Abstract
Background
Native vertebral osteomyelitis (NVO) caused by Staphylococcus aureus is associated with high risk of treatment failure and increased morbidity. The role of rifampin-based therapy for the treatment of this condition is controversial. The goal of this systematic review and meta-analysis is to explore the efficacy and safety of rifampin-based therapy for the treatment of S. aureus NVO.
Methods
We searched Cochrane, Embase, Medline, Scopus, and Web of Science databases for studies published up to May 2023, focusing on adults with NVO treated with or without rifampin-containing regimens. A random-effects model meta-analysis estimated relative risks and risk difference with 95% confidence intervals (CI).
Results
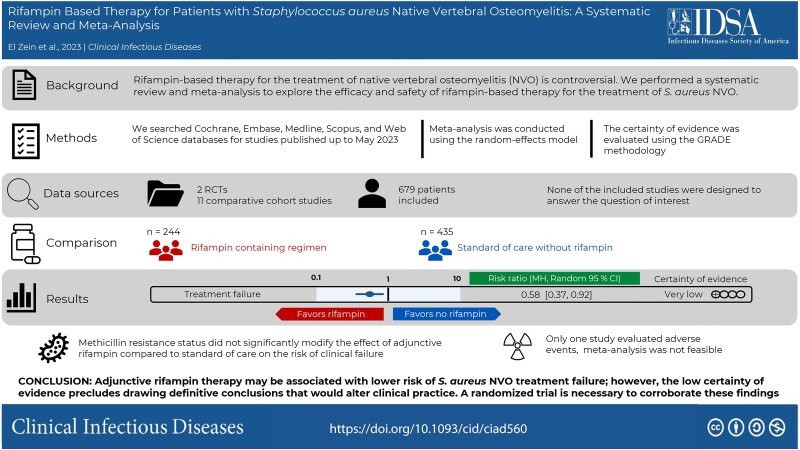
Thirteen studies (2 randomized controlled trials and 11 comparative cohort studies), comprising 244 patients with S. aureus NVO who received rifampin and 435 who did not, were analyzed. Meta-analysis showed that rifampin-based regimens were associated with lower risk of clinical failure (risk difference, −14%; 95% CI, −19% to −8%; P < .001; I 2 = 0%; relative risk, 0.58; 95% CI, .37–.92, P = .02, I 2 = 21%). Only 1 study reported on adverse events. All studies had a high or uncertain risk of bias, and the certainty of evidence was rated as very low.
Conclusions
Adjunctive rifampin therapy might be associated with lower risk of S. aureus NVO treatment failure; however, the low certainty of evidence precludes drawing definitive conclusions that would alter clinical practice. A randomized trial is necessary to corroborate these findings.
Keywords: rifampin, native vertebral osteomyelitis, NVO, rifampicin, spondylodiscitis
This meta-analysis showed that adjunctive rifampin therapy may be associated with lower risk of Staphylococcus aureus native vertebral osteomyelitis treatment failure. A randomized trial is necessary to corroborate these findings, given the very low certainty of evidence.
Graphical Abstract
Graphical Abstract.
Native vertebral osteomyelitis (NVO) is a potentially debilitating infection of the vertebrae, intervertebral discs, and adjacent soft tissues. The annual incidence of hospital admissions related to NVO increased in the United States from 2.9 to 5.4 per 100 000 patients between 1998 and 2013 [1] and from 6.1 to 11.3 per 100 000 patients in France between 2010 and 2019 [2]. Staphylococcus aureus is the most common pathogen causing NVO, accounting for 43% to 67% of the culture-positive cases [3–5]. Treatment failure in S. aureus NVO ranges between 10% and 48% in most clinical studies [5–9] and is associated with increased morbidity and disability [10]. There is an urgent need to identify antibiotic regimens that may improve outcomes in this patient population.
Although already explored in the context of implant-related infections [11], mounting evidence suggests that S. aureus biofilm formation of necrotic bone and intracellular bacterial persistence play an important role in the pathogenesis of chronic osteomyelitis and development of antibiotic tolerance [12, 13]. Rifampin, a semisynthetic rifamycin with high oral bioavailability, demonstrates excellent bone tissue penetration, achieving concentrations comparable to serum levels [14, 15]. Under appropriate conditions, rifampin exhibits activity against S. aureus in biofilms while retaining bactericidal properties against intracellular organisms [16–19]. Moreover, S. aureus isolates remain highly susceptible to rifampin, with less than 6% resistance reported in a US study [20] and 1% resistance observed at our institution in 2023 (unpublished data). However, worldwide data indicates higher rifampin resistance rates in methicillin-resistant isolates (12%–22%) compared with 1% in methicillin-susceptible S. aureus [21]. These characteristics make rifampin an attractive oral drug for the treatment of rifampin-susceptible S. aureus NVO.
In Europe, the combination of oral fluoroquinolones and rifampin is frequently used for the treatment of staphylococcal bone infection [22, 23]. The rationale for this dual treatment includes data on high bone penetration and resistance development prevention in comparison to fluoroquinolone or rifampin monotherapy [13]. Hence, this treatment combination is listed in the French guidelines published in 2007 [24] and, more recently, in an international consensus group statement on the treatment of osteomyelitis [25]. Extrapolation from cohort studies and randomized controlled trials (RCTs) that are not specifically designed to answer this clinical question show a possible trend toward improved outcomes with the use of adjunctive rifampin for the treatment of S. aureus NVO [5, 26, 27]; however, there have been no attempts to synthesize the literature to further examine this clinical question. To address this knowledge gap, we performed a systematic review to evaluate the efficacy and safety of adjunctive rifampin for the treatment of vertebral osteomyelitis caused by S. aureus.
METHODS
The presentation of findings in this systematic review and meta-analysis adheres to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [28].
Inclusion Criteria
We included studies that reported on adults with NVO treated with rifampin versus other treatments. Detailed inclusion and exclusion criteria are outlined in the Supplementary Appendix. The primary outcome was treatment failure. In the absence of a universally accepted definition of NVO treatment failure, we used the definitions as reported in the individual studies. The secondary outcome was adverse events associated with antibiotic therapy.
Search Methods
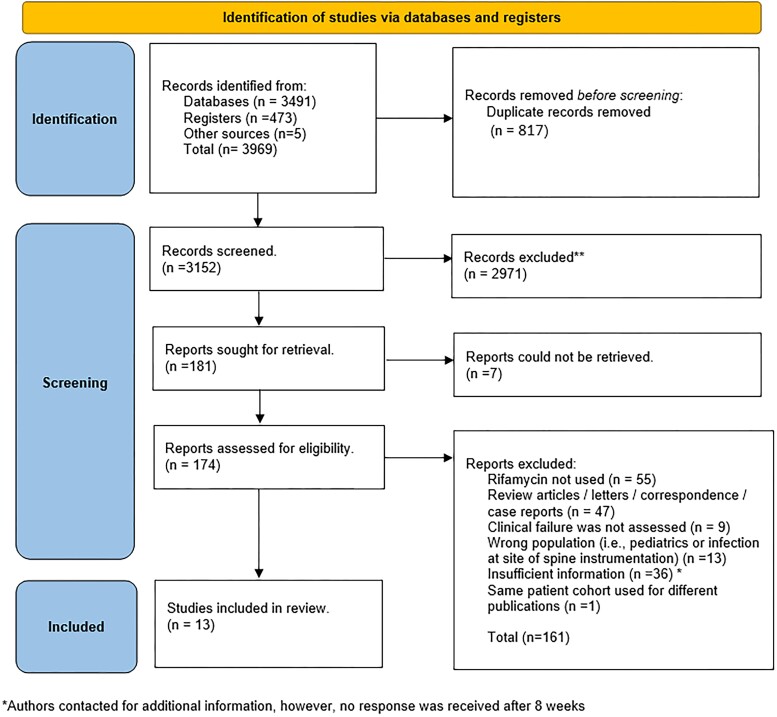
The literature was searched by a medical librarian (D. J. G.) for the concepts of vertebral osteomyelitis, S. aureus, rifampin, rifapentine, rifabutin, rifamycins, or other antibiotic treatments. Search strategies were created using a combination of keywords and standardized index terms. Searches were run on 13 January 2023, and updated on 23 May 2023, in the Ovid Cochrane Central Register of Controlled Trials (1991+), Ovid Embase (1974+), Ovid Medline (1946+ including E-pub ahead of print, in-process, and other non-indexed citations), Scopus (1788+), and Web of Science Core Collection (Science Citation Index Expanded 1975+ and Emerging Sources Citation Index 2015+). After removing case reports, animal and pediatric studies based on the exclusion criteria, a total of 3964 citations, were retrieved. Deduplication was performed in COVIDence before screening. The full search strategy is provided the Supplementary Material.
Study Selection, Data Extraction and Risk of Bias Assessment
Abstract and full-text screening was managed through the COVIDence Systematic Review Software (Veritas Health Innovation, Melbourne, Australia). Four reviewers (S. E. Z., M. P., F. P., and J. M.) were involved in abstract and full-text screening. Each article was reviewed by 2 reviewers. A third reviewer was consulted to resolve any emerging disagreements. Inter-rater reliability is shown in Supplementary Table 1. Forward citation chasing and manual screening of the reference lists of the included articles was performed to identify articles that were missed during the initial search. Two independent reviewers extracted relevant information from each article and recorded it in separate Excel sheets (Supplementary Appendix).
The risk of bias for comparative cohort studies was assessed using the Newcastle-Ottawa Scale. Each item on the Newcastle-Ottawa scale was judged as having “low risk,” “high risk,” or “unclear risk” of bias; however, global risk of bias judgments were made primarily based on the domain of comparability and adjustment for confounding. Two RCTs were included [29, 30]; however, these were not specifically designed to address this research question (Supplementary Table 2), and the randomization process was not anticipated to control for the confounding variables of interest. Consequently, for the purposes of this review, the RCTs were treated as cohort studies during risk of bias assessment.
The certainty of evidence was evaluated using the GRADE methodology and synthesized using GRADEpro [31] (accessed at: https://gdt.gradepro.org)
Statistical Analysis
Meta-analysis was conducted using the random-effects model because of anticipated heterogeneity in studies populations and settings. Meta-analysis estimated relative risks (RR) and risk difference (RD) with 95% confidence intervals (CI). RD was used to allow analysis of studies with zero events in both arms, although it may be associated with heterogeneity because of variation in baseline risk [32]. Heterogeneity was assessed through visual evaluation of point estimates and confidence interval overlap, and through using the I 2 statistic, where significant heterogeneity was considered as I 2 ≥ 50% [33]. Review Manager (RevMan), version 5.4, was used for analysis. Additional details on subgroup and sensitivity analyses are detailed in the Supplementary Appendix.
RESULTS
Search Results and Characteristics of the Included Studies
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram (Figure 1) presents the literature search results. In total, 13 studies were included in this systematic review, comprising 2 RCTs [29, 30] and 11 comparative cohort studies [5, 6, 23, 26, 27, 34–39] (Supplementary Table 2). Among these, authors of 7 studies supplied additional patient-level data specifically related to vertebral osteomyelitis without spine instrumentation caused by S. aureus, allowing their integration into the final systematic review [6, 23, 30, 37–40]. Supplementary Table 2 summarizes the characteristics of the included studies. The inclusion and exclusion criteria, as well as the definitions of clinical failure, varied across the studies. The mean duration of clinical follow-up in the included studies was 297.5 days (standard deviation, 226.9 days).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Outcome of Patients With S. aureus NVO Related to Adjunctive Rifampin Use
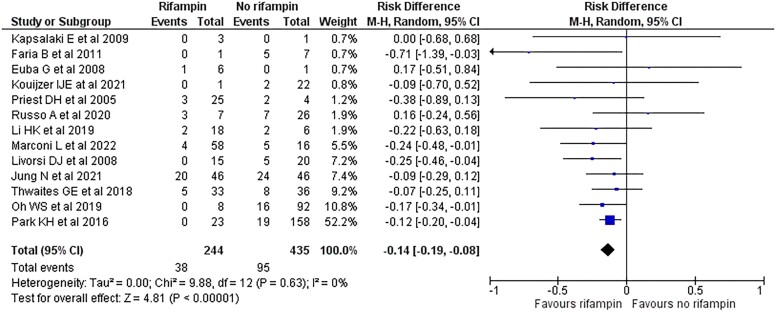
Overall, 244 patients with S. aureus NVO (35.9%) received a rifampin-containing regimen and 435 (64.1%) did not (Figure 2). In the 13 included studies, clinical failure occurred in 38 patients (15.6%) in the rifampin group and 95 patients (21.8%) in the standard-of-care group without rifampin. Meta-analysis including all 679 patients with S. aureus NVO showed a 14% absolute risk reduction in clinical failure in patients treated with a rifampin-based regimen compared with standard-of-care without rifampin (RD, −14%; 95% CI, −19 to −8; P < .001; I 2 = 0%) (Figure 2). RR of clinical failure in patients who received adjunctive rifampin was 0.58 (95% CI, .37–.92; P = .02; I 2 = 21%) (Supplementary Figure 1).
Figure 2.
Forest plot of 13 studies comparing the risk of treatment failure in patients with Staphylococcus aureus native vertebral osteomyelitis treated with adjunctive rifampin or not. Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
Data on blood culture results at the time of NVO diagnosis were available for 367 patients, of which 332 (90.5%) were positive for S. aureus. The antibiotics prescribed with rifampin are outlined in Supplementary Table 2. Fluoroquinolones were most commonly used. Others included glycopeptides, β-lactams, tetracyclines, and clindamycin, and less commonly daptomycin, trimethoprim-sulfamethoxazole, and aminoglycosides. Rifampin was administered for a median of 42 days (interquartile range, 27–56) [26, 30, 34–36], whereas the dose of rifampin ranged between 600 to 900 mg per day, often administered as 300 mg and 450 mg orally twice daily, respectively [6, 23, 29, 30]. Only the study by Thwaites et al [29] evaluated adverse events and therefore meta-analysis was not feasible. No studies using rifapentine or rifabutin were identified.
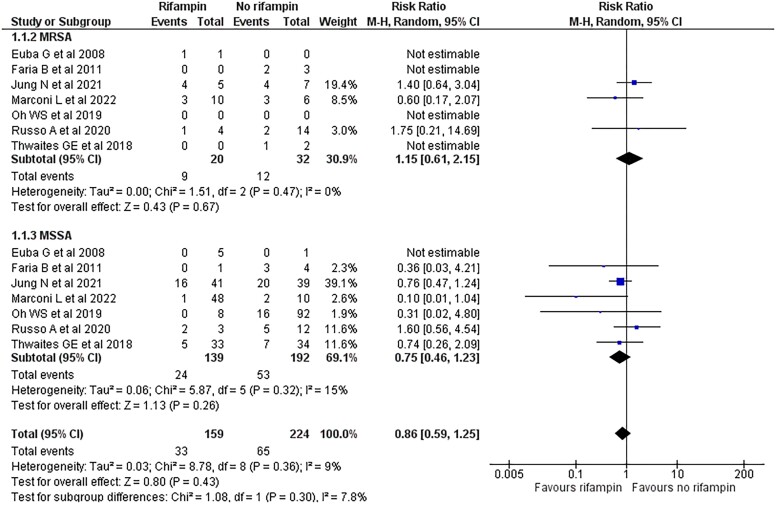
Among the 331 evaluable patients with methicillin-susceptible S. aureus and 52 with methicillin-resistant S. aureus NVO, rifampin was administered to 139 (42.0%) and 20 patients (38.5%), respectively. Methicillin resistance status did not significantly modify the effect of adjunctive rifampin compared with standard of care on the risk of clinical failure (P = .3) (Figure 3). Similarly, there was no statistically significant difference in the effect of rifampin on the subgroup of patients with low- or high-risk for recurrence (P = .85) (Supplementary Figure 2). The remainder of the preplanned subgroup analyses could not be performed because of insufficient data. Based on an exploratory subgroup analysis, studies predominantly using rifampin-fluoroquinolone combinations demonstrated a lower risk of treatment failure compared with those using other combinations (Table 1).
Figure 3.
Forest plot comparing the risk of treatment failure in patients with Staphylococcus aureus native vertebral osteomyelitis (NVO) treated with adjunctive rifampin or not, stratified by methicillin resistance. Methicillin resistance status did not significantly modify the effect of adjunctive rifampin compared with standard of care on the risk of clinical failure (P = .3). There is no significant heterogeneity between study results within each subgroup that require further exploration. However, a smaller number of studies and patients contributed data to the methicillin-resistant S. aureus (3 studies, 52 patients) compared with the methicillin-susceptible S. aureus (7 studies, 383 patients); therefore, the analysis may not be able to detect subgroup differences. Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; MRSA, methicillin resistant Staphylococcus aureus.
Table 1.
Exploratory Subgroup Analysis
| Subgroup | Number of Studies | Rifampin (Events/Total) |
SOC (Events/Total) |
Risk Ratio (95% CI) | Heterogeneity (I2) | P Valuea |
|---|---|---|---|---|---|---|
| Yearb | … | … | … | … | … | .15 |
| Before 2012 | 5 | 4/50 | 12/33 | 0.28 (.10–.81) | 0% | … |
| After 2012 | 8 | 34/194 | 83/402 | 0.66 (.40–1.11) | 28% | … |
| Number of patients | … | … | … | … | … | .9 |
| <50 patients/studies | 8 | 9/76 | 23/87 | 0.58 (.26–1.32) | 24% | … |
| ≥50 patients/studies | 5 | 29/168 | 72/348 | 0.55 (.29–1.02) | 33% | … |
| Overall RIF-FQ usec | ||||||
| High RIF-FQ use | 5 | 5/98 | 40/268 | 0.26 (.10–.68) | 0% | .06 |
| Low RIF-FQ use | 8 | 33/146 | 55/167 | 0.72 (.46–1.13) | 15% | … |
Abbreviations: CI, confidence interval; FQ, fluoroquinolone; RIF, rifampin; SOC, standard of care.
a P value for the test of subgroup differences. Statistical significance defined as < .1.
bA difference in the risk of treatment failure between the 2 subgroups could suggest that changes in diagnostic and therapeutic techniques over time may have confounded the clinical outcomes, acknowledging that a significant difference in the number of patients in the rifampin versus standard-of-care subgroups limits interpretability.
cPatient-level data not available. Exploratory subgroup analysis based on “overall” use of FQ—RIF combination in the entire study. A study was considered in the high RIF-FQ subgroup if this combination was used in >50% of the cases.
Finally, a sensitivity analysis was performed after excluding studies less than 5 patients in each arm [27, 34, 35, 39], less than 3 months’ mean follow-up [29, 34, 38], and/or only assessing the outcome of relapse but not death [27], still showed a significant absolute risk reduction in clinical failure with rifampin use (RD, −14%; 95% CI, −20 to −8%; P < .001; I 2 = 0%) (Supplementary Figure 3).
Methodological Quality of Included Studies and Certainty in the Evidence
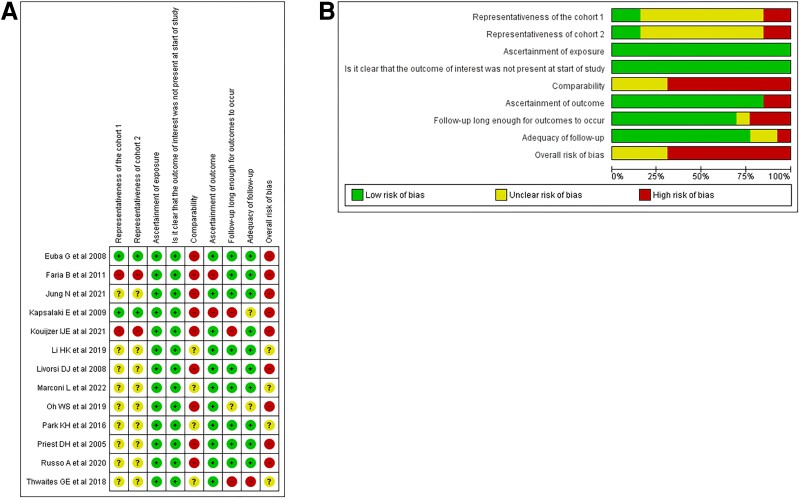
All studies were deemed to have a high or uncertain risk of bias (Figure 4A and 4B , and Supplementary Table 2). Potential confounders included age and comorbidities. Older and sicker patients may be inherently at an increased risk for treatment failure while being less likely to receive rifampin therapy because of intolerance or drug–drug interactions. Other potential confounders included source control, the dosage, timing, and overall duration of antibiotics and/or adjunctive rifampin therapy, as well as the potential effects of companion drugs. Additionally, S. aureus isolates resistant to rifampin may also be multidrug-resistant and associated with worse outcomes. The overall certainty of evidence was judged as very low because of serious concerns about methodological limitations and imprecision (small number of patients and events) (Supplementary Table 3).
Figure 4.
A, Risk of bias summary: review authors' judgments about each risk of bias item for each included study. B, Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
DISCUSSION
In this systematic review and meta-analysis, we identified an association between the use of adjunctive rifampin and a decreased risk of clinical failure in patients with S. aureus NVO. Subgroup analysis did not allow for conclusions to be drawn about the effect of rifampin-containing regimens on clinical failure in patients with methicillin-resistant S. aureus or those at high risk for recurrence. Although we were unable to assess the risk of adverse events, data from previous studies indicate no difference in serious adverse events between rifampin and standard-of-care regimens without rifampin. Nevertheless, drug-related adverse events and drug interactions complicating treatment are more frequent with rifampin [29, 41].
In contrast to its well-established role in the treatment of select patients with orthopedic device–related infections including spine implant [42, 43] and prosthetic joint infections [16, 44, 45], evidence supporting the use of rifampin for the treatment of S. aureus NVO stems primarily from pharmacokinetic and bone penetration studies, animal studies, heterogeneous human cohort studies, and 2 small RCTs published almost 40 years ago, which included patients with predominantly nonvertebral osteomyelitis caused by S. aureus [46, 47]. Recently, Wilson et al reported that the use of adjunctive rifampin was linked to significantly lower rates of death and amputation in patients with diabetic foot infections compared with those who did not receive rifampin [48]. This effect persisted after controlling for confounding variables even though rifampin was overall administered to younger patients with fewer comorbidities. A prospective randomized trial that aims to validate these findings is currently under way (NCT03012529). Despite these encouraging results, it should be emphasized that the prognosis for DFIs is typically poor, regardless of the antibiotic regimen. The complexity of these infections, often polymicrobial in nature and compounded by impaired peripheral vascular supply, often necessitates frequent surgical interventions. On the other hand, antibiotic therapy remains the mainstay of NVO treatment. In contrast to most other forms of osteomyelitis where surgical debridement is integral to achieving clinical cure, surgery in this population is reserved for the management of complications, such as spinal instability, neurological compromise or debilitating pain despite adequate medical therapy, or when antimicrobial therapy fails to eradicate infection [9]. Therefore, the objective of this systematic review and meta-analysis is to consolidate literature that focuses on a more homogeneous patient population in whom antimicrobial therapy alone is the primary factor influencing outcomes.
Rifampin is only used in combination with other antibiotics for the treatment of S. aureus infections resulting from the rapid emergence of resistance through a single mutation in the rpoB gene [49]. Fluoroquinolones are the most commonly used companion drugs. This is supported by pharmacokinetic studies showing good bone concentration achieved by fluoroquinolones [14] and literature from animal and human studies of implant-associated infections favoring rifampin-fluoroquinolone combinations [16, 44, 50]. Additionally, the excellent oral bioavailability of fluoroquinolones makes this combination especially appealing because it facilitates an early transition from intravenous to oral therapy with a fluoroquinolone-rifampin regimen [13]. A subgroup analysis indicated that the risk of clinical failure was lower in studies where rifampin-fluoroquinolone combinations were predominantly used, compared with those using other combinations. Although this analysis does not allow us to draw definitive conclusions, it underscores the need for additional studies specifically designed to investigate this observation. The discussion around rifampin-fluoroquinolone versus other rifampin combinations is inherently complex because of the multitude of antibiotics and their varying dosages and will not be explored in this review. Furthermore, the extrapolation of bone penetration and bioavailability studies to clinical outcomes lacks supportive clinical evidence, adding another layer of complexity and heterogeneity. Rifampin dosing in this systematic review ranged from 600 to 900 mg per day, typically administered as 300 mg and 450 mg twice per day, respectively (Supplementary Table 2). This dosing highly depends on tolerability and body weight and often reflects heterogeneity in clinical practice between various centers; consequently, we were unable to evaluate the effect of rifampin dosing on clinical outcomes. Rifampin dosing considerations, data on bone penetration of various antibiotics and theoretical limitations for other rifampin combinations have been explored elsewhere [14, 16, 25].
Finally, the timing of rifampin administration is an important consideration, as a high bacterial burden and older biofilms may increase the likelihood of treatment failure [16]. The high bacterial burden may partly explain the lack of benefit of adjunctive rifampin for the treatment of S. aureus bloodstream infection [29], including cases complicated by native valve infective endocarditis, osteoarticular, or deep foci of infection [41, 51–53]. In these studies, the definitions of osteoarticular and deep foci of infection were heterogeneous, encompassing vertebral and nonvertebral osteomyelitis, septic arthritis, orthopedic implant infection, in addition to pneumonia, urinary tract infections, infective endocarditis, and others, limiting the generalizability of the results to patients with NVO. In our systematic review, 90.5% of evaluable patients had positive S. aureus blood cultures at the time of NVO diagnosis; however, we could not assess the timing of rifampin initiation in relation to blood culture clearance because of a lack of patient-level data. Extrapolating from data on prosthetic joint infections, there are arguments supporting initiation of rifampin only after the bacterial load is reduced such as following surgery and an initial short course of parenteral antibiotic therapy [54].
To our knowledge, this study is the first to specifically examine this clinical question; however, it has several limitations. None of the studies included in this systematic review was designed to assess our question of interest. Consequently, the pooled results suffer a very high risk of bias because of low comparability. Patients treated with adjunctive rifampin may have been more likely to have an infectious disease specialty consultation, to be younger, with fewer comorbidities and higher baseline likelihood of achieving favorable outcomes compared with those who did not receive rifampin possibly from multiple underlying comorbidities and potential for drug–drug interactions [48]. Medication-related factors such as rifampin dosing, timing of initiation of adjunctive rifampin during the treatment course, duration of rifampin therapy, and the class and route (intravenous vs oral) of the companion drugs used may significantly affect clinical outcomes and warrant further research. We could not fully address these confounders through subgroup or sensitivity analyses because of the lack of sufficient patient-level data from the individual included studies. Finally, despite our attempts to contact the authors, several studies were not included because of insufficient patient-level data. The inclusion of these studies could have potentially altered the results of the meta-analysis.
In conclusion, this meta-analysis presents statistically significant findings in favor of using adjunctive rifampin therapy for treating NVO caused by S. aureus. Our findings align with existing literature on chronic osteomyelitis but provide a more focused examination of NVO. However, the limitations of the study preclude drawing definitive conclusions that would alter clinical practice, given the very low certainty in the evidence and the high risk of bias. Methodologically and statistically rigorous cohort studies and RCTs specifically designed to investigate this question are necessary to corroborate the findings of this systematic review. The decision to use adjunctive rifampin for the treatment of NVO should be individualized and carefully weighed against the risk for adverse events and complication of overall treatment until more definitive data supporting its benefit become available.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Said El Zein, Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Elie F Berbari, Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Matteo Passerini, Department of Infectious Disease, ASST FBF SACCO Fatebenefratelli, Milano, Lombardia, Italy.
Francesco Petri, Department of Infectious Disease, ASST FBF SACCO Fatebenefratelli, Milano, Lombardia, Italy.
Julian Maamari, St. Elizabeth's Medical Center, A Boston University Teaching Hospital, Brighton, Massachusetts, USA.
M Hassan Murad, Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Parham Sendi, Institute for Infectious Diseases, University of Bern, Bern, Switzerland.
Aaron J Tande, Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Notes
Acknowledgments. The authors thank Dr. Ilse J E Kouijzer, Dr. Sara Tedeschi, Dr. Alessandro Russo, Dr. Baek Nam Kim, Dr. Norma Jung, Dr. Guy Thwaites, the KEMRI Wellcome Trust Research Programme, and Dr. Matthew Scarborough for providing additional data that contributed to the development of this manuscript. The authors acknowledge Danielle J. Gerberi MLIS, AHIP, for conducting the literature search.
Financial support. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
References
- 1. Issa K, Diebo BG, Faloon M, et al. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg 2018; 31:E102–8. [DOI] [PubMed] [Google Scholar]
- 2. Conan Y, Laurent E, Belin Y, et al. Large increase of vertebral osteomyelitis in France: a 2010–2019 cross-sectional study. Epidemiol Infect 2021; 149:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009; 39:10–7. [DOI] [PubMed] [Google Scholar]
- 4. Kim DY, Kim UJ, Yu Y, et al. Microbial etiology of pyogenic vertebral osteomyelitis according to patient characteristics. Open Forum Infect Dis 2020; 7:ofaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park K-HC, Hyun O, Lee J-H, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016; 62:1262–9. [DOI] [PubMed] [Google Scholar]
- 6. Jung N, Ernst A, Joost I, et al. Vertebral osteomyelitis in patients with Staphylococcus aureus bloodstream infection: evaluation of risk factors for treatment failure. J Infect 2021; 83:314–20. [DOI] [PubMed] [Google Scholar]
- 7. Yagdiran A, Jochimsen D, Kernich N, et al. Treatment failure in vertebral osteomyelitis: is it all about Staphylococcus aureus? Spine (Phila Pa 1976) 2022; 47:E607–14. [DOI] [PubMed] [Google Scholar]
- 8. Arnold RR C, Croft LD, Gilliam BL, Morgan DJ. Factors associated with treatment failure in vertebral osteomyelitis requiring spinal instrumentation. Antimicrob Agents Chemother 2014; 58:880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berbari EFK, Souha S, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61:e26–46. [DOI] [PubMed] [Google Scholar]
- 10. Kehrer MH J, Bælum J, Jensen TG, Pedersen C, Lassen AT. Reduced ability to work both before and after infectious spondylodiscitis in working-age patients. Infect Dis (Lond) 2017; 49:95–103. [DOI] [PubMed] [Google Scholar]
- 11. Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 2018; 16:397–409. [DOI] [PubMed] [Google Scholar]
- 12. Gimza BD, Cassat JE. Mechanisms of antibiotic failure during Staphylococcus aureus osteomyelitis. Front Immunol 2021; 12:638085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zimmerli W, Sendi P. Systemic antibiotics. In: Stephen L, Kates OB, eds. Principles of orthopedic infection management. Davos: AO Publishing, 2017:63–76. [Google Scholar]
- 14. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis 2012; 54:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis 2019; 81:128–36. [DOI] [PubMed] [Google Scholar]
- 16. Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother 2019; 63:e01746-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renz N, Trampuz A, Zimmerli W. Controversy about the role of rifampin in biofilm infections: is it justified? Antibiotics 2021; 10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–19. [DOI] [PubMed] [Google Scholar]
- 19. Mandell GL, Vest TK. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis 1972; 125:486–90. [DOI] [PubMed] [Google Scholar]
- 20. Kanjilal S, Sater MRA, Thayer M, et al. Trends in antibiotic susceptibility in Staphylococcus aureus in Boston, Massachusetts, from 2000 to 2014. J Clin Microbiol 2018; 56:e01160-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 2019; 6(Suppl 1):S47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015; 385:875–82. [DOI] [PubMed] [Google Scholar]
- 23. Marconi L, Tedeschi S, Zamparini E, et al. Oral versus standard antimicrobial treatment for pyogenic native vertebral osteomyelitis: a single-center, retrospective, propensity score-balanced analysis. Open Forum Infect Dis 2022; 9:ofac366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [Primary infectious spondylitis, and following intradiscal procedure, without prothesis. Short text]. Med Mal Infect 2007; 37:554–72. [DOI] [PubMed] [Google Scholar]
- 25. Spellberg B, Aggrey G, Brennan MB, et al. Use of novel strategies to develop guidelines for management of pyogenic osteomyelitis in adults: a WikiGuidelines group consensus statement. JAMA Netw Open 2022; 5:e2211321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livorsi DJ, Daver NG, Atmar RL, Shelburne SA, White AC, Musher DM. Outcomes of treatment for hematogenous Staphylococcus aureus vertebral osteomyelitis in the MRSA ERA. J Infect 2008; 57:128–31. [DOI] [PubMed] [Google Scholar]
- 27. Priest DH, Peacock JE. Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J 2005; 98:854–62. [DOI] [PubMed] [Google Scholar]
- 28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thwaites GE, Scarborough M, Szubert A, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391:668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017; 87:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murad MH, Wang Z, Zhu Y, Saadi S, Chu H, Lin L. Methods for deriving risk difference (absolute risk reduction) from a meta-analysis. BMJ 2023; 381:e073141. [DOI] [PubMed] [Google Scholar]
- 33. Murad MH, Montori VM, Ioannidis JPA, Guyatt G, Rennie D, Meade MO. et al. In: Guyatt G, Rennie D, Meade MO, Cook DJ, et al., eds. Users' guides to the medical literature: a manual for evidence-based clinical practice. 3rd ed. New York: McGraw-Hill Education; 2015:9–10. [Google Scholar]
- 34. Euba G, Narváez JA, Nolla JM, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum 2008; 38:28–40. [DOI] [PubMed] [Google Scholar]
- 35. Faria B, Canto Moreira N, Sousa TC, et al. Spondylodiscitis in hemodialysis patients: a case series. Clin Nephrol 2011; 76:380–7. [DOI] [PubMed] [Google Scholar]
- 36. Kapsalaki E, Gatselis N, Stefos A, et al. Spontaneous spondylodiscitis: presentation, risk factors, diagnosis, management, and outcome. Int J Infect Dis 2009; 13:564–9. [DOI] [PubMed] [Google Scholar]
- 37. Oh WS, Moon C, Chung JW, et al. Antibiotic treatment of vertebral osteomyelitis caused by methicillin-susceptible Staphylococcus aureus: a focus on the use of oral β-lactams. Infect Chemother 2019; 51:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kouijzer IJE, van Leerdam EJ, Gompelman M, et al. Intravenous to oral switch in complicated Staphylococcus aureus bacteremia without endovascular infection: a retrospective single-center cohort study. Clin Infect Dis 2021; 73:895–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russo A, Ceccarelli G, Bellelli V, et al. Efficacy of daptomycin-containing regimen for treatment of staphylococcal or enterococcal vertebral osteomyelitis: a prospective clinical experience. Antibiotics (Basel) 2020; 9:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thwaites GES, Alexander J, Walker AS. Rifampicin in treating S aureus bacteraemia—authors' reply. Lancet 2018; 392:555–6. [DOI] [PubMed] [Google Scholar]
- 41. Ye CW C, Li Z, Li X, Pan J, Liu L, Wang Z. The effect of combination therapy on mortality and adverse events in patients with Staphylococcus aureus bacteraemia: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Ther 2021; 10:2643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cho OH, Bae IG, Moon SM, et al. Therapeutic outcome of spinal implant infections caused by Staphylococcus aureus: a retrospective observational study. Medicine (Baltimore) 2018; 97:e12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Köder K, Hardt S, Gellert MS, et al. Outcome of spinal implant-associated infections treated with or without biofilm-active antibiotics: results from a 10-year cohort study. Infection 2020; 48:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 45. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 46. Norden CW, Bryant R, Palmer D, Montgomerie JZ, Wheat J. Chronic osteomyelitis caused by Staphylococcus aureus: controlled clinical trial of nafcillin therapy and nafcillin-rifampin therapy. South Med J 1986; 79:947–51. [DOI] [PubMed] [Google Scholar]
- 47. Van der Auwera P, Klastersky J, Thys JP, Meunier-Carpentier F, Legrand JC. Double-blind, placebo-controlled study of oxacillin combined with rifampin in the treatment of staphylococcal infections. Antimicrob Agents Chemother 1985; 28:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson BM, Bessesen MT, Doros G, et al. Adjunctive rifampin therapy for diabetic foot osteomyelitis in the Veterans Health Administration. JAMA Netw Open 2019; 2:e1916003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aubry-Damon H, Soussy CJ, Courvalin P. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 1998; 42:2590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Greimel F, Scheuerer C, Gessner A, et al. Efficacy of antibiotic treatment of implant-associated Staphylococcus aureus infections with moxifloxacin, flucloxacillin, rifampin, and combination therapy: an animal study. Drug Des Devel Ther 2017; 11:1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rieg S, Ernst A, Peyerl-Hoffmann G, et al. Combination therapy with rifampicin or fosfomycin in patients with Staphylococcus aureus bloodstream infection at high risk for complications or relapse: results of a large prospective observational cohort. J Antimicrob Chemother 2020; 75:2282–90. [DOI] [PubMed] [Google Scholar]
- 52. Ma HC J, Peng L, Gao Y, Zhang G, Luo Z. Adjunctive rifampin for the treatment of Staphylococcus aureus bacteremia with deep infections: a meta-analysis. PLoS One 2020; 15:e0230383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Riedel DJ, Weekes E, Forrest GN. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob Agents Chemother 2008; 52:2463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beldman M, Löwik C, Soriano A, et al. If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin Infect Dis 2021; 73:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.