Abstract
Introduction:
Although the benefits of post-operative rehabilitation in cancer surgery are well established, the role of prehabilitation is less defined. Oesophagogastric cancers present a unique opportunity to study the impact of prehabilitation during the neoadjuvant window, whether with chemotherapy or chemoradiotherapy (NCT) in patients who are frequently nutritionally depleted. This trial examines the impact of a community-based exercise programme on patient fitness during and after the neoadjuvant window.
Methods:
A pragmatic, randomized controlled multicentre trial was undertaken in three centres. Inclusion criteria were patients aged at least 18 years planned for NCT and esophagectomy or gastrectomy. Participants were randomized 1:1 to an exercise prehabilitation group (EX) or to usual care (UC). The primary endpoint was cardiorespiratory fitness between baseline and pre-surgery time point using the 6-minute walk test (MVT). Secondary endpoints included a hand dynamometer, 10-s sit-to-stand, activity behaviour, body mass index, semi-structured interviews, questionnaires assessing the quality of life, surgical fear, general self-efficacy and mastery.
Results:
Between March 2019 and December 2020, 71 participants were recruited: EX (n=36) or UC (n=35). From baseline to pre-surgery, the difference-in-difference (DID) for EX showed a significant improvement in 6MWT of 50.7 m (P=0.05) compared to UC [mean (SD): 522.1 m (+/−104.3) to 582.1 m (+/−108) vs. 497.5 m (+/−106.3) to 506.0 m (+/−140.4). There was no statistically significant DID for secondary outcome measures.
Conclusions:
This community exercise prehabilitation programme significantly improves physical fitness for surgery, is feasible and provides a standardized framework for the prescription of exercise in oesophagogastric cancer patients undergoing NCT.
Keywords: gastric cancer, oesophageal cancer, prehabilitation
Introduction
Highlights
A community-based exercise programme can have a meaningful impact on physical fitness in patients undergoing neoadjuvant therapy for oesophagogastric cancer.
Exercise during the neoadjuvant window has no negative impact on body composition or sarcopenia scoring.
There are equivalent psychological outcomes for patients undergoing an exercise programme or usual care during the neoadjuvant window.
Patients in the exercise intervention cohort were more likely to identify physical activity as important to their well-being over the course of their treatment.
Oesophageal and gastric cancer remain important causes of gastrointestinal malignancies and collectively resulted in over 1 300 000 deaths worldwide in 20181. Although the global incidence of gastric cancer is slowly but consistently declining, the incidence of oesophageal cancer is increasing, with a steady increase in cases of oesophageal adenocarcinoma in Western populations1. Although both malignancies used to carry a dismal prognosis, improvements in perioperative therapies have impacted outcomes, with significant improvements in survival2. In oesophageal cancer, neoadjuvant cancer therapies (NCT) comprise either neoadjuvant chemoradiation, typically with the CROSS protocol, or perioperative chemotherapy with the FLOT protocol3,4. More recently, CheckMate 577 demonstrated a survival advantage with adjuvant nivolumab for patients undergoing CROSS chemoradiation who had residual disease on pathological examination, opening a new cohort of patients to post-operative adjuvant therapy5. Patients presenting with oesophagogastric malignancy are frequently malnourished due to dysphagia, with sarcopenia and poor physical fitness6. Despite their benefits, NCT can have deleterious impacts on patient fitness, with a proportion of patients not progressing to curative resection due to toxicities7–11. However, the neoadjuvant window also provides an opportunity for multidisciplinary intervention with a view to optimising patient fitness for surgery11–14.
Impaired physical fitness at the time of surgery is related to worse perioperative outcomes, which may impact overall survival15. Although there is evidence that post-operative rehabilitation improves patient outcomes in oesophageal cancer16, evidence on intervention in the neoadjuvant window is limited and may not be fully applicable to a modern cohort of oesophagogastric cancer patients, given recent changes in NCT and the advance of minimally invasive surgical approaches11–14,17,18.
To date, exercise programmes have been predominantly delivered with significant attendance in hospital settings17,18. Community-based exercise programmes reduce the necessity for patient attendance13. They are pragmatic and can facilitate engagement with exercise programmes during a busy neoadjuvant window while patients are undergoing NCT and who may be travelling significant distances due to centralised care19. Although studies on community-based programs are still sparse, they appear to have good compliance rates and can improve fitness and health-related quality of life13,19.
This comprehensive randomised controlled trial (RCT) is a multicentre trial determining the effect of a community-based exercise programme on cardiorespiratory fitness measured by the 6-minute walk test (6MWT) in the neoadjuvant, pre-operative and post-operative time periods with usual care for patients undergoing NCT and surgical resection of oesophagogastric cancers.
Methods
Study design
A pragmatic single-blind multicentre RCT was performed. The reporting was as per CONSORT guidelines (Supplemental Digital Content 1, http://links.lww.com/JS9/C861) and the full protocol has been published20,21. Participants were recruited at three university teaching hospitals. The methodology for the PERIOP-OG trial was based on experience gained from a feasibility study performed by our team, which was informed by our patient and public representatives. The protocol is published elsewhere21. The research ethics board approved the study at each participating site, and the study was registered at ClinicalTrials.gov with trial registration number NCT03807518. The date of the first registry was 17 January 17 2019.
Participants and randomisation
Patients aged at least 18 years planned for neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy prior to oesophagectomy or gastrectomy (total or subtotal) at any of the recruiting hospitals were eligible. Patients were identified at multidisciplinary cancer meetings, given oral information along with an information leaflet, and were then contacted 72 h later to confirm participation. A baseline assessment visit was scheduled, during which informed consent was obtained. Participants were randomized using a central random allocation sequence (1:1) using Stata version 17.1 (StataCorp. 2021, StatCorpLLC). As previously reported, due to the nature of the study, blinding patients, data collectors and physiological assessors were not possible, but the treating surgeons and their teams are blinded to randomisation, as is the primary analyst21.
Nutrition
All enrolled participants followed a standardized nutritional pathway of care, which was delivered by specialist dietitians dedicated to the care of oesophagogastric cancer patients at all sites.
Procedures
All patients were assessed at five different time points: baseline/pre-NCT, post-NCT, pre-surgery, post-surgery and 6 weeks later. Between March 2019 and March 2020, assessments were undertaken by trained staff. Due to COVID-19 government restrictions, after March 2020, assessments were undertaken by the participants at home using step-by-step instructions and a video demonstration of the assessments. Resources in the primary institution enabled baseline and pre-operative assessments to be conducted by trained staff throughout the trial period.
Patient comorbidities including documented prior respiratory disease, cardiovascular disease (including peripheral vascular disease), diabetes mellitus and documented orthopaedic disease (arthritis or prior joint replacement) were recorded.
Usual care (UC)
The UC group received routine care throughout their cancer pathway. No specific advice about exercise training was offered.
Exercise prehabilitation (EX)
The exercise training programme started before NCT (if time allowed), continued during NCT, and went up to the point of surgery. Following surgery, once patients were deemed clinically fit by the treating multidisciplinary team in the outpatient clinic (including a surgeon, physiotherapist, dietician, and nurse specialist), a 6-week post-operative exercise programme commenced. Participants in EX were offered an option to participate in either a centre-based exercise programme (CBEP) (in any of the seven exercise centres nationally), a home-based exercise programme (HBEP) or a combination. All participants in EX were provided with an exercise programme pack, which included a manual exercise handbook, a Fitbit, a rate of perceived exertion scale and a physical activity diary. They were also given a link to an online motivational video developed specifically for the PERIOP-OG trial. The exercise training programme is reported elsewhere21 and in Supplementary Appendix 1 (Supplemental Digital Content 2, http://links.lww.com/JS9/C862).
Outcomes
The primary outcome was measurement of change in cardiorespiratory fitness using the 6MWT assessed at the pre-operative time point22,23.
Secondary outcomes were:
Physical Health measured by:
Strength: Sit-to-stand test (×10 times)24 and hand dynamometer25.
Activity Behaviour: Physical activity and sedentary behaviour. Activity behaviour is assessed using a 7-day ActivPAL3 triaxial accelerometer. Participants in both groups are instructed to wear this device on the midpoint of the anterior aspect of the right thigh continuously for 7 days. The accelerometers do not provide participants with any feedback: data can only be analysed centrally by the lead researchers. Total activity counts per day, as well as time in sedentary behaviour are recorded for both groups21.
-
Body Composition: Body Mass Index (BMI)
Psychological Health measured by:
Life Orientation Test-Revised (LOT-R)26.
EQ-5D-5L health questionnaire27.
Functional Assessment of Cancer Therapy-Oesophageal (FACT-E) questionnaire28.
The Surgical Fear Questionnaire (SFQ)29.
General Self-Efficacy (GSE)30.
Pearlin Mastery Scale (PMS)31.
Semi-structured interviews: Pre-surgery and post-surgery to explore patients’ perceptions of the surgical pathway (Interview script is presented in Supplementary Appendix 2, Supplemental Digital Content 2, http://links.lww.com/JS9/C862).
Exploratory outcomes included data collection on:
Nutritional status: Glasgow Prognostic Score32 and Foodbook-24 dietary tool33.
Sarcopenia: using Horos software (www.horosproject.orgv3.3.5) to analyse computerised axial tomography (CT) imaging. Full imaging analysis was performed for 59 patients (26 intervention, 33 control). Imaging for the remaining patients was not available due to issues arising out of a national information technology systems security breach that occurred during Spring 2021. Skeletal muscle area was calculated using segmentation analysis at the level of the L3 vertebral body using density values of −29 to +150 Hounsfield units (HU)34. Visceral adiposity was calculated by segmentation analysis at the level of the L3/L4 intervertebral disc space using density values of −190 to −30 and manually removing subcutaneous fat34. To calculate subcutaneous fat, the reverse was performed with visceral fat removed34. Values were then calculated as cm2/m2 based on patient height34. Initial staging CT scans were compared with re-staging CT scans following the completion of NCT21.
Blood markers of inflammation: C-reactive protein and white cell count.
Post-operative Morbidity Score (POMS)35; the Clavien–Dindo classification36, and the Comprehensive Complication Index37. For patients undergoing oesophagectomy, post-operative morbidity was recorded as per the Oesophagectomy Complications Consensus Group38. Mortality was assessed at 30 days and 90 days. NCT toxicity, tolerance and compliance were recorded for all participants and adverse events in the relevant case report forms.
Estimated VO2peak (ml/kg/min) was calculated based on 6MWT using a standardised formula (VO2peak=0.03 × distance(m) + 3.98)39,40.
Sample size calculation
The sample size calculation was based on results from a recent publication13. Assuming a similar baseline 6MWT score, a 15% difference can be detected with a P value of 0.05 and a power of 80% with a sample of 26 participants in each group. Allowing for a 20% dropout, recruitment of 62 participants was required to demonstrate a 15% difference between groups with a P value of 0.05 and power of 80% with a sample of 26 participants in each group. Note: In response to the COVID-19 lockdown, the trial end date was extended from Summer 2020 to December 2020, and the final number recruited was 71 participants.
Statistical analysis
Descriptive analyses compared patient characteristics and outcome measures at different time points by group assignment. Group differences were tested using one-way ANOVA for numerical data and χ 2 tests for categorical data. A P≤0.05 was considered significant. The normality of the distribution of data was assessed using the Shapiro–Wilk test. Difference-in-differences (DID) assessments were conducted for group difference changes in outcomes between baseline/pre-NCT and pre-surgery and between post-surgery and 6 weeks later using multilevel regression models with the intervention group, time point and interaction of intervention and time points as covariates. As is standard practice for reporting RCTs, analysis was conducted without confounder adjustment. All analyses were performed as intention-to-treat analyses with all available observations. The primary outcome analysis tested DID in 6MWT. Similar analyses were conducted for secondary outcomes. Analysis was conducted using Stata version 17.1 (StataCorp. 2021, StatCorpLLC).
Results
Between 1 March 2019 and 31 December 2020, 117 patients were identified as eligible and 71 agreed to participate (Fig. 1). Fifty were recruited from centre A, 11 from centre B and 10 from centre C. Thirty-six were randomized to EX (following recruitment, one participant’s pathway changed and was therefore no longer eligible) and 35 to UC. The mean age was 62.8 years (+/−9.2) and 73% were male. Patient characteristics are presented in Table 1. For patients screened and not recruited, the mean age was 63.4 (10.6) years and 72% were male. Baseline characteristics were similar between the groups, with no differences in age, sex, BMI, smoking status, type of neoadjuvant therapy, American Society of Anaesthesiologists physical classification status (ASA status), Eastern Co-operative Oncology Group (ECOG) status or disease stage. Of note, there was a higher proportion of oesophagectomies included in the EX group (86% vs. 60%, P=0.01) and a higher number of cervical anastomoses (12% vs. 0%, P=0.04). There was also a higher LOT-R score in the EX group (21.8 vs. 18.1, P<0.001).
Figure 1.
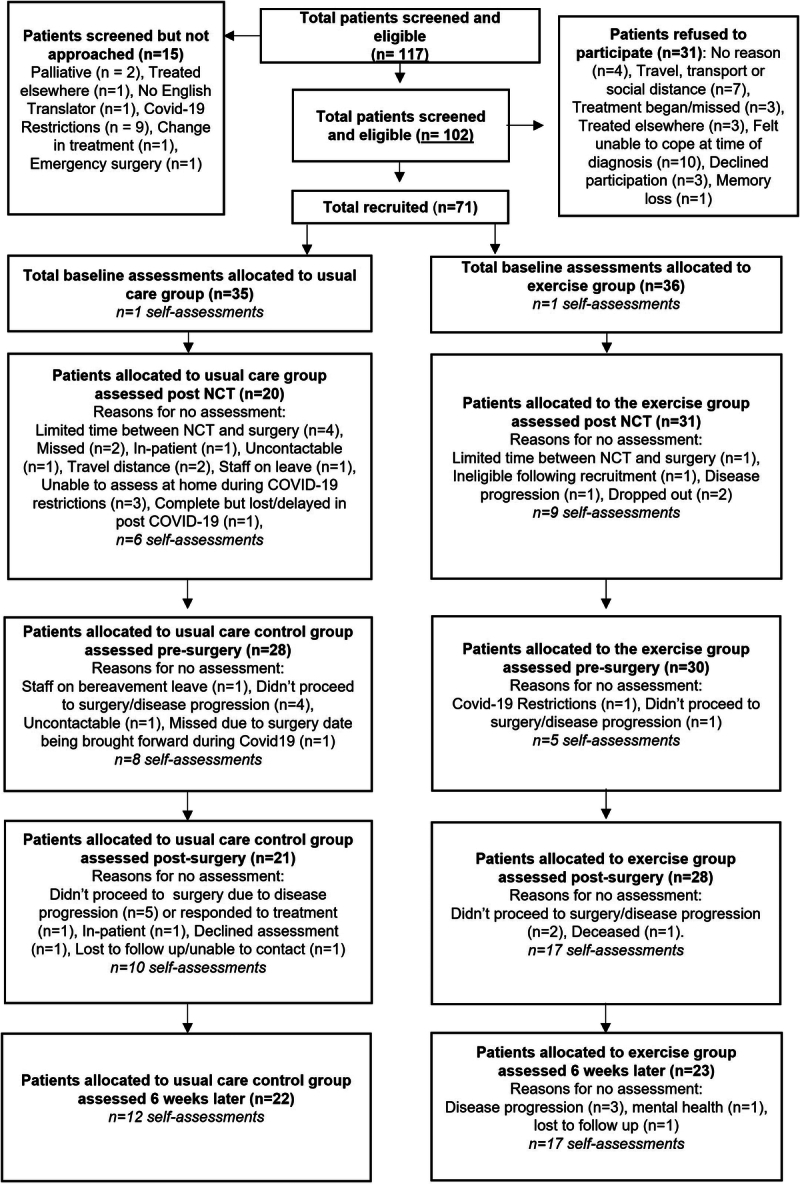
CONSORT diagram showing recruitment and assessment of patients at baseline, post-NCT, pre-surgery, post-surgery and at 6 weeks post-surgery. NCT, neoadjuvant cancer therapy.
Table 1.
Patient characteristics.
| Patient characteristics | Exercise (n=36) | Usual care (n=35) | All patients (n=71) | P |
|---|---|---|---|---|
| Age (years)a | 62.8 (9.2) | 61.5 (8.8) | 62.2 (9.0) | 0.53 |
| Genderb | ||||
| Male | 27 (75) | 25 (71) | 52 (73) | |
| Female | 9 (25) | 10 (29) | 19 (27) | 0.73 |
| Body mass index (kg/m2)a | 27.9 (5.5) | 27.7 (4.5) | 27.8 (5) | 0.91 |
| Frailty scorea | 25.7 (4.1) | 27.2 (5.3) | 26.4 (4.8) | 0.19 |
| Smoking statusb | ||||
| Current | 3 (9) | 5 (14) | 8 (11) | |
| Previous | 16 (44) | 17 (49) | 33 (47) | |
| Never | 16 (44) | 13 (37) | 29 (41) | |
| Unknown | 1 (3) | 0 (0) | 1 (1) | 0.66 |
| Dysphagia scoreb | ||||
| 0 | 12 (33) | 17 (49) | 29 (41) | |
| 1 | 8 (22) | 7 (20) | 15 (21) | |
| 2 | 7 (19) | 4 (11) | 11 (16) | |
| 3 | 6 (17) | 6 (17) | 12 (17) | |
| 4 | 1 (3) | 0 (0) | 1 (1) | |
| Unknown | 2 (6) | 1 (3) | 3 (4) | 0.6 |
| Nutritionb | ||||
| I | 20 (56) | 16 (46) | 36 (51) | |
| II | 6 (17) | 7 (20) | 13 (18) | |
| III | 8 (22) | 11 (31) | 19 (27) | |
| Unknown | 2 (5) | 1 (3) | 3 (4) | 0.61 |
| ECOG scoreb | ||||
| 0–1 | 34 (94) | 32 (91) | 66 (93) | |
| 2–3 | 2 (6) | 3 (9) | 5 (7) | 0.25 |
| ASA gradeb | ||||
| I | 6 (17) | 1 (3) | 7 (10) | |
| II | 18 (50) | 25 (71) | 43 (60) | |
| III | 12 (33) | 9 (26) | 21 (30) | 0.08 |
| Comorbidities | ||||
| Diabetes mellitus | 4 (11) | 3 (9) | 7 (10) | 0.72 |
| Respiratory | 6 (17) | 4 (11) | 10 (14) | 0.53 |
| Cardiovascular | 14 (39) | 21 (60) | 35 (49) | 0.08 |
| Orthopaedic | 3 (9) | 5 (14) | 8 (11) | 0.43 |
| Tumour locationb | ||||
| Oesophageal | 26 (72) | 18 (51) | 44 (62) | |
| Junctional | 6 (17) | 7 (20) | 13 (18) | |
| Gastric | 4 (11) | 10 (29) | 14 (20) | 0.18 |
| cT Categoryb | ||||
| T1 | 1 (3) | 1 (3) | 2 (3) | |
| T2 | 6 (16) | 2 (6) | 8 (11) | |
| T3 | 26 (72) | 26 (74) | 52 (73) | |
| T4 | 1 (3) | 4 (11) | 5 (7) | |
| Unknown | 2 (6) | 2 (6) | 4 (6) | 0.42 |
| cN Categoryb | ||||
| N0 | 14 (39) | 9 (25) | 23 (32) | |
| N1 | 14 (39) | 16 (46) | 30 (42) | |
| N2 | 6 (17) | 8 (23) | 14 (20) | |
| Unknown | 2 (5) | 2 (6) | 4 (6) | 0.47 |
| Neoadjuvant treatmentb | ||||
| CROSS | 29 (81) | 23 (66) | 52 (73) | |
| FLOT | 6 (16) | 12 (34) | 18 (25) | |
| No treatment (change of pathway) | 1 (3) | - | 1 (2) | 0.12 |
| Surgical characteristicsb | Exercise (n=29) | Usual care (n=25) | All patients (n=54) | |
| Surgery type | ||||
| Oesophagectomy | 25 (86) | 15 (60) | 40 (74) | |
| Gastrectomy | 4 (14) | 10 (40) | 14 (26) | 0.01c |
| Surgical procedure | ||||
| Oesophagectomy | ||||
| Transhiatial | 2 (8) | 0 (0) | 2 (4) | |
| Ivor Lewis | 22 (88) | 15 (100) | 37 (93) | |
| McKeown | 1 (4) | 0 (0) | 1 (3) | 0.47 |
| No. of minimally invasive approaches | 19 (76) | 9 (60) | 28 (70) | 0.16 |
| Gastrectomy | ||||
| Total gastrectomy | 2 (50) | 3 (30) | 5 (36) | |
| Extended total | 0 (0) | 2 (20) | 2 (14) | |
| Partial gastrectomy | 2 (50) | 5 (50) | 7 (50) | 0.41 |
| Oesophagectomy characteristics | Exercise (n=25) | Usual care (n=15) | All patients (n=40) | |
| Location of anastomosisb | ||||
| Cervical | 3 (12) | 0 (0) | 3 (7) | |
| Thoracic | 22 (88) | 15 (100) | 37 (93) | 0.04c |
| Thoracic phaseb | ||||
| Open | 4 (16) | 6 (40) | 10 (25) | |
| Thoracoscopic completed | 19 (76) | 9 (60) | 28 (70) | |
| Not applicable (transhiatial) | 2 (8) | 0 | 2 (5) | 0.16 |
| Abdominal phaseb | ||||
| Open | 3 (12) | 3 (20) | 6 (15) | |
| Lap converted to open | 1 (4) | 1 (7) | 3 (7) | |
| Lap completed | 12 (48) | 6 (40) | 18 (45) | |
| Lap assisted | 3 (12) | 0 (0) | 2 (5) | |
| Robotic | 6 (24) | 5 (33) | 11 (28) | 0.53 |
| Gastrectomy: surgical access | Exercise (n=4) | Usual care (n=10) | All patients (n=14) | |
| Lap completed | 4 (100) | 8 (80) | 12 (86) | |
| Lap converted to open | 0 (0) | 2 (20) | 2 (14) | |
| LOT-Ra | 21.8 (3) | 18.1 (7) | 20 (5.6) | <0.001c |
Note: Dysphasia is classified as follows: 0 – able to eat a normal diet/no dysphagia; 1 – able to swallow some solid foods; 2 – able to swallow only semi-solid foods; 3 – able to swallow liquids only; 4 – unable to swallow anything/total dysphagia. Nutrition is classified as follows: 1 – no additional supplementation needed; 2 – PO supplements and referred to a dietician; 3 – early dietetic intervention with assessment for supplement food feeding. ASA is classified as follows: 1 – a normal and healthy patient; 2 – a patient with mild systemic disease; 3 – a patient with severe and systemic disease; 4 – a patient with severe and systemic disease that is a constant threat to life; 5 – a moribund patient who is not expected to survive without the operation; 6 – a declared brain-dead patient whose organs are being removed for donation. ECOG is classified as follows: 0 – fully active and able to carry on all pre-disease performance without restriction; 1 – restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, for example, light housework and office work; 2 – ambulatory and capable of all self-care but unable to carry out any work activities and up and about more than 50% of waking hours; 3 – capable of only limited self-care and confined to bed or chair more than 50% of waking hours; 4 – completely disabled, cannot carry out any self-care, totally confined to bed or chair; 5 – death.
Data are presented as mean (SD).
n (%).
Was taken as statistically significant (P<0.05).
ASA, American Society of Anaesthesiology; ECOG, Eastern Cooperation Oncology Group; LOT-R, Revised Life Orientation Test Questionnaire.
Exercise prehabilitation
The mean (SD) duration of the pre-operative exercise training was 12.7 (+/−2.4) weeks. Four completed the CBEP, seven a mix of the CBEP and HBEP and the remaining 24 completed a HBEP (Supplementary Appendix 1, Supplemental Digital Content 2, http://links.lww.com/JS9/C862). Exercise diaries were returned for 29 (82.9%) participants in the intervention group pre-operatively and 25 (89.3%) post-operatively.
Primary outcome
There was a significant improvement in 6MWT (measured by DID) from baseline to pre-surgery for the EX group compared to UC [+50.7 m, P=0.05; EX mean (SD): 522.1 m (104.3) to 582.1 m (108) vs. UC mean 497.5 m (106) to 506 (140)]. From post-surgery to 6-week reassessment, there were no significant differences between the groups with both improving their 6MWT, although without a statistically significant difference in the degree of improvement (P=0.19) (Table 2 and Fig. 2).
Table 2.
Primary and secondary outcomes.
| Baseline, n | Post-NCT, n | Pre-surgery, n | Post-surgery, n | 6-Week reassessment, n | Diff-in-diff baseline to pre-surgery, P value (95% CI EX; UC) | Diff-in-diff post-surgery to 6 weeks, P value (95% CI EX; UC) | |
|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||
| 6 min walk test (m) | |||||||
| Exercise | 522.1 (104.3), 36 | 543.4 (108.7), 30 | 582.1 (108), 29 | 498.3 (99.5), 28 | 578.3 (104.7), 22 | 50.7, 0.05 (38.3–85.8; −8.5 to 48.0) | 31.8, 0.19 (18.5–85.6; 0.5–76.8) |
| Control | 497.5 (106.3), 34 | 511.2 (172), 19 | 506.0 (140.4), 24 | 515.0 (91.5), 17 | 547.6 (99.5), 17 | ||
| Secondary outcomes: Physical health | |||||||
| Handy dynamometer (kg) | |||||||
| Exercise | 31.1 (8.2), 34 | 31.1 (7.1), 22 | 28.2 (8.5), 23 | 28.0 (9.6), 11 | 31.6 (10.6), 6 | 0.4, 0.77 | −2.3, 0.23 |
| Control | 30.6 (9.1), 32 | 26.1 (7.9), 12 | 25.1 (8.7), 15 | 25.0 (8.5), 11 | 26.9 (9.1), 8 | (−5.1 to −1.3; −5.5 to −0.7) | (−1.4 to 3.4; −0.4 to 4.9) |
| Lower body strength (s) | |||||||
| Exercise | 18.2 (5.7), 36 | 17.7 (7.7), 29 | 15.5 (5.1), 28 | 17.9 (7.7), 26 | 15.3 (7.5), 22 | −2.5, 0.08 | −0.4, 0.80 |
| Control | 19.6 (7.2), 33 | 20.6 (8), 19 | 20.1 (6.7), 24 | 20.4 (5.1), 17 | 19.3 (5.3), 17 | (−4.5 to −0.9; −2.6 to 3.0) | (−6.5 to 0.7; −3.4 to 1.1) |
| Activity behaviour: Daily step count | |||||||
| Exercise | 7175.1 (4024.6), 29 | 6692.5 (3363.7), 25 | 7420.3 (4762.2), 19 | 5873.6 (3676.1), 21 | 5954.6 (3027), 21 | −63.4, 0.95 (−1106 to 2495; −1409 to 1799) | −711.6, 0.50 (−1698 to 2296; −231 to 1769) |
| Control | 6589.9 (4014.8), 27 | 5802.9 (3299.5), 19 | 7206.3 (2831.6), 19 | 6371.0 (3775.5), 18 | 6871.7 (4120.9), 17 | ||
| Sedentary behaviour (h) | |||||||
| Exercise | 19.1 (1.9), 29 | 19.8 (1.6), 25 | 18.4 (2.2), 19 | 20.0 (1.4), 21 | 19.7 (1.5), 21 | 0.7, 0.44 | 0.3, 0.61 |
| Control | 19.4 (2.3), 27 | 19.3 (1.9), 19 | 17.9 (3.2), 19 | 19.5 (2.1), 18 | 18.8 (2.5), 17 | (−1.4 to 0.9; −3.7 to 1.1) | (−1.1 to 0.6;−0.5 to 0.2) |
| Body mass index (kg/m2) | |||||||
| Exercise | 27.9 (5.5), 33 | 26.9 (4.3), 27 | 27.1 (5), 27 | 26.4 (4.7), 24 | 25.1 (4), 16 | −0.3, 0.77 | −0.7, 0.85 |
| Control | 27.7 (4.5), 30 | 26.4 (5.3),16 | 26.4 (4.9),,25 | 24.2 (4.3),,15 | 22.8 (2.4),,11 | (−1.7 to 0.4; −1.8 to 0.1) | (−1.1 to 0.4; −1.7 to −0.2) |
| Psychological health: EQ-5D Questionnaire | |||||||
| EQ-Utility | |||||||
| Exercise | 0.897 (0.165), 36 | 0.902 (0.132), 31 | 0.920 (0.119), 29 | 0.801 (0.172), 27 | 0.872 (0.113), 23 | 0.045, 0.27 (−0.1 to 0.0; −0.1 to 0.0) | 0.05, 0.23 (0.0–0.1; −0.1 to 0.2) |
| Control | 0.909 (0.115), 34 | 0.902 (0.122), 19 | 0.894 (0.128), 28 | 0.887 (0.103), 20 | 0.883 (0.158), 20 | ||
| EQ-VAS | |||||||
| Exercise | 78.8 (16.6), 36 | 76.7 (15.4), 31 | 83.6 (14.5), 29 | 70.4 (13.5), 27 | 76.6 (14.7), 23 | 5.4, 0.21 (−1.6 to 12.0; −8.1 to 3.6) | −1.2, 0.77 (−1.2 to 19.2; 3.4–12.5) |
| Control | 76.9 (19.2), 34 | 71.6 (19.2), 19 | 78.3 (15), 28 | 71.8 (15.2), 20 | 78.8 (11.8), 20 | ||
| FACT Questionnaire Total Score (0–176-best) | |||||||
| Exercise | 139.4 (17.2), 34 | 141.1 (17.4), 31 | 152.2 (16.2), 29 | 137.8 (14.9), 27 | 142.6 (19.5), 23 | 4.9, 0.23 (6.2–18.2; 0.4–18.8) | −0.5, 0.91 (−2.8 to 13.3; 12.7–13.1) |
| Control | 141.0 (22.8), 32 | 138.6 (27.1), 17 | 147.8 (25.4), 27 | 143.0 (20.5), 20 | 144.3 (16.1), 19 | ||
| FACT Physical well-being (0–28-best) | |||||||
| Exercise | 23.9 (4.2), 36 | 21.2 (5.4), 31 | 25.2 (3.7), 29 | 21.0 (4.2), 27 | 21.8 (5.6), 23 | 2.2, 0.08 | 0.1, 0.95 |
| Control | 23.6 (4.9), 34 | 18.8 (7.9), 19 | 22.6 (7.1), 28 | 21.6 (4.9), 20 | 21.9 (5), 20 | (−0.6 to 2.9; −2.2 to 0.7) | (−1.9 to 2.4; −1.2 to 2.4) |
| FACT Social/family well-being (0–28-best) | |||||||
| Exercise | 26.3 (2.6), 36 | 25.5 (4.1), 31 | 25.6 (4.1), 29 | 26.2 (3.6), 27 | 26.0 (3.8), 23 | 0.2, 0.86 | −0.6, 0.40 |
| Control | 27.0 (2), 34 | 26.9 (1.5), 19 | 26.1 (2.9), 28 | 26.8 (2.7), 20 | 27.2 (1.3), 20 | (−1.2 to 0.8; −2.6 to 0.4) | (−1.2 to 0.5; −1.4 to 1.7) |
| FACT Emotional well-being (0–24-best) | |||||||
| Exercise | 19.1 (4.7), 36 | 20.2 (3), 31 | 19.5 (4.1), 29 | 20.5 (3), 27 | 20.9 (3.3), 23 | −0.7, 0.38 | 0.1, 0.92 |
| Control | 18.5 (4.7), 34 | 20.9 (3), 19 | 19.4 (3.6), 28 | 20.8 (4.3), 20 | 21.0 (3.3), 20 | (−0.8 to 1.2; −0.1 to 2.4) | (−1.1 to 2.5; −1.4 to 1.8) |
| FACT Functional well-being (0–28-best) | |||||||
| Exercise | 21.2 (6.2), 36 | 21.6 (4.4), 31 | 22.6 (4.9), 29 | 18.4 (5.5), 27 | 20.0 (4.5), 23 | 1.5, 0.25 | −0.7, 0.59 |
| Control | 22.1 (6), 34 | 18.6 (7.2), 19 | 21.8 (6.1), 28 | 20.9 (5.5), 20 | 21.8 (4.9), 19 | (−1.0 to 3.2; −2.1 to 1.4) | (−1.0 to 2.77; 0.5–3.7) |
| FACT Additional concerns (0–68-best) | |||||||
| Exercise | 49.4 (10.2), 34 | 52.7 (9.7), 31 | 59.3 (7.5), 29 | 51.7 (6.3), 27 | 53.9 (7.5), 23 | 2.0, 0.47 | 0.7, 0.75 |
| Control | 49.4 (13), 32 | 52.5 (13.6), 17 | 57.8 (12.5), 27 | 52.9 (8.8), 20 | 53.0 (7.3), 20 | (5.9–14.3; 3.7–12.7) | (0.3–4.4; −3.6 to 6.4) |
| Surgical Fear Questionnaire | |||||||
| Surgical fear Short-term (0–40-very afraid) | |||||||
| Exercise | 12.5 (10.1), 36 | 13.3 (10.1), 31 | 11.9 (11.7), 30 | — | — | −1.5, 0.50 | — |
| Control | 9.7 (9.7), 35 | 10.0 (9.1), 20 | 10.1 (10.6), 28 | (−4.0 to 2.4; −1.7 to 6.7) | |||
| Surgical fear Long-term (0–40-very afraid) | |||||||
| Exercise | 17.8 (14.1),6 | 16.6 (14.1),1 | 15.2 (13.9),0 | — | — | −2.0, 0.45 | — |
| Control | 13.1 (12.1), 35 | 14.3 (13.3), 20 | 12.3 (13.8), 28 | (−4.3 to 0.5; −2.7 to 2.5) | |||
| Surgical fear Overall (0–80-very afraid) | |||||||
| Exercise | 30.3 (23.1), 36 | 29.9 (23.3), 31 | 27.1 (24), 30 | — | — | −3.6, 0.43 | — |
| Control | 22.8 (19.5), 35 | 24.3 (21), 20 | 22.4 (22.2), 28 | (−7.8 to 2.5; −3.7 to 8.5) | |||
| General Self-efficacy (0–40-best) | |||||||
| Exercise | 34.4 (4.5), 34 | 34.1 (4.8), 31 | 34.9 (4.1), 29 | 34.5 (3.9), 27 | 35.7 (4), 23 | 1.3, 0.20 | −0.0, 0.99 |
| Control | 35.1 (4.2), 34 | 33.5 (5.5), 17 | 34.3 (4.5), 27 | 34.5 (4.9), 20 | 35.0 (4.2), 20 | (−0.7 to 2.2; −2.4 to 0.9) | (−0.3 to 2.3; −0.7 to 2.8) |
| Pearlin Mastery Scale (7–48-best) | |||||||
| Exercise | 30.5 (8.2), 36 | 32.4 (7), 31 | 30.9 (6.1), 29 | 33.6 (6.9), 27 | 33.7 (5.3), 23 | 1.3, 0.55 | −3.0, 0.18 |
| Control | 31.4 (6.3), 34 | 30.4 (7.3), 19 | 29.9 (8.3), 28 | 30.6 (7.4), 20 | 33.5 (6.2), 20 | (−3.5 to 1.9; −4.5 to 2.9) | (−3.8 to 2.4; −1.1 to 6.6) |
Figure 2.
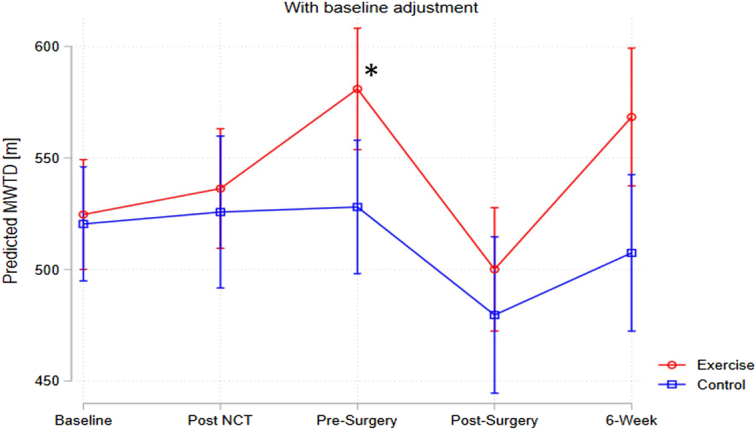
Changes in 6-minute walk test at post-NCT, pre-surgery, post-surgery and 6-week post-surgery time points, comparing exercise group and usual care group. *P=0.05.
There were no significant differences between the groups from baseline to the final assessment (P=0.41).
Secondary outcomes
Physical health
Strength: From baseline to pre-surgery, there were no significant DID in the sit-to-stand test between the groups: −2.5 s (P=0.078). From post-surgery to 6-week reassessment, there were no significant DID between the groups: 0.4 s (P=0.797). From baseline to pre-surgery, there were no significant DID in the hand dynamometer: +0.4 kg (P=0.77). From post-surgery to 6-week reassessment, there were no significant DID between the groups: −2.3 kg (P=0.233) (Table 2). Data are graphically presented in Figure 3.
Figure 3.
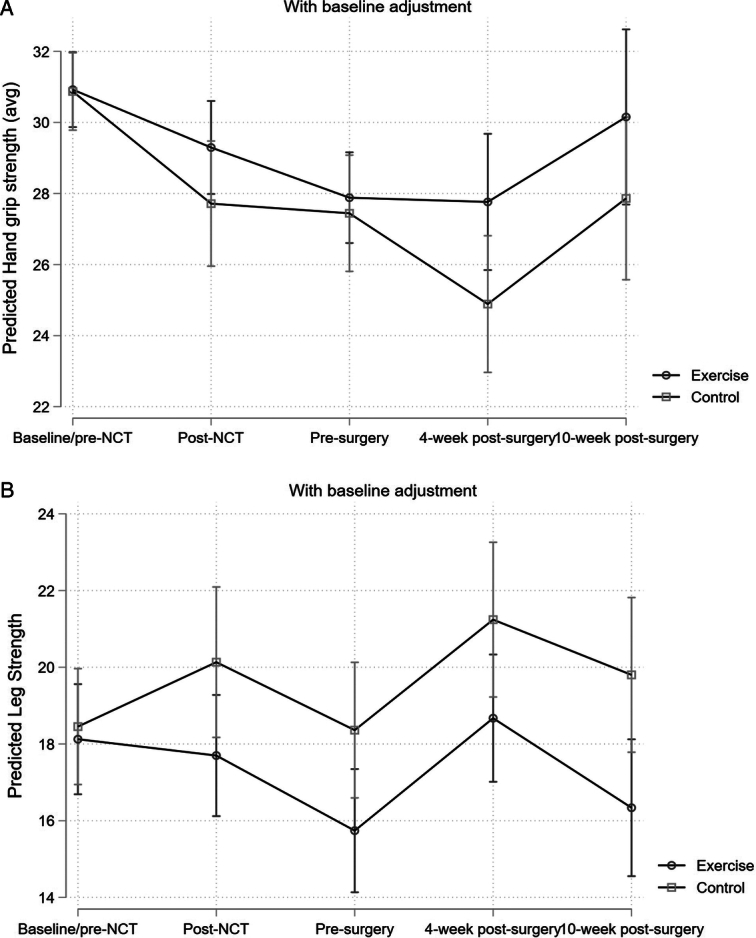
(A) Changes in hand dynamometer grip strength in exercise and control group pre-NCT and post-NCT, pre-surgery and post-surgery and 10 weeks post-surgery; (B) changes in sit-to-stand test in exercise and control group pre-NCT and post-NCT, pre-surgery and post-surgery and 10 weeks post-surgery.
Activity Behaviour: From baseline to pre-surgery, there was no significant DID in the daily step count between the groups: −63.4 steps (P=0.952). From post-surgery to 6-week reassessment, there were no significant DID between the groups: −711.6 steps (P=0.5). From baseline to pre-surgery, there was no significant DID in sedentary behaviour between the groups: 0.7 h (P=0.444). From post-surgery to 6-week reassessment, there were no significant DID between the groups: 0.3 h (P=0.614) (Table 2).
Body Composition (BMI): From baseline to pre-surgery, there were no significant DID between the groups: −0.3 kg/m2 (P=0.765). From post-surgery to 6-week reassessment, there were no significant DID between the groups: −0.7 (P=0.846) (Table 2).
VO 2peak : From baseline to pre-surgery there was a significant DID between the groups: 1.9 ml/kg/min (P=0.02). From post-surgery to 6-week reassessment, there were no significant DID between the groups: 0.6 ml/kg/min (P=0.4) (Supplementary Appendix 7, Supplemental Digital Content 2, http://links.lww.com/JS9/C862).
Psychological health
EQ-5D-5L health questionnaire: From baseline to pre-surgery, there were no significant DID in EQ-5D Utility between the groups: 0.045 (P=0.271). From post-surgery to 6-week reassessment, there were no significant DID between the groups: 0.050 (P=0.227). From baseline to pre-surgery, there was no significant DID in EQ-5D VAS between the groups: 5.4 (P=0.207). From post-surgery to 6-week reassessment, there were no significant DID between the groups: −1.2 (P=0.768) (Table 2). Data are graphically presented in Supplementary Appendix 3 (Supplemental Digital Content 2, http://links.lww.com/JS9/C862).
Functional Assessment of Cancer Therapy-Oesophageal (FACT-E) questionnaire: From baseline to pre-surgery, there were no significant DID between the groups: 4.9 (P=0.228). From post-surgery to 6-week reassessment, there were no significant DID between the groups: −0.5 (P=0.911) (Table 2). Data are graphically presented in Supplementary Appendix 4 (Supplemental Digital Content 2, http://links.lww.com/JS9/C862).
From baseline to pre-surgery, there were no significant DID between the groups in overall surgical fear (P=0.429), general self-efficacy (P=0.195) or the Pearlin Mastery Scale (0.547) (Table 2).
The semi-structured interviews revealed three main themes (each involving subthemes): pre-operatively included optimism and recovery, anxiety and hopes for the future. Whilst the post-surgery interviews revealed activity awareness and the new normal, journey of recovery and trauma of diagnosis. There were differences between EX vs. UC. Themes, subthemes and quotes pre-surgery and post-surgery are presented in Table 3.
Table 3.
Thematic summary of post-operative interviews.
| Theme | Subtheme | Quotations | ||
|---|---|---|---|---|
| Pre-surgery | ||||
| (1) Activity awareness and the “new normal” | (a) Health behaviour changea
(b) Lifestyle adjustment |
“..keep up as much physical activity if I can, and also to keep a good diet and also to get enough sleep”
“have to adjust to …like..I was thinking you’re going to have to go around with …like a money belt..for something to put your food Awareness they will have to adjust to a new lifestyle” |
||
| (c) Physical activity-recovery linka | “At my age I have to be fit, physically fit. There’s a no brainer at any age” | |||
| (2) Journey of Recovery | (a) Optimism and acceptance | “recovery but it is understandable but I am happy with the outcome and I am happy the way things are progressing for me. Overall content with outcome and how things are progressing” | ||
| (b) Reflectiona | “live life to the full really everyday because life is short, you do realise you know that your health is your wealth” | |||
| (c) Return to normalitya | “I would like to be able to play music again, and even as I said the accordion is very relaxing” | |||
| (d) Ups and downsa | “I went through a real rough stage em, It wasn’t easy” | |||
| (3) Trauma of diagnosis | (a) Post-operative complications | “I lost a bit of confidence and I was in a lot of pain for a while but we are getting there” | ||
| (b) Overwhelmed | “Cancer just sort of takes over your whole life you know?” | |||
| (c) Mental health | “it just kinda felt, oh god, I don’t know, a bit hopeless” | |||
| Post-surgery | ||||
| (1) Optimism and recovery | (a) Confidence vs. uncertaintya | “But, eh, I’ve great confidence in terms of, eh, the team I’ve dealt with, and, eh, the experience they” | ||
| (b) Long-term recovery | “Like then I would see no reason why I shouldn’t get back to full health eventually, might take a couple of months” | |||
| (c) Physical activity to promote post-op recoverya | “Ah, healthy, I suppose is exercise and healthy eating-” | |||
| (2) Anxiety | (a) Internal anxietya | “The pain yea after, after the operation and……if I’ll be able to get back to myself the way I was” | ||
| (b) Moment of realizationa | “it hasn’t been over the last few months actually, it’s only when we met Professor __, and when he went through the extent of the operation, that’s when the fear and the thoughts and the anxiety really kicked in.” | |||
| (c) External factors | “particularly given the Covid situation at the moment in hospitals and so on. Em they understand it’s a difficult operation as well, so yeah there is a natural kind of anxiety about it” | |||
| Hopes for the future maintenancea | (a) Physical activity | “Fitness training…in the sense, in a gym, training sessions with a trainer” | ||
| (b) Return to daily activities | “I’m involved with a few charities, and to get back with them and working with them and to get back to mass, to daily mass, and just get back to having the holiday every year, myself and my friend go on a holiday, sometimes two. And just to get back to normality” | |||
| (c) Re-evaluation and realisation | “To appreciate life, that’s the main one (laughs)..the things you take for granted..and when your sick, oh god you look out the window and see people passing by and think how lucky are they” | |||
Indicates subthemes where a significant group difference was evidenced between control and intervention conditions.
Exploratory outcomes
Post-operative outcomes, toxicity, tolerance, compliance to NCT and sarcopenia data are presented in Table 4. There were no significant differences in toxicities, failure to proceed to surgery, post-operative complications, or pathological outcomes between the groups. There were no differences in radiological assessments of sarcopenia between groups. Glasgow Prognostic Scale and haematological markers of inflammation are presented in Supplementary Appendix 5 (Supplemental Digital Content 2, http://links.lww.com/JS9/C862). Nutritional intake data is presented in Supplemental Appendix 6 (Supplemental Digital Content 2, http://links.lww.com/JS9/C862). Of note, there was no significant difference in time to surgery from enrollment for the oesophageal cancer or gastric cancer patients (P=0.49 and 0.12, respectively; Supplementary Appendix 8, Supplemental Digital Content 2, http://links.lww.com/JS9/C862).
Table 4.
Rates of neoadjuvant treatment toxicity, tolerance and compliance, post-operative morbidity and pathological data.
| Exercise (n=36) | Usual care (n=35) | All patients (n=71) | P | |
|---|---|---|---|---|
| Neoadjuvant treatmenta | ||||
| Dose reduction | 5 (14) | 5 (14) | 10 (14) | 0.29 |
| Hospitalisation during treatment | 7 (19) | 10 (29) | 17 (24) | |
| CTC toxicity grade | ||||
| 0–1 | 28 (78) | 25 (71) | 53 (75) | |
| 2–3 | 6 (17) | 6 (17) | 12 (17) | 0.86 |
| Failure to proceed to surgery | 4 (11) | 10 (29) | 14 (20) | |
| Disease progression | 4 (100) | 9 (90) | 13 (93) | |
| Complete clinical response | 0 (0) | 1 (10) | 1 (7) | |
| Exercise (n=29) | Usual care (n=25) | All patients (n=54) | ||
| Post-operative follow-upa | ||||
| No. patients without complications | 11 (40) | 13 (52) | 24 (44) | 0.44 |
| Clavien–Dindo classification | ||||
| Grades 1–2 | 10 (34) | 3 (9) | 13 (24) | |
| Grades 3–4 | 6 (21) | 7 (28) | 13 (24) | |
| Grade 5 | 1 (3) | 0 | 1 (2) | 0.37 |
| Comprehensive Complication Indexb | 16 (20.3) | 13.9 (17.9) | 15 (19.1) | 0.70 |
| POMS day 3 | 17 (59) | 15 (60) | 32 (59) | 0.92 |
| POMS day 5 | 13 (45) | 11 (44) | 24 (44) | 0.92 |
| Pulmonary | ||||
| Day 3 | 5 (17) | 11 (44) | 16 (30) | |
| Day 5 | 4 (14) | 4 (16) | 8 (15) | |
| Infectious | ||||
| Day 3 | 7 (24) | 7 (28) | 14 (26) | |
| Day 5 | 7 (24) | 6 (24) | 13 (24) | |
| Renal | ||||
| Day 3 | 4 (14) | 5 (20) | 9 (17) | |
| Day 5 | 0 (0) | 3 (12) | 3 (6) | |
| Gastrointestinal | ||||
| Day 3 | 9 (31) | 6 (24) | 15 (28) | |
| Day 5 | 8 (28) | 5 (20) | 13 (24) | |
| Cardiovascular | ||||
| Day 3 | 6 (21) | 2 (8) | 8 (15) | |
| Day 5 | 4 (14) | 1 (4) | 5 (9) | |
| Neurological | ||||
| Day 3 | 0 (0) | 0 (0) | 0 (0) | |
| Day 5 | 0 (0) | 0 (0) | 0 (0) | |
| Haematological | ||||
| Day 3 | 0 (0) | 0 (0) | 0 (0) | |
| Day 5 | 0 (0) | 2 (8) | 2 (4) | |
| Wound | ||||
| Day 3 | 0 (0) | 0 (0) | 0 (0) | |
| Day 5 | 0 (0) | 0 (0) | 0 (0) | |
| Pain | ||||
| Day 3 | 1 (3) | 2 (8) | 3 (6) | |
| Day 5 | 1 (3) | 1 (4) | 2 (4) | |
| Unplanned readmission post-surgery (%) | 2 (7) | 2 (8) | 4 (7) | 0.91 |
| Length of stay, days | 18 (19) | 14 (10) | 16 (15) | 0.32 |
| Oesophagectomy complications (n=25) | (n=15) | (n=40) | ||
| Pulmonary complication | 5 (20) | 5 (33) | 10 (25) | 0.79 |
| Chyle leak | 1 (4) | 3 (20) | 4 (10) | 0.23 |
| Anastomotic leak | 2 (8) | 0 (0) | 2 (5) | 0.18 |
| Recurrent laryngeal nerve injury | 1 (4) | 0 (0) | 1 (2.5) | 0.37 |
| Exercise (n=29) | Usual care (n=25) | All patients (n=54) | ||
| Pathological dataa | ||||
| ypT Category | ||||
| T0 | 3 (10) | 7 (28) | 10 (19) | |
| T1 | 5 (17) | 3 (12) | 8 (15) | |
| T2 | 3 (10) | 2 (8) | 5 (9) | |
| T3 | 12 (41) | 10 (40) | 22 (41) | |
| T4 | 6 (21) | 3 (12) | 9 (17) | 0.52 |
| ypN Category | ||||
| N0 | 17 (59) | 16 (64) | 33 (61) | |
| N1 | 5 (17) | 2 (12) | 8 (15) | |
| N2 | 5 (17) | 0 | 5 (9.3) | |
| N3 | 2 (7) | 6 (24) | 8 (15) | 0.06 |
| cPR (T0N0) | ||||
| Yes | 3 (10) | 6 (24) | 9 (17) | |
| No | 26 (90) | 19 (76) | 45 (83) | 0.26 |
| Tumour regression gradea | ||||
| 1–2 | 9 (31) | 10 (40) | 19 (35) | |
| 3–5 | 20 (69) | 15 (60) | 35 (65) | 0.49 |
| Resection radicalitya | ||||
| R0 | 25 (86) | 22 (88) | 47 (87) | |
| R1 | 4 (14) | 2 (8) | 6 (11) | |
| Unknown | 0 | 1 (4) | 1 (2) | 0.53 |
| Lymph nodes resectedb | 25.5 (12.3) | 30.3 (9.4) | 27.7 (11.2) | 0.13 |
| Lymph nodes involvedb | 2.1 (3.6) | 3.6 (6.9) | 2.8 (5.3) | 0.31 |
| Exercise (n=26) | Usual care (n=33) | All patients (n=59) | ||
| Sarcopenia scoring data | ||||
| Skeletal muscle area (cm2/m2)b | ||||
| Baseline | 53.4 (9.6) | 54.8 (11.1) | 54.2 (10.4) | 0.62 |
| Pre-operative | 49.3 (7.9) | 50.4 (10.8) | 49.9 (9.6) | 0.66 |
| Difference-in-difference | 4.1 (3.3) | 4.4 (3.5) | 4.2 (3.4) | 0.75 |
| Visceral adiposity area (cm2/m2)b | ||||
| Baseline | 56.2 (33.9) | 52.8 (18.8) | 54.3 (26.2) | 0.67 |
| Pre-operative | 55.5 (31.7) | 50.0 (23.7) | 52.4 (27.3) | 0.51 |
| Difference-in-difference | 0.7 (12.7) | 2.7 (12.4) | 1.9 (12.5) | 0.58 |
| Subcutaneous adiposity area (cm2/m2)b | ||||
| Baseline | 95.0 (45.4) | 95.0 (43.8) | 95.0 (43.9) | 0.99 |
| Pre-operative | 87.4 (43.6) | 84.3 (38.6) | 85.7 (40.6) | 0.79 |
| Difference-in-difference | 7.5 (16.9) | 10.3 (14.7) | 9.1 (15.6) | 0.54 |
| Total fat area (cm2/m2)b | ||||
| Baseline | 158 (68.6) | 154 (53.1) | 156 (59.8) | 0.84 |
| Pre-operative | 150 (62.3) | 140 (53.6) | 144 (57.2) | 0.54 |
| Difference-in-difference | 7.96 (25.0) | 14.5 (21.7) | 11.6 (23.2) | 0.34 |
Values for sarcopenia scores are cm2/m2.
n (%).
Data are presented as mean (SD).
cPR, complete pathological response; POMS, post-operative morbidity score.
Mortality at 30 days was recorded for one patient in EX and no further mortality at day 90 was recorded. There were no adverse events during the perioperative training programme.
Discussion
PERIOP-OG is a comprehensive multicentre RCT examining the physiological and psychological impacts of exercise throughout the neoadjuvant window and in the post-operative, rehabilitation phase for patients undergoing curative resection of locally advanced oesophagogastric malignancies. The study achieved its primary outcome, showing a significant improvement in cardiorespiratory fitness following the introduction of a community-based exercise programme during the neoadjuvant window, and continued to the time of surgical resection for patients undergoing oesophagogastric resection. This provides valuable, real-world evidence that a low-cost intervention can have a material impact on patient fitness prior to major resectional surgery.
Secondary outcomes showed improved VO2peak during the neoadjuvant window and a trend towards difference in sit-to-stand, although other physical parameters were not significantly different between groups, either pre-surgery or in the post-operative rehabilitation setting. Of note, there were no differences in BMI between groups at the testing points, but also no significant change in radiological assessment of sarcopenia scores (where available). This suggests that a prescribed exercise programme does not have adverse outcomes on body composition and is not related to perioperative weight loss. This has not been specifically examined in other studies in this area12–14.
There were no significant differences in psychological outcomes between groups, although these results should be interpreted with caution. A significant portion of this RCT took place during severe COVID-19 restrictions early in the pandemic, and it is impossible to control how that may have affected feelings of fear and anxiety around NCT and significant resectional surgery. The semi-structured interviews do, however, give common themes and identify the EX group as being more focussed on their physical health and fitness in the perioperative period. Furthermore, there were significant and prolonged restrictions on movement, travel and meeting in person. This may have contributed to the similarities in activity levels and sedentary behaviour between the groups, as cancer patients were advised by public health experts to isolate and reduce social contact. Due to the inability to blind participants to the trial interventions, it may also be that the UC group increased their own exercise regimen due to perceived benefit. The intervention group also had a higher proportion of patients with oesophageal cancer. The increased physiological insult from neoadjuvant radiation, transthoracic resection and oesophageal cancer may contribute to the lack of improvement in other physiological domains.
Patients in the exercise group demonstrated good compliance with the prescribed exercise programme, with 80.5% and 77.7% completing satisfactory exercise logs in the pre-operative and post-operative periods, respectively. These data are presented in Supplementary Appendix 1 (Supplemental Digital Content 2, http://links.lww.com/JS9/C862, section 13).
There were no significant differences in perioperative complications between the groups, although with the caveat that the EX group had more patients with oesophageal cancer, with the accompanying risks of single lung ventilation, and with more intra-thoracic anastomoses. This trial was not adequately powered to investigate the influence of improved perioperative fitness on post-operative complications, although this is something that may be of interest either in a future trial or, indeed, in a large observational study.
Physical fitness is an important prognostic marker for patients undergoing major cancer resections. Patients with poor cardiorespiratory fitness have reduced overall survival at 1 year8. Others have previously attempted to examine this influence of cardiorespiratory fitness on patient outcomes in those undergoing resection of upper gastrointestinal malignancies.
Minnella et al.13, in a single-centre study, demonstrated that a prehabilitation programme resulted in improved 6MWT in their intervention cohort pre-surgery for oesophagogastric cancers, with a sustained effect in the post-operative period. Similar to this study, there was no impact on perioperative outcomes. There was no prescribed post-operative rehabilitation programme, and only a minority of patients (42% intervention, 44% control) underwent minimally invasive procedures. Thirty-two per cent of patients did not receive NCT, and over 75% of patients in their cohort underwent oesophagectomy with the added surgical insult of either a laparotomy or thoracotomy. In contrast, all patients in this study underwent NCT and the majority were completed with a minimally invasive approach. This shows that even in patients with potential treatment toxicities but with reduced surgical insult, a prehabilitation programme can improve physical fitness, which in turn may lead to improved long-term outcomes.
Valkenet et al.12 conducted a multicentre RCT looking at the impact of inspiratory muscle training (IMT) on patient outcomes in those undergoing oesophagectomy, with a primary endpoint of post-operative pulmonary complications. Although they did show an improvement in inspiratory function, this did not translate into any meaningful impact on post-operative complications. Again however, not all patients underwent neoadjuvant therapy, and a significant number of patients underwent both transhiatal and left-sided thoracotomy for resection. IMT should form only one aspect of a prehabilitation intervention, and again, it is difficult to draw firm inferences on how a modern cohort of patients undergoing minimally invasive abdominal and thoracic resections may respond.
Allen et al.14 reported a single-centre RCT examining the impact of a prehabilitation programme for oesophageal cancer patients on pre-operative anaerobic threshold (AT) while also examining grip strength, sarcopenia, and psychological outcomes. They showed an improvement in anaerobic threshold and grip strength in their cohort, with improved psychological outcomes in the intervention group. They did not show an improvement in perioperative outcomes. However, all patients but one received perioperative chemotherapy without radiation and underwent open resections, with the majority having a thoracotomy. Although debate persists about the optimal approach both in terms of NCT and surgical technique, a significant number of patients still receive chemoradiation preferentially over chemotherapy. Determining the impact of prehabilitation on these patients requires a pragmatic trial run in real-world conditions, which facilitates decision-making. This contrasts with PERIOP-OG, which recruited patients with both oesophageal and gastric cancers, with a significant proportion receiving thoracic oesophageal radiation. This better reflects current practise, and is unlikely to change in the near future, notwithstanding results from RCTs comparing chemoradiation and chemotherapy for oesophageal cancer.
There are limitations to this study. As previously mentioned, a significant portion of this trial took place under severe COVID-19 restrictions. This impacted in-person assessment but likely helped facilitate accessing either a community or hybrid programme, and the provision of remote assessment. The close supervision including regular calls for compliance, supervision and troubleshooting any difficulties with the exercise programme helped to ensure a standardised approach for those undergoing supervised and home-based exercise. In addition, some of the assessments were taken at home due to pandemic-related restrictions; however, there was standardised training and phone follow-up to troubleshoot assessments as required. For patients living remotely from their NCT, a community-based or home-based programme can minimise the frequency of commuting for treatment or support. It is impossible to say how the pandemic, with isolation and restrictions, may have impacted psychological outcomes. Within this study, patients were not perfectly matched for disease location and surgical approach. However, this reflects the undifferentiated pattern of presentation to upper gastrointestinal services. Furthermore, as there was a higher rate of oesophageal cancer and transthoracic resection in the oesophageal group, it may be that patients undergoing only transabdominal resection will have improved outcomes compared to a similar exercise group. The study did not assess activity levels prior to diagnosis in detail, although these would require retrospective self-reporting and bring a large risk of bias. The preference was to establish patients’ baseline functioning with standardised testing.
PERIOP-OG represents, to our knowledge, the most comprehensive RCT looking at the influence of fitness on outcomes to date. PERIOP-OG examined in tandem pre-operative and post-operative interventions, and multiple domains of physical and psychological well-being. As such, it provides a framework for community-based exercise programs that can improve physical fitness during the neoadjuvant window prior to major oesophagogastric resection. Although PERIOP-OG did not demonstrate significant differences beyond the primary outcome, there was a trend toward improved well-being in other domains. Oesophagogastric malignancies are complex, with significant impacts on physical, nutritional and psychological well-being. A pragmatic exercise intervention can form one element of care for patients undergoing treatment for locally advanced oesophagogastric cancer.
Conclusion
A pragmatic community-based exercise programme can significantly improve pre-operative fitness in patients undergoing neoadjuvant therapy and oesophagogastric resection, with no evidence of any negative impact in physical or psychological domains. This low-cost intervention may help improve perioperative outcomes.
Ethical approval
The study was approved by the research ethics board of Beaumont Hospital (Ref 18/58) as the central site, with secondary approval at University Hospital Galway and Mercy University Hospital. It was registered with ClinicalTrials.gov, NCT03807518, registered on 01/01/19. https://clinicaltrials.gov/ct2/show/NCT03807518
Source of funding
Funding for this trial was provided by the Beaumont Hospital Foundation Trust and the Oesophageal Cancer Fund (the Irish National Charity for Oesophageal Cancer: www.ocf.ie).
Author contribution
L.L., J.B., R.T., N.McC., and W.R.: conceptualization; L.L., R.T., J.B., N.McC., J.S., and M.B.: methodology; L.L., R.T., P.C., T.M., W.R., and M.A.: validation; J.S., L.L., R.T., J.B., and M.B.: formal analysis; L.L., R.T., W.R., M.A., T.M., P.C., and N.McC.: investigation; WR, PC, M.A., T.M., N.McC., and L.L.: resources; L.L., R.T., and J.S.: data curation; L.L., J.B., R.T., and W.R.: writing; W.R., M.A., P.C., N.McC., and T.M.: supervision; W.R. and J.B.: project administration; W.R.: funding acquisition. All authors were involved in writing and revising.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: ClinicalTrials.gov.
Unique identifying number or registration ID: NCT03807518, registered on 01/01/19.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/ct2/show/NCT03807518
Guarantor
William Robb and Lisa Loughney.
Data availability statement
Data will be made available upon reasonable request.
Provenance and peer review
No commissioned, externally peer-reviewed.
Collaborators
Wendy Hickey, Department of Upper GI Surgery, Beaumont Hospital, Dublin, Ireland; Claire Coleman, Department of Upper GI Surgery, Beaumont Hospital, Dublin, Ireland; Orla Brett, Department of Upper GI Surgery, Beaumont Hospital, Dublin, Ireland; Oliver McAnena, Department of Upper GI Surgery, University Hospital, Galway, Ireland; Chris G. Collins, Department of Upper GI Surgery, University Hospital, Galway, Ireland; Claire M. Timon, School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland; Louise Buckley, Department of Upper GI Surgery, Mercy University Hospital, Cork, Ireland; Pamela Gallagher, School of Psychology, Dublin City University, Dublin, Ireland; Aoife Quinn, Department of Upper GI Surgery, University Hospital, Galway, Ireland; Emma Houlihan, Cancer Care West, Galway, Ireland; D.J. O’Dwyer, HeartWise Clonmel, Co Tipperary, Ireland; Mark McManus, Cork Leisure World, Cork, Ireland; Austin Toomy, Cork Leisure World, Cork, Ireland; Niall Moyna, School of Health & Human Performance, Dublin City University, Dublin, Ireland; Kate Murphy, School of Health & Human Performance, Dublin City University, Dublin, Ireland; Fiona Skelly, School of Health & Human Performance, Dublin City University, Dublin, Ireland; Micheal Bailey, Patient & Public Representatives; Edwina McCabe, Patient & Public Representatives.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 27 June 2024
Contributor Information
Lisa Loughney, Email: lisa.loughney@gmail.com.
Jarlath Bolger, Email: jarbolger@rcsi.ie.
Roisin Tully, Email: roisintully@rcsi.ie.
Jan Sorensen, Email: jansorensen@rcsi.ie.
Marie Bambrick, Email: marie.bambrick@gmail.com.
Paul A. Carroll, Email: paulcarroll@rcsi.ie.
Mayilone Arumugasamy, Email: mayilone.aru@gmail.com.
Thomas J. Murphy, Email: tmurphy@muh.ie.
Noel McCaffrey, Email: noel.mccaff@gmail.com.
William B. Robb, Email: robb.will@gmail.com.
References
- 1. Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020;159:335–349.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolger JC, Donohoe CL, Lowery M, et al. Advances in the curative management of oesophageal cancer. Br J Cancer 2022;126:706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 4. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393(10184):1948–1957. [DOI] [PubMed] [Google Scholar]
- 5. Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 2021;384:1191–1203. [DOI] [PubMed] [Google Scholar]
- 6. Park A, Orlandini MF, Szor DJ, et al. The impact of sarcopenia on esophagectomy for cancer: a systematic review and meta-analysis. BMC Surg 2023;23:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jack S, West MA, Raw D, et al. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol 2014;40:1313–1320. [DOI] [PubMed] [Google Scholar]
- 8. West MA, Anastasiou Z, Ambler G, et al. The effects of cancer therapies on physical fitness before oesophagogastric cancer surgery: a prospective, blinded, multi-centre, observational, cohort study [version 1; peer review: 2 approved]. NIHR Open Res 2021;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015;12:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sinclair R, Navidi M, Griffin SM, et al. The impact of neoadjuvant chemotherapy on cardiopulmonary physical fitness in gastro-oesophageal adenocarcinoma. Ann R Coll Surg Engl 2016;98:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolger JC, Loughney L, Tully R, et al. Perioperative prehabilitation and rehabilitation in esophagogastric malignancies: a systematic review. Dis Esophagus 2019;32:doz058. [DOI] [PubMed] [Google Scholar]
- 12. Valkenet K, Trappenburg JCA, Ruurda JP, et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg 2018;105:502–511. [DOI] [PubMed] [Google Scholar]
- 13. Minnella EM, Awasthi R, Loiselle SE, et al. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg 2018;153:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen SK, Brown V, White D, et al. Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: effect on cardiopulmonary exercise test performance, muscle mass and quality of life-a pilot randomized clinical trial. Ann Surg Oncol 2022;29:1839–1850. [DOI] [PubMed] [Google Scholar]
- 15. Halliday LJ, Doganay E, Wynter-Blyth VA, et al. The impact of prehabilitation on post-operative outcomes in oesophageal cancer surgery: a propensity score matched comparison. J Gastrointest Surg 2021;25:2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Neill LM, Guinan E, Doyle SL, et al. The RESTORE randomized controlled trial: impact of a multidisciplinary rehabilitative program on cardiorespiratory fitness in esophagogastric cancer survivorship. Ann Surg 2018;268:747–755. [DOI] [PubMed] [Google Scholar]
- 17. Dettling DS, van der Schaaf M, Blom RLGM, et al. Feasibility and effectiveness of pre-operative inspiratory muscle training in patients undergoing oesophagectomy: a pilot study. Physiother Res 2013;18:16–26. [DOI] [PubMed] [Google Scholar]
- 18. van Adrichem EJ, Meulenbroek RL, Plukker JTM, et al. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Ann Surg Oncol 2014;21:2353–2360. [DOI] [PubMed] [Google Scholar]
- 19. Loughney L, Cahill R, O’Malley K, et al. Compliance, adherence and effectiveness of a community-based pre-operative exercise programme: a pilot study. Perioper Med 2019;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tully R, Loughney L, Bolger J, et al. The effect of a pre- and post-operative exercise programme versus standard care on physical fitness of patients with oesophageal and gastric cancer undergoing neoadjuvant treatment prior to surgery (the PERIOP-OG trial): study protocol for a randomised controlled trial. Trials 2020;21:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg 2011;91:1494–1500. [DOI] [PubMed] [Google Scholar]
- 23. Lyden K, Kozey Keadle SL, Staudenmayer JW, et al. Validity of two wearable monitors to estimate breaks from sedentary time. Med Sci Sports Exerc 2012;44:2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Netz Y, Ayalon M, Dunsky A, et al. “The multiple-sit-to-stand” field test for older adults: what does it measure? Gerontology 2004;50:121–126. [DOI] [PubMed] [Google Scholar]
- 25. Yu R, Ong S, Cheung O, et al. Reference values of grip strength, prevalence of low grip strength, and factors affecting grip strength values in chinese adults. J Am Med Dir Assoc 2017;18:551.e9–551.e16. [DOI] [PubMed] [Google Scholar]
- 26. Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67:1063–1078. [DOI] [PubMed] [Google Scholar]
- 27. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 28. Darling G, Eton DT, Sulman J, et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer 2006;107:854–863. [DOI] [PubMed] [Google Scholar]
- 29. Theunissen M, Peters ML, Schouten EGW, et al. Validation of the surgical fear questionnaire in adult patients waiting for elective surgery. PLoS One 2014;9:e100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwarzer R, Jerusalem M, Weinman J, et al. Generalized self-efficacy scale. Measures in Health Psychology: A User’s Portfolio. NFER-NELSON; 1995:35–37. [Google Scholar]
- 31. Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19:2–21. [PubMed] [Google Scholar]
- 32. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 33. Timon CM, van den Barg R, Blain RJ, et al. A review of the design and validation of web- and computer-based 24-h dietary recall tools. Nutr Res Rev 2016;29:268–280. [DOI] [PubMed] [Google Scholar]
- 34. Murray TÉ, Williams D, Lee MJ. Osteoporosis, obesity, and sarcopenia on abdominal CT: a review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom Radiol 2017;42:2376–2386. [DOI] [PubMed] [Google Scholar]
- 35. Grocott MPW, Browne JP, Van der Meulen J, et al. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J Clin Epidemiol 2007;60:919–928. [DOI] [PubMed] [Google Scholar]
- 36. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 37. Slankamenac K, Nederlof N, Pessaux P, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–762; discussion 762–763. [DOI] [PubMed] [Google Scholar]
- 38. Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286–294. [DOI] [PubMed] [Google Scholar]
- 39. Schumacher AN, Shackelford DYK, Brown JM, et al. Validation of the 6-min walk test for predicting peak V˙O2 in cancer survivors. Med Sci Sports Exerc 2019;51:271–277. [DOI] [PubMed] [Google Scholar]
- 40. Cahalin LP, Mathier MA, Semigran MJ, et al. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996;110:325–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.


