Abstract
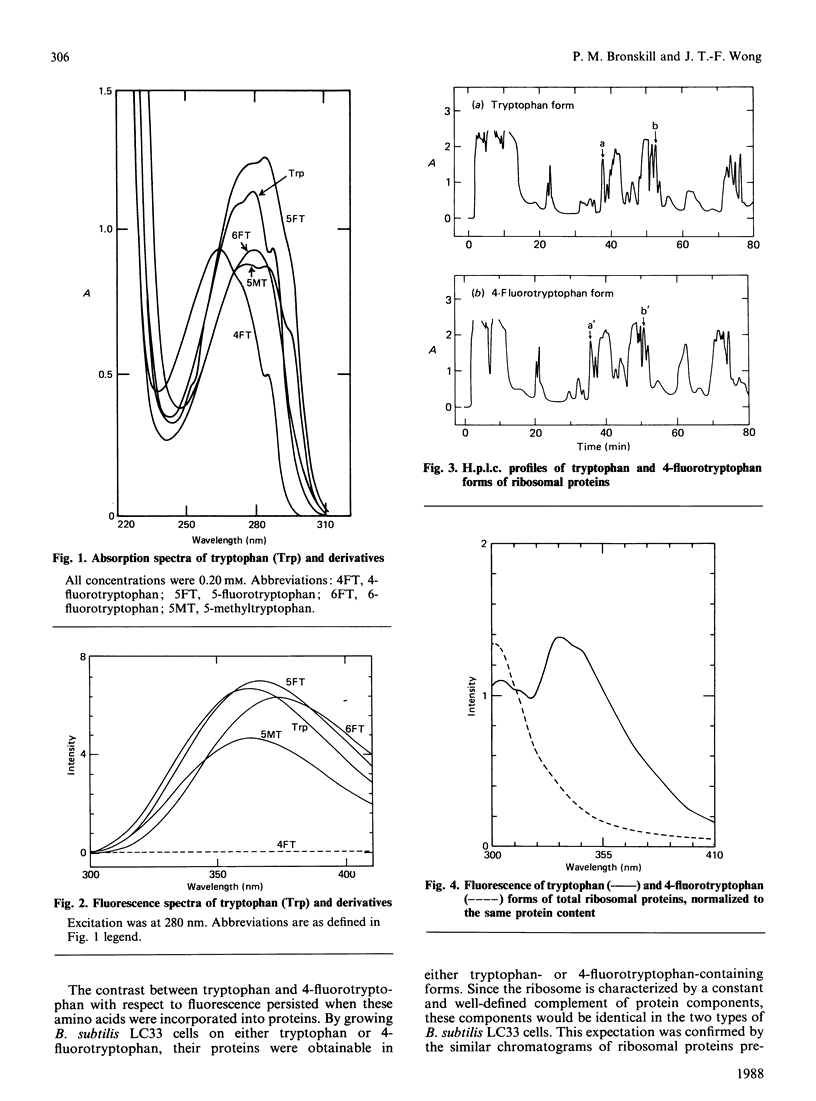
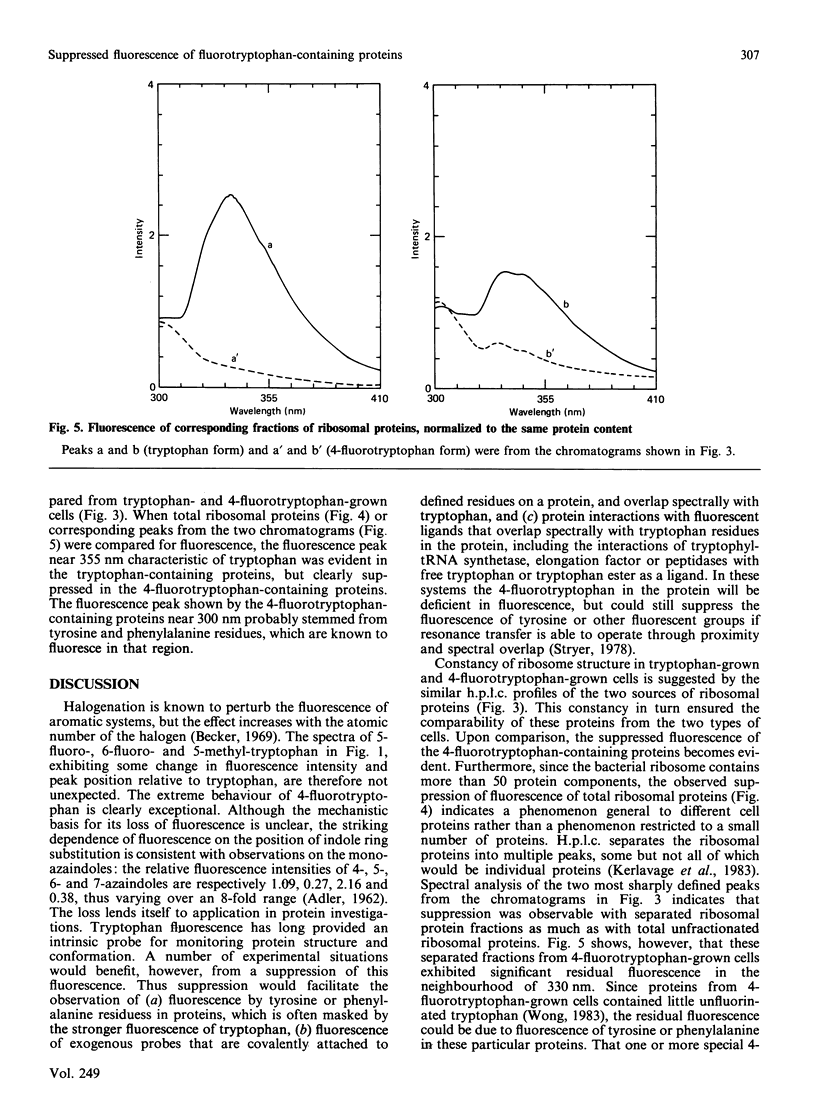
The tryptophan-auxotrophic Bacillus subtilis LC33 mutant strain utilizes either tryptophan or 4-fluorotryptophan for growth. Proteins therefore could be isolated from these cells in either tryptophan-containing or 4-fluorotryptophan-containing forms. Since 4-fluorotryptophan is non-fluorescent, tryptophan fluorescence would be suppressed in the 4-fluorotryptophan-containing proteins, facilitating the investigation of other chromophores either on the proteins or interacting with the proteins. This approach, potentially applicable to any protein endogenous to or clonable into B. subtilis, was illustrated by an examination of the fluorescence of B. subtilis ribosomal proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browne D. R., Kenyon G. L., Hegeman G. D. Incorporation of monoflurotryptophans into protein during the growth of Escherichia coli. Biochem Biophys Res Commun. 1970 Apr 8;39(1):13–19. doi: 10.1016/0006-291x(70)90750-3. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MUNIER R. Effets des analogues structuraux d'aminoacides sur la croissance, la synthèse de protéines et la synthèse d'enzymes chez Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):347–356. doi: 10.1016/0006-3002(59)90007-1. [DOI] [PubMed] [Google Scholar]
- Ferris R. J., Cowgill C. A., Traut R. R. Separation of ribosomal proteins from Escherichia coli and rabbit reticulocytes using reverse-phase high-performance liquid chromatography. Biochemistry. 1984 Jul 17;23(15):3434–3442. doi: 10.1021/bi00310a009. [DOI] [PubMed] [Google Scholar]
- Kerlavage A. R., Hasan T., Cooperman B. S. Reverse phase high performance liquid chromatography of Escherichia coli ribosomal proteins: standardization of 70 S, 50 S, and 30 S protein chromatograms. J Biol Chem. 1983 May 25;258(10):6313–6318. [PubMed] [Google Scholar]
- Milne A. N., Mak W. W., Wong J. T. Variation of ribosomal proteins with bacterial growth rate. J Bacteriol. 1975 Apr;122(1):89–92. doi: 10.1128/jb.122.1.89-92.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt E. A., Ho C. Incorporation of fluorotryptophans into proteins of escherichia coli. Biochemistry. 1975 Jul;14(13):3035–3040. doi: 10.1021/bi00684a037. [DOI] [PubMed] [Google Scholar]
- RENNERT O. M., ANKER H. S. ON THE INCORPORATION OF 5',5',5'-TRIFLUOROLEUCINE INTO PROTEINS OF E. COLI. Biochemistry. 1963 May-Jun;2:471–476. doi: 10.1021/bi00903a013. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Sykes B. D., Weingarten H. I., Schlesinger M. J. Fluorotyrosine alkaline phosphatase from Escherichia coli: preparation, properties, and fluorine-19 nuclear magnetic resonance spectrum. Proc Natl Acad Sci U S A. 1974 Feb;71(2):469–473. doi: 10.1073/pnas.71.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. T. Membership mutation of the genetic code: loss of fitness by tryptophan. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6303–6306. doi: 10.1073/pnas.80.20.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A. Studies on the mechanism of protein synthesis; incorporation of p-fluorophenylalanine into alpha-amylase of Bacillus subtilis. Biochim Biophys Acta. 1960 Jun 17;41:98–103. doi: 10.1016/0006-3002(60)90373-5. [DOI] [PubMed] [Google Scholar]



