Abstract
Purpose of review
The purpose of this review is to critically evaluate the effects of vitamin D on muscle mass and physical/muscle function in middle-aged and older adults, based on recent human studies, including cross-sectional, observational, and intervention studies. Vitamin D, beyond its well established role in bone health, has shown potential in influencing muscle physiology, making it a nutrient of interest in the context of sarcopenia and related chronic conditions.
Recent findings
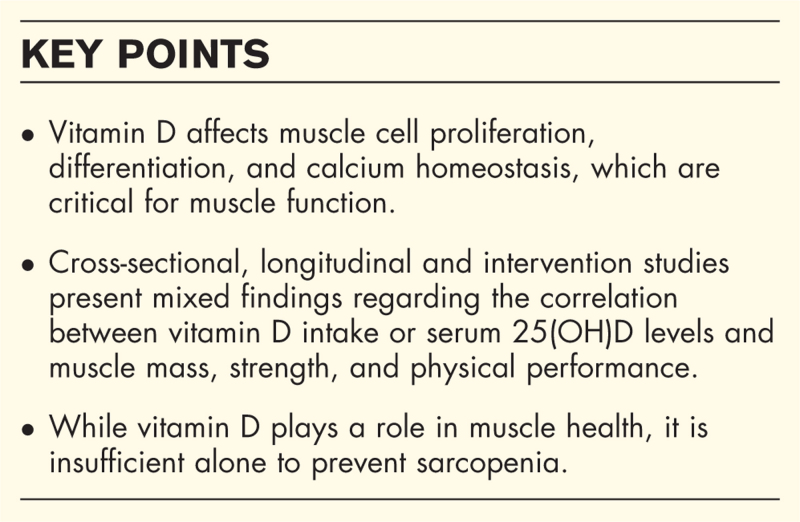
The review states how vitamin D affects muscle function, emphasizing its role in muscle cell proliferation, differentiation, and key signaling pathways. Additionally, the review of recent human studies revealed an inconsistent relationship between vitamin D and sarcopenia and related indices, with mixed results regarding muscle mass and strength. Variability in supplementation dose, duration, and baseline 25-hydroxyvitamin D levels may contribute to these inconsistencies.
Summary
While animal studies indicate vitamin D's effectiveness in muscle growth, cross-sectional, observational, and intervention studies do not show clear benefits of maintaining efficient vitamin D levels on muscle mass or function in humans. Although vitamin D impacts muscle health, it is insufficient alone, emphasizing the need for a multifaceted approach to sarcopenia prevention and management.
Keywords: muscle mass, muscle strength, physical function, sarcopenia, vitamin D
INTRODUCTION
Vitamin D, a crucial nutrient for maintaining bone health, has garnered significant attention for its potential impact on muscle mass and function. Given the rising interest in optimizing muscle health to combat sarcopenia and its associated chronic diseases (i.e. type 2 diabetes and cardiovascular diseases), an updated, evidence-based review is essential. This study aims to explore the latest findings on vitamin D status and its effects on skeletal muscle in middle-aged and older individuals. By synthesizing recent clinical trials and studies, we seek to provide a comprehensive understanding of vitamin D's role in muscle physiology, offering insights that could inform clinical practices and public health strategies to improve muscle health across populations.
Box 1.
no caption available
Vitamin D metabolism
Vitamin D is a fat-soluble nutrient primarily obtained through sunlight exposure and to a lesser extent from dietary sources and supplements. In humans, vitamin D exists in two primary forms: vitamin D2 (ergocalciferol), derived from plant sources, and vitamin D3 (cholecalciferol), synthesized in the skin through sun exposure and also found in animal-based foods. Once ingested or produced, vitamin D2/D3 is metabolized in the liver to 25-hydroxyvitamin D (25(OH)D), the main circulating form, by cytochrome P450 oxidases, primarily CYP2R1. This compound is then further hydroxylated in the kidneys to its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D) by 25-hydroxylase (CYP27B1) [1].
Role of vitamin D in the modulation of skeletal muscle mass and function
1,25(OH)2D works with the vitamin D receptor (VDR) to induce both genomic and nongenomic actions in skeletal muscle. Genomically, 1,25(OH)2D binds to VDR, which then forms a complex with the retinoid X receptor (RXR) and modulates gene expression by binding to vitamin D response elements (VDREs) in DNA. Nongenomically, 1,25(OH)2D influences intracellular signaling pathways, including the activation of c-Src and MAPK pathways, and modulates calcium homeostasis, impacting muscle function and proteostasis. Vitamin D treatment in-vitro setting has been shown to influence key aspects of muscle cell behavior, including myoblast proliferation, differentiation, and myotube formation. Notably, high doses of 1,25(OH)2D can inhibit myoblast proliferation but promote hypertrophy in differentiated myotubes. For example, recent study demonstrated that 1,25(OH)2D3 significantly enhances myogenic differentiation in C2C12 muscle cells by increasing myogenin expression through a functional VDRE on the myogenin promoter. This effect of vitamin D also resulted in larger myotube diameters and greater expression of myosin heavy chain isoforms, indicating its critical role in muscle cell development [2].
In animal studies, the role of vitamin D in muscle function and regeneration has been extensively explored using various models. Whole-body and tissue-specific VDR knockout models exhibit significant reductions in grip strength and muscle fiber size, along with increased expression of atrophy-related genes, underscoring the importance of VDR signaling in maintaining muscle integrity. Additionally, in injury models, the administration of vitamin D postinjury enhances muscle regeneration by promoting cell proliferation, decreasing apoptosis, and improving overall muscle repair [3]. These findings collectively suggest that vitamin D plays a crucial role in preserving muscle health and enhancing recovery following muscle damage or disease. Aside from the morphological changes, vitamin D influences muscle function by modulating calcium transport in skeletal muscle through nongenomic responses. It facilitates rapid calcium and phosphate movement across cell membranes, essential for muscle contraction. VDR regulates calcium flow when activated, further influencing muscle contraction. These mechanisms highlight vitamin D's role in calcium handling and muscle contraction [4].
Review of human studies
In focusing on the relationship between vitamin D and muscle health, sarcopenia – which increases the risk of clinical outcomes such as disability, falls, and mortality – is an important issue. Therefore, we reviewed articles published in the last 18 months that examined the relationship between vitamin D nutritional status and/or serum 25-hydroxyvitamin D [25(OH)D] levels and sarcopenia and related indicators such as muscle mass, muscle strength, and physical performance, in independent middle-aged and older adults. A systematic search was conducted using Medline (PubMed), with a detailed search strategy provided in Supplementary information.
We excluded RCT studies in which vitamin D was supplemented with other nutrients except for calcium. The results of the review are presented in Tables 1 and 2.
Table 1.
The association between vitamin D intake or serum concentration and physical performance and sarcopenia in cross-sectional and longitudinal studies
Table 2.
Baseline characteristics of the included studies: effects of Vitamin D supplementation on physical function, performance test, and activity indexes
| References | Group | Mean age in years (no. of individuals) | BMI | Vitamin D added, ng/ml | Intervention duration | Baseline 25 (OH)D, ng/ml, mean (SD) | After intervention 25 (OH)D, ng/ml, mean(SD) | Outcomes | Main outcome in the intervention group |
| Chou et al.[20▪] | Intervention | 64.7 (6.3) [520] | 28.1 (5.3) | 50 μg/day | 24 months | 27.6 (8.8) | 40.0 (9.0) | Grip strength, TUG, walking speed, standing balance, repeated chair stands, SPPB | No significant improvement in physical performance |
| Control | 65.1 (6.6) [534] | 28.3 (5.4) | 0 μg/day (placebo) | 28.7 (9.3) | No significant change | ||||
| Houston et al.[22] | Intervention | 73.7 (6.3) [66] | 30.2 (4.3) | 50 μg/day | 12 months | 19.4 (4.2) | 28.6 (6.7) | Lower-extremity leg power, leg and grip strength, SPPB, health ABC physical performance battery, TUG, postural sway, gait velocity | No significant improvement in leg power, strength, or physical performance |
| Control | 73.1 (6.3) [70] | 30.4 (4.6) | 0 μg/day (placebo) | 19.9 (4.9) | 20.2 (5.0) | ||||
| Schrack et al.[24] | Intervention | 77.2 (5.3) [293] | 30.6 (5.8) | ≥25 μg/day(25 μg/d, 50 μg/d, 100 μg/d) | 24 months | 22.0 (4.9) | No description | SPPB, physical activity outcomes (total activity counts per day, active minutes per day, and activity fragmentation) | No significant improvement in physical activity outcomes |
| Control | 77.2 (5.3) [278] | 30.6 (6.4) | 5 μg/day | 22.0 (5.2) | No description | ||||
| Draxler et al.[23] | Intervention | VD daily: No description [27]VD monthly: No description [31] | VD daily: 28.3 (4.4)VD monthly: 26.8 (4.3) | VD daily: 20 μg/dVD monthly: 1250 μg/m | 17 weeks | VD daily: 23.9 (6.0)VD monthly: 23.7 (6.6) | VD daily: 28.0 (7.6)VD monthly: 32.9 (7.6) | Arm curl test, TUG, 6-min walk test, 30- second chair stand test, the handgrip strength | No significant improvement in physical performance |
| Control | No description [27] | 26.2 (4.9) | 0 μg/day (placebo) | 20.8 (4.8) | 23.6 (8.3) | ||||
| Dawson-Hughes et al.[25] | Intervention | 71 (5) [185] | 26.9 (4.0) | 17.5 μg/d | 36 months | 22.2 (7.7) | 30.8 (7.5) | Muscle performance (timed walk, grip strength, and chair-rise), two balance tests, the one-leg stand and tandem stand | The dominant hand grip strength significantly decreased in the VD group compared with the placebo group [-4.4 (18.9) vs -1.0 (17.1), P = 0.024].No significant improvement in other physical performance and balance measure |
| Control | 71 (5) [201] | 26.8 (4.2) | 0 μg/day (placebo) | 21.3 (7.0) | 22.7 (6.3) | ||||
| Haghighi et al.[21] | Intervention | VD: 57.4 (4.8) [11]VD+resistance training: 55.4 (3.8) [11] | VD: 29.9 (5.0)VD+resistance training: 28.6 (3.1) | 1250 μg/2 wks(Equivalent to 75 μg/d) | 12 weeks | VD: 22.59 (14.67)VD+resistance training: 32.85 (12.49) | VD: 28.39 (18.05)VD+resistance training: 44.63 (17.4) | Leg press, chest press, leg extension, leg curl, shoulder press exercises | No significant improvement in upper- and lower-extremities muscle strength |
| Control | Placebo+resistance training: 55.4 (0.0) [11]Placebo: 55.8 (4.7) [11] | Placebo+resistance training: 29.8 (3.7)Placebo: 30.11 (5.3) | 0 μg/day (placebo) | Placebo+resistance training: 32.73 (6.74)Placebo: 19.91 (9.43) | Placebo+resistance training: 19.94 (9.1)Placebo: 20.2 (14.02) |
25(OH)D, 25-hydroxyvitamin D; SPPB, Short Physical Performance Battery; TUG, Timed-Up and Go; VD, Vitamin D.
The relationship of dietary vitamin D intake and sarcopenia indicators (cross-sectional studies)
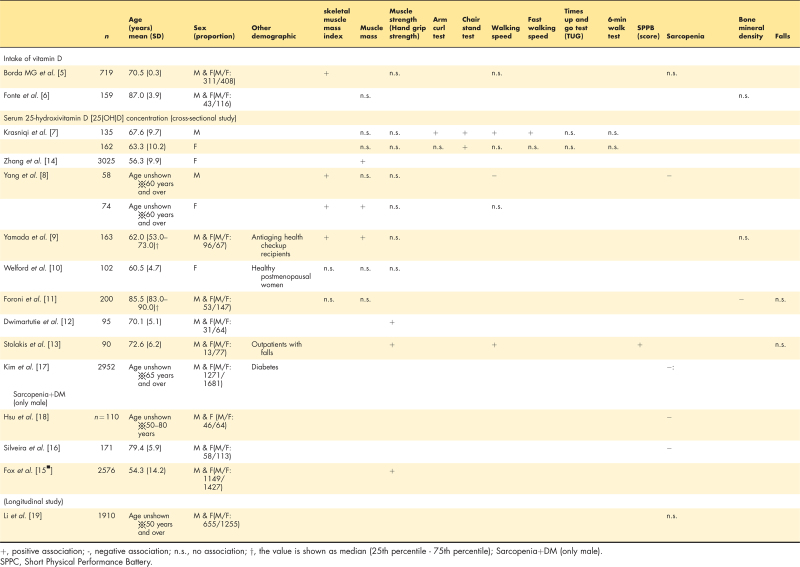
The studies about the relationship between vitamin D intake and indicator of sarcopenia diagnosis are two studies, but the results are not consistent. Among 719 older adults aged 70 years in Sweden, vitamin D intake assessed by diet history method showed a significant positive correlation with limb skeletal muscle mass index by DXA method (r = 0.219, P = 0.004), but no significant relationship with walking speed or grip strength [5]. On the other hand, there was no significant difference in the association between vitamin D intake by one-day dietary record including supplement and the muscle mass by DXA method among 159 Brazilian older adults aged 80 years or older [6].
The relationship of serum 25(OH)D concentration and muscle mass and physical/muscle function (cross-sectional studies)
Nine studies examined the relationship between serum 25(OH)D concentration and muscle mass, strength or function. Four investigated the relationship between serum 25(OH)D concentration and both of skeletal muscle mass and muscle strength [7–10]. There were four studies that only included older people [8,11–13]. Skeletal muscle mass was assessed by BIA in three studies [7,8,14] and by DXA in three studies [9–11]. Seven, two and three studies assessed muscle strength (hand grip strength) [7–10,12,13,15▪] and walking speed [7,8,13], respectively. For all indicators, the results were not consistent by sex and age group. In a study measuring serum 25(OH)D concentration in four seasons (spring, summer, autumn and winter), the results differed from season to season [10]. Considering that serum 25(OH)D concentration is also affected by sunlight, the season in which the study was conducted may have influenced the results.
The relationship between 25(OH)D concentration and falls (cross-sectional studies)
There are two studies about the relationship between 25(OH)D concentration and falls [11,13]. Participants were asked about their history of falls in the past 12 months. While participants with falls had a significantly lower serum 25(OH)D concentration than those without falls among people aged 65 years and over (24.3 ± 8.9 vs. 30.6 ± 9.3 ng/ml, P = 0.001) [13], no association was observed in the study for participants aged 80 years and over [11].
The relationship between sarcopenia and serum 25(OH)D concentration (cross-sectional studies)
Four studies examined the relationship between sarcopenia and serum 25(OH)D concentration. The diagnosis of sarcopenia used was the European Working Group on Sarcopenia in Older People (EWGSOP2) criteria in two articles [8,16] and the Asian Sarcopenia Working Group 2019 (AWGS2019) criteria in two articles [17,18].
The participants in all studies had a relatively high range of serum 25(OH)D concentrations. However, only one study found no association between sarcopenia diagnosis and serum 25(OH)D concentration [17]. However, in the study, the group with both sarcopenia and type 2 diabetes had the lowest serum 25(OH)D concentrations compared to those with sarcopenia alone, diabetes alone, and neither condition. A study conducted in Taiwan [18] included participants with type 2 diabetes, categorized into nonsarcopenia, possible sarcopenia, and sarcopenia groups. The findings demonstrated a significant inverse association between diabetes diagnosis and serum 25(OH)D levels. Furthermore, participants with both sarcopenia and osteoporosis had the lowest levels of vitamin D, which was generally consistent with previous study [16]. Future studies on the associations between serum 25(OH)D concentrations and sarcopenia should consider the involvement of other metabolic diseases.
Longitudinal study
In a cohort study involving 1910 Chinese men and women aged 50 years and older, the association between changes in sarcopenia status over 4 years and serum 25(OH)D levels was investigated. Sarcopenia was diagnosed using AWGS 2019 criteria. Although the assay method for determining serum 25(OH)D concentrations was not specified, no significant association was found after adjusting for covariates [19].
Vitamin D supplementation (intervention studies)
In this section, we review randomized controlled trials (RCTs), all of which were double-blind, investigating the effects of continuous vitamin D supplementation on physical functions and performance tests (Table 2). Six RCTs were selected [20▪,21–25]. Three studies used vitamins alone, one included calcium, one involved resistance exercise, and one combined vitamin D with both calcium and resistance exercise. Vitamin D dosages ranged from 17.5 to 50 μg per day, with interventions lasting from 12 weeks to 3 years. Individuals were aged 50 years or older, and five out of six studies included both men and women.
Three studies investigated the effects of vitamin D supplementation on physical performance, function, and activity. Two studies examined the effect of only supplemental vitamin D3 (cholecalciferol, 50 μg/day) on physical function and performance tests. Over a 2-year period in middle to older adults [20▪] and a 12-month period in older adults [22], both studies consistently reported no significant effects on physical function and performance tests. Another study [24] observed the effect of 50 μg/day vitamin D3 supplementation over 24 months on physical activity during free-living conditions in individuals over 70 years old. The results showed no significant changes in overall physical activity levels, suggesting that vitamin D supplementation alone does not influence daily physical activity patterns in older adults.
One study examined the effect of combining vitamin D and calcium on muscle strength and physical performance [25]. This study found no significant improvement in muscle performance measures such as walking speed, grip strength, and chair-rise time, or balance measures like the one-leg stand and tandem stand compared to placebo. In fact, despite the supplementation with vitamin D3 and calcium, grip strength of the dominant hand decreased in the supplemented group compared to the placebo group. On the contrary, higher 3-year mean 25(OH)D levels were positively associated with improved one-leg stand time, suggesting a potential benefit for balance.
Another study investigated the combined effects of vitamin D supplementation and resistance training over 12 weeks [23]. The results indicated significant improvements in muscle strength and power with resistance training. However, vitamin D supplementation alone did not provide any additional benefit. This highlights the importance of physical exercise in improving muscle function and suggests that vitamin D alone is not sufficient.
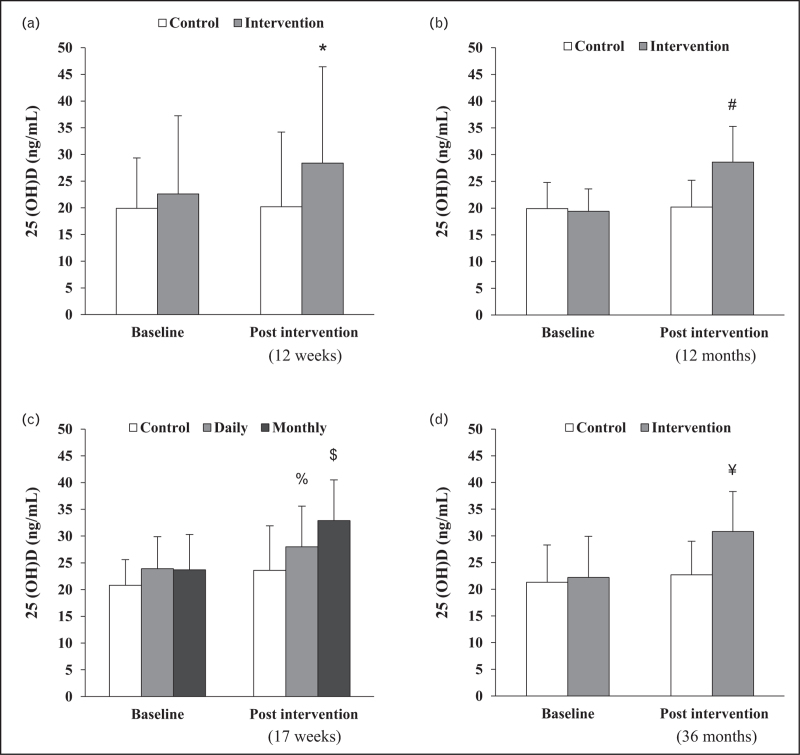
Overall, recent intervention studies suggest that vitamin D supplementation does not significantly enhance physical performance, muscle function, or daily physical activity patterns in middle-aged to older adults regardless of the frequency, dose, or duration of the intervention. One contributing factor is that the study was conducted in populations with vitamin D sufficiency (25(OH)D level ≥ 20 ng/ml) (Fig. 1). A previous review indicated that the effectiveness of vitamin D intervention on clinical outcomes is poor in vitamin D sufficient individuals with baseline circulating 25(OH)D levels of at least 20 ng/ml [26].
FIGURE 1.
Changes in Serum 25-hydroxyvitamin D [25(OH)D] concentrations before and after Vitamin D supplementation across four randomized controlled trials (RCTs). Panels (a: Ref. [21], b: Ref. [22], c: Ref. [23], and d: Ref. [25]) correspond to different RCTs. ∗ significantly different from baseline, # significant difference in change from pre to postintervention between groups (P < 0.0001), % P = 0.01 vs. baseline, $P < 0.001 vs. baseline, ¥P < 0.001 vs. control.
In addition, a recent meta-analysis further complicates this picture, revealing that vitamin D supplementation might negatively affect muscle health, including increased time for timed up and go (TUG) tests, decreased maximal muscle strength during knee flexion, and a trend toward lower Short Physical Performance Battery (SPPB) scores [27]. These findings suggest that vitamin D alone may not provide clear benefits for muscle health and may even have adverse effects under certain conditions. Furthermore, inconsistent findings regarding vitamin D's effects on muscle mass and function may stem from variations in supplementation dose, duration, baseline 25(OH)D levels, and genetic factors. Polymorphisms in the VDR gene, such as BsmI and FokI, might influence individual responses to vitamin D, leading to varied outcomes [28]. However, it is too early to draw conclusions due to the small number of studies reviewed.
CONCLUSION
The relationship between vitamin D and muscle health in middle-aged and older adults is complex and not fully conclusive. Recent human studies have shown inconsistent results regarding the benefits of vitamin D status on muscle mass, strength, and function. These mixed outcomes may be due to differences in study design, ingested dosages, and participants’ baseline 25(OH)D levels. While in-vitro and animal studies indicate that vitamin D plays a significant role in muscle physiology, these findings are not always reflected in human trials. It is clear that vitamin D alone is not sufficient to prevent sarcopenia, emphasizing the need for a multifaceted approach that includes resistance training and takes into account individual genetic differences. Future research should focus on unraveling the detailed interactions between vitamin D, muscle function, and other influencing factors to improve muscle health strategies for aging populations.
Acknowledgements
The authors thank Yu Cai for assistance in creating the evidence table.
Financial support and sponsorship
This research was supported by AMED under Grant Number JP 24dk0110051h0001 to Y.H., JSPS KAKENHI Grant Number JP21KK0177 to S.F.
Conflicts of interest
All authors have no conflict of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Giustina A, Bilezikian JP, Adler RA, et al. Consensus statement on Vitamin D status assessment and supplementation: whys, whens, and hows. Endocr Rev 2024; 27:bnae009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alliband KH, Parr T, Jethwa PH, Brameld JM. Active vitamin D increases myogenic differentiation in C2C12 cells via a vitamin D response element on the myogenin promoter. Front Physiol 2023; 14:1322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratos I, Schleese S, Rinas I, et al. Effect of calcitriol and Vitamin D receptor modulator 2 on recovery of injured skeletal muscle in Wistar rats. Biomedicines 2023; 11:2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sist M, Zou L, Galloway SDR, Rodriguez-Sanchez N. Effects of vitamin D supplementation on maximal strength and power in athletes: a systematic review and meta-analysis of randomized controlled trials. Front Nutr 2023; 10:1163313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borda MG, Samuelsson J, Cederholm T, et al. Nutrient intake and its association with appendicular total lean mass and muscle function and strength in older adults: a population-based study. Nutrients 2024; 16:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonte FK, Spinoza ED, Carvalho VA, et al. Relationship of protein, calcium and vitamin D consumption with body composition and fractures in oldest-old independent people. Clinical nutrition ESPEN 2024; 59:398–403. [DOI] [PubMed] [Google Scholar]
- 7.Krasniqi E, Boshnjaku A, Ukëhaxhaj A, et al. Association between vitamin D status, physical performance, sex, and lifestyle factors: a cross-sectional study of community-dwelling Kosovar adults aged 40 years and older. Eur J Nutr 2024; 63:821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Dai Y, Li Z, et al. Relationship of serum 25-hydroxyvitamin D levels with sarcopenia and body composition in community-dwelling older adults: a paired case-control study. J Am Med Direct Assoc 2023; 24:1213–1219. [DOI] [PubMed] [Google Scholar]
- 9.Yamada C, Kuwabara A, Sakai Y, et al. Usefulness of Vitamin D deficiency questionnaire for Japanese (VDDQ-J) for screening of Vitamin D deficiency and low muscle mass in relatively healthy Japanese anti-aging health checkup examinees. J Nutr Sci Vitaminol (Tokyo) 2023; 69:435–443. [DOI] [PubMed] [Google Scholar]
- 10.Welford AE, Darling AL, Allison SJ, et al. Lack of significant seasonal association between serum 25(OH)D concentration, muscle mass and strength in postmenopausal women from the D-FINES longitudinal study. J Nutr Sci 2022; 11:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foroni MZ, Cendoroglo MS, Sakane EN, et al. Serum 25 hydroxyvitamin D concentrations in individuals over 80 years old and their correlations with musculoskeletal and health parameters. Endocrine 2023; 79:559–570. [DOI] [PubMed] [Google Scholar]
- 12.Dwimartutie N, Setiati S, Tamin TZ, et al. Vitamin D levels in prefrail older adults and its correlation with hand grip strength. Acta Med Indones 2023; 55:172–179. [PubMed] [Google Scholar]
- 13.Stolakis K, Megas P, Panagiotopoulos E, et al. Association of vitamins B12 and D3 with balance and falls in a sample of Greek older people. J Musculoskelet Neuronal Interact 2023; 23:205–214. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Cheng Y, Chen C, et al. Interaction of estradiol and vitamin D with low skeletal muscle mass among middle-aged and elderly women. BMC Womens Health 2023; 23:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Fox FAU, Koch L, Breteler MMB, et al. 25-hydroxyvitamin D level is associated with greater grip strength across adult life span: a population-based cohort study. Endocr Connect 2023; 12:e220501. [DOI] [PMC free article] [PubMed] [Google Scholar]; The dose-response relationship between vitamin D levels and grip strength was approximately 20--40 ng/ml, suggesting an increased risk of adverse effects at excess levels of circulating 25-OHD levels above 50 ng/ml.
- 16.Silveira EA, Vinícius-Souza G, Pereira CC, et al. Osteosarcopenia later in life: prevalence and associated risk factors. Clin Nutr ESPEN 2023; 58:213–220. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Kobori T. Association of a combination of sarcopenia and Type 2 diabetes with blood parameters, nutrient intake, and physical activity: a nationwide population-based study. Nutrients 2023; 15:4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu YT, Lin JY, Lin CJ, et al. Association of possible sarcopenia or sarcopenia with body composition, nutritional intakes, serum Vitamin D levels, and physical activity among patients with Type 2 diabetes mellitus in Taiwan. Nutrients 2023; 15:3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Xiang Q, Dong B, et al. Transitional dynamics of sarcopenia and associations of nutritional indices with state transitions in Chinese aged ≥ 50. J Nutr Health Aging 2023; 27:741–751. [DOI] [PubMed] [Google Scholar]
- 20▪.Chou SH, Cook NR, Kotler G, et al. Effects of supplemental Vitamin D3, omega-3 fatty acids on physical performance measures in VITamin D and OmegA-3 TriaL. J Clin Endocrinol Metab 2024; dgae150.doi: 10.1210/clinem/dgae150. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a secondary analysis of the large RCT, VITamin D, and OmegA-3 TriaL (VITAL), which negated the risk-reducing effects of various clinical outcomes as well as physical function.
- 21.Haghighi AH, Shojaee M, Askari R, et al. The effects of 12 weeks resistance training and vitamin D administration on neuromuscular joint, muscle strength and power in postmenopausal women. Physiol Behav 2024; 274:114419. [DOI] [PubMed] [Google Scholar]
- 22.Houston DK, Marsh AP, Neiberg RH, et al. Vitamin D supplementation and muscle power, strength and physical performance in older adults: a randomized controlled trial. Am J Clin Nutr 2023; 117:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draxler A, Franzke B, Kelecevic S, et al. The influence of vitamin D supplementation and strength training on health biomarkers and chromosomal damage in community-dwelling older adults. Redox Biol 2023; 61:102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrack JA, Cai Y, Urbanek JK, et al. The association of vitamin D supplementation and serum vitamin D levels with physical activity in older adults: results from a randomized trial. J Am Geriatr Soc 2023; 71:2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson-Hughes B, Wang J, Barger K, Ceglia L. Effects of Vitamin D with calcium and associations of mean 25-hydroxyvitamin D levels with 3-year change in muscle performance in healthy older adults in the Boston STOP IT Trial. Calcif Tissue Int 2022; 111:580–586. [DOI] [PubMed] [Google Scholar]
- 26.Bouillon R, Manousaki D, Rosen C, et al. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 2022; 18:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bislev LS, Grove-Laugesen D, Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials. J Bone Miner Res 2021; 36:1651–1660. [DOI] [PubMed] [Google Scholar]
- 28.Bollen SE, Bass JJ, Wilkinson DJ, et al. The impact of genetic variation within the vitamin D pathway upon skeletal muscle function: a systematic review. J Steroid Biochem Mol Biol 2023; 229:106266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.