Abstract
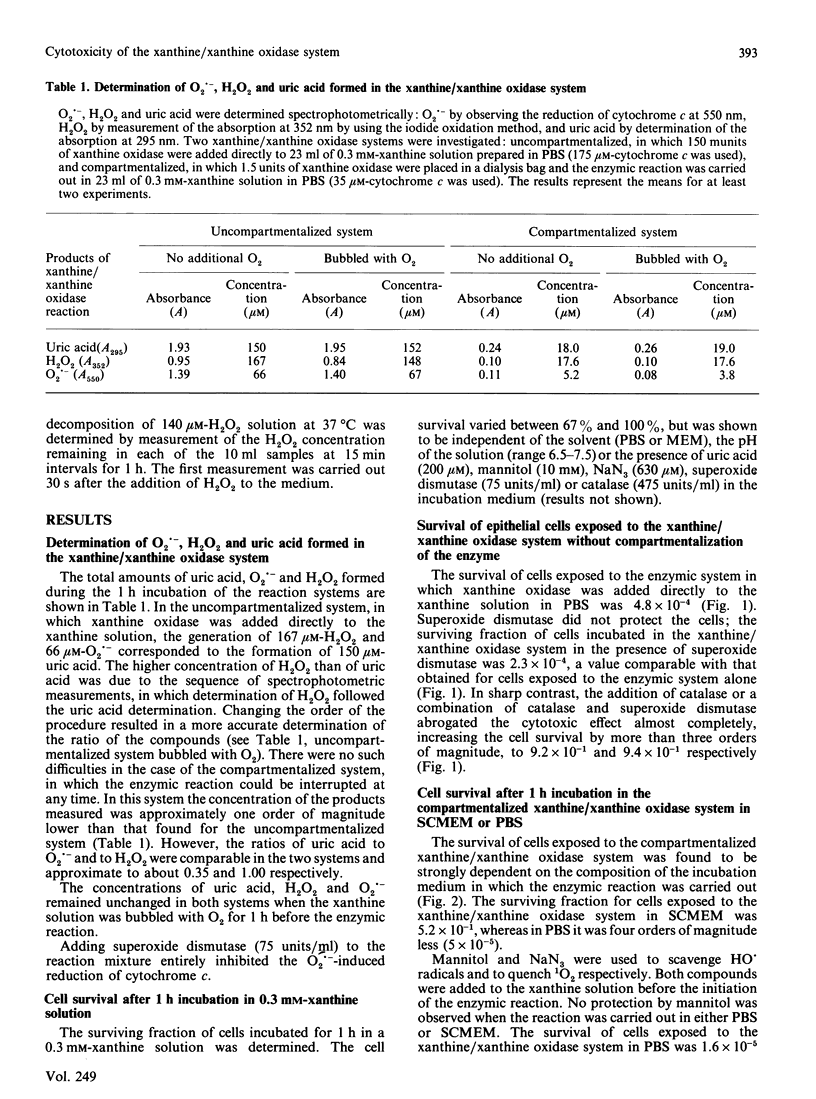
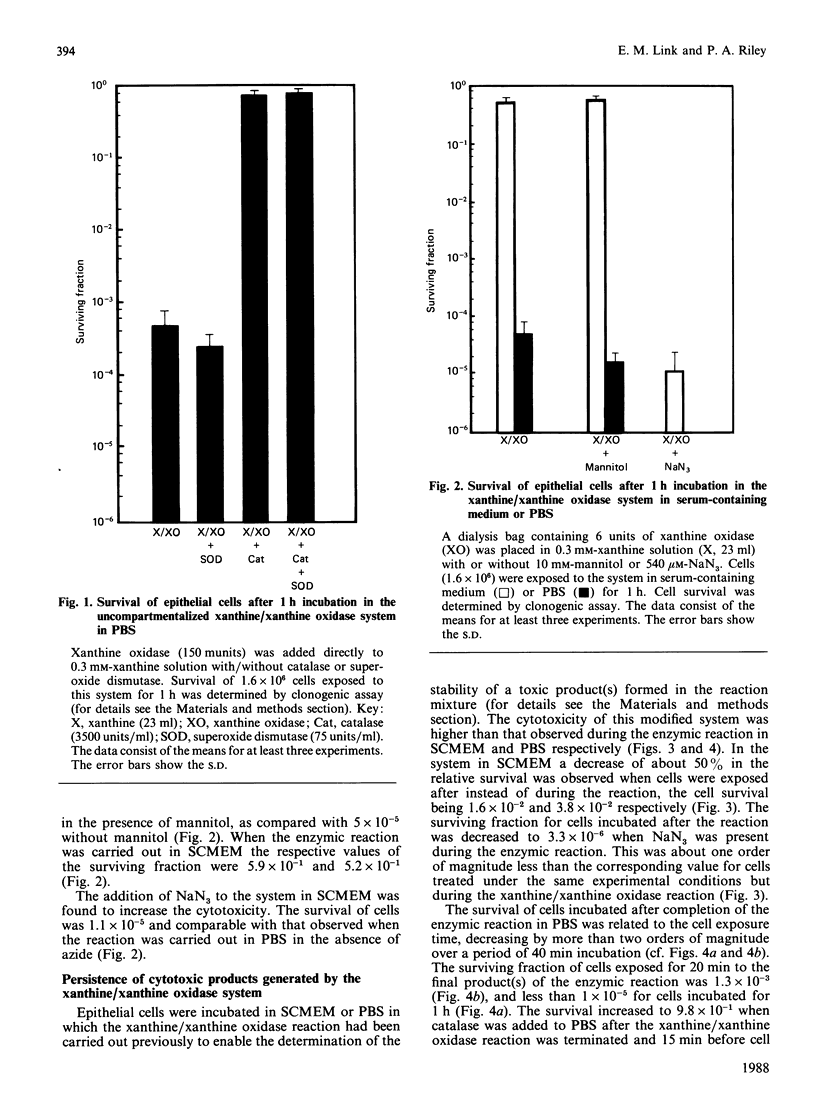
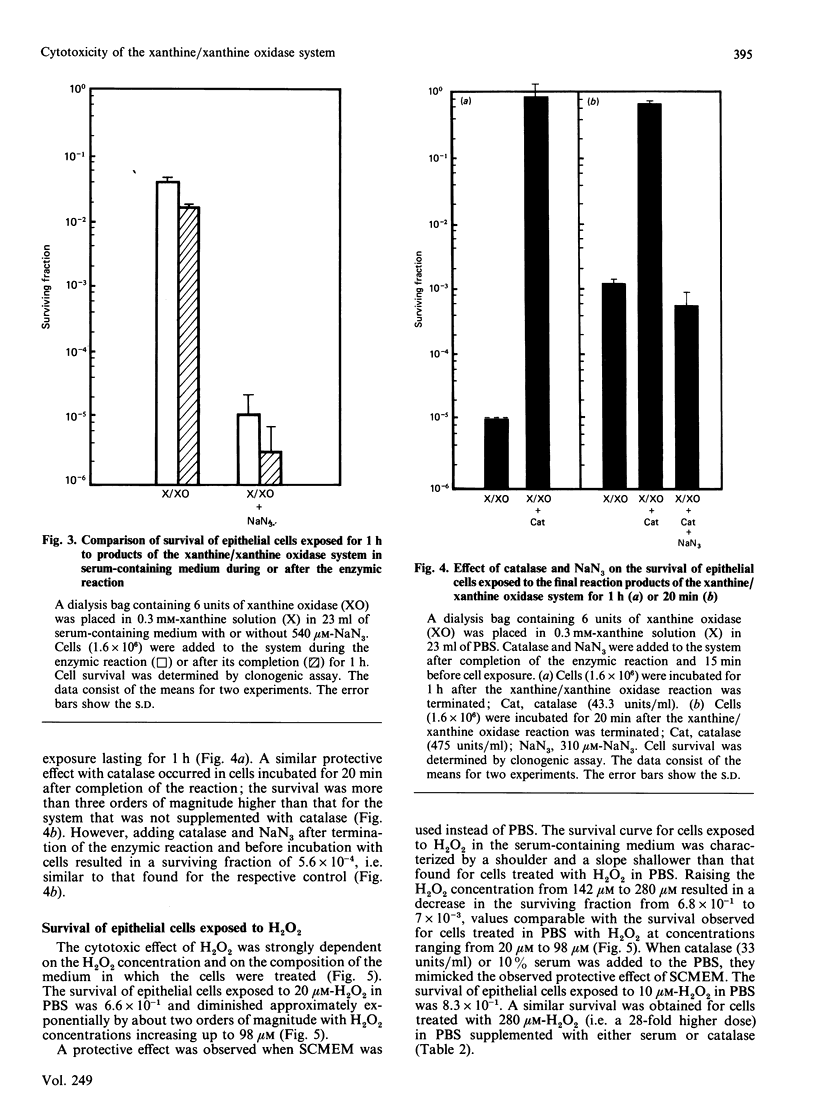
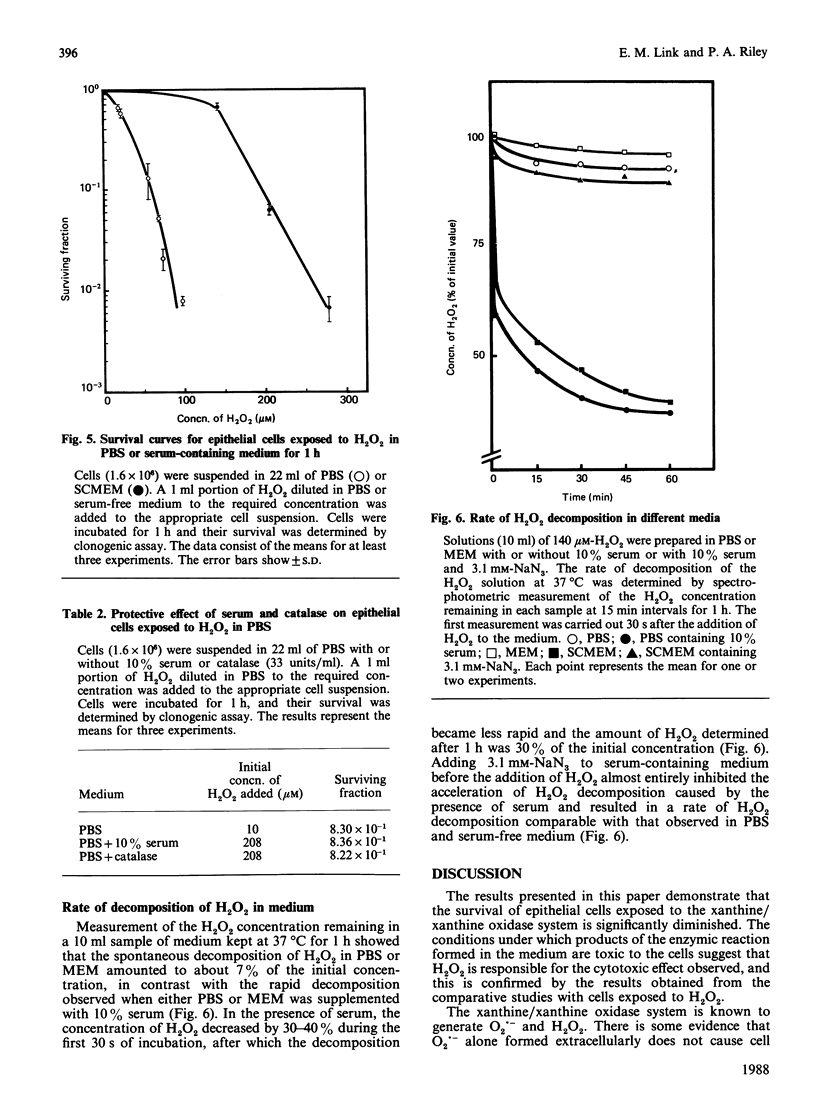
1. The survival of mammalian epithelial cells exposed in vitro to the xanthine/xanthine oxidase system in phosphate-buffered saline (PBS) or serum-containing medium (SCMEM) was investigated. 2. The cytotoxic effect observed depended on the composition of the medium in which the enzymic reaction was carried out; a surviving fraction of 5 x 10(-5) was found for cells exposed in PBS and 5.2 x 10(-1) for those in SCMEM. 3. The cytotoxic product(s) formed by the xanthine/xanthine oxidase system was relatively stable in PBS; survival of cells incubated after completion of the enzymic reaction was always less than that found for cells exposed during the reaction in the same system. 4. Superoxide dismutase or mannitol present during the enzymic reaction did not inhibit the cytotoxic effect. 5. NaN3 (a single-oxygen quencher and a catalase inhibitor) added to the system in SCMEM caused a reduction in survival to the level observed for cells exposed to the enzymic reaction in PBS. 6. Catalase completely protected cells, but no protection was observed when both catalase and NaN3 were present in the reaction mixture. 7. A similar cytotoxic effect was produced when cells were treated with H2O2 alone. 8. The rate of H2O2 decomposition in medium was accelerated by the presence of serum, but this was completely inhibited by NaN3. 9. It is concluded that H2O2 is the major cytotoxic product formed by the xanthine/xanthine oxidase system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneson R. M. Substrate-induced chemiluminescence of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1970 Feb;136(2):352–360. doi: 10.1016/0003-9861(70)90205-5. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielski B. H., Arudi R. L., Sutherland M. W. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J Biol Chem. 1983 Apr 25;258(8):4759–4761. [PubMed] [Google Scholar]
- Bucher J. R., Tien M., Morehouse L. A., Aust S. D. Redox cycling and lipid peroxidation: the central role of iron chelates. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):222–226. doi: 10.1016/s0272-0590(83)80130-4. [DOI] [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Demple B., Johnson A., Fung D. Exonuclease III and endonuclease IV remove 3' blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D., Ballou D., Van Heuvelen A., Palmer G., Massey V. Kinetic studies on the substrate reduction of xanthine oxidase. J Biol Chem. 1973 Sep 10;248(17):6135–6144. [PubMed] [Google Scholar]
- Floyd R. A., Nagy I. Formation of long-lived hydroxyl free radical adducts of proline and hydroxyproline in a Fenton reaction. Biochim Biophys Acta. 1984 Oct 9;790(1):94–97. doi: 10.1016/0167-4838(84)90337-6. [DOI] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Superoxide dismutase inhibits the superoxide-driven Fenton reaction at two different levels. Implications for a wider protective role. FEBS Lett. 1985 Jun 3;185(1):19–23. doi: 10.1016/0014-5793(85)80732-8. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille R., Fee J. A., Massey V. Equilibrium properties of xanthine oxidase containing FAD analogs of varying oxidation-reduction potential. J Biol Chem. 1981 Sep 10;256(17):8933–8940. [PubMed] [Google Scholar]
- Hille R., Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J Biol Chem. 1981 Sep 10;256(17):9090–9095. [PubMed] [Google Scholar]
- Hochstein P., Hatch L., Sevanian A. Uric acid: functions and determination. Methods Enzymol. 1984;105:162–166. doi: 10.1016/s0076-6879(84)05022-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. E., Mello-Filho A. C., Meneghini R. Correlation between cytotoxic effect of hydrogen peroxide and the yield of DNA strand breaks in cells of different species. Biochim Biophys Acta. 1984 Apr 5;781(3):234–238. doi: 10.1016/0167-4781(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Joshi P. C., Pathak M. A. The role of active oxygen (1O2 and O(2)) induced by crude coal tar and its ingredients used in photochemotherapy of skin diseases. J Invest Dermatol. 1984 Jan;82(1):67–73. doi: 10.1111/1523-1747.ep12259146. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- King M. M., Lai E. K., McCay P. B. Singlet oxygen production associated with enzyme-catalyzed lipid peroxidation in liver microsomes. J Biol Chem. 1975 Aug 25;250(16):6496–6502. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Samis H. V., Baird M. B. The kinetics of degradation of DNA and RNA by H 2 O 2 . Biochim Biophys Acta. 1972 Jul 31;272(4):539–548. doi: 10.1016/0005-2787(72)90509-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Miller A. R. Oxidation of cell wall polysaccharides by hydrogen peroxide: a potential mechanism for cell wall breakdown in plants. Biochem Biophys Res Commun. 1986 Nov 26;141(1):238–244. doi: 10.1016/s0006-291x(86)80359-x. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Gram T. E., Trush M. A. Stimulation of mouse heart and liver microsomal lipid peroxidation by anthracycline anticancer drugs: characterization and effects of reactive oxygen scavengers. J Pharmacol Exp Ther. 1983 Sep;226(3):806–816. [PubMed] [Google Scholar]
- Nagy I., Floyd R. A. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim Biophys Acta. 1984 Nov 9;790(3):238–250. doi: 10.1016/0167-4838(84)90028-1. [DOI] [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. S., Ballou D. P., Palmer G., Massey V. The mechanism of action of xanthine oxidase. J Biol Chem. 1974 Jul 25;249(14):4363–4382. [PubMed] [Google Scholar]
- Olson J. S., Ballow D. P., Palmer G., Massey V. The reaction of xanthine oxidase with molecular oxygen. J Biol Chem. 1974 Jul 25;249(14):4350–4362. [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. The mechanism of liver microsomal lipid peroxidation. Biochim Biophys Acta. 1975 Apr 7;385(2):232–241. doi: 10.1016/0304-4165(75)90351-7. [DOI] [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1071–1078. doi: 10.1016/0006-291x(73)91047-4. [DOI] [PubMed] [Google Scholar]
- Porras A. G., Olson J. S., Palmer G. The reaction of reduced xanthine oxidase with oxygen. Kinetics of peroxide and superoxide formation. J Biol Chem. 1981 Sep 10;256(17):9006–9103. [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):476–490. [PubMed] [Google Scholar]
- Roots R., Okada S. Estimation of life times and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat Res. 1975 Nov;64(2):306–320. [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide and iron salts by superoxide- and ascorbate-dependent mechanisms: relevance to the pathology of rheumatoid disease. Clin Sci (Lond) 1983 Jun;64(6):649–653. doi: 10.1042/cs0640649. [DOI] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Sugioka K., Nakano M. A possible mechanism of the generation of singlet molecular oxygen in nadph-dependent microsomal lipid peroxidation. Biochim Biophys Acta. 1976 Feb 16;423(2):203–216. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- Ward J. F., Evans J. W., Limoli C. L., Calabro-Jones P. M. Radiation and hydrogen peroxide induced free radical damage to DNA. Br J Cancer Suppl. 1987 Jun;8:105–112. [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Shasby D. M., Husted R. M. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest. 1985 Sep;76(3):1155–1168. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Gery I., Kessler D., Kinoshita J. H. Macrophage mediated damage to rat lenses in culture: a possible model for uveitis-associated cataract. Invest Ophthalmol Vis Sci. 1983 May;24(5):651–654. [PubMed] [Google Scholar]
- Zigler J. S., Jr, Jernigan H. M., Jr, Garland D., Reddy V. N. The effects of "oxygen radicals" generated in the medium on lenses in organ culture: inhibition of damage by chelated iron. Arch Biochem Biophys. 1985 Aug 15;241(1):163–172. doi: 10.1016/0003-9861(85)90372-8. [DOI] [PubMed] [Google Scholar]


