Abstract
Background
Influenza circulation during the 2022–2023 season in the United States largely returned to pre–coronavirus disease 2019 (COVID-19)-pandemic patterns and levels. Influenza A(H3N2) viruses were detected most frequently this season, predominately clade 3C.2a1b.2a, a close antigenic match to the vaccine strain.
Methods
To understand effectiveness of the 2022–2023 influenza vaccine against influenza-associated hospitalization, organ failure, and death, a multicenter sentinel surveillance network in the United States prospectively enrolled adults hospitalized with acute respiratory illness between 1 October 2022, and 28 February 2023. Using the test-negative design, vaccine effectiveness (VE) estimates against influenza-associated hospitalization, organ failures, and death were measured by comparing the odds of current-season influenza vaccination in influenza-positive case-patients and influenza-negative, SARS-CoV-2–negative control-patients.
Results
A total of 3707 patients, including 714 influenza cases (33% vaccinated) and 2993 influenza- and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–negative controls (49% vaccinated) were analyzed. VE against influenza-associated hospitalization was 37% (95% confidence interval [CI]: 27%–46%) and varied by age (18–64 years: 47% [30%–60%]; ≥65 years: 28% [10%–43%]), and virus (A[H3N2]: 29% [6%–46%], A[H1N1]: 47% [23%–64%]). VE against more severe influenza-associated outcomes included: 41% (29%–50%) against influenza with hypoxemia treated with supplemental oxygen; 65% (56%–72%) against influenza with respiratory, cardiovascular, or renal failure treated with organ support; and 66% (40%–81%) against influenza with respiratory failure treated with invasive mechanical ventilation.
Conclusions
During an early 2022–2023 influenza season with a well-matched influenza vaccine, vaccination was associated with reduced risk of influenza-associated hospitalization and organ failure.
Keywords: influenza, vaccine effectiveness, attenuation, organ failure, acute respiratory illness
During an early 2022–2023 influenza season with well-matched influenza vaccine, substantial vaccine effectiveness was observed against influenza-associated hospitalization and a range of severe influenza-associated outcomes, including organ failure, intensive care unit (ICU) admission, and need for invasive mechanical ventilation.
The adoption of non-pharmaceutical interventions intended to reduce the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (eg, masking, social distancing) during the coronavirus disease 2019 (COVID-19) pandemic likely contributed to the low influenza circulation observed during the 2020–2021 and 2021–2022 seasons [1]. Influenza vaccine effectiveness (VE) was low during the 2021–2022 season, during which influenza A(H3N2) viruses predominated. Although interim and mid-season VE estimates for 2021–2022 showed almost no effectiveness against influenza virus infection in outpatient settings [2, 3], end-of-year estimates showed some protection against influenza-associated hospitalization for adults aged <65 years without immunocompromising conditions [4]. The 2022–2023 season in the United States was early compared with past seasons, beginning in October 2022 and peaking in December 2022, and marked a return to influenza circulation consistent with influenza seasons prior to the COVID-19 pandemic [1, 5, 6].
Viruses belonging to the influenza A(H3N2) 3C.2a1b.2a clade predominated during the 2022–2023 season, accounting for about two-thirds of influenza cases, followed by those from the influenza A(H1N1)pdm09 virus 6B.1A.5a clade [1]. The 2022–2023 influenza vaccine matched these 2 viruses well; it contained an influenza A/Darwin/9/2021 (H3N2)-like virus, similar to the influenza A(H3N2) 3C.2a1b.2a clade, and an influenza A/Victoria/2570/2019 (H1N1)pdm09-like virus, similar to the influenza A(H1N1) 6B.1A.5a clade [7, 8]. With notable exceptions [9–11], few studies have measured VE against severe outcomes in hospitalized or severely ill patients. Data on the effectiveness of the 2022–2023 influenza vaccine for preventing the full spectrum of influenza-associated severe disease are therefore useful.
The Investigating Respiratory Viruses in the Acutely Ill (IVY) Network is a multistate network of hospitals in the United States that enrolls adults hospitalized with acute respiratory illness (ARI) to evaluate the effectiveness of respiratory virus vaccines [12–14]. The objectives of this analysis were to evaluate the effectiveness of the 2022–2023 influenza vaccine against influenza-associated hospitalization, organ failures, and death.
METHODS
Participants and Sites
Twenty-four hospitals from 19 US states within the IVY Network prospectively enrolled patients into this multi-pathogen ARI surveillance program between 1 October 2022, and 28 February 2023 (Appendix A). The IVY Network is funded by the Centers for Disease Control and Prevention (CDC) and coordinated from Vanderbilt University Medical Center. This program was determined to be a non-research public health surveillance activity by CDC and each participating site and was conducted in a manner consistent with applicable federal law and CDC policy.
Personnel at all sites were trained to follow a common protocol outlining eligibility criteria, data collection, and specimen collection procedures. Patient eligibility criteria included: (1) age ≥18 years; (2) hospital admission; (3) clinical presentation consistent with ARI, defined as having ≥1 of the following: fever, cough, shortness of breath, new hypoxemia, or new pulmonary findings on chest imaging consistent with pneumonia; and (4) a clinically obtained molecular or antigen test for influenza virus, SARS-CoV-2, or respiratory syncytial virus (RSV) within 10 days of symptom onset (Appendix B, section 1).
For this analysis, patients who tested positive for influenza were classified as cases; those who tested negative for influenza and SARS-CoV-2 were classified as controls. Site personnel attempted to enroll all eligible influenza, SARS-CoV-2, and RSV cases and enrolled eligible controls in a 1:1 ratio with locally enrolled cases. Controls were enrolled within 2 weeks of a corresponding case.
Centralized Viral Testing
In addition to local clinical viral testing, upper respiratory specimens were collected from participants at enrollment and shipped to Vanderbilt University Medical Center (Nashville, Tennessee, USA) for centralized reverse transcription–polymerase chain reaction (RT-PCR) testing for influenza virus, SARS-CoV-2, and RSV using a standardized process (Appendix B, section 2) [12]. Influenza cases tested positive for influenza virus and negative for SARS-CoV-2 by local clinical testing and/or centralized RT-PCR testing. Controls, however, had to test negative for both influenza virus and SARS-CoV-2 by all completed clinical tests and centralized tests. Specimens that tested positive for influenza virus by RT-PCR at the central laboratory underwent viral whole genome sequencing at University of Michigan (Ann Arbor, Michigan, USA) (Appendix B, section 2).
Influenza Vaccination Status Ascertainment
Current-season influenza vaccination status was determined by electronic medical record and local Immunization Information System (IIS) searches and by plausible self-report that included the date and location of vaccine receipt (Appendix B, section 3) [15]. A patient was classified as vaccinated if they received ≥1 influenza vaccination on or after 1 August 2022, and at least 14 days before illness onset. Patients were classified as unvaccinated if they received no influenza vaccine doses between 1 August 2022, and the date of illness onset. Data on vaccinations during previous seasons were not collected.
Outcomes
The primary outcome for evaluation of influenza vaccine effectiveness was influenza-associated hospitalization, defined as hospital admission for symptomatic, laboratory-confirmed influenza virus infection. Vaccine effectiveness against more severe influenza-associated disease was assessed using secondary outcomes of symptomatic, laboratory-confirmed influenza with: (1) hypoxemia treated with supplemental oxygen therapy at any flow rate or by any device; (2) acute respiratory, cardiovascular or renal failure treated with organ support (a composite of new receipt of high-flow nasal cannula, non-invasive ventilation, invasive mechanical ventilation, vasopressor use, or renal replacement therapy); (3) respiratory failure treated with invasive mechanical ventilation; (4) intensive care unit (ICU) admission; and (5) death (detailed definitions of each in Appendix B, section 4).
Analytical Exclusions
The analysis excluded enrolled patients who: (1) were identified as not meeting eligibility criteria; (2) tested positive for SARS-CoV-2; (3) were enrolled as controls before the first influenza case or after the last influenza case at the local site (which ensured all included controls were enrolled during local circulation of influenza); (4) had unknown influenza vaccination status; or (5) were missing data required for the VE against influenza-associated hospitalization model. Patients with SARS-CoV-2 infection were not included as controls in this analysis due to potential confounding stemming from an association between influenza and COVID-19 vaccine receipt and consequent downward bias on influenza VE estimates [16, 17]; recent analysis from our network estimated VE to be 7 percentage points lower with the inclusion of SARS-CoV-2-positive controls than without [4].
Statistical Analysis
We described demographic and clinical characteristics of influenza case-patients and control-patients, as well as vaccinated and unvaccinated patients, using counts and percentages or medians and interquartile ranges. Case versus control and vaccinated versus unvaccinated groups were compared using the Pearson χ2 test for categorical variables or Wilcoxon rank-sum testing for continuous variables.
Consistent with a test negative design for evaluation of vaccine effectiveness [18–20], logistic regression was used to calculate the odds of vaccination (vaccinated vs unvaccinated status) among cases with the outcomes of interest (the primary outcome and each secondary outcome) versus controls. Logistic regression models were adjusted for potential confounders, including age (continuous, in years); sex (male/female), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, Other, Unknown), Census region, month/year of enrollment, number of categories of medical comorbidities (0, 1, 2, 3, ≥4), and the following yes/no variables: residence in a long-term care facility, hospital admission in the last year and immunocompromised status (Appendix B, section 5). Vaccine effectiveness for protection against the outcome of interest was then calculated as: (1 − adjusted odds ratio for vaccination) × 100%.
VE against influenza-associated hospitalization (primary outcome) was calculated for the overall population, patient age groups (18–64 years; ≥65 years), immunocompromised status (immunocompetent; immunocompromised), and subtype of influenza virus (A[H3N2]; A[H1N1]). Immunocompromised status was classified based on the definition of moderate-to-severe immunosuppression by the National Institutes of Health (Appendix B, section 6) [21].
The proportions of patients experiencing each of the secondary severe outcomes were compared between vaccinated cases and unvaccinated cases using unadjusted χ2 tests. Additionally, peak severity of respiratory illness experienced during the first 28 days of hospital admission was classified by assigning each case to 1 of 5 ordinal categories based on the highest level of respiratory support received: (1) no supplemental oxygen; (2) standard flow supplemental oxygen (flow rate <30 liters/minute); (3) high-flow nasal cannula or non-invasive ventilation; (4) invasive mechanical ventilation; or (5) death. We developed a multivariable proportional odds regression model where vaccination status was the primary exposure variable, the five-category ordinal respiratory severity scale was the outcome variable, and the same covariates used in the VE models were included (Appendix B, section 5).
Finally, VE against each of the 5 secondary outcomes was calculated using the same approach described for VE against hospitalizations. Separate logistic regression models were constructed in which cases were defined as influenza patients who met each of the outcome definitions. The same control group (adults hospitalized with ARI and testing negative for influenza virus and SARS-CoV-2) were used in VE models for all outcomes. Analyses were conducted using R (Vienna, Austria) [22].
RESULTS
Enrollment and Patient Characteristics
Overall, 7129 patients were enrolled between 1 October 2022, and 28 February 2023. Of these, 3422 were excluded from the analysis, with the most common reasons being testing positive for SARS-CoV-2 (2496), incomplete data on influenza vaccination status (690), and enrollment as a control before the first or after the last influenza case at the local site (232) (Appendix C, Supplementary Figure 1). A total of 3707 patients comprised the analytic data set, including 714 influenza cases and 2993 controls.
Among 714 influenza-case patients, influenza type was determined by RT-PCR for 431 patients, including 286/431 (67%) with influenza A(H3N2) and 145/431 (33%) with influenza A(H1N1). Among the 286 patients with influenza A(H3N2) confirmed by RT-PCR, viral whole genome sequencing identified a clade in 219 patients, all of which were clade 3C.2a1b.2a. Among the 145 patients with influenza A(H1N1), viral whole genome sequencing identified a clade in 100 patients, all of which were clade 6B.1A.5a.2. No influenza B was detected.
Influenza vaccination was lower among influenza case patients (235/714; 33%) than control patients (1462/2993, 49%) (P < .001) (Table 1). Among vaccinated patients, case patients had shorter times from influenza vaccination to illness onset (median [interquartile range {IQR}]: 63 days [43–83 years]) compared with control patients (74.5 days [47–104 days], P < .001). Additionally, case patients, compared with control patients, were younger (median age: 63 vs 65 years, P = .006), more likely to be non-Hispanic Black (30% vs 23%; P < .001), and less likely to have had ≥1 hospitalization in the previous year (51% vs 62%, P < .001). Patients who received influenza vaccination, compared with unvaccinated patients, were older (median age: 68 vs 62 years, P < .001), more likely to be non-Hispanic White (65% vs 49%; P < .001), more likely to be immunocompromised (19% vs 14%; P < .001), and more likely to have had ≥1 hospitalization in the previous year (63% vs 57%; P < .001) (Appendix D, Supplementary Table 1).
Table 1.
Characteristics of Influenza-positive Patients (Cases) and Influenza-negative/SARS-CoV-2-negative Patients (Controls)
| Overall | Influenza-Positive Case Patients | Influenza-Negative and SARS-CoV-2-Negative Control Patients | P | |
|---|---|---|---|---|
| No. (%) (row percentage) | 3707 (100%) | 714 (19%) | 2993 (81%) | |
| Census region | .002 | |||
| Northeast | 848 (23%) | 148 (21%) | 700 (23%) | |
| Midwest | 1126 (30%) | 243 (34%) | 883 (30%) | |
| South | 978 (26%) | 206 (29%) | 772 (26%) | |
| West | 755 (20%) | 117 (16%) | 638 (21%) | |
| Vaccination characteristics | ||||
| Vaccinated | 1700 (46%) | 235 (33%) | 1462 (49%) | |
| Days from influenza vaccination to illness onset, median (IQR) | 73 (47–101) | 63 (43–83) | 74.5 (47–103) | <.001 |
| Vaccination group | <.001 | |||
| 14–90 d from vaccination | 961 (26%) | 158 (22%) | 803 (27%) | |
| >90 d from vaccination | 484 (13%) | 31 (4%) | 453 (15%) | |
| Demographic characteristics | ||||
| Age, median (IQR), y | 65 (53–75) | 62 (51–73) | 65 (53–75) | .006 |
| Age group | .007 | |||
| 18–49 y | 768 (21%) | 170 (24%) | 598 (20%) | |
| 50–64 y | 1030 (28%) | 213 (30%) | 817 (27%) | |
| ≥65 y | 1909 (51%) | 331 (46%) | 1578 (53%) | |
| Female | 1854 (50%) | 390 (55%) | 1464 (51%) | .006 |
| Race/ethnicity | <.001 | |||
| White, non-Hispanic | 2081 (56%) | 353 (49%) | 1728 (58%) | |
| Black, non-Hispanic | 898 (24%) | 213 (30%) | 685 (23%) | |
| Hispanic, any race | 439 (12%) | 94 (13%) | 345 (11%) | |
| Other race, non-Hispanic | 195 (5%) | 40 (6%) | 155 (5%) | |
| Unknown | 94 (3%) | 14 (2%) | 80 (3%) | |
| Health status indicators | ||||
| Insured | 3548 (96%) | 667 (93%) | 2881 (96%) | <.001 |
| Current tobacco smoking | 685 (18%) | 165 (23%) | 520 (17%) | .003 |
| ≥1 Hospitalization in prior year | 2209 (60%) | 362 (51%) | 1847 (62%) | <.001 |
| Chronic respiratory condition | 1593 (43%) | 300 (42%) | 1293 (43%) | .566 |
| Moderate to severely immunocompromised | 603 (16%) | 103 (14%) | 500 (17%) | .138 |
| No. of chronic underlying medical conditions categories | .007 | |||
| 0 | 242 (7%) | 61 (9%) | 181 (6%) | |
| 1 | 644 (17%) | 146 (20%) | 498 (17%) | |
| 2 | 946 (26%) | 178 (25%) | 768 (26%) | |
| 3 | 880 (24%) | 178 (25%) | 722 (24%) | |
| ≥4 | 995 (27%) | 171 (24%) | 824 (28%) | |
| Admission characteristics | ||||
| Days from illness onset to influenza testing, median (IQR) | 2 (0–3) | 2 (1–3) | 2 (0–3) | <.001 |
| Days from illness onset to hospitalization, median (IQR) | 2 (0–3) | 2 (1–3) | 2 (0–3) | <.001 |
Abbreviations: IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
VE Against Influenza-associated Hospitalization
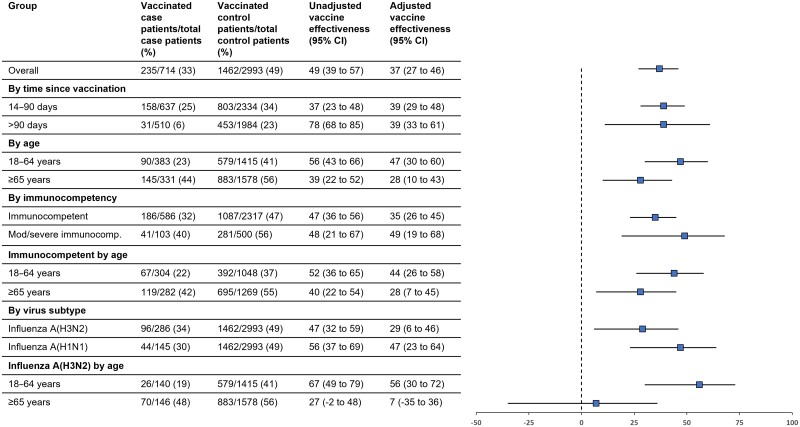
Overall, VE against influenza-associated hospitalization was 37% (95% confidence interval [CI]: 27%–46%) and was similar among those vaccinated 14–90 days prior to illness onset (39% [95% CI: 29%–48%]) and those vaccinated >90 days before onset (39% [95% CI: 33%–61%]) (Figure 1). Stratified by age group, VE was 47% (95% CI: 30%–60%) among adults aged 18–64 years, and 28% (95% CI: 10%–43%) among adults aged ≥65 years. By immunocompromised status, VE was 35% (95% CI: 23%–45%) for immunocompetent patients and 49% (95% CI: 19%–68%) for immunocompromised patients; VE differed between immunocompetent adults aged 18–64 years (44% [95% CI: 25%–58%]) and aged ≥65 years (28% [95% CI: 28% [7%–45%]). Stratified by virus subtype, VE against influenza-associated hospitalization was 29% (95% CI: 6%–46%) for influenza A(H3N2) and 47% (95% CI: 23%–64%) for influenza A(H1N1). VE against influenza A(H3N2) differed between patients aged 18–64 years (56% [95% CI: 36%–72%]) and ≥65 years (7% [95% CI: −35% to 36%]).
Figure 1.
Vaccine effectiveness (VE) against influenza-associated hospitalization for the 2022–2023 seasonal influenza vaccine overall and by subgroups. Abbreviation: CI, confidence interval.
Illness Severity Among Vaccinated and Unvaccinated Influenza Cases
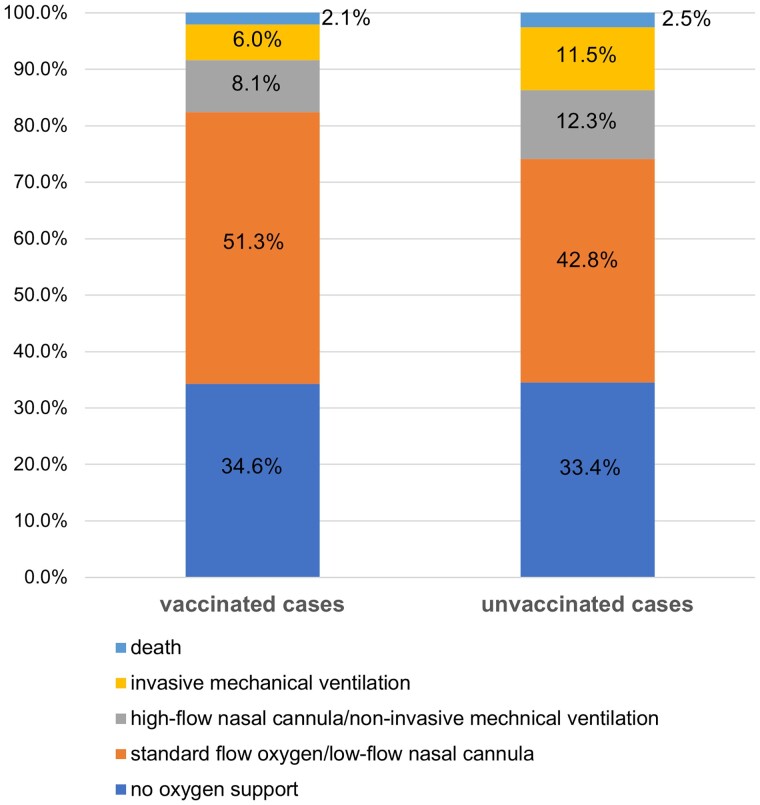
In unadjusted comparisons, a lower proportion of vaccinated cases compared with unvaccinated cases experienced many of the severe outcomes, including invasive mechanical ventilation (6% vs 11%, P = .03), vasopressor use (5% vs 11%, P = .02), organ support (15% vs 25%, P < .001), and intensive care unit (ICU) admission (11% vs 22%, P < .001) (Table 2). Based on a 5-category ordinal scale for respiratory disease severity, vaccinated cases had lower peak severity in the hospital compared with unvaccinated cases (adjusted odds ratio: 0.59 [95% CI: .50–.70]) (Figure 2).
Table 2.
In-hospital Outcomes Through Hospital day 28 Among Patients Hospitalized With Influenza Infection (Cases), by Vaccination Status
| Outcome | Vaccinated Cases (n = 235) | Unvaccinated Cases (n = 479) | P |
|---|---|---|---|
| Supplemental oxygen therapy (standard flow oxygen, high-flow nasal cannula, non-invasive ventilation, or invasive mechanical ventilation) | 155 (66%) | 319 (67%) | .93 |
| High-flow nasal cannula and/or non-invasive ventilation | 19 (8%) | 61 (13%) | .08 |
| Invasive mechanical ventilation | 14 (6%) | 54 (11%) | .03 |
| Acute vasopressor use | 11 (5%) | 49 (10%) | .02 |
| Acute renal replacement therapy | 0/226a (0%) | 3/458a (1%) | .55 |
| Acute organ support (high-flow nasal cannula, non-invasive ventilation, invasive mechanical ventilation, renal replacement therapy, or vasopressor use) | 35 (15%) | 122 (25%) | <.001 |
| Intensive care unit admission | 27 (11%) | 107 (22%) | <.001 |
| Death | 5 (2%) | 12 (3%) | .96 |
aPatients on chronic renal replacement therapy before their influenza illness were not eligible for acute renal replacement therapy and hence were removed from the denominator for this outcome. Chronic renal replacement therapy was received by 9 vaccinated cases and 21 unvaccinated cases.
Figure 2.
Peak respiratory illness severity experienced during the first 28 d of hospitalization among vaccinated and unvaccinated influenza cases. Peak respiratory illness severity was classified based on a 5-level scale, including: (1) no oxygen therapy; (2) standard flow supplemental oxygen (flow rate <30 L/min); (3) high flow nasal cannula or non-invasive ventilation; (4) invasive mechanical ventilation; (5) death. Overall, vaccinated cases experienced lower peak severity than unvaccinated cases (adjusted odds ratio: 0.59 [95% CI: .50–.70]). Abbreviation: CI, confidence interval.
VE Against Organ Failure and Death Outcomes
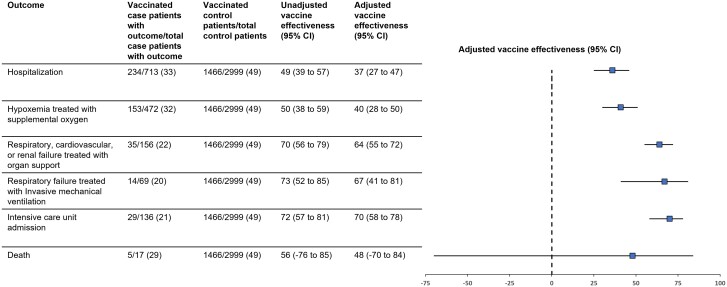
Influenza vaccination demonstrated benefit against: influenza with hypoxemia treated with supplemental oxygen (VE: 41% [95% CI: 29%–50%]); influenza with respiratory, cardiovascular, or renal failure treated with acute organ support (VE: 65% [95% CI: 56%–72%]); influenza with respiratory failure treated with invasive mechanical ventilation (VE: 66% [95% CI: 40%–81%]); and influenza-associated ICU admission (VE: 69% [95% CI: 59%–83%]) (Figure 3). VE against influenza-associated death was 48% (95% CI: −70% to 84%); however, the number of influenza-associated deaths was limited (5 vaccinated and 12 unvaccinated influenza cases died), resulting in a wide confidence interval.
Figure 3.
Vaccine effectiveness (VE) of the 2022–2023 seasonal influenza vaccine against influenza-associated organ failure and death outcomes. Abbreviation: CI, confidence interval.
DISCUSSION
During the 2022–2023 US influenza season, we found significant VE against adult influenza-associated hospitalization overall and across subgroups based on age, immunocompromised status, and virus type. Furthermore, we found strong VE against severe manifestations of influenza, including organ failure, need for invasive mechanical ventilation, and ICU admission.
The VE against influenza-associated hospitalization reported here was higher than from the same network during the prior year (the 2021–2022 influenza season), when overall VE against hospitalization was 26% (95% CI: −14% to 52%) [4]. Higher VE during 2022–2023 compared with the 2021–2022 season may be attributable to the relative earliness of the 2022–2023 season, which peaked during early December 2022 with a hospitalization rate of about 9 per 100 000 [1]. In contrast, the largest peak for the 2021–2022 season occurred in early April 2022 (4 months later), with a hospitalization rate of about 2 per 100 000 [1]. Due to the early influenza season during 2022–2023, the median days from last vaccination to illness onset in this analysis was 72.5 days (IQR: 47–103 days) compared with 161 days (IQR: 122–196 days) during 2021–2022 in the same network [4]. Waning protection from influenza vaccination has been observed in previous seasons, particularly those with late onset and peaks [23]. Although this study found no difference in VE between patients vaccinated 14–90 versus ≥90 days before illness onset, it is possible that substantial waning does not begin until later (eg, 150 days), and few patients during the 2022–2023 season were vaccinated this long before illness onset. Although the early influenza season during 2022–2023 likely played a role in the relatively high observed VE during this year compared to prior years, low VE in many seasons suggests an ongoing need for improved influenza vaccines, including messenger RNA (mRNA) vaccines.
Influenza surveillance networks [1] and individual studies [5, 6], including the current analysis, found that influenza A(H1N1) comprised about one third of circulating viruses during an A(H3N2) predominant 2022–2023 season. As observed in this tudy, VE is typically higher for A(H1N1) than A(H3N2) viruses, which likely contributed to higher VE against influenza A during this season compared with prior seasons with lower proportions of A(H1N1) cases [8, 24, 25]. Lower VE against A(H3N2) may be due to mutations in A(H3N2) that result in escape of the virus from the immune response to vaccination [26, 27].
The lower VE observed for adults ≥65 years old, especially against A(H3N2), may be due to immunological factors in this subgroup [9, 10, 28, 29]. Adults ≥65 years old were not exposed to A(H3N2) early in life, potentially reducing the effectiveness of vaccination later in life against A(H3N2) via the phenomenon of immune imprinting. In this analysis A(H3N2) cases were slightly more frequent in patients aged ≥65 years compared with those aged 18–64 years (68% vs 65% of cases). Additionally, lower VE in older adults might be partially related to immune senescence [30], “inflamm-aging” (ie, a chronic low-level state of inflammation from multiple underlying conditions and frailty suspected to impair immune responses in older adults) [31], and potential blunting effects of repeated vaccination [32].
Interestingly, we found similar VE for immunocompromised and immunocompetent adults. Although lower VE against severe influenza in immunocompromised patients has been observed in previous studies [33], including within this network during prior years [4, 9], patients with immunocompromising conditions are a heterogenous group where varying composition from season to may affect seasonal VE in the immunocompromised population compared to the concurrent immunocompetent population. A recent meta-analysis across multiple seasons found no difference in influenza VE between immunocompromised and immunocompetent persons [34], supporting that influenza VE may not be reduced in some immunocompromised populations. In addition, the potentially higher likelihood of both being vaccinated and being hospitalized with low-severity illness among immunocompromised patients could lead to residual confounding [35].
Importantly, influenza vaccination was associated with a beneficial effect even for patients hospitalized with influenza, as evidenced by better in-hospital outcomes among vaccinated compared with unvaccinated cases. These findings suggest that vaccination can attenuate influenza disease severity and prevent the most severe manifestations of influenza even in those infected despite vaccination. Our findings of substantial VE against influenza-associated ICU admission and a range of organ failures quantify the benefit of vaccination for the prevention of these severe outcomes.
This analysis was subject to limitations. First, sample size limited VE estimation or precision for some subgroups. Second, data on the type of influenza vaccine received were not collected, precluding comparative effectiveness analyses by vaccine type. Of note, most US adults aged ≥65 years receive a higher-dose or adjuvanted vaccine (compared with standard dose vaccine); thus, adverse biological and virological factors associated with influenza in older adults may offset the benefits of enhanced vaccines [35]. Third, the low number of influenza-associated deaths in this analysis limited our ability to make conclusions about VE against influenza-associated mortality. Finally, although the analysis controlled for several relevant potential confounders, residual confounding is possible.
CONCLUSIONS
During the 2022–2023 US influenza season, characterized by an early peak of influenza activity and a good match between the vaccine and circulating viruses, we observed significant protection from vaccination against influenza-associated hospitalizations overall, for both A(H3N2) and A(H1N1) subtypes, and for key patient subgroups at increased risk for severe illness, including those ≥65 years old and those with immunocompromising conditions. Substantial VE was observed against a range of severe influenza-associated outcomes, including organ failures and ICU admission, demonstrating that vaccination can be beneficial in preventing the most severe manifestations of influenza even when infection is not averted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Nathaniel M Lewis, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Yuwei Zhu, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Ithan D Peltan, Department of Medicine, Intermountain Medical Center, Murray, Utah, and University of Utah, Salt Lake City, Utah, USA.
Manjusha Gaglani, Baylor Scott and White Health, Temple and Dallas, Texas, and Texas A&M University College of Medicine, Temple, Texas, USA.
Tresa McNeal, Baylor Scott and White Health, and Baylor College of Medicine, Temple, Texas, USA.
Shekhar Ghamande, Baylor Scott and White Health, Texas A&M University College of Medicine, Temple, Texas, USA.
Jay S Steingrub, Department of Medicine, Baystate Medical Center, Springfield, Massachusetts, USA.
Nathan I Shapiro, Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Abhijit Duggal, Department of Medicine, Cleveland Clinic, Cleveland, Ohio, USA.
William S Bender, Department of Medicine, Emory University, Atlanta, Georgia, USA.
Leyla Taghizadeh, Department of Emergency Medicine, Hennepin County Medical Center, Minneapolis, Minnesota, USA.
Samuel M Brown, Department of Medicine, Intermountain Medical Center, Murray, Utah, and University of Utah, Salt Lake City, Utah, USA.
David N Hager, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michelle N Gong, Department of Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, USA.
Amira Mohamed, Department of Medicine, The Ohio State University, Columbus, Ohio, USA.
Matthew C Exline, Department of Medicine, The Ohio State University, Columbus, Ohio, USA.
Akram Khan, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon, USA.
Jennifer G Wilson, Department of Emergency Medicine, Stanford University School of Medicine, Stanford, California, USA.
Nida Qadir, Department of Medicine, University of California-Los Angeles, Los Angeles, California, USA.
Steven Y Chang, Department of Medicine, University of California-Los Angeles, Los Angeles, California, USA.
Adit A Ginde, Department of Emergency Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Nicholas M Mohr, Carver College of Medicine, University of Iowa, Iowa City, Iowa, USA.
Christopher Mallow, Department of Medicine, University of Miami, Miami, Florida, USA.
Adam S Lauring, Departments of Internal Medicine and Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan, USA.
Nicholas J Johnson, Department of Emergency Medicine and Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington, Seattle, Washington, USA.
Kevin W Gibbs, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
Jennie H Kwon, Department of Medicine, Washington University, St.Louis, Missouri, USA.
Cristie Columbus, Baylor, Scott and White Health, Dallas, Texas, USA.
Robert L Gottlieb, Baylor University Medical Center Dallas, Baylor, Scott & White Heart and Vascular Hospital, Baylor, Scott and White Research Institute, Dallas, Texas, USA.
Catherine Raver, Baylor, Scott and White Health, Dallas, Texas, USA.
Ivana A Vaughn, Department of Public Health Sciences, Henry Ford Health, Detroit, Michigan, USA.
Mayur Ramesh, Division of Infectious Diseases, Henry Ford Health, Detroit, Michigan, USA.
Cassandra Johnson, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Lois Lamerato, Department of Public Health Sciences, Henry Ford Health, Detroit, Michigan, USA.
Basmah Safdar, Emergency Medicine, Yale University School of Medicine, New Haven, Connecticut, USA.
Jonathan D Casey, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Todd W Rice, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Natasha Halasa, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
James D Chappell, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carlos G Grijalva, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
H Keipp Talbot, Departments of Medicine and Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Adrienne Baughman, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kelsey N Womack, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Sydney A Swan, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Elizabeth Harker, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Ashley Price, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Jennifer DeCuir, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Diya Surie, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Sascha Ellington, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Wesley H Self, Department of Emergency Medicine, Vanderbilt Institute for Clinical and Translational Research, and Vanderbilt University Medical Center, Nashville, Tennessee, USA.
for the Investigating Respiratory Viruses in the Acutely Ill (IVY) Network:
Nicole Calhoun, Kempapura Murthy, Joselyn Cravens, Judy Herrick, Amanda McKillop, Eric Hoffman, Ashley Graves, Martha Zayed, Michael Smith, Baylor Scott, White Health, Tammy Fisher, Mariana Hurutado-Rodriguez, Taryn Kruse, Symone Dunkley, Gabriela Perez, Ashley Bychkowski, Therissa Grefsrud, Nicole Calhoun, Kempapura Murthy, Judy Herrick, Michael Smith, Jay Steingrub, Lori-Ann Kozikowski, Lesley De Souza, Scott Ouellette, Nathan I Shapiro, Michael Bolstad, Brianna Coviello, Robert Ciottone, Arnaldo Devilla, Ana Grafals, Conor Higgins, Carlo Ottanelli, Kimberly Redman, Douglas Scaffidi, Alexander Weingart, Diya Surie, Meredith McMorrow, Jennifer DeCuir, Nathaniel Lewis, Elizabeth Harker, Sascha Ellington, Omar Mehkri, Megan Mitchell, Zachary Griffith, Connery Brennan, Kiran Ashok, Bryan Poynter, Abhijit Duggal, Laurence Busse, Caitlin ten Lohuis, Nicholas Stanley, Sophia Zhang, Matthew E Prekker, Anne E P Frosch, Audrey Hendrickson, Stephen Douglas, Kowsar Hurreh, Ithan Peltan, Cassie Smith, Hunter Marshall, David N Hager, Harith Ali, Minh Phan, Michelle Gong, Amira Mohamed, Rahul Nair, Jen-Ting (Tina) Chen, Matthew Exline, Sarah Karow, Maryiam Khan, Madison So, Connor Snyder, Gabrielle Swoope, David Smith, Brooke Lee, Amanie Rasul, Manisha Pathak, Zachery Lewald, Reece Wilson, Akram Khan, José Peña, Genesis Briceno, Cassandra Ahmed, Jesus Martinez, Edvinas Pocius, Minn Oh, Jessica Hyde, Sherie Gause, Jennifer G Wilson, Alexandra June Gordon, Cynthia Perez, Lily Lau, Ismail Hakki Bekiroglu, Cody Tran, Trevor Frankel, Omai Garner, Sukantha Chandrasekaran, Adit Ginde, David Douin, Amanda Martinez, David Huynh, Aimee Steinwand, Amy Sullivan, Cori Withers, Nicholas Mohr, Anne Zepeski, Paul Nassar, Shannon Landers, Karin Nielsen, Noble Briggs, Cathy Fairfield, Alex Peebles, Chris Mallow, Carolina Rivas, Emily Martin, Arnold Monto, Adam Lauring, E J McSpadden, Rachel Truscon, Anne Kaniclides, Lara Thomas, Ramsay Bielak, Weronika Damek Valvano, Rebecca Fong, William J Fitzsimmons, Christopher N Blair, Julie Gilbert, Leigh Papalambros, Ankur Holz, Nicholas Johnson, Vasisht Srinivasan, Christine D Crider, Kyle A Steinbock, Thomas C Paulsen, Layla A Anderson, Wesley H Self, H Keipp Talbot, Carlos Grijalva, Ian Jones, Natasha Halasa, James Chappell, Kelsey Womack, Jillian Rhoads, Adrienne Baughman, Christy Kampe, Jakea Johnson, Sydney Swan, Cassandra Johnson, Yuwei Zhu, Todd Rice, Jonathan Casey, William B Stubblefield, Yuwei Zhu, Laura L Short, Lauren J Ezzell, Margaret E Whitsett, Rendie E McHenry, Samarian J Hargrave, Marcia Blair, Jennifer L Luther, Claudia Guevara Pulido, Bryan P M Peterson, D Clark Files, Kevin Gibbs, Leigha Landreth, Madeline Hicks, Lisa Parks, Jennie Kwon, Bijal Parikh, David McDonald, Carleigh Samuels, Lucy Vogt, Caroline O’Neil, Alyssa Valencia, Tiffany Hink, Ashley Bychkowski, Symone Dunkley, Tammy Fisher, Therissa Grefsrud, Mariana H Hurutado-Rodriguez, Gabriela Cantu Perez, Kim Beney, Rachna Jayaprakash, Sindhuja Koneru, Jean Ashley Lava, Zina Pinderi, Melissa Resk, Anirudh Goyal, Lauren DeLamielleure, Michael Kosover, and Carolyn Brokowski
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the US Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (contract numbers 75D30122C12914 and 75D30122C14944 to W. H. S.).
References
- 1. CDC . Fluview Past Weekly Surveillance Reports 2017–2023. Available at: https://www.cdc.gov/flu/weekly/pastreports.htm. Accessed 18 March 2023.
- 2. Chung JR, Kim SS, Kondor RJ, et al. Interim estimates of 2021–22 seasonal influenza vaccine effectiveness —United States, February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delahoy MJ, Mortenson L, Bauman L, et al. Influenza A(H3N2) outbreak on a university campus—Michigan, October–November 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1712–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tenforde MW, Patel M, Lewis NM, et al. Vaccine effectiveness against influenza A(H3N2)–associated hospitalized illness: United States. Clin Infect Dis 2022; 76:1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLean HQ, Petrie JG, Hanson KE, et al. Interim estimates of 2022–23 seasonal influenza vaccine effectiveness—Wisconsin, October 2022–February 2023. MMWR Morb Mortal Wkly Rep 2023; 72:201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skowronski DM, Chuang ESY, Sabaiduc S, et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Euro Surveill 2023; 28:2300043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Recommendations announced for influenza vaccine composition for the 2022–2023 northern hemisphere influenza season. 25 February 2022. Available at: https://www.who.int/news/item/25-02-2022-recommendations-announced-for-influenza-vaccine-composition-for-the-2022-2023-northern-hemisphere-influenza-season. Accessed 18 March 2023.
- 8. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022–23 influenza vaccine—United States, 2021–22 season. MMWR Morb Mortal Wkly Rep 2022; 71:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grijalva CG, Feldstein LR, Talbot HK, et al. Influenza vaccine effectiveness for prevention of severe influenza-associated illness among adults in the United States, 2019–2020: a test-negative study. Clin Infect Dis 2021; 73:1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018; 36:5916–25. [DOI] [PubMed] [Google Scholar]
- 11. Arriola C, Garg S, Anderson EJ, et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 2017; 65:1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 13. Surie D, DeCuir J, Zhu Y, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated hospitalization among immunocompetent adults aged ≥65 years—IVY network, 18 states, September 8–November 30, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams K, Rhoads JP, Surie D, et al. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: living test negative design study. BMJ 2022; 379:e072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Surie D, Bonnell LN, DeCuir J, et al. Comparison of mRNA vaccine effectiveness against COVID-19-associated hospitalization by vaccination source: immunization information systems, electronic medical records, and self-report-IVY network, February 1–August 31, 2022. Vaccine 2023; 41:4249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doll MK, Pettigrew SM, Ma J, Verma A. Effects of confounding bias in COVID-19 and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis 2022; 75:e564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leuchter RK, Jackson NJ, Mafi JN, Sarkisian CA. Association between COVID-19 vaccination and influenza vaccination rates. N Engl J Med 2022; 386:2531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 19. Feldstein LR, Self WH, Ferdinands JM. Incorporating real-time influenza detection into the test-negative design for estimating influenza vaccine effectiveness: the real-time test-negative design (rtTND). Clin Infect Dis 2021; 72:1669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foppa IM, Ferdinands JM, Chaves SS, et al. The case test-negative design for studies of the effectiveness of influenza vaccine in inpatient settings. Intl J Epidemiology 2016; 45:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institutes of Health . Special considerations in people who are immunocompromised. Available at: https://www.covid19treatmentguidelines.nih.gov/special-populations/immunocompromised/. Accessed 18 March 2023.
- 22. R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2023. Available at: https://www.R-project.org/. Accessed 18 March 2023.
- 23. Ferdinands JM, Gaglani M, Martin ET, et al. Waning vaccine effectiveness against influenza-associated hospitalizations among adults, 2015–2016 to 2018–2019, United States hospitalized adult influenza vaccine effectiveness network. Clin Infect Dis 2021; 73:726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizzo C, Gesualdo F, Loconsole D, et al. Moderate vaccine effectiveness against severe acute respiratory infection caused by A(H1N1)pdm09 influenza virus and no effectiveness against A(H3N2) influenza virus in the 2018/2019 season in Italy. Vaccine 2020; 8:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valenciano M, Kissling E, Reuss A, et al. The European I-MOVE Multicentre 2013–2014 Case-Control Study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2). Vaccine 2015; 33:2813–22. [DOI] [PubMed] [Google Scholar]
- 26. Flannery B, Zimmerman RK, Gubareva LV, et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis 2016; 214:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin ET, Cheng C, Petrie JG, et al. Low influenza vaccine effectiveness against A(H3N2)-associated hospitalizations in 2016–2017 and 2017–2018 of the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2021; 223:2062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gostic KM, Bridge R, Brady S, Viboud C, Worobey M, Lloyd-Smith JO. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog 2019; 15:e1008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis 2017; 215:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McElhaney JE, Verschoor CP, Andrew MK, Haynes L, Kuchel GA, Pawelec G. The immune response to influenza in older humans: beyond immune senescence. Immun Ageing 2020; 17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 2009; 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:723–36. [DOI] [PubMed] [Google Scholar]
- 33. Hughes K, Middleton DB, Nowalk MP, et al. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in immunocompromised adults. Clin Infect Dis 2021; 73:e4353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beck CR, McKenzie BC, Hashim AB, et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis from a public health policy perspective. PLoS One 2011; 6:e29249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among US medicare beneficiaries ages 65 years and older during the 2019–2020 season. Clin Infect Dis 2021; 73:e4251–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.