Abstract
Background
Sotrovimab is an anti-spike neutralization monoclonal antibody developed to reduce the risk of coronavirus disease 2019 (COVID-19) progression and advancement to hospitalization in high-risk patients. Currently, there is limited research describing the association of sotrovimab treatment in patients with hematologic malignancy and the predictive factors of hospitalization.
Methods
We performed an observational study of 156 consecutive cancer patients who received sotrovimab at Memorial Sloan Kettering Cancer Center in New York City during the BA.1 Omicron surge. We evaluated the demographic, clinical, and laboratory characteristics of the patients who had subsequent COVID-19–related hospitalization(s) compared to those who did not.
Results
Among the 156 study patients, 17 (11%) were hospitalized, of whom 4 were readmitted for COVID-19–related complications; 3 deaths were attributed to COVID-19. Results from multivariable logistic regression show that significant factors associated with hospitalization include patients on anti-CD20 therapy (adjusted odds ratio [aOR], 5.59 [95% confidence interval {CI}, 1.73–18.12]; P = .004) and with relapse/refractory disease (aOR, 5.69 [95% CI, 1.69–19.16]; P = .005). Additionally, whole genome sequencing of severe acute respiratory syndrome coronavirus 2 detected high occurrences of mutations in the spike gene associated with treatment-related resistance longitudinal samples from 11 patients treated with sotrovimab.
Conclusions
While sotrovimab is effective at reducing COVID-19 hospitalization and disease severity in patients with hematologic malignancy when administered early, patients who received anti-CD20 antibodies showed substantial morbidity. Due to the high potential for resistance mutation to sotrovimab and increased morbidity in patients on anti-CD20 therapy, combination treatment should be explored to determine whether it provides added benefits compared to monotherapy.
Keywords: COVID-19, hematologic malignancy, monoclonal antibodies, SARS-CoV-2
Treatment with anti-CD20 therapy within the past year and relapse/refractory disease were associated with significantly higher odds of COVID-19–related hospitalization. Furthermore, a high occurrence of sotrovimab-related mutations was detected in the longitudinal evaluation of respiratory samples of hospitalized patients.
The use of monoclonal antibodies (mAbs) that retain activity against circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants has been the standard of treatment for patients at high risk for progression to severe coronavirus disease 2019 (COVID-19) [1]. The United States (US) Food and Drug Administration (FDA) has issued emergency use authorization (EUA) over the course of the COVID-19 pandemic of several mAbs for the treatment of mild to moderate COVID-19 in adult and pediatric patients aged ≥12 years. Among these, sotrovimab was given EUA designation in May 2021 based on a prespecified interim analysis of a multicenter randomized controlled trial (RCT) that demonstrated an 85% risk reduction in disease progression in nonhospitalized patients with symptomatic COVID-19 before Omicron [2]. In addition, no patients treated with sotrovimab required oxygen support or mechanical ventilation [3]. Most patients in this trial did not have cancer but had other high-risk conditions including obesity, diabetes, and 2 or more medical conditions in 45% of the participants.
The Omicron variant that emerged in November 2021 encodes several amino acid substitutions in the spike protein, 15 of which are in the receptor-binding domain [4]. Because BA.1-specific mutations only had a marginal effect on sotrovimab binding [5, 6], this led to its preferential use during the BA.1 surge in the US. The FDA has since suspended sotrovimab EUA due to a reduction in neutralization against Omicron sublineages [7]. There is limited knowledge on the effectiveness of mAb treatment in specific patient groups. A critical gap in the pivotal trials arises from the minimal representation of high-risk cancer patients, such as those with hematologic malignancy (HM), who are among those at the highest risk for COVID-19 hospitalization and complications. Chronic infection that could perpetuate in host viral evolution due to antibody evasion is a primary concern in this population [8, 9]. Furthermore, the high-risk patient characteristics that predict hospitalization despite mAb treatment are unknown. Identifying predictors of COVID-19 progression after mAb treatment will help optimize treatment strategies for those at the highest risk for poor COVID-19 outcomes. The present study describes the predictors for hospitalization, clinical characteristics, and virologic outcomes in sotrovimab-treated patients with HM.
METHODS
Study Population
Memorial Sloan Kettering Cancer Center is a 574-bed tertiary cancer center in New York City with approximately 25 000 admissions and 173 000 patient-days annually.
From 21 December 2021 to 27 January 2022 during the BA.1 Omicron surge, all consecutive adult and pediatric patients with SARS-CoV-2 infection who received sotrovimab were included in the study. Patients who received sotrovimab on the day of hospital admission were excluded (n = 8). A retrospective analysis of 156 patients was conducted. All patients were followed up for at least 60 days after sotrovimab infusion until 30 April 2022. Identification of case patients and their medical background and clinical course during COVID-19 illness were extracted from electronic medical records. The Memorial Sloan Kettering Cancer Center Institutional Review Board granted a Health Insurance Portability and Accountability Act waiver of authorization to conduct this study.
Laboratory Methods
SARS-CoV-2 RNA Test
The viral RNA was detected in nasopharyngeal swabs or saliva samples as previously described [10]. In brief, real-time reverse-transcription polymerase chain reaction testing for SARS-CoV-2 RNA was performed using several commercial assays including the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific, Waltham, Massachusetts) targeting the N, S, and ORF genes; the Cobas SARS-CoV-2 test (Roche Molecular Diagnostics, Indianapolis, Indiana) targeting the ORF1 a/b and E genes; the BioFire Respiratory Panel 2.1 (bioMérieux, Salt Lake City, Utah); and the ePlex Respiratory Panel 2 (GenMark/Roche Molecular Diagnostics, Indianapolis, Indiana). Samples were reported as positive per the manufacturers’ instructions. When available, the cycle threshold (Ct) value was retrieved from each instrument.
SARS-CoV-2 Whole Genome Sequencing
Whole genome sequencing (WGS) was performed on available samples with a Ct value <30 to increase likelihood of successful sequencing. Samples tested on platforms that do not provide Ct value (eg, BioFire RP 2.1 and ePlex 2) were WGS without knowledge of approximate viral loads. WGS was performed as previously described using the Artic protocol with version 4.1 primers (Integrated DNA Technologies, Coralville, Iowa) [11]. Pangolin software (https://github.com/cov-lineages/pangolin) was used to assign lineages for each consensus sequence using the Pango nomenclature for all sequences passing sequencing quality check. Mutation calling was performed by uploading all FASTA files to Nextclade version 2.2.0 (https://clades.nextstrain.org) [12].
Anti–SARS-CoV-2 spike immunoglobulin G antibody assay was performed as previously described [13].
Statistical Analysis
The 156 study patients were stratified into 2 groups based on COVID-19–related hospitalization. Clinical and demographic characteristics were examined for all patients. Univariate and multivariable logistic regression models were used to study risk factors for COVID-19–related hospitalizations. Initial univariate analysis was performed to screen the potential prognostic factors with a P value of < .05. The multivariable model was then developed by incorporating variables that were statistically significant in the univariate model. For variables with zero cell frequency, the Firth bias-correction method was used to address separation issues [14]. Multicollinearity was not a significant factor among the independent variables included in the multivariable model as the variance inflation factor (VIF) for each variable was <2 (data not shown). Final selection of variables included in the multivariable model was done by applying a stepwise approach using logistic regression. Results from the models were expressed as odds ratio (OR) with 95% confidence interval (CI) and P value for statistical significance. Assumptions of model fit were assessed using the Hosmer-Lemeshow test, which confirmed the adjusted model was a good fit for the data (P = .288). The threshold for statistical significance was set at a 2-sided P < .05. All calculations were performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
Baseline Clinical Characteristics of the Study Cohort
During the study period, 156 high-risk patients with HM received sotrovimab. The median age was 61.5 years (range, 12–91 years) and 78 patients were male. The underlying cancers were lymphoma (62), leukemia and myelodysplastic syndrome (66), and myeloma/amyloidosis (28). Twenty-four patients had received hematopoietic stem cell transplant (HCT) or chimeric antigen receptor T-cell (CAR-T) therapy (10 autologous transplant, 11 allogeneic transplant, and 3 CAR-T recipients). The median time from cellular therapy to SARS-CoV-2 infection was 93.5 days (interquartile range, 54.5–127.8 days). Eighty-three patients had at least 1 additional comorbid condition (Table 1). By the end of the study period, 152 were still alive.
Table 1.
Univariable and Multivariable Logistic Regression Analyses of Risk Factors for Coronavirus Disease 2019–Related Hospitalizations in 156 High-Risk Patients With Hematologic Malignancy Who Received Early Treatment With Sotrovimab a
| Characteristic | Admits Cases (No Admits) (n = 156) | Univariate Modelb | Multivariable Modelb | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value* | OR (95% CI) | P Value* | ||
| Age ≥65 y | 7 (60) | .92 (.33–2.56) | .876 | … | |
| Age <65 y | 10 (79) | 1.00 | … | ||
| Female sex | 8 (70) | .87 (.32–2.40) | .797 | … | |
| Male sex | 9 (69) | 1.00 | … | ||
| Days to sotrovimab from symptom onsetc | |||||
| ȃ>10 d | 2 (4) | 4.68 (.77–28.54) | .094 | … | |
| ȃ6–10 d | 3 (20) | 1.41 (.36–5.49) | .625 | … | |
| ȃ0–5 d | 11 (103) | 1.00 | NA | … | |
| Creatinine ≥1.1 mg/dLd | 3 (38) | .56 (.15–2.05) | .380 | … | |
| Creatinine <1.1 mg/dLd | 14 (99) | 1.00 | … | ||
| Lymphocyte count ≥500 cell/µLe | 13 (116) | .616 (.18–2.07) | .433 | … | |
| Lymphocyte count <500 cell/µLe | 4 (22) | 1.00 | … | ||
| CD3+CD4+ (T4 helper) cells, blood ≥200 cell/µLf | 4 (27) | .37 (.09–1.52) | .169 | … | |
| CD3+CD4+ (T4 helper) cells, blood <200 cells/µLf | 6 (15) | 1.00 | … | ||
| CD19 ≥50 cells/µLf | 2 (16) | .39 (.07–2.15) | .281 | … | |
| CD19 <50 cells/µLf | 7 (22) | 1.00 | … | ||
| Neutrophils ≥0.5 k/µLg | 16 (118) | .54 (.06–5.16) | .595 | … | |
| Neutrophils <0.5 k/µLg | 1 (4) | 1.00 | … | ||
| Total IgG ≥500 mg/dL | 8 (64) | 0.94 (0.26–3.36) | .921 | … | |
| Total IgG <500 mg/dL | 4 (30) | 1.00 | … | ||
| Anti-spike SARS-CoV-2 antibody level (Abbott)h | |||||
| ȃ ≥500 AU/mL | 3 (22) | 1.14 (.26–4.96) | .865 | … | |
| ȃ <500 AU/mL | 6 (50) | 1.00 | … | ||
| COVID-19 vaccination statusi | |||||
| ȃFully vaccinated | 14 (112) | 1.13 (.30–4.19) | .861 | … | |
| ȃNot fully vaccinated | 3 (27) | 1.00 | … | ||
| Comorbiditiesj | |||||
| ȃAt least 1 comorbidity | 11 (72) | 1.71 (.60–4.87) | .318 | … | |
| ȃNo comorbidity | 6 (67) | 1.00 | … | ||
| ȃAtrial fibrillation (yes) | 5 (10) | 5.38 (1.58–18.31) | .007* | 3.68 (.77–17.54) | .102 |
| ȃAtrial fibrillation (no) | 12 (129) | 1.00 | 1.00 | ||
| ȃCOPD (yes) | 2 (7) | 2.52 (.48–13.22) | .276 | … | |
| ȃCOPD (no) | 15 (132) | 1.00 | … | ||
| ȃHeart failure (yes) | 3 (3) | 9.71 (1.79–52.76) | .009* | 6.89 (.85–56.11) | .071 |
| ȃHeart failure (no) | 14 (136) | 1.00 | 1.00 | ||
| ȃHIV positive | 0 (2) | 1.57 (.04–67.0) | .814 | … | |
| ȃHIV negative | 17 (137) | 1.00 | … | ||
| ȃDiabetes (yes) | 4 (22) | 1.64 (.49–5.49) | .425 | … | |
| ȃDiabetes (no) | 13 (117) | 1.00 | … | ||
| ȃHypertension (yes) | 7 (49) | 1.29 (.46–3.59) | .631 | … | |
| ȃHypertension (no) | 10 (90) | 1.00 | … | ||
| ȃRenal failure (yes) | 4 (23) | 1.55 (.46–5.18) | .475 | … | |
| ȃRenal failure (no) | 13 (116) | 1.00 | … | ||
| Systemic steroids (yes)k | 8 (57) | 1.28 (.47–3.51) | .631 | … | |
| Systemic steroids (no)k | 9 (82) | 1.00 | … | ||
| Anti-CD20 therapy (yes)l | 9 (29) | 4.27 (1.51–12.03) | .006* | 5.59 (1.73–18.12) | .004* |
| Anti-CD20 therapy (no)l | 8 (110) | 1.00 | 1.00 | ||
| Cancer type | |||||
| ȃLeukemia/MDS | 5 (61) | .48 (.15–1.53) | .216 | … | |
| ȃMyeloma/amyloidosis | 3 (25) | .71 (.18–2.84) | .625 | … | |
| ȃLymphoma | 9 (53) | 1.00 | NA | … | |
| Chemotherapy (yes)m | 12 (82) | 1.67 (.56–5.00) | .36 | … | |
| Chemotherapy (no)m | 5 (57) | 1.00 | … | ||
| Relapse/refractory disease (yes) | 8 (28) | 3.52 (1.25–9.96) | .017* | 5.69 (1.69–19.16) | .005* |
| Relapse/refractory disease (no) | 9 (111) | 1.00 | 1.00 | ||
| BMT CAR-T (yes)n | 2 (22) | .71 (.15–3.34) | .663 | … | |
| BMT CAR-T (no)n | 15 (117) | 1.00 | … | ||
| BMI, kg/m2o | |||||
| ȃUnderweight (<18.5) | 0 (4) | .71 (.02–20.6) | .842 | … | |
| ȃNormal weight (18.5–24.9) | 6 (41) | 1.00 | NA | … | |
| ȃOverweight (25.0–29.9) | 8 (46) | 1.17 (.38–3.56) | .786 | … | |
| ȃObese (≥30.0) | 1 (40) | .24 (.04–1.50) | .126 | … | |
Abbreviations: Ab, antibody; BMI, body mass index; BMT, bone marrow transplant; CAR-T, chimeric antigen receptor T-cell; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; IgG, immunoglobulin G; MDS, myelodysplastic syndrome; NA, not applicable; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data include 2 patients who received pre-exposure prophylaxis with Evusheld prior to COVID-19 diagnosis.
ORs and P values calculated from logistic regression applying Firth correction, where appropriate.
Symptom onset is COVID-19 related. Onset date not available for 13 patients. Symptom onset to sotrovimab administration ranged from 0 to 16 days (median, 3 days).
Creatinine and IgG: Most recent values before COVID-19 diagnosis within the last 6 months.
Lymphocytes: Most recent 3 laboratory values within 12 months prior to COVID-19 diagnosis. All 3 counts must be <500 cells/µL to be in the “<500” category. At least 1 count of value ≥500 cells/µL is considered in the “≥500” category. One patient did not have 3 laboratory values prior to COVID-19 diagnosis.
CD4 and CD19: Most recent values before COVID-19 diagnosis within the last year.
Neutrophils: Most recent values before COVID-19 diagnosis within the last month.
Anti-spike antibody: Most recent test value before COVID-19 diagnosis within 1 year. Excludes 2 patients who received Evusheld prior to sotrovimab.
Study patients were considered fully vaccinated for COVID-19 ≥2 weeks after the final dose of a primary vaccination series. Not fully vaccinated: No vaccination records were available for these patients.
Comorbidities (atrial fibrillation, COPD, heart failure, HIV, hypertension, diabetes, renal failure) are based on International Classification of Diseases, Tenth Revision diagnosis codes within 12 months of study period.
Systemic steroids include patients who were on steroids within 30 days prior to COVID-19 diagnosis. Includes dexamethasone, methylprednisolone, hydrocortisone, and prednisone.
Anti-CD20 therapy within previous 12 months from receipt of sotrovimab. This includes rituximab, obinutuzumab, tafasitamab, hyaluronidase-rituximab, and ofatumumab.
Chemotherapy during study period.
BMT and CAR-T therapy combined. Service dates are prior to COVID-19 diagnosis and sotrovimab treatment and are within 1 year of study period.
Most recent value during past 12 months; 10 patients did not have BMI values.
Statistical significance at α = .05.
Twenty-nine unique patients required all-cause hospital admission during the study follow-up period; 17 were hospitalized for COVID-19 management, and 4 patients contributed to 5 COVID-19–related readmissions (Table 2). The remaining hospitalizations were unrelated to COVID-19. Of the 156 study patients, 155 had no prior COVID-19 infection, and 2 patients received preexposure prophylaxis with Evusheld 5 and 8 days before COVID-19 diagnosis.
Table 2.
Clinical Characteristics and Outcomes During Index Hospitalization for 17 Patients Hospitalized After Receiving Sotrovimab
| Characteristic | No. (%)a |
|---|---|
| Age, y, median | 63 |
| Sex, female | 9 (52.9) |
| Unvaccinated | 2 (11.8) |
| Symptoms at hospitalization | |
| ȃFever | 13 (76.5) |
| ȃDyspnea | 9 (52.9) |
| ȃCough | 11 (64.7) |
| ȃNasal congestion | 2 (11.8) |
| ȃPoor oral intake | 7 (41.2) |
| ȃChest pain | 3 (17.6) |
| ȃFatigue | 5 (29.4) |
| CT chest findings (n = 9) | |
| ȃBilateral consolidative opacities | 2 (22.2) |
| ȃBilateral patchy ground-glass opacities | 8 (89.0) |
| CXR findings (n = 8) | |
| ȃUnilateral opacities | 3 (37.5) |
| ȃBilateral opacities | 3 (37.5) |
| ȃClear lungs | 2 (25) |
| Oxygen requirement | |
| ȃNo oxygen requirement or <24 h | 11 (64.7) |
| ȃNasal cannula (>24 h) | 4 (23.5) |
| ȃHigh-flow oxygen | 1 (5.9) |
| ȃMechanical ventilation | 1 (5.9) |
| COVID-19 severityb | |
| ȃMild | 11 (64.7) |
| ȃModerate | 4 (23.5) |
| ȃSevere/critical | 2 (11.8) |
| COVID-19 treatment | |
| ȃAny COVID-19 treatment | 14 (82.4) |
| ȃRemdesivir | 13 (76.5) |
| ȃDexamethasone | 6 (35.3) |
| ICU-level care for COVID-19 | 1 (5.9) |
| Outcomes | |
| ȃCOVID-19–related deathc | 3 (17.6) |
| ȃCOVID-19–related readmission | 4 (23.5) |
| ȃChronic infectiond | 3 (17.6) |
| Length of stay, d, median (range) | 8 (1–64) |
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest radiograph; ICU, intensive care unit.
Patients can be in >1 category.
COVID-19 severity based on maximum oxygen requirement through admission. Mild infections remained on room air or required nasal cannula <24 hours; moderate infections required nasal cannula >24 hours; severe/critical infections required high-flow oxygen or intubation.
COVID-19–related deaths are based on review of electronic medical records and clinical judgment of patient presentation/illness in relation to the patients’ primary disease and time from COVID-19 positivity.
Chronic COVID-19 infection is defined as patients who have had progressive or recurrent COVID-19–related symptoms (or require home oxygen) with imaging consistent with COVID-19 and, in the absence of an alternate etiology, with or without evidence of viral persistence.
The median time to sotrovimab administration from symptom onset was 3 days (range, 0–16 days), and from laboratory diagnosis, 0–9 days. One hundred twenty six patients with available records were fully vaccinated. Study patients were considered fully vaccinated for COVID-19 ≥2 weeks after the final dose of a primary vaccination series. Serological assessment (anti–SARS-CoV-2 spike antibody levels, Abbott) before and after COVID-19 diagnosis and sotrovimab treatment were available for 89 COVID-19–vaccinated patients (Supplementary Figure 1).
Comparison Between Hospitalized Versus Nonhospitalized Sotrovimab Recipients
As shown in Table 1, notable findings on univariable analysis showed higher odds of hospitalization among those with refractory or relapsed cancer (OR, 3.52 [95% CI, 1.25–9.96]; P = .017) and patients who received anti-CD20 therapy within the previous 12 months (OR, 4.27 [95% CI, 1.51–12.03]; P = .006). Patients with atrial fibrillation (P = .007) and heart failure (P = .009) were also significant in univariable analysis. Multivariable logistic regression adjusting for significant factors associated with hospitalization on univariable analysis demonstrated that the ORs for anti-CD20 therapy (adjusted OR [aOR], 5.59 [95% CI, 1.73–18.12]; P = .004) and relapse/refractory disease (aOR, 5.69 [95% CI, 1.69–19.16]; P = .005) increased, with significant associations maintained. Atrial fibrillation and heart failure were not significant after covariate adjustment. Among the laboratory findings, neutropenia and lymphopenia (absolute lymphocyte count <500 cells/µL) prior to COVID-19 diagnosis were not significant predictors of hospitalization. Other notable factors that did not predict hospitalization included age; time to sotrovimab administration from symptom onset; vaccination status; comorbidities; underlying cancer type; previous cellular therapy; or HCT, systemic chemotherapy, or steroids within 30 days prior to COVID-19 diagnosis. The median time to hospitalization from symptom onset and sotrovimab administration was 17.0 and 3.0 days, respectively (Supplementary Figure 2). Symptom onset dates were not available for 13 patients.
Clinical Outcome of Hospitalized Patients, Duration of Viral Shedding, and SARS-CoV-2 Viral Evolution
The clinical presentation and outcomes of the 17 patients during their index hospitalization are summarized in Table 2. Fever and dyspnea were the most common presenting symptoms at hospitalization, 6 patients required oxygen support, and 15 had radiographic evidence of lower airway involvement. Most patients received standard of care therapy with remdesivir and dexamethasone. The illness severity was mild to moderate for 15 of the 17 patients. One patient was intubated and died after 2 days of ventilatory support. Overall, 3 deaths were attributed to COVID-19 where 1 occurred at index admission and 2 upon readmission.
Four of the 17 hospitalized patients were readmitted for COVID-19–related issues with 1 patient readmitted twice. The COVID-19 severity for these 5 readmissions was either moderate (3/5 [60%]) or severe/critical (2/5 [40%]). All had received anti-CD20 therapy within the preceding year. One unvaccinated patient with mantle cell lymphoma with rituximab-based therapy (last dose 4 months prior) had 3 admissions characterized by fevers and worsening shortness of breath despite antibiotics and 2 courses of steroids and remdesivir. Additionally, the patient also received treatment with baricitinib. Despite this, the patient remained oxygen-dependent for 3 months. Of the 3 other readmitted patients, 1 developed chronic COVID-19 infection and 2 died from progressive respiratory failure attributed to COVID-19. Chronic COVID-19 infection is defined as patients who have had progressive or recurrent COVID-19–related symptoms (or require home oxygen) with imaging consistent with COVID-19 and, in the absence of an alternate etiology, with or without evidence of viral persistence.
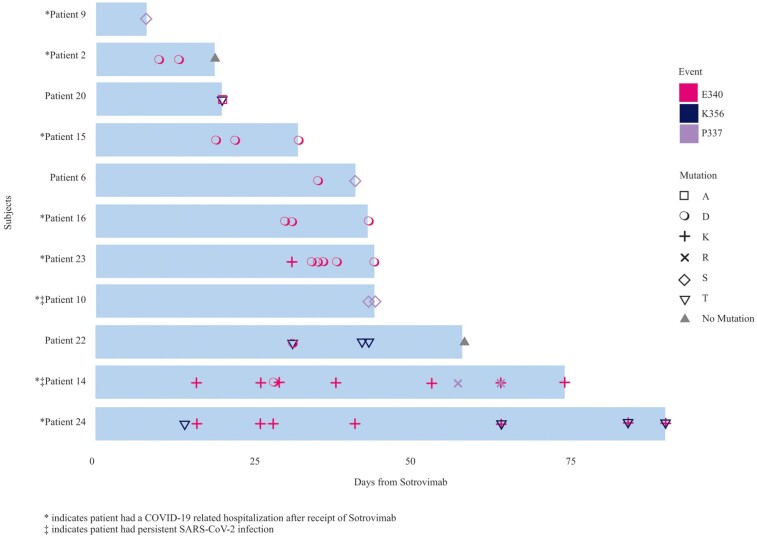
Time from viral detection to viral clearance for the 156 study patients before and after sotrovimab showed 62 patients with negative results by the end of the study period with a median time to viral clearance at 62.5 days (range, 8–133 days) (Supplementary Figure 3). Only patients with 3 or more samples were included for longitudinal mutational analysis. Overall, 80 samples were available for WGS from 13 patients, including 57 specimens from 8 hospitalized patients and 23 samples from 5 nonhospitalized patients. Samples before and after sotrovimab were available for 12 of the 13 patients. The following Omicron subvariants were identified among these: BA.1, BA.1.1, BA.1.1.10, BA.1.15, and BA.1.17.2. The mutational analysis for the available samples is shown in Figure 1 by hospitalization status. WGS detected mutations in the spike gene associated with resistance to sotrovimab (P337R/S, E340A/K/D, K356T) in 11 patients; 8 of 8 hospitalized patients with available samples developed treatment-related mutations. Additionally, 3 nonhospitalized patients also showed evidence of treatment-related mutations. Mutations were first detected in samples collected between 8 and 43 days following initiation of treatment with sotrovimab (median, 20 days); 10 of 11 patients showed no evidence of mutations before day 8 from receipt of sotrovimab. One patient did not have available sequencing results until day 34 following sotrovimab when E340D was detectable. The maximum duration of detection of a viral escape variant was 90 days following sotrovimab.
Figure 1.
Various spike protein mutations associated with resistance identified following receipt of sotrovimab in 11 patients, including 8 hospitalized cases and 2 persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. *Patient had a coronavirus disease 2019–related hospitalization after receipt of sotrovimab. ‡Patient had a persistent SARS-CoV-2 infection.
DISCUSSION
In our study cohort of highly vaccinated high-risk hematologic malignancy patients during the Omicron BA.1 surge, 11% of sotrovimab recipients were hospitalized for COVID-19 management, 1.2% required intensive care unit (ICU) level of care, and 3 patients died due to COVID-19. Refractory or relapsed malignancy and anti-CD20 therapy were significant predictors of hospitalization, but recent chemotherapy and steroid treatment did not influence this risk. Longitudinal evaluation of respiratory samples from hospitalized patients showed a high frequency of sotrovimab-related mutations that emerged within 8 to 43 days of treatment.
Early treatment with mAb reduces the risk of hospitalization and other COVID-19–related complications. In a pivotal international RCT, 1% of the sotrovimab-treated patients required hospitalization by day 29 compared to 7% in the placebo group [2]. The postauthorization experience among immunocompromised patients has most commonly been reported in solid organ transplant (SOT) recipients, with hospitalization rates ranging from 3% to 16% [15, 16]. Solera et al found a 30-day COVID-19–related hospitalization rate of 16% in SOT recipients who received sotrovimab; 4.7% required oxygen with no COVID-19–related ICU admissions, and no patients required mechanical ventilation [17]. Compared to these reports, our COVID-19–related hospitalization risk in patients with HM is 11% (17 of 156), with most (n = 15) hospitalized patients having mild to moderate manifestations.
Experience with sotrovimab in high-risk patients with HM, especially patients who have received anti-CD20 therapies, has not been extensively reported. Before the availability of vaccines and COVID-19 treatments, HM was consistently associated with higher hospitalization rates and complications in multiple reports [18, 19]. Moreover, although the risk of adverse outcomes is mitigated in the post–vaccine era, COVID-19–related complications remain common among patients undergoing treatment for HM [20, 21]. For example, in the study by Mittelman et al, the relative risk of hospitalization and severe disease was 3.13 and 2.27, respectively, in vaccinated patients with HM compared to matched controls without HM [21]. The overall risk was highest for those on active treatment.
Low CD8+ T-cell responses during acute infection predict severe disease, and B-cell depletion in hematologic cancers has been linked with chronic persistent COVID-19 [9]. Early mAb treatment can overcome some of these viral-specific immune deficits and improve patient outcomes. In our cohort, anti-CD20 therapy was a significant predictor of hospitalization: 38 patients received anti-CD20 therapies in our study cohort within the preceding 12 months of sotrovimab treatment (median, 5 months). Among these, 30 were fully vaccinated against COVID-19, 9 required hospitalization, 3 were readmitted (with 1 patient readmitted twice), and 3 died due to COVID-19 complications. Two of the 38 anti-CD20 recipients had evidence of persistent infection. Despite the early use of sotrovimab, there remains substantial morbidity in this population. Additional assessment of therapeutic approaches, including combination treatments, is warranted.
While there is an advantage in reducing hospitalization with the rapid administration of mAb, attention to the emergence of resistant viral escape variants is paramount, especially among immunocompromised hosts. Our data corroborate the phenomenon reported in Rockett et al and Destras et al in that sotrovimab use in immunocompromised patient populations may promote the rapid emergence of escape mutations [8, 22]. A recent analysis of 16 sotrovimab-treated patients, mostly SOT recipients and some on B-cell–depleting therapies, showed evidence of mutations in 6 of the 16 analyzed patients, although not all had serial samples available to follow the emergence of mutations [23]. Whether patients with HM harbor treatment resistance mutations to a higher degree than other high-risk groups and the public health implications of these findings are unclear at this time.
There are several limitations to our study. The most important limitation is the small sample size and a small number of COVID-19–related hospitalizations, which did not allow for a robust multivariable analysis. Of note, the heterogeneity of the transplant or CAR-T recipients and its small sample size with only 2 hospitalizations preclude additional analyses to identify predictors within this group. As a result, we could have missed detecting other predictors of disease progression. Additionally, our report includes the most extensive cohort experience from a homogenous high-risk hematologic malignancy group. We did not have a contemporary group of non-mAb-treated hematologic malignancy patients, so we were therefore unable to measure the effectiveness of mAb treatment in this population; however, this was not the primary goal of our study. Furthermore, relevant immune parameters such as CD19+ B cells and CD4 lymphocyte counts were not entirely available for analysis, nor can we confirm whether patients without vaccination records were immunized elsewhere. In addition, we did not perform viral sampling and serological assessments at predetermined intervals. Nonetheless, there was still a substantial number of analyzable samples to draw meaningful conclusions. Last, because our cohort had a high rate of COVID-19 vaccination (>80%), findings cannot be extrapolated to unvaccinated high-risk patients with HM.
In summary, early sotrovimab use reduced COVID-19–related hospitalization and severity in high-risk hematologic malignancy patients. However, there is a high occurrence of treatment-related mutations. Most importantly, patients treated with anti-CD20 therapies remain at higher risk for COVID-19–related adverse outcomes. Due to the high potential for resistance mutation to sotrovimab and increased morbidity in patients on anti-CD20 therapy, combination treatment should be explored to determine whether it provides added benefits compared to monotherapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Judy Yan, Infection Control, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Samantha N Steiger, Department of Pharmacy, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Rich Kodama, Infectious Disease Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Jerome Fender, Infection Control, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Digital Informatics and Technology Solutions, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Carrie Tan, Department of Pharmacy, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Justin Laracy, Infection Control, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Infectious Disease Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Joan and Sanford Weill Medical College of Cornell University, New York, New York, USA.
Nina Cohen, Department of Pharmacy, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Tracy McMillen, Clinical Microbiology Service, Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Krupa Jani, Clinical Microbiology Service, Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Elizabeth V Robilotti, Infection Control, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Infectious Disease Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Joan and Sanford Weill Medical College of Cornell University, New York, New York, USA.
N Esther Babady, Clinical Microbiology Service, Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Susan K Seo, Infectious Disease Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Joan and Sanford Weill Medical College of Cornell University, New York, New York, USA.
Mini Kamboj, Infection Control, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Infectious Disease Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York, USA; Department of Medicine, Joan and Sanford Weill Medical College of Cornell University, New York, New York, USA.
Notes
Author contributions. J. Y. and M. K. were responsible for the conception and design of the study, interpretation of the data, and preparation of the manuscript. M. K. managed the study. J. Y., S. N. S., J. F., C. T., J. L, N. C., and S. K. S. acquired the data. J. Y., E. V. R., N. E. B., and R. K. analyzed the data. J. Y. performed statistical analysis. J. Y., E. V. R., and R. K. produced the figures and tables. T. M., N. E. B., and K. J. analyzed laboratory samples. All authors reviewed the final draft, critically reviewed and contributed to the writing of the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments. The authors thank all the healthcare workers within Memorial Sloan Kettering Cancer Center for their collective effort against the coronavirus disease 2019 (COVID-19) pandemic.
Data availability. All sequences were uploaded to GISAID. For original data, please contact yanj7@mskcc.org.
Financial support. N. E. B. reports support for this work in the form of research grants from GenMark Diagnostics and ArcBio/Canta.
References
- 1. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 3. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu L, Zhou L, Mo M, et al. SARS-CoV-2 Omicron RBD shows weaker binding affinity than the currently dominant Delta variant to human ACE2. Signal Transduct Target Ther 2022; 7:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 2022; 185:447–56.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022; 602:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration . FDA updates sotrovimab emergency use authorization. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization. Accessed 28 July 2022.
- 8. Rockett R, Basile K, Maddocks S, et al. Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N Engl J Med 2022; 386:1477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee CY, Shah MK, Hoyos D, et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov 2022; 12:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Babady NE, McMillen T, Jani K, et al. Performance of severe acute respiratory syndrome coronavirus 2 real-time RT-PCR tests on oral rinses and saliva samples. J Mol Diagn 2021; 23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chow K, Aslam A, McClure T, et al. Risk of healthcare-associated transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospitalized cancer patients. Clin Infect Dis 2022; 74:1579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aksamentov I, Roemer C, Hodcroft EB, Neher RA. Nextclade: clade assignment, mutation calling and quality control for viral genomes. J Open Source Softw 2021; 6:3773. [Google Scholar]
- 13. Tamari R, Politikos I, Knorr DA, et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov 2021; 2:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27–38. [Google Scholar]
- 15. Yetmar ZA, Beam E OJC, et al. Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the Omicron epoch. Transpl Infect Dis 2022; 24:e13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chavarot N, Melenotte C, Amrouche L, et al. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection. Kidney Int 2022; 101:1290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solera JT, Árbol BG, Alshahrani A, et al. Impact of vaccination and early monoclonal antibody therapy on coronavirus disease 2019 outcomes in organ transplant recipients during the Omicron wave. Clin Infect Dis 2022; 75:2193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020; 26:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 2020; 10:935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 2022; 160:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mittelman M, Magen O, Barda N, et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood 2022; 139:1439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Destras G, Bal A, Simon B, Lina B, Josset L. Sotrovimab drives SARS-CoV-2 omicron variant evolution in immunocompromised patients. Lancet Microbe 2022; 3:e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huygens S, Oude Munnink B, Gharbharan A, et al. Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the severe acute respiratory syndrome coronavirus 2 Omicron variant. Clin Infect Dis 2023; 76:e507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.