from Italy63,64 addressed safety and tolerability of vaccinations in MS. Pain at the injection site (57.1%) and fatigue (37.9%) were the most frequently observed AEs in a single centre study on 140 patients treated with different DMTs63; no patient experienced severe side effects requiring hospitalization. A similar good safety and tolerability profile was observed in another study that recruited 40 healthy controls and 47 patients with MS, of whom 28 were on treatment with ocrelizumab and 19 with fingolimod.64
Overall, COVID-19 vaccines are well tolerated by patients with MS and the risk of relapse after vaccination is negligible and lesser than the infection itself. Yet, vaccine hesitancy involved a relevant proportion of patients with MS (10–20%), although their propensity to be vaccinated was even higher than that found in the general population and increased over the pandemic course.65 Vaccine hesitancy in MS has been related to several factors, including education level, personality trait, emotional status, promotion by healthcare professionals, level of knowledge and misconceptions about vaccination.66,67 Safety data may encourage those who remain hesitant about COVID-19 vaccinations, and help physicians in directing their patients towards accepting vaccination.
Synthesis: available evidence does not support an increased risk of MS relapses following COVID-19 vaccination; overall, there is no special concern about the safety and tolerability of vaccination in patients with MS as compared to general population. A comprehensive evaluation of individual medical history remains crucial.
Efficacy of COVID-19 vaccines in people with MS under DMTs
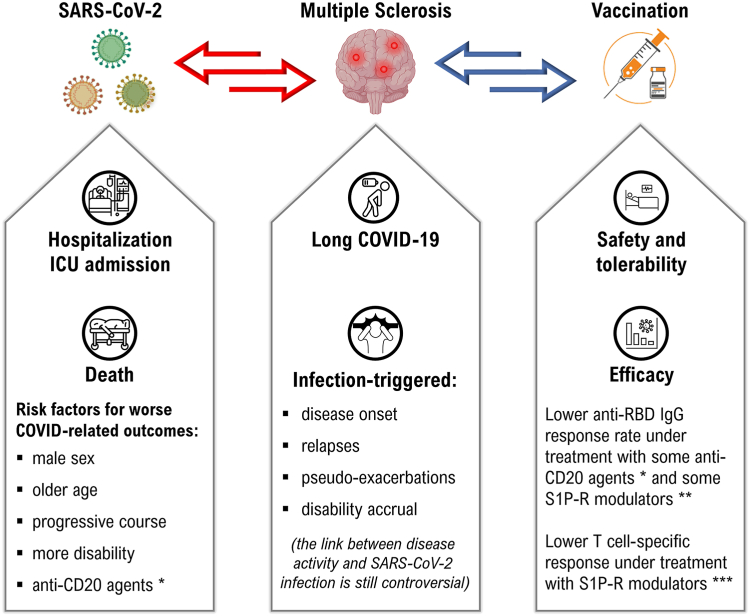
Currently approved COVID-19 vaccines target SARS-CoV-2 spike protein68 and include mRNA-based vaccines (BNT162b2, Pfizer-BioNTech; mRNA-1273, Moderna), viral vector-based vaccines (Ad26.COV2.S, Janssen/Johnson & Johnson; AZD1222, AstraZeneca), and protein-based vaccines (NVX-CoV2373, Novavax). The currently available intramuscular-based vaccines induce IgG and IgM anti-receptor-binding domain (RBD) antibodies, neutralizing antibodies and CD4+ and CD8+ T cell responses.69 Besides several important functions, antibodies can neutralize the virus, whereas T cells kill viral-infected cells, contribute to B cell activation and, consequently, to antibody production. Neutralising antibodies play an essential role in viral containment and are considered a correlate of protection.70 Moreover, T cells can recognize SARS-CoV-2 variants that partially escape humoral-based immunity, as shown in both healthy individuals71 and vulnerable subjects,72 including patients with MS.73 Patients with MS under DMTs targeting T and B cell immunity are potentially at risk of an impaired ability to mount an efficient antibody- and cell-mediated immune response after infections or vaccinations, in terms of both lower amount and shorter durability, compared with that generated in the healthy controls, as described for SARS-CoV-2 vaccination73,74 (Fig. 1).
Fig. 1.
The interplay between multiple sclerosis, SARS-CoV-2 infection and anti-SARS-CoV-2 vaccination. ∗an increased risk of severe COVID-19 outcomes and reduced humoral response to anti-SARS-CoV-2 vaccination were observed in patients under treatment with ocrelizumab and rituximab in observational studies with mixed methods and designs, whereas data on other anti-CD20 agents (i.e. ofatutumab) are insufficient to draw similar conclusions. ∗∗sufficient antibody titres and seroconversion rate were observed after primary and booster vaccination in patients under treatment with selective S1P-R modulators (ozanimod, siponimod), whereas response to anti-SARS-CoV-2 vaccination is relevantly decreased with unselective S1P-R modulators (fingolimod), but without implying an increased risk of severe COVID-19 disease. ∗∗∗reduced T-cell response to anti-SARS-CoV-2 vaccination was observed in patients under treatment with sphingosine-1-phosphate receptor modulators, regardless of their selectivity, but without implying an increased risk of severe COVID-19 disease.
A meta-analysis including data from 2203 individuals with MS and 864 healthy controls revealed no adequate antibody response to vaccination in 56% and 28% of patients under treatment with anti-CD20 agents and S1P-R modulators, respectively, as compared with 7% of those who were treated with different DMTs.75
Booster mRNA-vaccine doses reinforce specific immunity, although this is dependent on the type of therapy used.76 Fingolimod, a S1P-R modulator which hampers B and T cell egress from the lymph nodes and reduces the circulating lymphocytes levels, diminishes both T cell-mediated and antibody-mediated response to vaccination74 and boosters.76 A more efficient immune response to SARS-CoV-2 vaccines has been recently reported with selective (ozanimod, ponesimod, siponimod) than unselective (fingolimod) S1P-R modulators.77 However, although T cell responses are reduced, fingolimod is not associated with an increased risk of severe COVID-19 disease,10,16,17,19,20 likely because a viral-specific immunity is established and present at the lymphoid tissues level.75 Anti-CD20 agents, such as ocrelizumab and rituximab, reduce the ability to develop a sufficient antibody response to SARS-CoV-2 vaccination, whereas cellular responses are usually preserved.73, 74, 75, 76 In this regard, the measurement of SARS-CoV-2-specific T-cell responses might represent a strategy to assess the efficacy of vaccination in patients treated with such DMTs.74
Particular attention is thereby needed to patients under anti-CD20 treatments due to the antibody impairment76 which may lead to set-up a specifically tailored strategy to provide a primary prophylaxis to prevent disease onset before and after SARS-CoV-2 exposure (pre-and post-exposure prophylaxis) and, eventually, during infection to prevent worse COVID-19 outcomes.78 Available data are insufficient to draw similar conclusions for other anti-CD20 treatments (ofatumumab). Other DMTs such as interferon β, glatiramer acetate, teriflunomide, dimethyl/diroximel Fumarate, and natalizumab do not seem to dramatically affect vaccine responses in terms of induction of antibody response,75 although they are quantitatively reduced compared to that induced by healthy controls.76 Immune studies in patients with MS characterizing the T cell-specific response showed that the booster vaccine dose further increases both the CD4+ and CD8+ effector memory T cells (TEM) and the CD8+ terminally differentiated memory T cells (TEMRA).79 The increased memory T cells can be important to prevent the onset of severe COVID-19 disease as they expand if re-challenged and contribute to prompt immune responses controlling the initial viral replication, including that of the current variants,73 and spread in the host. In patients with MS, a full vaccination was associated with a significant reduction in the hospitalization rate for COVID-19, compared to the pre-vaccination time for all DMTs, including fingolimod but not ocrelizumab.80,81
Recommendation: the available evidence supports the SARS-CoV-2 vaccination with mRNA-based vaccines in patients with MS; the vaccination schedule should be tailored to the type and timing of DMT administration in patients treated with (or just about to start) depletive agents and S1P-R modulators.
MS care in the pandemic
The first wave of the COVID-19 pandemic led to widespread disruptions in healthcare systems worldwide that impacted MS care.82 Medical and nursing staff were either re-deployed to COVID-19 activities or acquired the infection: beds in regular wards and intensive care units were at some point mostly allocated to patients with COVID-19, non-emergency medical appointments and routine check-ups were postponed or shifted to telemedicine platforms to reduce the risk of viral transmission and suspension of routine blood tests and magnetic resonance imaging (MRI) scans happened in many countries.83 In some instances, access to DMTs was also affected at the beginning of the pandemic. Supply chain disruptions and prioritization of resources towards COVID-19-related healthcare resulted in delays or difficulties in obtaining DMTs.84 Some patients experienced interruptions in their treatment schedules, raising concerns about disease progression and relapses. In one study, the most common type of medication change was delay in infusion (71.9%), of which only 51.2% was advised by a healthcare provider, with most patients citing fear of contracting COVID-19 as the main reason,85 due to traveling and hospital visits rather than for exposure to immunosuppressant treatments.86 In another study, patients with MS declared to be more concerned about the risks of experiencing relapses during re-infection by novel SARS-CoV-2 variants.87 Compared to the previous year, adherence to DMTs decreased by approximately 10% in some regions.88 In response, national professional bodies and patient organizations were swift to respond to this problem by providing specific guidance and recommendations to minimize the risk of infection. This included maintaining social distancing, practicing strict hygiene measures, and prioritizing vaccination against COVID-19.89 Guidelines primarily focused on DMTs indicated that continuing or initiating MS treatment was much more beneficial in the long-term than interrupting or postponing it.83,89 Yet, there were some modifications in the pattern of DMT prescription, especially during the first months of the pandemic, mainly involving a reduction of S1P-R modulators and immune cell-depleting agents, such as alemtuzumab, cladribine, ocrelizumab, rituximab. In this regard, some Authors have raised concern about the growing prescription of less effective DMTs during the pandemic in the absence of evidence-based data on the consequences on long-term disability of such an approach.90
Natalizumab initiation was favoured in patients with high inflammatory activity, also using the opportunity of extended dose intervals, and at-home infusions in infected patients.83,89 On-demand re-dosing based on CD19 cell count or extended interval dosing were taken into account as potential risk mitigation strategy for the anti-CD20 agents ocrelizumab and rituximab.91 Observational studies on ocrelizumab-treated patients suggested that delaying re-infusion by 3–8 weeks with respect to the standard 6-month interval was associated with neither breakthrough disease activity nor disability progression.92, 93, 94 Moreover, it has been suggested that an extended interval dosing might probably increase the response rate to the current COVID-19 vaccines.92,95 Change in administration schedule or delay starting were also recommended for specific DMTs before getting vaccination, including anti-CD20 agents, S1P-R modulators, alemtuzumab, cladribine.95
After lockdown, DMT prescriptions quickly returned to pre-pandemic levels, as revealed by an increase in both initial prescription and any treatment switch, especially from moderate to high-efficacy DMTs.96 On the other hand, other authors found that the use of DMTs and co-prescribed antidepressants was stable in Germany from 2019 to 2021, maybe reflecting the different impact of COVID-19 pandemic across countries.97
Recommendation: available data suggest that change in administration schedule of specific DMTs, such as extended interval dosing for anti-CD20 agents, might be considered as a relatively safe option for patients with MS in particular circumstances.
COVID-19 and impact on MS rehabilitation
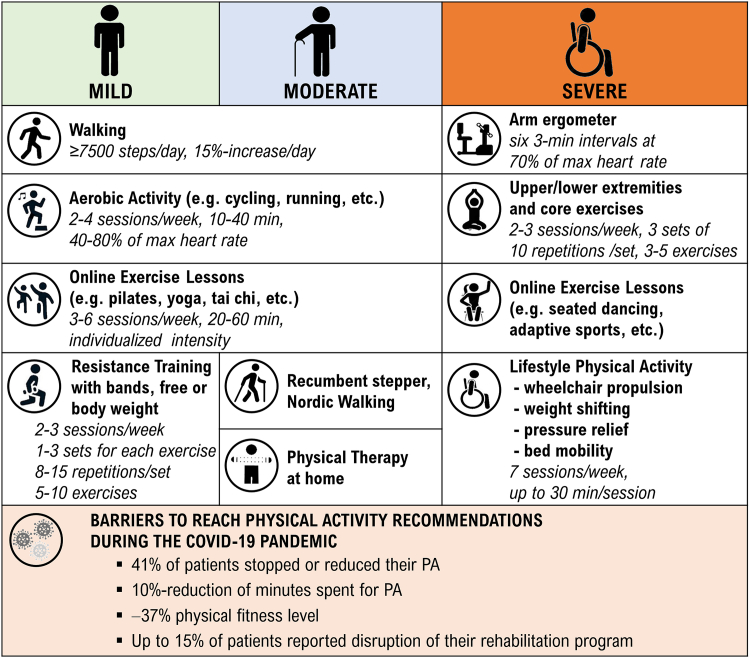
Rehabilitation, including physical activity (PA), cognitive training and occupational training, is an integral component of comprehensive MS management. The recent MS guidelines recommend at least 150 min per week of PA.98 It is known that PA can positively affect physical and mental health, reduce MS symptoms and, overall, improve the quality of life.99 Exercise, a subset of PA, has even been found to have a modest beneficial effect on relapse rates.100 Walking has emerged as the most prevalent PA practiced across different disability levels in multiple studies, with its popularity even increasing during the COVID-19 pandemic.101 However, despite this rise in walking activity, the need to mitigate the spread of the SARS-CoV-2, particularly among this high-risk population, has limited the access to regular and structured PA and rehabilitation.101 During the pandemic there were not only reductions in PA, but also deterioration in sleep quality,102 as well as detrimental neuropsychiatric and cognitive consequences, especially on patients adopting maladaptive coping strategies and those more disabled who lost social support.103 Patients with MS experienced more severe symptoms of depression and at least the same level of anxiety than the general populations, with subsequent worsening of mental health dimensions of quality of life.104
In an international observational study involving 3028 patients with MS, 15.5% reported disruption to their rehabilitative therapy as a result of the COVID-19 pandemic.85 In a Czech study, 41% of 297 included patients stopped or reduced PA during 2020 and 37% reported that their level of physical fitness decreased during the pandemic.105 An international cross-sectional survey designed by the European Network for Best Practice and Research in Multiple Sclerosis Rehabilitation (RIMS), completed by 215 physiotherapists, confirmed that accessibility, the average number, length and perceived effectiveness of rehabilitative sessions (physical therapy, occupational therapy, social service, speech and language therapy, psychological support, dietary interventions, medical management, vocational rehabilitation and cognitive training) provided to patients with MS were significantly reduced during the COVID-19 pandemic.106 This phenomenon has not been even reversed by the fact that physiotherapists increased the usage of mobile apps, rehabilitation videos and exercise websites. Home-based motor or cognitive training delivered by handheld application for smartphone or tablets, computer software, off-the-shelf video games and exergames (e.g. RehaCom, BrainHQ, COGNI-TRAcK, Nintendo Wii, Xbox Kinect, etc.) gathered lots of attention as user-friendly, easily-accessible, and effective to promote fitness and healthy behaviour and to promote social integration via online multiplayer mode.106 Although technology has played a crucial role in maintaining rehabilitation delivery, only 14% of representatives of MS rehabilitation services and 10% of healthcare professionals planned to use technologies after the pandemic.105 Lastly, a large-scale international survey showed a reduction in PA during the pandemic among 3725 respondents: 60% met the weekly 150-min PA guidelines before the pandemic, but during the pandemic, there was a 10% reduction across all disability groups (mild, moderate, severe).98 Nonetheless, in the context of the numerous challenges posed by the pandemic, this relatively moderate reduction in activity amongst patients with MS who had pre-existing exercise habits can be interpreted as a positive outcome, serving as a testament to their resilience and sustained motivation. Thus, with the declining incidence of COVID-19 and increasing availability of PA options and generally rehabilitation, it is crucial to build upon this motivation. The aim should not only be to return to the original PA levels, but also to optimize PA and exercise treatments in terms of frequency, duration, and intensity, which can be supported by technology (Fig. 2). Psychosocial support for MS patients also underwent significant changes during the pandemic. As for the general population, isolation, limited social interactions, and disrupted routines negatively impacted the mental health of individuals with MS,102 particularly those with pre-existing activity limitations.104 Support groups and in-person counselling sessions were replaced by virtual alternatives, although these may not have provided the same level of emotional support.83
Fig. 2.
Physical activity (PA) as an integral component of comprehensive MS management: recommendations and barriers during the COVID-19 pandemic.
Recommendation: alternative strategy to conventional rehabilitation, including home-based tele-rehabilitation or training supported by emerging technologies, should be implemented to support and enhance access to rehabilitation and healthy lifestyle in patients with MS.
A perspective for the future: role of telemedicine
When COVID-19 severely challenged national health care systems, telemedicine proved to be a powerful ally of neurologists in the management of the patient with MS throughout the lockdown period.107 From that moment the Digital Health, in all its declinations, has undergone an exponential growth, but the pandemic revealed also the gap to be filled in this field, owing to not only patient-related factors (age, socio-economic disparities, level of digital literacy), but also regulatory, legal and reimbursement barriers, cyber-security and privacy issue due to use of Internet-based platforms.108 Nevertheless, the COVID-19 pandemic has merely accelerated a process that had already been ongoing for decades, doubling in a few years the use of digital devices and other telemedicine services.109 An international consensus statement highlights the main fields of telemedicine deployment in MS, which now extend beyond the “simple” remote examination of MS patients at high risk from COVID-19 infection.107 Through telemedicine, healthcare providers can conduct virtual consultations, enabling them to assess patients' overall health, review their symptoms, and make treatment recommendations.110 Telemedicine could also facilitate access to multidisciplinary care for individuals with MS, as healthcare professionals (neurologists, physical therapists, occupational therapists, psychologists, etc.) could collaborate remotely, share information, and provide comprehensive care to patients, all while minimizing the need for in-person visits.111
Long-term non-invasive remote assessment of different types of parameters (e.g. range and speed of motion, meters or number of steps in a day, heart rate, hours of sleep, etc.) can provide the so-called “Digital Biomarkers”112 useful to evaluate disease activity and response to therapy as well as a prognostic and progression predictor integrated in a concept of a digital MS twin through artificial intelligence-based analysis.113
However, it is important to acknowledge that telemedicine does have limitations. Not all aspects of MS care can be effectively delivered remotely, such as certain diagnostic procedures or physical examinations. In such cases, in-person visits may still be necessary and therefore telemedicine can be considered as a complementary, rather than alternative, approach to MS management.
In the context of the COVID-19 pandemic, the need for digital therapeutics has arguably never been greater.114 Tele-neurorehabilitation can provide continuity of treatment as well as a highly tailored intervention plan. Digital therapeutics, treatments delivered remotely and enabled by modern technology, facilitate the provision of personalized, evidence-based, interdisciplinary interventions to manage the complexities associated with MS.
Recommendation: in patients with MS, telemedicine should be considered for follow-up visits, certificates, prescription renewal, neurorehabilitation, disease monitoring; specific guidelines to govern telehealth, together with ameliorating of digital infrastructures and enhancing cybersecurity, are mandatory.
Conclusions
The COVID-19 pandemic represented a challenge for neurologists and patients with MS, who have faced the uncertainty related to the interplay between the SARS-CoV-2, the neuro-immunological disease and the DMT-induced alteration of immune-homeostasis. Failure in medical management, challenges in accessing treatments, increased vulnerability to COVID-19, safety of vaccines against SARS-CoV-2, and psychological effects of pandemic were among the key concerns for people with MS.
Big data sharing initiative and large disease registries helped identify patient- and DMT-related risk factors for worse COVID-19 outcomes.
Despite their indisputable efficacy, the anti-SARS-CoV-2 vaccines raised concerns about their safety and their effectiveness in the context of immune suppression induced by some anti-CD20 agents. However, the development and distribution of COVID-19 vaccines provided hope and protection for patients with MS, thus reinforcing pre-existing recommendations for collecting the immunisation history in all patients and for tailoring on an individual basis the timing of vaccine administration,115 especially in those under S1P-R modulators and immune cell-depleting DMTs.
Patients who faced difficulties accessing healthcare facilities obtained great benefits from the increased adoption of home-based solution for rehabilitation and telemedicine for remote assessments. However, despite new advances in technology and a heightened interest due to the pandemic, digital therapeutics need to be further developed and utilized to trigger a real “Digital Revolution” that paves the future for new opportunities.109
The disruption of medical and neurorehabilitation services occurring during the earlier pandemic phases highlighted the importance of more adaptable healthcare systems and the resilience of patients with MS and their support networks in the context of such global challenges. To conclude, the COVID-19 outbreak was a valuable lesson that compelled us to elaborate a framework for mitigating any future emergency and improving the standard of care of patients with MS (Table 1).
Table 1.
Lesson learnt from COVID-19 for improving MS care.
| Problem | Challenge | Opportunity |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contributors
Conceptualization: LP, MDF. Visualization: LP, MDF. Supervision: LP, GA, EGC, DG, JK, DK, LL, CL, MPS, DS, TZ, MDF. Writing original draft, review and editing: LP, MDF (Introduction, Effect of DMTs on COVID-19 outcomes, Conclusions); CL, MPS (Course of COVID-19 in people with MS, Effect of COVID-19 on clinical course of MS); EGC (Long COVID: implications for people with MS); JK, MDF (COVID-19 vaccines and MS: tolerability and safety issues); DG (Efficacy of COVID-19 vaccines in people with MS under DMTs); GA, TZ (MS care in the pandemic); DK, DS (COVID-19 and impact on MS rehabilitation); LL, TZ (A perspective for the future: role of telemedicine).
Declaration of interests
LP has received personal fees and non-financial support from Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Viatris.
GA has received compensation for consulting services, speaking honoraria or participation in advisory boards from Merck, Roche, and Horizon Therapeutics; travel support for scientific meetings from Novartis, Roche, ECTRIMS and EAN. She serves as editor for Europe of the Multiple Sclerosis Journal—Experimental, Translational and Clinical journal; and as a member of the editorial and scientific committee of Acta Neurológica Colombiana. She is a member of the International Women in Multiple Sclerosis (iWiMS) network executive committee, of the European Biomarkers in Multiple Sclerosis (BioMS-eu) steering committee, and of the MOGAD Eugene Devic European Network (MEDEN) steering group.
EGC has received educational and/or consultancy fees from Alexion, Almirall, Biogen, Bristol-Myers Squibb, Janssen, Sanofi, Merck, Novartis, Roche and Teva.
DG has received educational and consultancy fees from Amgen, Almirall, Biogen, Biomerieux, Diasorin, Eli Lilly, Janssen, PDB Biotec, Qiagen, Quidel. She was partially supporedt by the Italian Ministry of Health, Ricerca Corrente, Linea 1.
JK has received grants and consulting fees from F. Hoffmann-La Roche Ltd, Biogen, Teva, Merck, Novartis, Immunic, Celgene and Sanofi (payments to institution); reports speaker relationships with F. Hoffmann-La Roche Ltd, Biogen, Celgene, Teva, Merck, Novartis, Sanofi and Viatris (payments to institution).
DK has received educational and consultancy fees from Biogen, Teva, Merck and Roche.
LL has received educational and consultancy fees from Biogen, Teva, Merck, Roche, Novartis, Bristol-Myers Squibb, Almirall, Horizon, Alexion, Sanofi.
CL has received consulting or travel fees from Biogen, Novartis, Roche, Sanofi, Teva and Merck, and research grant from Biogen.
MPS has received consulting fees from Biogen, Novartis, Roche, Sanofi, Merck, Alexion, Bristol-Myers Squibb, Immunic.
DS has received financial support for conference travel and/or speaker honoraria from. Novartis, Biogen, Merck, Teva, Janssen-Cilag, and Roche and is also supported by the Charles University: Cooperation Program in Neuroscience and by the National Institute for. Neurological Research project funded by the European Union–Next Generation EU. (Programme EXCELES, ID Project No. LX22NPO5107).
TZ has received consulting or serving on speaker bureaus for Alexion, Biogen, Bristol-Myers Squibb, Roche, Sandoz, Novartis, Merck, Teva and Sanofi, as well as research support from Biogen, Novartis, Merck, Roche and Sanofi.
MDF participated on advisory boards and steering committees for and received speaker or writing honoraria, research support and funding for travelling from Alexion, BMS, Bayer, Biogen Idec, Genzyme, Horizon, Janssen, Merck, Mylan, Novartis, Roche, Siemens Healthineers, Teva and Viatris.
Acknowledgements
No specific grant from any funding agency in the public, commercial, or not-for-profit sectors were involved in the preparation of this paper.
Contributor Information
Luca Prosperini, Email: luca.prosperini@gmail.com.
Georgina Arrambide, Email: garrambide@cem-cat.org.
Elisabeth G. Celius, Email: elisabethgulowsen.celius@ulleval.no.
Delia Goletti, Email: delia.goletti@inmi.it.
Joep Killestein, Email: j.killestein@amsterdamumc.nl.
Daphne Kos, Email: daphne.kos@kuleuven.be.
Luigi Lavorgna, Email: luigi.lavorgna@policliniconapoli.it.
Celine Louapre, Email: celine.louapre@aphp.fr.
Maria Pia Sormani, Email: mariapia.sormani@unige.it.
Dominika Stastna, Email: dominika.stastna@vfn.cz.
Tjalf Ziemssen, Email: tjalf.ziemssen@uniklinikum-dresden.de.
Massimiliano Di Filippo, Email: massimiliano.difilippo@unipg.it.
References
- 1.Pugliatti M., Berger T., Hartung H.P., Oreja-Guevara C., Bar-Or A. Multiple sclerosis in the era of COVID-19: disease course, DMTs and SARS-CoV2 vaccinations. Curr Opin Neurol. 2022;35(3):319–327. doi: 10.1097/WCO.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 2.Stefanou M.I., Palaiodimou L., Theodorou A., et al. Safety of COVID-19 vaccines in multiple sclerosis: a systematic review and meta-analysis. Mult Scler. 2023;29(4-5):585–594. doi: 10.1177/13524585221150881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etemadifar M., Nouri H., Pitzalis M., et al. Multiple sclerosis disease-modifying therapy and COVID-19 vaccines: a practical review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022;93(9):986–994. doi: 10.1136/jnnp-2022-329123. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhry F., Jageka C., Levy P.D., Cerghet M., Lisak R.P. Review of the COVID-19 risk in multiple sclerosis. J Cell Immunol. 2021;3(2):68–77. doi: 10.33696/immunology.3.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louapre C., Collongues N., Stankoff B., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1–10. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sormani M.P., Schiavetti I., Carmisciano L., et al. COVID-19 severity in multiple sclerosis: putting data into context. Neurol Neuroimmunol Neuroinflammation. 2022;9(1) doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garjani A., Middleton R.M., Nicholas R., Evangelou N. Recovery from COVID-19 in multiple sclerosis: a prospective and longitudinal cohort study of the United Kingdom multiple sclerosis register. Neurol Neuroimmunol Neuroinflammation. 2022;9(1) doi: 10.1212/NXI.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prosperini L., Tortorella C., Haggiag S., et al. Increased risk of death from COVID-19 in multiple sclerosis: a pooled analysis of observational studies. J Neurol. 2022;269(3):1114–1120. doi: 10.1007/s00415-021-10803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiavetti I., Carmisciano L., Ponzano M., et al. Signs and symptoms of COVID-19 in patients with multiple sclerosis. Eur J Neurol. 2022;29(12):3728–3736. doi: 10.1111/ene.15554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosperini L., Tortorella C., Haggiag S., et al. Determinants of COVID-19-related lethality in multiple sclerosis: a meta-regression of observational studies. J Neurol. 2022;269(5):2275–2285. doi: 10.1007/s00415-021-10951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson-Yap S., Pirmani A., Kalincik T., et al. Updated results of the COVID-19 in MS global data sharing initiative: anti-CD20 and other risk factors associated with COVID-19 severity. Neurol Neuroimmunol Neuroinflammation. 2022;9(6) doi: 10.1212/NXI.0000000000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barzegar M., Mirmosayyeb O., Gajarzadeh M., et al. COVID-19 among patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2021;8(4) doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oncel I., Alici N., Solmaz I., et al. The outcome of COVID-19 in pediatric-onset multiple sclerosis patients. Pediatr Neurol. 2022;134:7–10. doi: 10.1016/j.pediatrneurol.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salter A., Cross A.H., Cutter G.R., et al. COVID-19 in the pregnant or postpartum MS patient: symptoms and outcomes. Mult Scler Relat Disord. 2022;65 doi: 10.1016/j.msard.2022.104028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvert C., Brockway M.M., Zoega H., et al. Changes in preterm birth and stillbirth during COVID-19 lockdowns in 26 countries. Nat Hum Behav. 2023;7(4):529–544. doi: 10.1038/s41562-023-01522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sormani M.P., De Rossi N., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sormani M.P., Salvetti M., Labauge P., et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol. 2021;8(8):1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salter A., Fox R.J., Newsome S.D., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spelman T., Forsberg L., McKay K., Glaser A., Hillert J. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler. 2022;28(7):1051–1059. doi: 10.1177/13524585211026272. [DOI] [PubMed] [Google Scholar]
- 20.Longinetti E., Bower H., McKay K.A., et al. COVID-19 clinical outcomes and DMT of MS patients and population-based controls. Ann Clin Transl Neurol. 2022;9(9):1449–1458. doi: 10.1002/acn3.51646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Januel E., Hajage D., Labauge P., et al. Association between anti-CD20 therapies and COVID-19 severity among patients with relapsing-remitting and progressive multiple sclerosis. JAMA Netw Open. 2023;6(6) doi: 10.1001/jamanetworkopen.2023.19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiner T., Wilson-Murphy M., Mendelt-Tillema J., et al. Characteristics of pediatric patients with multiple sclerosis and related disorders infected with SARS-CoV-2. Mult Scler. 2023;29(4–5):576–584. doi: 10.1177/13524585231151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng K., Li X., Yang D., et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. eClinicalMedicine. 2023;63 doi: 10.1016/j.eclinm.2023.102154. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(23)00331-0/fulltext Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun D., Du Q., Wang R., et al. COVID-19 and the risk of neuromyelitis optica spectrum disorder: a Mendelian randomization study. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellucci G., Rinaldi V., Buscarinu M.C., et al. Multiple sclerosis and SARS-CoV-2: has the interplay started? Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.755333. https://www.frontiersin.org/articles/10.3389/fimmu.2021.755333 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelena G., Casas M., Eizaguirre M.B., et al. Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult Scler Relat Disord. 2022;57 doi: 10.1016/j.msard.2021.103368. [DOI] [PubMed] [Google Scholar]
- 28.Rahmani M., Moghadasi A.N., Shahi S., et al. COVID-19 and its implications on the clinico-radiological course of multiple sclerosis: a case–control study. Med Clin. 2023;160(5):187–192. doi: 10.1016/j.medcli.2022.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters G., Van Remoortel A., Nagels G., Van Schependom J., D’haeseleer M. Occurrence and severity of coronavirus disease 2019 are associated with clinical disability worsening in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2023;10(3) doi: 10.1212/NXI.0000000000200089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bsteh G., Assar H., Gradl C., et al. Long-term outcome after COVID-19 infection in multiple sclerosis: a nation-wide multicenter matched-control study. Eur J Neurol. 2022 doi: 10.1111/ene.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etemadifar M., Abhari A.P., Nouri H., et al. Does COVID-19 increase the long-term relapsing-remitting multiple sclerosis clinical activity? A cohort study. BMC Neurol. 2022;22(1):64. doi: 10.1186/s12883-022-02590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghajanian S., Shafiee A., Akhondi A., et al. The effect of COVID-19 on Multiple Sclerosis relapse: a systematic review and meta-analysis. Mult Scler Relat Disord. 2023;81 doi: 10.1016/j.msard.2023.105128. [DOI] [PubMed] [Google Scholar]
- 33.Montini F., Nozzolillo A., Tedone N., et al. COVID-19 has no impact on disease activity, progression and cognitive performance in people with multiple sclerosis: a 2-year study. J Neurol Neurosurg Psychiatry. 2023;95(4):342–347. doi: 10.1136/jnnp-2023-332073. [DOI] [PubMed] [Google Scholar]
- 34.Babtain F., Bajafar A., Nazmi O., et al. The disease course of multiple sclerosis before and during COVID-19 pandemic: a retrospective five-year study. Mult Scler Relat Disord. 2022;65 doi: 10.1016/j.msard.2022.103985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soriano J.B., Murthy S., Marshall J.C., et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monje M., Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110(21):3484–3496. doi: 10.1016/j.neuron.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rousseau B.A., Bhaduri-McIntosh S. Inflammation and epstein-barr virus at the crossroads of multiple sclerosis and post-acute sequelae of COVID-19 infection. Viruses. 2023;15(4):949. doi: 10.3390/v15040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein S.R., Ramelli S.C., Grazioli A., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verde F., Milone I., Bulgarelli I., et al. Serum neurofilament light chain levels in Covid-19 patients without major neurological manifestations. J Neurol. 2022;269(11):5691–5701. doi: 10.1007/s00415-022-11233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Y.-J., Liu S.-H., Manachevakul S., et al. Biomarkers in long COVID-19: a systematic review. Front Med. 2023;10 doi: 10.3389/fmed.2023.1085988. https://www.frontiersin.org/articles/10.3389/fmed.2023.1085988 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waltz E. Could long COVID be linked to herpes viruses? Early data offer a hint. Nature. 2022 doi: 10.1038/d41586-022-02296-5. https://www.nature.com/articles/d41586-022-02296-5 Available at: [DOI] [PubMed] [Google Scholar]
- 43.Su Y., Yuan D., Chen D.G., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjornevik K., Münz C., Cohen J.I., Ascherio A. Epstein-Barr virus as a leading cause of multiple sclerosis: mechanisms and implications. Nat Rev Neurol. 2023;19(3):160–171. doi: 10.1038/s41582-023-00775-5. [DOI] [PubMed] [Google Scholar]
- 45.Etter M.M., Martins T.A., Kulsvehagen L., et al. Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: a prospective cross-sectional study. Nat Commun. 2022;13(1):6777. doi: 10.1038/s41467-022-34068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cervia-Hasler C., Brüningk S.C., Hoch T., et al. Persistent complement dysregulation with signs of thromboinflammation in active long Covid. Science. 2024;383(6680) doi: 10.1126/science.adg7942. [DOI] [PubMed] [Google Scholar]
- 47.Mizrahi B., Sudry T., Flaks-Manov N., et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023;380 doi: 10.1136/bmj-2022-072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abramoff B.A., Hentschel C., Dillingham I.A., et al. The association of multiple sclerosis, traumatic brain injury, and spinal cord injury to acute and long COVID-19 outcomes. PM R. 2023 doi: 10.1002/pmrj.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayward W., Buch E.R., Norato G., et al. Procedural motor memory deficits in patients with long-COVID. Neurology. 2024;102(3) doi: 10.1212/WNL.0000000000208073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Filippo M., Portaccio E., Mancini A., Calabresi P. Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci. 2018;19(10):599–609. doi: 10.1038/s41583-018-0053-9. [DOI] [PubMed] [Google Scholar]
- 51.Menculini G., Mancini A., Gaetani L., et al. Psychiatric symptoms in multiple sclerosis: a biological perspective on synaptic and network dysfunction. J Neurol Neurosurg Psychiatry. 2023;94(5):389–395. doi: 10.1136/jnnp-2022-329806. [DOI] [PubMed] [Google Scholar]
- 52.Zrzavy T., Kollaritsch H., Rommer P.S., et al. Vaccination in multiple sclerosis: friend or foe? Front Immunol. 2019;10:1883. doi: 10.3389/fimmu.2019.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ismail I.I., Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022;362 doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S., Muccilli A., Schneider R., Selchen D., Krysko K. Acute central nervous system demyelination following COVID-19 vaccination. Neurology. 2022;99(23_Supplement_2):S34–S35. [Google Scholar]
- 55.Rinaldi V., Bellucci G., Buscarinu M.C., et al. CNS inflammatory demyelinating events after COVID-19 vaccines: a case series and systematic review. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.1018785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Filippo M., Cordioli C., Malucchi S., et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(4):448–450. doi: 10.1136/jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- 58.Di Filippo M., Ferraro D., Ragonese P., et al. SARS-CoV-2 vaccination and multiple sclerosis: a large multicentric study on relapse risk after the third booster dose. J Neurol. 2023;271(1):24–31. doi: 10.1007/s00415-023-12034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boekel L., Steenhuis M., Hooijberg F., et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in The Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3(11):e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wieske L., Kummer L.Y.L., van Dam K.P.J., et al. Risk factors associated with short-term adverse events after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases. BMC Med. 2022;20(1):100. doi: 10.1186/s12916-022-02310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frahm N., Fneish F., Ellenberger D., et al. SARS-CoV-2 vaccination in patients with multiple sclerosis in Germany and the United Kingdom: gender-specific results from a longitudinal observational study. Lancet Reg Health Eur. 2022;22 doi: 10.1016/j.lanepe.2022.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q., Dudley M.Z., Chen X., et al. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19(1):173. doi: 10.1186/s12916-021-02059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capone F., Rossi M., Cruciani A., et al. Safety, immunogenicity, efficacy, and acceptability of COVID-19 vaccination in people with multiple sclerosis: a narrative review. Neural Regen Res. 2023;18(2):284–288. doi: 10.4103/1673-5374.346539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Capuano R., Altieri M., Conte M., et al. Humoral response and safety of the third booster dose of BNT162b2 mRNA COVID-19 vaccine in patients with multiple sclerosis treated with ocrelizumab or fingolimod. J Neurol. 2022;269(12):6185–6192. doi: 10.1007/s00415-022-11296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yap S.M., Al Hinai M., Gaughan M., et al. Vaccine hesitancy among people with multiple sclerosis. Mult Scler Relat Disord. 2021;56 doi: 10.1016/j.msard.2021.103236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalron A., Dolev M., Menascu S., et al. Overcoming COVID-19 vaccine hesitancy in people with multiple sclerosis. Mult Scler. 2021;27(Suppl 2):218. [Google Scholar]
- 67.Proietti F., Landi D., Ponzano M., et al. COVID-19 vaccine hesitancy among Italian people with multiple sclerosis. Neurol Sci. 2023;44(3):803–808. doi: 10.1007/s10072-022-06559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suthar A.B., Wang J., Seffren V., et al. Public health impact of covid-19 vaccines in the US: observational study. BMJ. 2022;377 doi: 10.1136/bmj-2021-069317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sette A., Sidney J., Crotty S. T cell responses to SARS-CoV-2. Annu Rev Immunol. 2023;41:343–373. doi: 10.1146/annurev-immunol-101721-061120. [DOI] [PubMed] [Google Scholar]
- 70.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 71.Tarke A., Coelho C.H., Zhang Z., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847–859.e11. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrone L., Picchianti-Diamanti A., Sebastiani G.D., et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis. 2022;121:24–30. doi: 10.1016/j.ijid.2022.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrone L., Tortorella C., Aiello A., et al. Humoral and cellular response to spike of delta SARS-CoV-2 variant in vaccinated patients with multiple sclerosis. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.881988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tortorella C., Aiello A., Gasperini C., et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gombolay G.Y., Dutt M., Tyor W. Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis. Ann Clin Transl Neurol. 2022;9(8):1321–1331. doi: 10.1002/acn3.51628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conway S., Saxena S., Baecher-Allan C., et al. Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine. Mult Scler Exp Transl Clin. 2023;9(2) doi: 10.1177/20552173231165196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proschmann U., Mueller-Enz M., Woopen C., et al. Differential effects of selective versus unselective sphingosine 1-phosphate receptor modulators on T- and B-cell response to SARS-CoV-2 vaccination. Mult Scler. 2023;29(14):1849–1859. doi: 10.1177/13524585231200719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vita S., Rosati S., Ascoli Bartoli T., et al. Monoclonal antibodies for pre- and postexposure prophylaxis of COVID-19: review of the literature. Pathog Basel Switz. 2022;11(8):882. doi: 10.3390/pathogens11080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruggieri S., Aiello A., Tortorella C., et al. Dynamic evolution of humoral and T-cell specific immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis followed until the booster dose. Int J Mol Sci. 2023;24(10):8525. doi: 10.3390/ijms24108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schiavetti I., Cordioli C., Stromillo M.L., et al. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult Scler. 2022;28(13):2106–2111. doi: 10.1177/13524585221102918. [DOI] [PubMed] [Google Scholar]
- 81.Capuano R., Prosperini L., Altieri M., et al. Symptomatic COVID-19 course and outcomes after three mRNA vaccine doses in multiple sclerosis patients treated with high-efficacy DMTs. Mult Scler. 2023;29(7):856–865. doi: 10.1177/13524585231167515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciccarelli O., Cohen J.A., Thompson A. Response of the multiple sclerosis community to COVID-19. Mult Scler. 2020;26(10):1134–1136. doi: 10.1177/1352458520948748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sastre-Garriga J., Tintoré M., Montalban X. Keeping standards of multiple sclerosis care through the COVID-19 pandemic. Mult Scler. 2020;26(10):1153–1156. doi: 10.1177/1352458520931785. [DOI] [PubMed] [Google Scholar]
- 84.Manacorda T., Bandiera P., Terzuoli F., et al. Impact of the COVID-19 pandemic on persons with multiple sclerosis: early findings from a survey on disruptions in care and self-reported outcomes. J Health Serv Res Policy. 2021;26(3):189–197. doi: 10.1177/1355819620975069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moss B.P., Mahajan K.R., Bermel R.A., et al. Multiple sclerosis management during the COVID-19 pandemic. Mult Scler. 2020;26(10):1163–1171. doi: 10.1177/1352458520948231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baba C., Yigit P., Dastan S., et al. Challenges of persons with multiple sclerosis on ocrelizumab treatment during COVID-19 pandemic. Neurol Clin Neurosci. 2022;10(1):3–8. doi: 10.1111/ncn3.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moghadasi A.N., Vaheb S., Hamtaei-Ghashti S., et al. Fear of re-infection, relapse, and anxiety during COVID-19 pandemic in patients with multiple sclerosis: a multi-center study. Curr J Neurol. 2023;22(2):82–86. doi: 10.18502/cjn.v22i2.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colais P., Cascini S., Balducci M., et al. Impact of the COVID-19 pandemic on access to healthcare services amongst patients with multiple sclerosis in the Lazio region, Italy. Eur J Neurol. 2021;28(10):3403–3410. doi: 10.1111/ene.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brownlee W., Bourdette D., Broadley S., Killestein J., Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- 90.Giovannoni G., Hawkes C., Lechner-Scott J., et al. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Lierop Z.Y., Toorop A.A., van Ballegoij W.J., et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult Scler. 2022;28(7):1121–1125. doi: 10.1177/13524585211028833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rolfes L., Pawlitzki M., Pfeuffer S., et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol Neuroinflammation. 2021;8(5) doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zanghì A., Avolio C., Signoriello E., et al. Is it time for ocrelizumab extended interval dosing in relapsing remitting MS? Evidence from an Italian multicenter experience during the COVID-19 pandemic. Neurotherapeutics. 2022;19(5):1535–1545. doi: 10.1007/s13311-022-01289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guerrieri S., Bucca C., Nozzolillo A., et al. Ocrelizumab extended-interval dosing in multiple sclerosis during SARS-CoV-2 pandemic: a real-world experience. Eur J Neurol. 2023;30(9):2859–2864. doi: 10.1111/ene.15891. [DOI] [PubMed] [Google Scholar]
- 95.Otero-Romero S., Lebrun-Frénay C., Reyes S., et al. European committee for treatment and research in multiple sclerosis and European academy of neurology consensus on vaccination in people with multiple sclerosis: improving immunization strategies in the era of highly active immunotherapeutic drugs. Eur J Neurol. 2023;30(8):2144–2176. doi: 10.1111/ene.15809. [DOI] [PubMed] [Google Scholar]
- 96.Affinito G., Trama U., Palumbo L., et al. Impact of COVID-19 and system recovery in delivering healthcare to people with multiple sclerosis: a population-based Study. Neurol Sci. 2023;44(11):3771–3779. doi: 10.1007/s10072-023-07052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orschiedt J., Jacyshyn-Owen E., Kahn M., et al. The influence of the COVID-19 pandemic on the prescription of multiple sclerosis medication in Germany. Biomed Pharmacother. 2023;158 doi: 10.1016/j.biopha.2022.114129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pedullà L., Santoyo-Medina C., Novotna K., et al. Physical activity in multiple sclerosis: meeting the guidelines at the time of the COVID-19 pandemic. J Neurol Phys Ther. 2023;47(2):112–121. doi: 10.1097/NPT.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 99.Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as medicine in multiple sclerosis-time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. 2019;19(11):88. doi: 10.1007/s11910-019-1002-3. [DOI] [PubMed] [Google Scholar]
- 100.Pilutti L.A., Platta M.E., Motl R.W., Latimer-Cheung A.E. The safety of exercise training in multiple sclerosis: a systematic review. J Neurol Sci. 2014;343(1–2):3–7. doi: 10.1016/j.jns.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Jonsdottir J, Santoyo-Medina C, Kahraman T, et al. Changes in physiotherapy services and use of technology for people with multiple sclerosis during the COVID-19 pandemic. Mult Scler Relat Disord. 2023;71:104520. doi: 10.1016/j.msard.2023.104520. [DOI] [PubMed] [Google Scholar]
- 102.Strober L, Weber E, Lequerica A, Chiaravalloti N. Surviving a global pandemic: the experience of depression, anxiety, and loneliness among individuals with multiple sclerosis. Mult Scler Relat Disord. 2022;58:103497. doi: 10.1016/j.msard.2022.103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costabile T, Carotenuto A, Lavorgna L, et al. COVID-19 pandemic and mental distress in multiple sclerosis: implications for clinical management. Eur J Neurol. 2021;28(10):3375–3383. doi: 10.1111/ene.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Altieri M., Capuano R., Bisecco A., et al. The psychological impact of Covid-19 pandemic on people with Multiple Sclerosis: a meta-analysis. Mult Scler Relat Disord. 2022;61:103774. doi: 10.1016/j.msard.2022.103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novotná K., Větrovská R., Struskova E., et al. Despite the COVID-19 pandemic, people with chronic neurological disease (Multiple Sclerosis) are trying to maintain physical activity. Stud Sport. 2022;16(2):46–52. [Google Scholar]
- 106.Brichetto G., Tacchino A., Leocani L., Kos D. Impact of Covid-19 emergency on rehabilitation services for Multiple Sclerosis: an international RIMS survey. Mult Scler Relat Disord. 2022;67 doi: 10.1016/j.msard.2022.104179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reyes S., Cunningham A.L., Kalincik T., et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: an international consensus statement. J Neuroimmunol. 2021;357 doi: 10.1016/j.jneuroim.2021.577627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lieneck C., McLauchlan M., Phillips S. Healthcare cybersecurity ethical concerns during the COVID-19 global pandemic: a rapid review. Healthcare. 2023;11(22):2983. doi: 10.3390/healthcare11222983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lavorgna L., Brigo F., Moccia M., et al. e-Health and multiple sclerosis: an update. Mult Scler. 2018;24(13):1657–1664. doi: 10.1177/1352458518799629. [DOI] [PubMed] [Google Scholar]
- 110.Keszler P., Maloni H., Miles Z., Jin S., Wallin M. Telemedicine and multiple sclerosis: a survey of health care providers before and during the COVID-19 pandemic. Int J MS Care. 2022;24(6):266–270. doi: 10.7224/1537-2073.2021-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sadeghi N., Eelen P., Nagels G., et al. Innovating care in multiple sclerosis: feasibility of synchronous internet-based teleconsultation for longitudinal clinical monitoring. J Pers Med. 2022;12(3):433. doi: 10.3390/jpm12030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dillenseger A., Weidemann M.L., Trentzsch K., et al. Digital biomarkers in multiple sclerosis. Brain Sci. 2021;11(11):1519. doi: 10.3390/brainsci11111519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voigt I., Inojosa H., Dillenseger A., et al. Digital twins for multiple sclerosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.669811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.d'Arma A., Rossi V., Pugnetti L., et al. Managing chronic disease in the COVID-19 pandemic: an e-learning application to promote a healthy lifestyle for persons with multiple sclerosis. Psychol Health Med. 2022;27(2):428–435. doi: 10.1080/13548506.2021.1939072. [DOI] [PubMed] [Google Scholar]
- 115.Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: a Delphi consensus statement. Mult Scler. 2021;27(3):347–359. doi: 10.1177/1352458520952310. [DOI] [PubMed] [Google Scholar]