Abstract
Background
Many clinical guidelines recommend that clinicians use antibiograms to inform empiric antimicrobial therapy. However, hospital antibiograms are typically generated by crude aggregation of microbiologic data, and little is known about an antibiogram's reliability in predicting antimicrobial resistance (AMR) risk at the patient-level. We aimed to assess the diagnostic accuracy of antibiograms as a tool for selecting empiric therapy for Escherichia coli and Klebsiella spp. for individual patients.
Methods
We retrospectively generated hospital antibiograms for the nationwide Veterans Health Administration (VHA) facilities from 2000 to 2019 using all clinical culture specimens positive for E. coli and Klebsiella spp., then assessed the diagnostic accuracy of an antibiogram to predict resistance for isolates in the following calendar year using logistic regression models and predefined 5-step interpretation thresholds.
Results
Among 127 VHA facilities, 1 484 038 isolates from 704 779 patients for E. coli and 671 035 isolates from 340 504 patients for Klebsiella spp. were available for analysis. For E. coli and Klebsiella spp., the discrimination abilities of hospital-level antibiograms in predicting individual patient AMR were mostly poor, with the areas under the receiver operating curve at 0.686 and 0.715 for ceftriaxone, 0.637 and 0.675 for fluoroquinolones, and 0.576 and 0.624 for trimethoprim-sulfamethoxazole, respectively. The sensitivity and specificity of the antibiogram varied widely by antimicrobial groups and interpretation thresholds with substantial trade-offs.
Conclusions
Conventional hospital antibiograms for E. coli and Klebsiella spp. have limited performance in predicting AMR for individual patients, and their utility in guiding empiric therapy may be low.
Keywords: hospital antibiogram, diagnostic accuracy, antimicrobial resistance, empiric therapy, gram-negative rods
We conducted a comprehensive evaluation of diagnostic accuracies for conventional antibiograms to predict antimicrobial resistance (AMR) among Escherichia coli and Klebsiella spp. We found that antibiograms had mostly poor accuracy in predicting AMR, indicating limited utility for guiding empiric therapy.
(See the Editorial Commentary by MacDougall on pages 1501–3.)
Hospital antibiograms, a facility-level summary of antimicrobial susceptibility data of various organisms isolated from patients, is a common local reference tool. Antibiogram is mainly used for 2 purposes at hospitals [1]. First, it is used to monitor antimicrobial resistance (AMR) trends for epidemiologic investigations and to compare resistance rates across institutions [2, 3]. Second, clinicians may refer to an antibiogram when selecting initial empiric antimicrobial therapy. For this second purpose, antimicrobial stewardship and clinical practice guidelines recommend using an antibiogram, and multiple federal agencies and accreditation organizations incorporate the antibiogram in their recommendations or requirements [4–11].
Despite its frequent appearance in guidelines and requirements, using antibiograms to guide empiric antimicrobial therapy has potential limitations given the crude, population-level data aggregation. The Clinical & Laboratory Standards Institute (CLSI) provides a guideline on how to create an antibiogram at each hospital that makes recommendations on the selection of organisms to be reported (only species with testing data for ≥30 isolates should be included), selection of antimicrobials (only microorganism-antimicrobials combinations routinely tested should be included), and frequency of reports (at least annually). However, the CLSI guideline includes no isolate- or patient-level information as a routine data presentation practice. Therefore, if the volume of a hospital's clinical practice is overrepresented by a few areas (eg, high-volume surgical centers), an antibiogram could be skewed and may not represent an accurate risk of resistance for an individual patient. In addition, only 40%–60% of hospitals adopted the CLSI recommendations, and the proportion of hospitals that adhered to the guideline when producing antibiograms was reported to be 10%–50%, making the consistent evaluation of antibiogram challenging [12–15].
To date, little is known about the accuracy of antibiograms as a tool to predict AMR when deciding on empiric antimicrobial therapy for an individual patient. Some studies have evaluated this issue; however, they were limited by their single-center nature, care settings (mostly from academic centers), and short study durations [1, 16].
In this study, we aimed to quantitatively assess the diagnostic accuracy of standardized hospital antibiograms from a previous calendar year as a prediction tool for AMR at the patient-level for the 2 most common species in Enterobacteriaceae: Escherichia coli and Klebsiella spp. This study was performed using 20 years of nationwide Veterans Health Administration (VHA) System data, including hospitals with a diverse geographic distribution, care settings, and facility sizes.
METHODS
Study Design and Population
For this nationwide retrospective cohort study, we obtained microbiologic and patient-level data from the Corporate Data Warehouse (CDW), an integrated infrastructure system from VHA's electronic medical records. We focused on two major Gram-negative rods (GNRs) species, E. coli and Klebsiella spp. (excluding Klebsiella aerogenes) a priori because these 2 species are the most commonly encountered in serious GNR infections in community and nosocomial settings [17, 18]. K. aerogenes was excluded because some facilities reported Enterobacter spp. only at the genus level before 2017, when K. aerogenes was reclassified from Enterobacter spp. to Klebsiella spp. [19].
Data for all clinical isolates for E. coli and Klebsiella spp. reported at 127 VHA hospitals (located in 48 continental states and the District of Columbia), and >1400 outreach clinics from January 2000 to December 2020, and their susceptibility reports were extracted regardless of age, gender, race, ethnicity, residential address, or socioeconomic status. Within the VHA system, each community-based outpatient clinic was assigned to its affiliated hospital, and a small number of hospitals (1 in most cases) and affiliated clinics form a regional healthcare system were also considered as a single “facility.” The data were stratified at the facility level, and we used this data to create standardized antibiograms for all facility/year and patient-level datasets for diagnostic accuracy calculations. We did not use antibiograms released by each facility because we did not have access to all locally created antibiograms at all facilities during the study period, and there might have been variation in practice for the creation of antibiograms across different facilities.
The median number of operating beds per facility (including acute care, long-term care, mental health, and rehabilitation beds) was 232 (interquartile range [IQR]: 131–359). Acute inpatient capacities at VHA facilities ranged from 0 to 266 beds (IQR: 37–109; 2 facilities had provided only outpatient care, and 3 other facilities had only long-term care units and/or mental health inpatient units). Among 127 facilities with inpatient beds, 24 (18.9%) were located in rural areas. Total acute inpatient capacity was ∼10 000 acute-care beds (including 1900 authorized intensive care unit beds) and ∼12 000 long-term care beds.
During 2002–2020, approximately 90% of all facilities had on-site microbiology laboratories (based on a VHA-internal survey conducted by the VA Healthcare Analysis and Information Group), and they are required to conduct quality control/quality assessment routinely per requirements of VHA-designated accreditation organizations and to use methods and equipment approved by the Food and Drug Administration (FDA) [20].
Variable Definition
We categorized all antimicrobials with clinical importance for the two GNRs into 25 groups based on their antimicrobial activities and therapeutic equivalence (Supplementary Table 1) [21, 22]. If there were conflicting results within a group reported for the same isolate, we included the most resistant result from a report. For example, an E. coli that tested intermediate or resistant to moxifloxacin and susceptible to ciprofloxacin would be considered non-susceptible to fluoroquinolones for this study. Susceptibility reported as “S (susceptible)” was regarded as susceptible isolates, and reported as “SDD (susceptible-dose dependent),” “I (intermediate),” or “R (resistant)” were regarded as non-susceptible isolates. Minimal inhibitory concentrations (MICs) or zone diameter measurements of Kirby-Bauer disk diffusion tests were not routinely available in the data source. If only MICs or zone diameter measurements were reported without interpretation, the susceptibility was determined according to the CLSI guidelines [22].
The Creation Process of Standardized Antibiograms
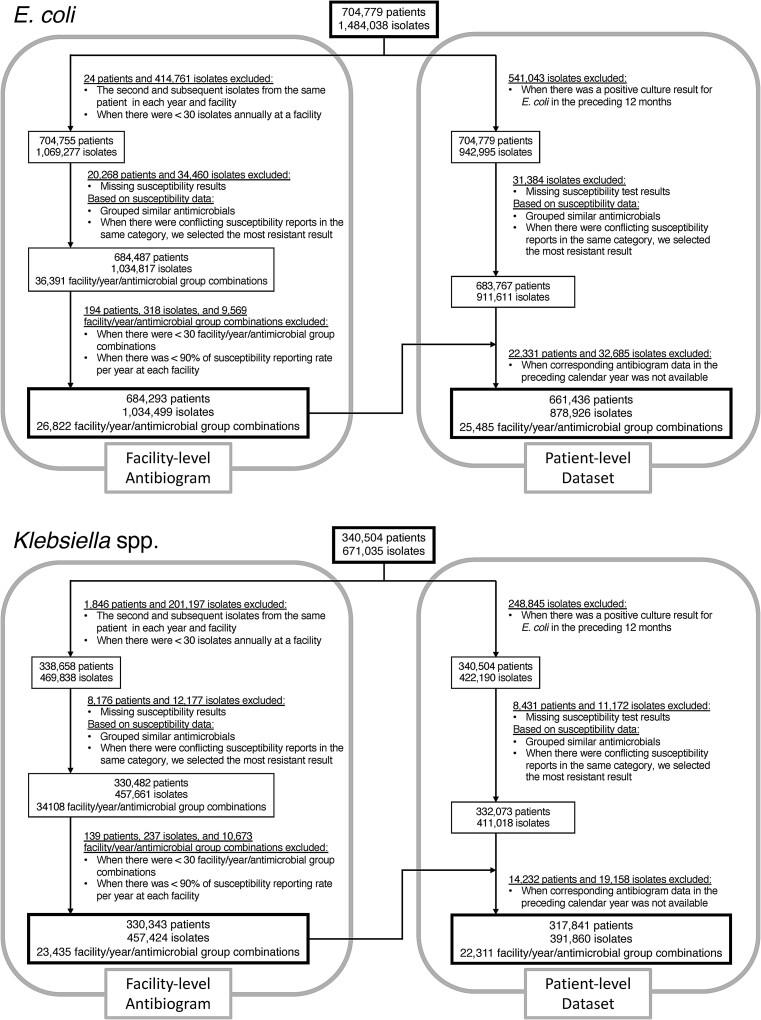
Following CLSI guidelines, we included only the first isolate per patient in each calendar year and facility for E. coli and Klebsiella spp. We then created standardized antibiograms from 2000 to 2019 at all facilities, based on phenotypic susceptibility (susceptible vs non-susceptible) for antimicrobial categories for each calendar year and facility, where ≥30 isolate-antimicrobial combinations a year were available (Figure 1). To be consistent with the CLSI recommendation to include only routinely tested antimicrobial agents in the antibiogram, we excluded facility-year-antimicrobial combinations when antimicrobial susceptibility was reported for <90% of isolates in a respective calendar year.
Figure 1.
The creation process of standardized antibiograms and a patient-level data set.
The Creation Process of Patient-level Dataset
To evaluate the diagnostic accuracies of antibiograms, we created a patient-level data set of isolates from patients who did not have any positive cultures of the same organism at the same facility in the preceding 12 months (Figure 1). We assumed clinicians would use the patients’ positive cultures from the prior year when guiding empiric therapy instead of their local antibiogram. Reported susceptibility test results (susceptible vs non-susceptible) for these isolates were included as the target for the prediction. Isolates from 2001 to 2020 were included in this patient-level dataset to assess the diagnostic accuracies of antibiograms from the previous calendar year, as typically used in actual clinical practice.
Statistical Analyses
Logistic regression models with susceptibility test results as the dependent variable (non-susceptible as the target outcome) and antimicrobial susceptibility rates reported in antibiograms from the previous calendar year as the independent variable were fit to assess the prediction performance of antibiograms for individual patients. We also considered polynomial (squared and cubic) terms for antimicrobial susceptibility rates to accommodate possible non-linear relationships and selected variables by logistic least absolute shrinkage and selection operator (LASSO), utilizing cross-validation with 5-fold. However, neither squared nor cubic terms were selected through this variable selection process, and the inclusion of polynomial terms did not significantly improve the models’ performance. Therefore, we decided to include susceptibility rates as the sole independent continuous variable.
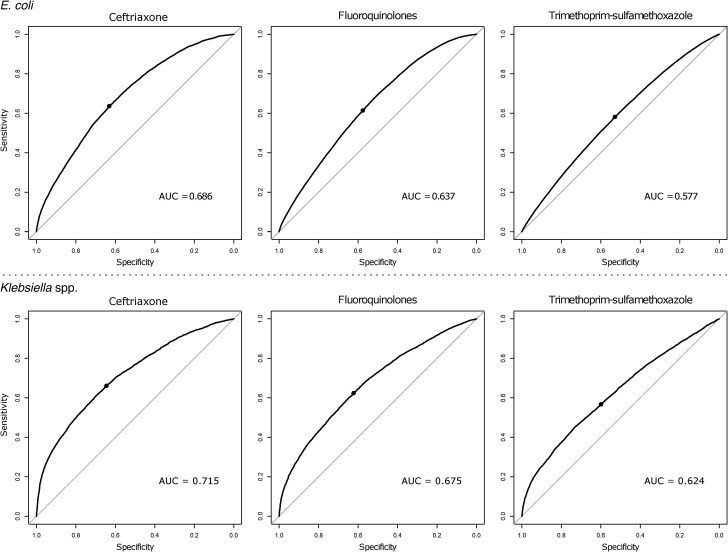
To assess prediction performance, we generated 2 × 2 contingency tables and reported sensitivities and specificities for all antimicrobial groups. We also created receiver operating characteristic (ROC) curves for the 3 commonly used antimicrobial classes for GNR infections, ceftriaxone, fluoroquinolones, and trimethoprim-sulfamethoxazole, to visualize the prediction performances and estimate the area under the curve (AUC) of the receiver operating characteristic (ROC). We interpreted AUC at 0.5–0.7 as poor, 0.7–0.8 as moderate, 0.8–0.9 as good, and >0.9 as excellent discriminative abilities [23, 24]. The best interpretation threshold was determined to maximize classification accuracy for each organism-antimicrobial combination. The model predicted non-susceptible results on an isolate when the prevalence of susceptible isolates reported in the antibiogram from the previous calendar year was below this threshold, and we calculated sensitivity and specificity based on the model-driven threshold.
We set predefined interpretation thresholds with 5 step-wise increments at <80%, <85%, <90%, <95%, and <98% (ie, 2%–20% prevalence of AMR). These thresholds were set based on a pilot study our group conducted in 2022 [25] that demonstrated that clinicians use 85%–95% thresholds for the interpretation of antibiogram when making empiric therapy decisions, and they might not feel comfortable using certain antibiotics when the susceptibility prevalence in antibiogram was below these thresholds, depending on the clinical situations and contexts. In this analysis with predefined thresholds, the model predicted non-susceptible results when the prevalence of susceptible isolates reported in the antibiogram from the previous calendar year was below these thresholds. For example, if the prevalence of susceptible isolates reported in the antibiogram from the previous calendar year was 88%, the model predicted non-susceptible with <90%, <95%, and <98% thresholds but predicted otherwise with <80% and <85% thresholds. This could simulate clinicians avoiding certain antimicrobials for empiric therapy when the reported proportions of susceptible isolates are low in antibiogram.
CLSI had revised breakpoints on major antimicrobial classes for GNRs over the years, and we conducted a stratified analysis of each category by whether the data was from before and after the revisions of CLSI standards. We performed evaluations only when more than ten facility/year combinations were available for each antimicrobial group.
All statistical analyses were performed with R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics
The institutional review board at the University of Iowa and the Research and Development Committee at the Iowa City Veterans Affairs Health Care System approved this study and granted a waiver for informed consent for this retrospective cohort study.
Role of the Funding Source
The funding source had no role in the design of this study and did not have any role during its collection, management, analysis, interpretation of the data, or decision to publish results.
RESULTS
During the study period, the VHA provided care to 16 411 896 unique patients through 250 185 551 patient-days of care (acute, long-term, mental health, and rehabilitation) and over 1.6 billion clinic visits. Table 1 summarizes the demographic characteristics of patients and the proportion of susceptible isolates in antibiograms for each antimicrobial. During 2000–2020, 1 484 038 isolates from 704 779 patients and 671 035 isolates from 340 504 were available for analysis for E. coli and Klebsiella spp., respectively. Figure 1 shows the creation process of standardized antibiograms and a patient-level data set. For E. coli, 1 034 499 specimens from 684 293 patients contributed to the facility-level antibiograms (26 822 facility/year/antimicrobial group combinations), and 878 926 specimens from 661 436 patients were included in the patient-level dataset. For Klebsiella spp, 457 424 specimens from 330 343 patients contributed to the facility-level antibiograms (23 435 facility/year/antimicrobial group combinations), and 391 860 specimens from 317 841 patients were included in the patient-level data set. The median and IQR for the proportion of susceptible isolates varied widely for each antimicrobial (Table 1). Discrepancies of susceptibility test results within antibiotic groups among isolates from the same patient on the same day occurred only infrequently (Supplementary Table 2).
Table 1.
Demographic Characteristics of Patients and Proportion of Susceptible Isolates for E. coli and Klebsiella spp
| Demographics | E. coli | Klebsiella spp. |
|---|---|---|
| Number of included patients | 704 779 | 340 504 |
| Number of isolates | 1 484 038 | 671 035 |
| Blood | 89 684 (6.0%) | 52 189 (7.8%) |
| Urine | 1 237 929 (83.4%) | 484 209 (72.2%) |
| Feces/Rectal | 6685 (0.5%) | 878 (0.1%) |
| Lower respiratory tracta | 4377 (0.3%) | 6030 (0.9%) |
| Cerebrospinal fluid | 59 (<0.1%) | 83 (<0.1%) |
| Other/Unknown | 145 304 (9.8%) | 127 646 (19.0%) |
| Gender, male | 565 055 (80.1%) | 306 784 (90.1%) |
| Age, y | 67 [57–77] | 69 [61–78] |
| Proportion of susceptible isolates in facility antibiograms | ||
| Ampicillin-Sulbactam/Amoxicillin-Clavulanate | 64.9% [58.7%–72.1%] | 80.4% [75.9%–85.6%] |
| Carbapenems | 100% [99.7%–100%] | 99.6% [98.7%–100%] |
| Cefazolin | 87.0% [82.6%–90.7%] | 82.7% [78.0%–87.0%] |
| Ceftriaxone | 95.9% [93.1%–98.3%] | 95.3% [92.1%–97.8%] |
| Fluoroquinolones | 76.8% [69.0%–86.0%] | 92.9% [88.7%–95.7%] |
| Piperacillin-Tazobactam | 96.9% [95.5%–98.2%] | 94.2% [90.9%–96.6%] |
| Trimethoprim-Sulfamethoxazole | 79.4% [75.1%–84.0%] | 91.1% [88.0%–93.7%] |
Data presented as median [interquartile range].
aLower respiratory tract specimens include bronchoalveolar lavage, pleural effusion, and lung biopsy specimens.
Prediction Performance and Diagnostic Accuracy of Hospital Antibiograms for E. coli and Klebsiella spp.
Table 2 summarizes the prediction performance of hospital antibiograms from prior calendar year to predict antimicrobial non-susceptibilities of E. coli and Klebsiella spp. isolates for selected major antimicrobial groups. ROC curves for antibiograms to predict non-susceptibilities for the 3 commonly used antimicrobial groups (ceftriaxone, fluoroquinolones, and trimethoprim-sulfamethoxazole) are shown in Figure 2. For E. coli, the discriminative abilities of antibiograms to predict non-susceptible results for 3 major antimicrobial groups based on AUCs were all poor (AUC: 0.686 for ceftriaxone, 0.637 for fluoroquinolones, and 0.576 for trimethoprim-sulfamethoxazole). For Klebsiella spp., discriminative abilities were moderate (AUC: 0.715) for ceftriaxone and poor for fluoroquinolones and trimethoprim-sulfamethoxazole (AUCs: 0.675 and 0.624, respectively). Best interpretation thresholds to predict non-susceptible results estimated from logistic regression models for both organisms largely mirrored the prevalences of non-susceptible isolates (Tables 2 and 3). Supplementary Table 3 summarizes the coefficients of logistic regression models and discriminative abilities for all antimicrobial groups evaluated. A stratified analysis of antimicrobial groups according to the revisions of CLSI standards with lower thresholds for ceftriaxone, cefepime, carbapenems, and fluoroquinolones showed slightly lower prediction performances of hospital antibiograms with revised breakpoints (Supplementary Table 3).
Table 2.
Performance of Hospital Antibiograms to Predict Non-Susceptibility of E. coli and Klebsiella spp. Isolates
| Antimicrobial Groups | Number of Facilities Included | Number of Facility/Year Combinations | Number of Isolates Evaluated | AUC | Prediction Performance | Best Interpretation Threshold to Predict Non-Susceptibilitya | Sna | Spa |
|---|---|---|---|---|---|---|---|---|
| E. coli | ||||||||
| Ampicillin-Sulbactam/Amoxicillin-Clavulanate | 115 | 1801 | 644 813 | 0.599 | Poor | <64.1% | 58.6% | 55.4% |
| Carbapenems | 105 | 1547 | 579 098 | 0.761 | Moderate | <99.7% | 68.1% | 72.8% |
| Cefazolin | 124 | 2100 | 699 874 | 0.645 | Poor | <86.1% | 59.5% | 60.2% |
| Ceftriaxone | 118 | 1894 | 679 959 | 0.686 | Poor | <94.9% | 63.6% | 63.1% |
| Fluoroquinolones | 127 | 2354 | 834 707 | 0.637 | Poor | <76.1% | 61.4% | 57.6% |
| Piperacillin-Tazobactam | 114 | 1448 | 540 827 | 0.610 | Poor | <96.6% | 56.8% | 59.4% |
| Trimethoprim- Sulfamethoxazole |
122 | 2314 | 824 503 | 0.576 | Poor | <79.2% | 56.9% | 53.8% |
| Klebsiella spp. | ||||||||
| Ampicillin-Sulbactam/Amoxicillin-Clavulanate | 120 | 1794 | 307 293 | 0.624 | Poor | <81.0% | 59.4% | 57.2% |
| Carbapenems | 105 | 1475 | 261 236 | 0.815 | Good | <98.7% | 71.9% | 76.6% |
| Cefazolin | 123 | 1955 | 305 070 | 0.619 | Poor | <82.9% | 57.7% | 58.3% |
| Ceftriaxone | 119 | 1755 | 300 655 | 0.715 | Moderate | <94.4% | 66.1% | 64.5% |
| Fluoroquinolones | 126 | 2214 | 371 778 | 0.675 | Poor | <92.1% | 62.4% | 62.3% |
| Piperacillin- Tazobactam |
116 | 1427 | 248 733 | 0.656 | Poor | <93.4% | 57.9% | 64.2% |
| Trimethoprim- Sulfamethoxazole |
121 | 2167 | 366 397 | 0.624 | Poor | <90.7% | 56.7% | 59.9% |
Abbreviations: AUC, area under the curve; Sn, sensitivity; Sp, specificity.
aValues at the highest AUC.
Figure 2.
ROC curves of antibiograms to predict non-susceptibility for 3 major antimicrobial groups. Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic.
Table 3.
Performance of Hospital Antibiograms to Predict Non-Susceptibility of E. coli and Klebsiella spp. Isolates With Predefined Thresholds
| Antimicrobial Group | Threshold for Interpretation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <80% | <85% | <90% | <95% | <98% | ||||||
| Sn | Sp | Sn | Sp | Sn | Sp | Sn | Sp | Sn | Sp | |
| E. coli | ||||||||||
| Ampicillin-Sulbactam/Amoxicillin-Clavulanate | 98.7% | 3.7% | 99.9% | 0.3% | 100% | 0% | 100% | 0% | 100% | 0% |
| Carbapenems | 11.3% | 99.9% | 11.3% | 99.9% | 11.3% | 99.9% | 15.6% | 99.8% | 15.6% | 99.8% |
| Cefazolin | 25.9% | 90.2% | 49.2% | 70.7% | 95.1% | 11.7% | 100% | 0% | 100% | 0% |
| Ceftriaxone | 2.9% | 99.6% | 6.0% | 98.9% | 10.8% | 97.5% | 65.0% | 61.4% | 100% | 0% |
| Fluoroquinolones | 76.4% | 42.2% | 91.8% | 22.8% | 98.8% | 7.2% | 100% | 0% | 100% | 0% |
| Piperacillin-Tazobactam | 0.5% | 99.9% | 0.9% | 99.8% | 2.6% | 99.3% | 10.8% | 95.2% | 100% | 0% |
| Trimethoprim-Sulfamethoxazole | 64.4% | 46.2% | 93.5% | 11.5% | 100% | 0.1% | 100% | 0% | 100% | 0% |
| Klebsiella spp. | ||||||||||
| Ampicillin-Sulbactam/Amoxicillin-Clavulanate | 50.9% | 65.3% | 86.8% | 25.3% | 99.6% | 1.5% | 100% | 0% | 100% | 0% |
| Carbapenems | 10.4% | 99.6% | 17.0% | 99.2% | 26.8% | 98.6% | 33.0% | 97.9% | 50.9% | 94.5% |
| Cefazolin | 36.3% | 79.2% | 78.0% | 34.9% | 99.8% | 0.3% | 100% | 0% | 100% | 0% |
| Ceftriaxone | 19.1% | 97.6% | 22.1% | 96.7% | 29.7% | 93.7% | 80.1% | 45.2% | 100% | 0% |
| Fluoroquinolones | 15.2% | 97.1% | 20.5% | 95.1% | 39.8% | 82.8% | 99.1% | 3.7% | 100% | 0% |
| Piperacillin-Tazobactam | 12.5% | 98.2% | 14.7% | 97.5% | 21.8% | 93.4% | 91.4% | 19.2% | 100% | 0% |
| Trimethoprim-Sulfamethoxazole | 13.9% | 96.7% | 19.9% | 93.9% | 45.8% | 72.2% | 100% | 0% | 100% | 0% |
Abbreviations: Sn, sensitivity; Sp, specificity.
Table 3 summarizes the diagnostic accuracy of antibiograms for the 7 major antimicrobials. There were substantial trade-offs between sensitivity and specificity that depended on the threshold for the 7 major antimicrobials and other antimicrobial groups (Supplementary Table 4). In most combinations, specificities were <10% when thresholds were set to achieve reasonable sensitivities (eg, ≥90%). Along with AUC, these results suggested mostly poor prediction performance and limited value when antibiograms are used as a prediction tool for individual patients.
DISCUSSION
In this nationwide retrospective cohort study, we found that standardized hospital-level antibiograms for the 2 major species of GNR, E. coli and Klebsiella spp., have limited performance in predicting the risk of AMR for individual patients when they are used as a decision-making tool. The sensitivity and specificity varied by antimicrobial groups and thresholds with substantial trade-offs, and no combination of organism and antimicrobials achieved both reasonably high sensitivity and specificity. Notably, the specificities of most combinations were extremely low (<10% in most cases and near 0% in some cases) when thresholds were set to achieve reasonably high sensitivities (eg, ≥90%).
This study is the most comprehensive evaluation of antibiograms as a diagnostic tool to predict AMR to date. Overall, our results suggested that the performance of antibiograms as a tool to predict AMR at the individual patient level is mostly poor, and its potential to contribute to appropriate therapeutic decision making may be limited. The value of hospital antibiograms may also be limited when used as a tool to monitor resistance trends and to compare resistance prevalences across institutions.
Antibiograms have been used in clinical practice for decades without critical validation. Many clinical guidelines suggest that hospitals create an antibiogram to optimize empiric antimicrobial therapy. For example, clinical guidelines published by the Infectious Diseases Society of America (IDSA), the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and also the Surviving Sepsis Campaign guideline recommended that local antimicrobial-prescribing guidelines based on the local epidemiology of AMR should guide decisions on empiric therapy [4, 5, 7–9, 26]. Our study demonstrated that antibiograms might have only minimal value in predicting AMR and “optimizing” empiric therapy. Current guidelines incorporating antibiograms in their recommendations as a key tool to make empiric therapy decisions should be reconsidered.
Several previous studies proposed stratified antibiograms according to the setting (eg, inpatient vs. outpatient) [27, 28], clinical syndromes [16, 27, 29], or age [5, 30, 31]. The CLSI guideline includes these approaches as an“enhanced antibiogram.” Although enhanced antibiograms are reported to have improved diagnostic accuracies in single-center studies conducted at academic institutions, it is difficult for a stratified antibiogram to present data for patients with multiple risk factors, and it may underestimate the risk of resistance for medically complex patients. In addition, their generalizability, applicability, and practicality are questionable at smaller hospitals.
On the other hand, antibiograms may overestimate the risk of AMR in relatively healthy patients with fewer risk factors and sway clinicians to choose more broad-spectrum agents for those patients. Mostly poor prediction capabilities and drastic trade-offs between sensitivity and specificity also raise a concern that antibiograms may be contributing to the overuse of broad-spectrum antimicrobials among patients with low risks of AMR. With the advent of advanced predictive analytics (eg, machine learning), personalized predictions for the risk of AMR may provide more useful guidance to clinicians [32].
This study had several limitations. First, more than 80% of patients were adult males, and this gender imbalance might result in a small proportion of isolates from uncomplicated urinary tract infections, of which the most common pathogens are E. coli and Klebsiella spp. [33]. This may potentially limit the generalizability to populations outside the VHA systems. However, the present study included data from diverse settings at various hospitals throughout the United States over 20 years, which should increase the generalizability to various geographic locations and clinical settings. Second, we focused on assessing standardized antibiograms based on the CLSI guidelines. Our results do not necessarily deny the usefulness of “enhanced antibiograms,” such as stratified antibiograms by clinical locations, patient populations, or clinical syndromes. Although our results indicated a poor value of standardized antibiograms, it is still possible that these enhanced antibiograms may perform better (as suggested in several previous studies) and be useful to clinicians when making empiric therapy decisions. However, we are skeptical that adding only 1 or 2 dimensions of clinical data are adequate to overcome the fundamental limitation of antibiograms, which originate from their crude data aggregation. Finally, we did not test the performance of antibiogram use in combination with a good medical history that could augment and possibly improve antibiotic selection.
In conclusion, conventional hospital antibiograms for the 2 most common GNR species, E. coli and Klebsiella spp., have poor performance in predicting the risk of AMR for individual patients without recent culture isolates when they are used as a sole prediction tool, and their contribution to empiric therapy decision making may be limited. Clinicians should also consider other clinical and epidemiologic data when making empiric therapy decisions, and guideline statements that suggest hospital antibiograms are a valuable tool for decision making in empiric therapy may need to be reconsidered. With the continued evolution of machine learning or artificial intelligence algorithms, the future of antibiograms may include individualized patient-level prediction for improved performance and optimized empiric antimicrobial therapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Shinya Hasegawa, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa, Iowa City, Iowa, USA.
Daniel J Livorsi, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa, Iowa City, Iowa, USA.
Eli N Perencevich, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa, Iowa City, Iowa, USA.
Jonas N Church, Department of Internal Medicine, University of Iowa, Iowa City, Iowa, USA.
Michihiko Goto, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa, Iowa City, Iowa, USA.
Notes
Financial support. This work was supported by the Agency for Healthcare Research and Quality (AHRQ; grant number K08HS027472 to M. G.).
References
- 1. Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2007; 27:1306–12. [DOI] [PubMed] [Google Scholar]
- 2. Schulz LT, Fox BC, Polk RE. Can the antibiogram be used to assess microbiologic outcomes after antimicrobial stewardship interventions? A critical review of the literature. Pharmacotherapy 2012; 32:668–76. [DOI] [PubMed] [Google Scholar]
- 3. Kohlmann R, Gatermann SG. Analysis and presentation of cumulative antimicrobial susceptibility test data: the influence of different parameters in a routine clinical microbiology laboratory. PLoS One 2016; 11:e0147965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:147–59. [DOI] [PubMed] [Google Scholar]
- 7. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 8. Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect 2022; 28:521–47. [DOI] [PubMed] [Google Scholar]
- 9. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e143. [DOI] [PubMed] [Google Scholar]
- 10. Agency for Healthcare Research and Quality . Toolkit 3. The nursing home antibiogram program toolkit: how to develop and implement an antibiogram program. Available at: https://www.ahrq.gov/nhguide/toolkits/help-clinicians-choose-the-right-antibiotic/toolkit3-develop-implement-antibiogram-program.html. Accessed 3 May 2023.
- 11. Centers for Disease Control and Prevention . The core elements of hospital antibiotic stewardship programs: 2019. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf. Accessed 3 May 2023.
- 12. Xu R, Polk RE, Stencel L, et al. Antibiogram compliance in university HealthSystem consortium participating hospitals with clinical and laboratory standards institute guidelines. Am J Health Syst Pharm 2012; 69:598–606. [DOI] [PubMed] [Google Scholar]
- 13. Ernst EJ, Diekema DJ, BootsMiller BJ, et al. Are United States hospitals following national guidelines for the analysis and presentation of cumulative antimicrobial susceptibility data? Diagn Microbiol Infect Dis 2004; 49:141–5. [DOI] [PubMed] [Google Scholar]
- 14. Lautenbach E, Nachamkin I. Analysis and presentation of cumulative antimicrobial susceptibility data (antibiograms): substantial variability across medical centers in the United States. Infection Control Hosp Epidemiol 2006; 27:409–12. [DOI] [PubMed] [Google Scholar]
- 15. Moehring RW, Hazen KC, Hawkins MR, Drew RH, Sexton DJ, Anderson DJ. Challenges in preparation of cumulative antibiogram reports for community hospitals. J Clin Microbiol 2015; 53:2977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabs N, Wieczorkiewicz SM, Costello M, Zamfirova I. Development of a urinary-specific antibiogram for gram-negative isolates: impact of patient risk factors on susceptibility. Am J Infect Control 2014; 42:393–400. [DOI] [PubMed] [Google Scholar]
- 17. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2019; 63:e00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holmes CL, Anderson MT, Mobley HLT, Bachman MA. Pathogenesis of gram-negative bacteremia. Clin Microbiol Rev 2021; 34:e00234-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tindall BJ, Sutton G, Garrity GM. Enterobacter aerogenes Hormaeche and Edwards 1960 (approved lists 1980) and Klebsiella mobilis Bascomb et al. 1971 (approved lists 1980) share the same nomenclatural type (ATCC 13048) on the approved lists and are homotypic synonyms, with consequences for the name Klebsiella mobilis Bascomb et al. 1971 (approved lists 1980). Int J Syst Evol Microbiol 2017; 67:502–4. [DOI] [PubMed] [Google Scholar]
- 20. Department of Veterans Affairs Veterans Health Administration . VHA Directive 1106, pathology and laboratory medicine service. Available at: https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=7477. Accessed 3 May 2023.
- 21. Grayson ML, Cosgrove SE, Crowe S, et al. Kucers’ the use of antibiotics: a clinical review of antibacterial, antifungal, antiparasitic and antiviral drugs, seventh edition. Boca Raton: CRC, 2017. [Google Scholar]
- 22. (CLSI) TCLSI . M100 performance standards for antimicrobial susceptibility testing, 32nd ed. Berwyn, PA: Clinical and Laboratory Standards Institute, 2022. [Google Scholar]
- 23. de Hond AAH, Steyerberg EW, van Calster B. Interpreting area under the receiver operating characteristic curve. Lancet Digit Health 2022; 4:e853–e5. [DOI] [PubMed] [Google Scholar]
- 24. Meurer WJ, Tolles J. Logistic regression diagnostics: understanding how well a model predicts outcomes. JAMA 2017; 317:1068–9. [DOI] [PubMed] [Google Scholar]
- 25. Hasegawa S, Perencevich EN, Dukes KC, Goto M. Physicians’ acceptable treatment failure rates and interpretation of antibiogram for gram-negative infections: a pilot survey study of infectious diseases specialists. Open Forum Infect Dis 2022; 9:ofac492.807. [Google Scholar]
- 26. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 27. Randhawa V, Sarwar S, Walker S, Elligsen M, Palmay L, Daneman N. Weighted-incidence syndromic combination antibiograms to guide empiric treatment of critical care infections: a retrospective cohort study. Critical Care 2014; 18:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swami SK, Banerjee R. Comparison of hospital-wide and age and location—stratified antibiograms of S. aureus, E. coli, and S. pneumoniae: age- and location-stratified antibiograms. Springerplus 2013; 2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grodin L, Conigliaro A, Lee SY, Rose M, Sinert R. Comparison of UTI antibiograms stratified by ED patient disposition. Am J Emerg Med 2017; 35:1269–75. [DOI] [PubMed] [Google Scholar]
- 30. Lin WP, Huang YS, Wang JT, Chen YC, Chang SC. Prevalence of and risk factor for community-onset third-generation cephalosporin-resistant Escherichia coli bacteremia at a medical center in Taiwan. BMC Infect Dis 2019; 19:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mueller MR, Hayden MK, Fridkin SK, et al. Nosocomial acquisition of Pseudomonas aeruginosa resistant to both ciprofloxacin and imipenem: a risk factor and laboratory analysis. Eur J Clin Microbiol Infect Dis 2008; 27:565–70. [DOI] [PubMed] [Google Scholar]
- 32. Corbin CK, Sung L, Chattopadhyay A, et al. Personalized antibiograms for machine learning driven antibiotic selection. Commun Med 2022; 2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.