Abstract
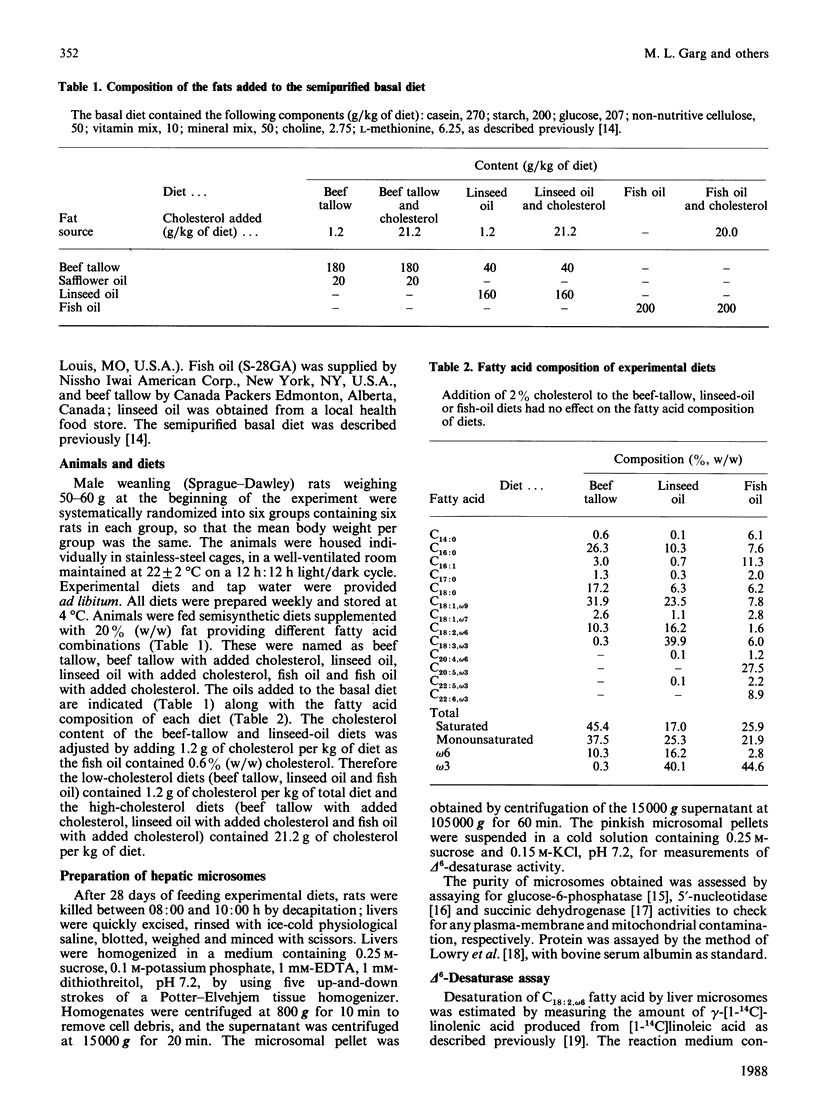
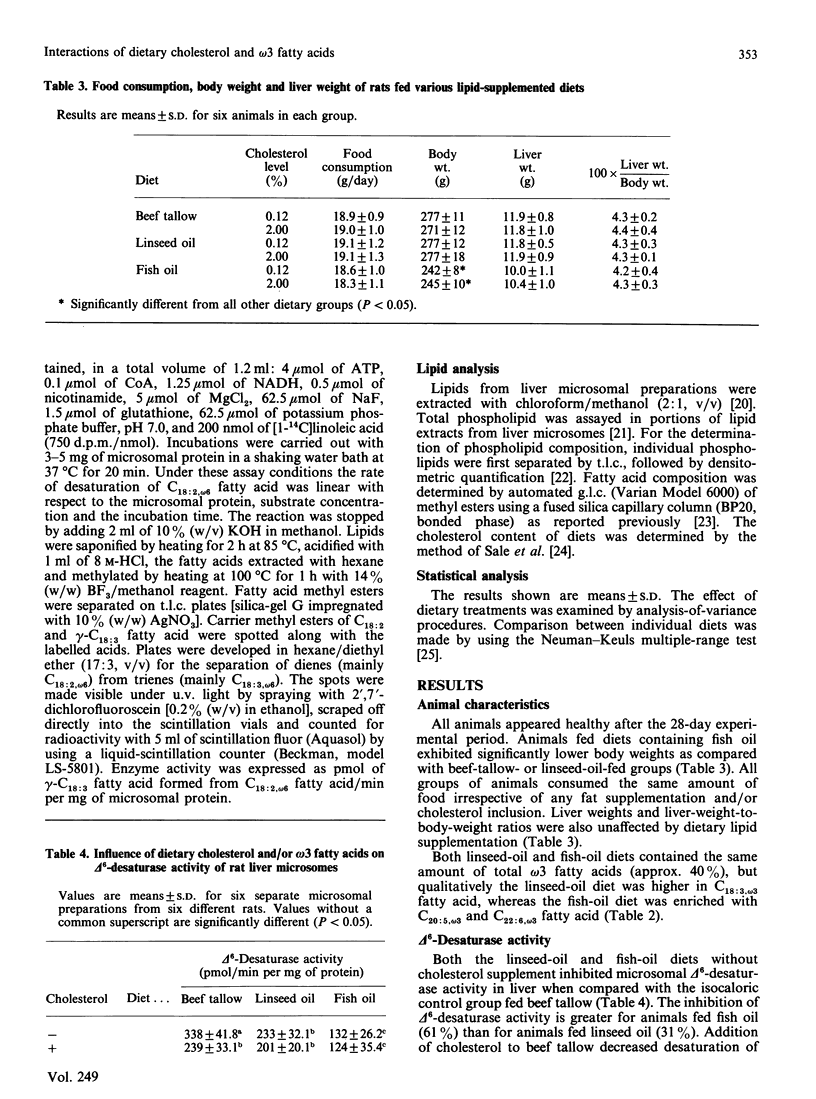
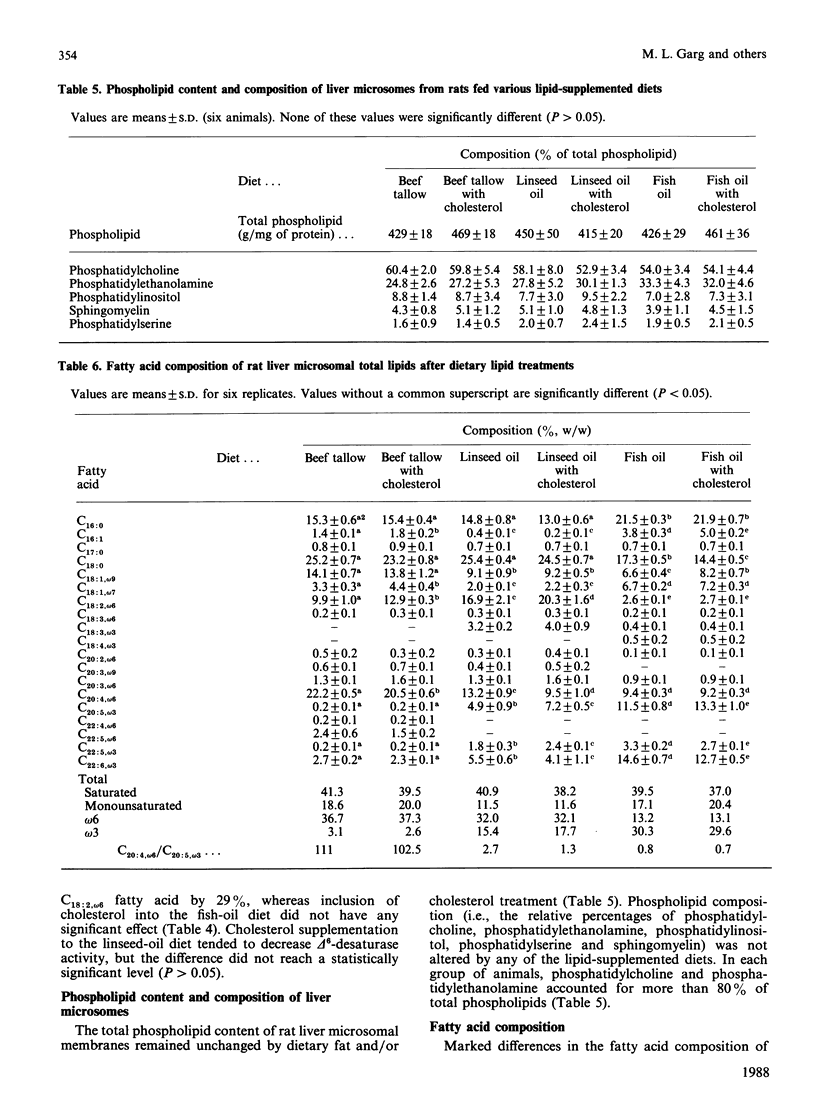
The effect of feeding semipurified diets enriched in linseed (rich in C18:3, omega 3 fatty acid) or fish (rich in C20:5, omega 3 and C22:6, omega 3 fatty acid) oil with and without cholesterol supplementation on the desaturation of linoleic acid (C18:2, omega 6) by rat liver microsomal fractions was investigated. Animals fed diets supplemented with beef tallow were used as equal-energy controls. Both linseed-oil and fish-oil diets, without added cholesterol, decrease conversion of C18:2, omega 6 fatty acid to gamma-linolenic acid (C18:3, omega 6). Reduction in delta 6-desaturation was significantly greater for animals fed the diet containing fish oil than with animals fed the linseed-oil diet. The major effect of cholesterol supplementation was to decrease the rate of desaturation of C18:2, omega 6, when fed in combination with the beef-tallow diet, whereas delta 6-desaturation was unaffected when cholesterol was fed along with diets high in omega 3 fatty acids (linseed oil or fish oil). The activity of the delta 6-desaturase in vitro is consistent with the fatty acid composition observed for the microsomal membranes on which this enzyme is localized. Dietary linseed oil and fish oil lowered the arachidonic (C20:4, omega 6) acid content of rat liver microsomes, with an accompanying increase in membrane eicosapentaenoic (C20:5, omega 3) and docosahexaenoic (C22:6, omega 3) acid content, in comparison with the group fed beef tallow. Inclusion of cholesterol into the beef-tallow or linseed-oil diets resulted in decreased membrane C20:4, omega 6-fatty-acid content, with concomitant increase in C18:2, omega 6-fatty-acid content. However, addition of cholesterol to the fish-oil diet did not alter the microsomal membrane content of C20:4, omega 6 fatty acid. Thus it is suggested that (1) the decrease in prostaglandin E2, thromboxane and prostacyclin levels generally observed after fish-oil consumption may be at least partly due to inhibition of C20:4, omega 6-fatty-acid synthesis from C18:2, omega 6 fatty acid; and (2) consumption of fish oil prevents the further decrease in C20:4, omega 6-fatty-acid levels by dietary cholesterol that is apparent when cholesterol is fed in combination with diets high in saturated fat or C18:3, omega 3 fatty acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS J. S., GRIFFITH W. H., MEAD J. F., STEIN R. A. Toxicity of air-oxidized soybean oil. J Nutr. 1960 Feb;70:199–210. doi: 10.1093/jn/70.2.199. [DOI] [PubMed] [Google Scholar]
- Ahmed A. A., Holub B. J. Alteration and recovery of bleeding times, platelet aggregation and fatty acid composition of individual phospholipids in platelets of human subjects receiving a supplement of cod-liver oil. Lipids. 1984 Aug;19(8):617–624. doi: 10.1007/BF02534720. [DOI] [PubMed] [Google Scholar]
- Brenner R. R., Peluffo R. O. Inhibitory effect of docosa-4,7,10,13,16,19-hexaenoic acid upon the oxidative desaturation of linoleic into gamma-linolenic acid and of alpha-linolenic into octadeca-6,9,12,15-tetraenoic acid. Biochim Biophys Acta. 1967 Feb 14;137(1):184–186. doi: 10.1016/0005-2760(67)90024-0. [DOI] [PubMed] [Google Scholar]
- Brenner R. R. The oxidative desaturation of unsaturated fatty acids in animals. Mol Cell Biochem. 1974 Mar 8;3(1):41–52. doi: 10.1007/BF01660076. [DOI] [PubMed] [Google Scholar]
- Bruckner G. G., Lokesh B., German B., Kinsella J. E. Biosynthesis of prostanoids, tissue fatty acid composition and thrombotic parameters in rats fed diets enriched with docosahexaenoic (22:6n3) or eicosapentaenoic (20:5n3) acids. Thromb Res. 1984 Jun 15;34(6):479–497. doi: 10.1016/0049-3848(84)90253-6. [DOI] [PubMed] [Google Scholar]
- Clandinin M. T., Yamashiro S. Dietary factors affecting the incidence of dietary fat-induced myocardial lesions. J Nutr. 1982 Apr;112(4):825–828. doi: 10.1093/jn/112.4.825. [DOI] [PubMed] [Google Scholar]
- Croft K. D., Beilin L. J., Vandongen R., Mathews E. Dietary modification of fatty acid and prostaglandin synthesis in the rat. Effect of variations in the level of dietary fat. Biochim Biophys Acta. 1984 Sep 12;795(2):196–207. doi: 10.1016/0005-2760(84)90066-3. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O. A hypothesis on the development of acute myocardial infarction in Greenlanders. Scand J Clin Lab Invest Suppl. 1982;161:7–13. [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Dyerberg J. Platelet - vessel wall interaction: influence of diet. Philos Trans R Soc Lond B Biol Sci. 1981 Aug 18;294(1072):373–381. doi: 10.1098/rstb.1981.0113. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Garg M. L., Snoswell A. M., Sabine J. R. Influence of dietary cholesterol on desaturase enzymes of rat liver microsomes. Prog Lipid Res. 1986;25(1-4):639–644. doi: 10.1016/0163-7827(86)90131-1. [DOI] [PubMed] [Google Scholar]
- Glastris B., Pfeiffer S. E. Mammalian membrane marker enzymes: sensitive assay for 5'-nucleotidase and assay for mammalian 2',3'-cyclic-nucleotide-3'-phosphohydrolase. Methods Enzymol. 1974;32:124–131. doi: 10.1016/0076-6879(74)32015-0. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY P. W., PELUFFO R., WAKIL S. J. ON THE BIOSYNTHESIS OF DIENOIC FATTY ACID BY ANIMAL TISSUES. Biochem Biophys Res Commun. 1963 Aug 1;12:300–304. doi: 10.1016/0006-291x(63)90300-0. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K. M., Clandinin M. T. Phosphatidylethanolamine methyltransferase: evidence for influence of diet fat on selectivity of substrate for methylation in rat brain synaptic plasma membranes. Biochim Biophys Acta. 1987 Apr 3;918(2):97–105. doi: 10.1016/0005-2760(87)90183-4. [DOI] [PubMed] [Google Scholar]
- Harris W. S., Connor W. E., McMurry M. P. The comparative reductions of the plasma lipids and lipoproteins by dietary polyunsaturated fats: salmon oil versus vegetable oils. Metabolism. 1983 Feb;32(2):179–184. doi: 10.1016/0026-0495(83)90226-3. [DOI] [PubMed] [Google Scholar]
- Herold P. M., Kinsella J. E. Fish oil consumption and decreased risk of cardiovascular disease: a comparison of findings from animal and human feeding trials. Am J Clin Nutr. 1986 Apr;43(4):566–598. doi: 10.1093/ajcn/43.4.566. [DOI] [PubMed] [Google Scholar]
- Johnson M. R., Mathur S. N., Coffman C., Spector A. A. Dietary fat saturation and hepatic acylcoenzyme A:cholesterol acyltransferase activity. Effect of n-3 polyunsaturated and long-chain saturated fat. Arteriosclerosis. 1983 May-Jun;3(3):242–248. doi: 10.1161/01.atv.3.3.242. [DOI] [PubMed] [Google Scholar]
- Kamath S. A., Narayan K. A. Interaction of Ca 2+ with endoplasmic reticulum of rat liver: a standardized procedure for the isolation of rat liver microsomes. Anal Biochem. 1972 Jul;48(1):53–61. doi: 10.1016/0003-2697(72)90169-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lokesh B. R., Bruckner G., Kinsella J. E. Reduction in thromboxane formation by n-3 fatty acids enriched lung microsomes from rat and guinea pig following the ingestion of dietary menhaden oil. Prostaglandins Leukot Med. 1984 Sep;15(3):337–348. doi: 10.1016/0262-1746(84)90133-1. [DOI] [PubMed] [Google Scholar]
- Mounié J., Faye B., Magdalou J., Goudonnet H., Truchot R., Siest G. Modulation of UDPglucuronosyltransferase activity in rats by dietary lipids. J Nutr. 1986 Oct;116(10):2034–2043. doi: 10.1093/jn/116.10.2034. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J. Fish oil attenuates the cholesterol induced rise in lipoprotein cholesterol. Am J Clin Nutr. 1986 May;43(5):752–757. doi: 10.1093/ajcn/43.5.752. [DOI] [PubMed] [Google Scholar]
- Omodeo Salè F., Marchesini S., Fishman P. H., Berra B. A sensitive enzymatic assay for determination of cholesterol in lipid extracts. Anal Biochem. 1984 Nov 1;142(2):347–350. doi: 10.1016/0003-2697(84)90475-5. [DOI] [PubMed] [Google Scholar]
- Phillipson B. E., Rothrock D. W., Connor W. E., Harris W. S., Illingworth D. R. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985 May 9;312(19):1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- Raheja R. K., Kaur C., Singh A., Bhatia I. S. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973 Nov;14(6):695–697. [PubMed] [Google Scholar]
- STOFFEL W. Biosynthesis of polyenoic fatty acids. Biochem Biophys Res Commun. 1961 Nov 29;6:270–273. doi: 10.1016/0006-291x(61)90376-x. [DOI] [PubMed] [Google Scholar]
- Sanders T. A., Vickers M., Haines A. P. Effect on blood lipids and haemostasis of a supplement of cod-liver oil, rich in eicosapentaenoic and docosahexaenoic acids, in healthy young men. Clin Sci (Lond) 1981 Sep;61(3):317–324. doi: 10.1042/cs0610317. [DOI] [PubMed] [Google Scholar]
- Siess W., Roth P., Scherer B., Kurzmann I., Böhlig B., Weber P. C. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet. 1980 Mar 1;1(8166):441–444. doi: 10.1016/s0140-6736(80)90995-2. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Kaduce T. L., Dane R. W. Effect of dietary fat saturation on acylcoenzyme A:cholesterol acyltransferase activity of rat liver microsomes. J Lipid Res. 1980 Feb;21(2):169–179. [PubMed] [Google Scholar]
- de Gomez Dumm I. N., de Alaniz M. J., Brenner R. R. Effect of dietary fatty acids on delta 5 desaturase activity and biosynthesis of arachidonic acid in rat liver microsomes. Lipids. 1983 Nov;18(11):781–788. doi: 10.1007/BF02534636. [DOI] [PubMed] [Google Scholar]


