Abstract
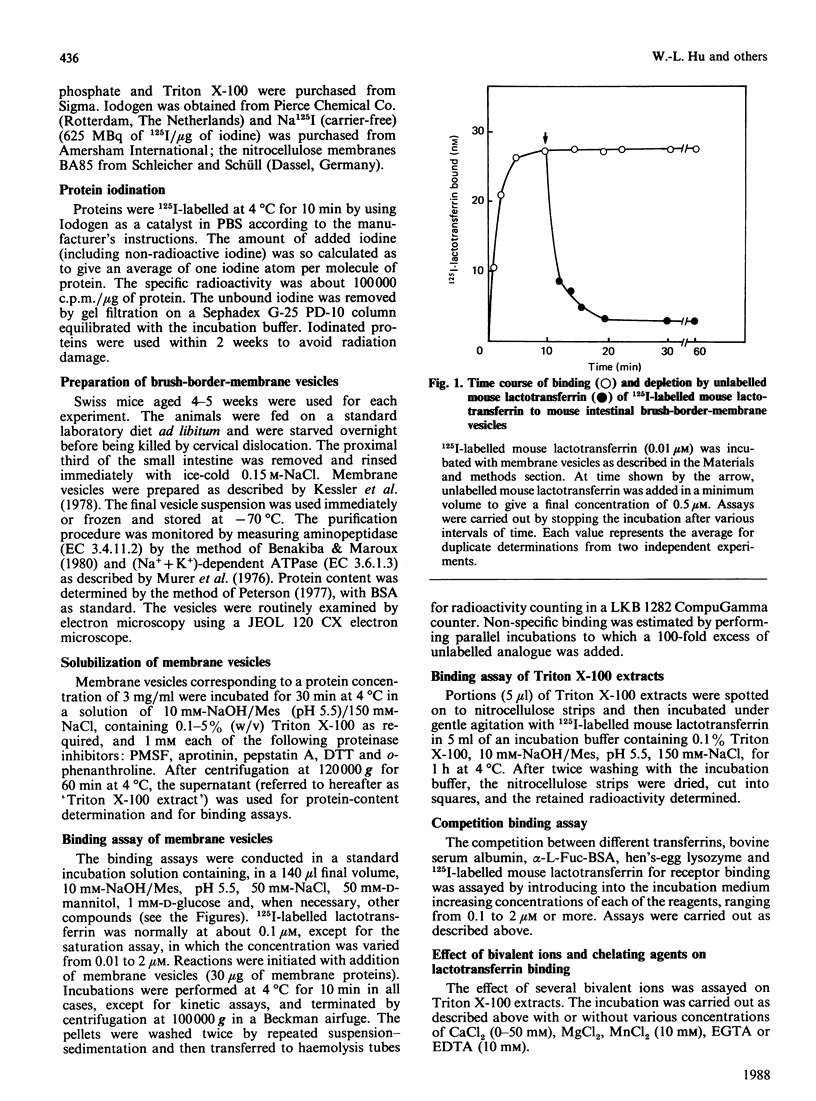
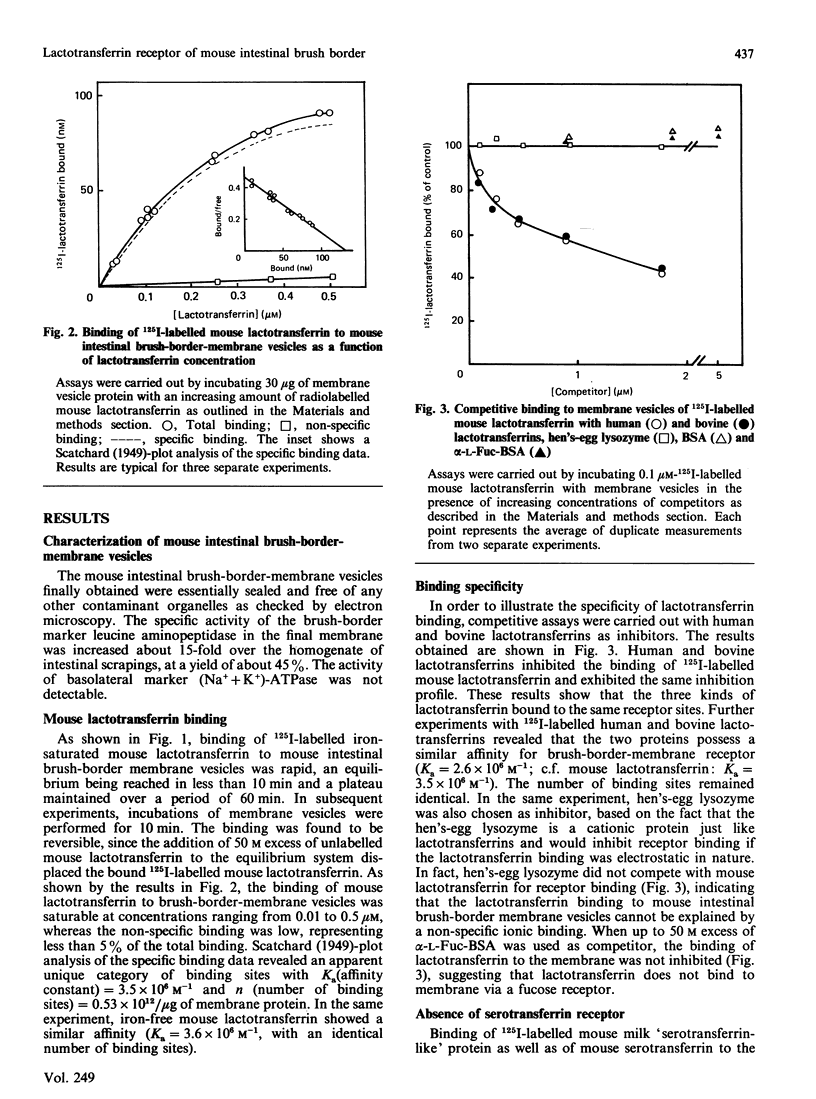
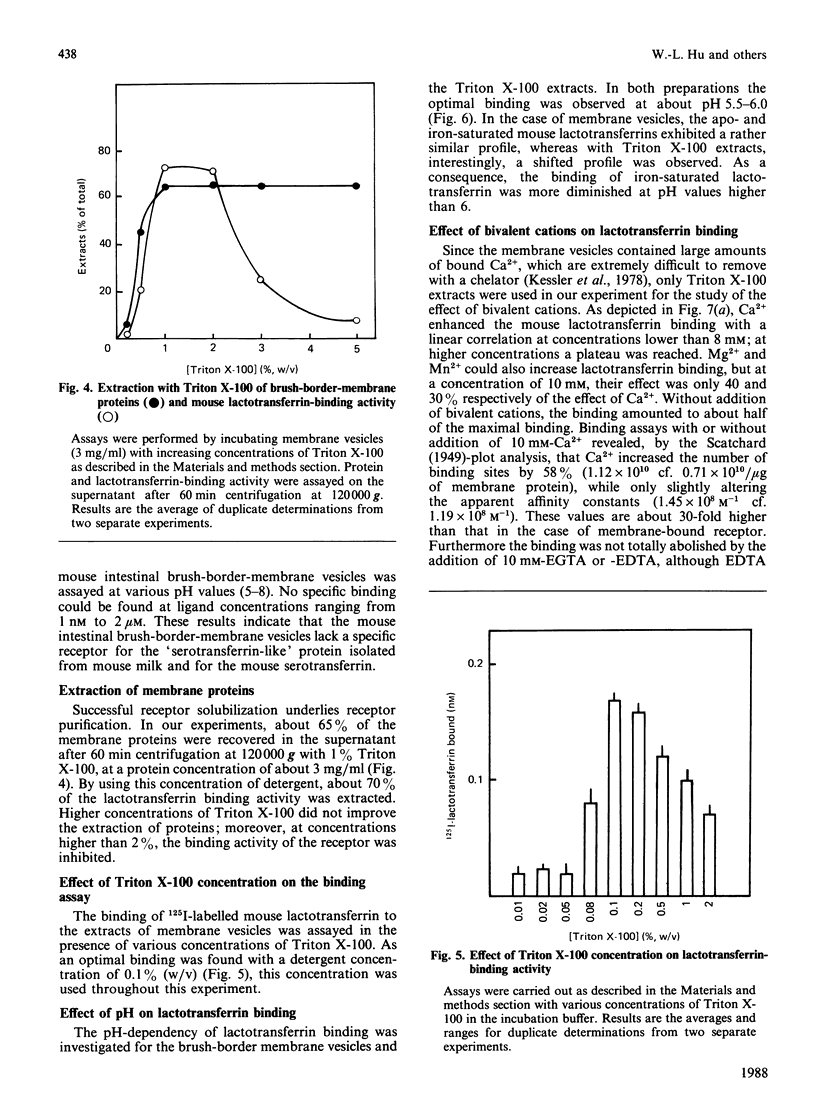
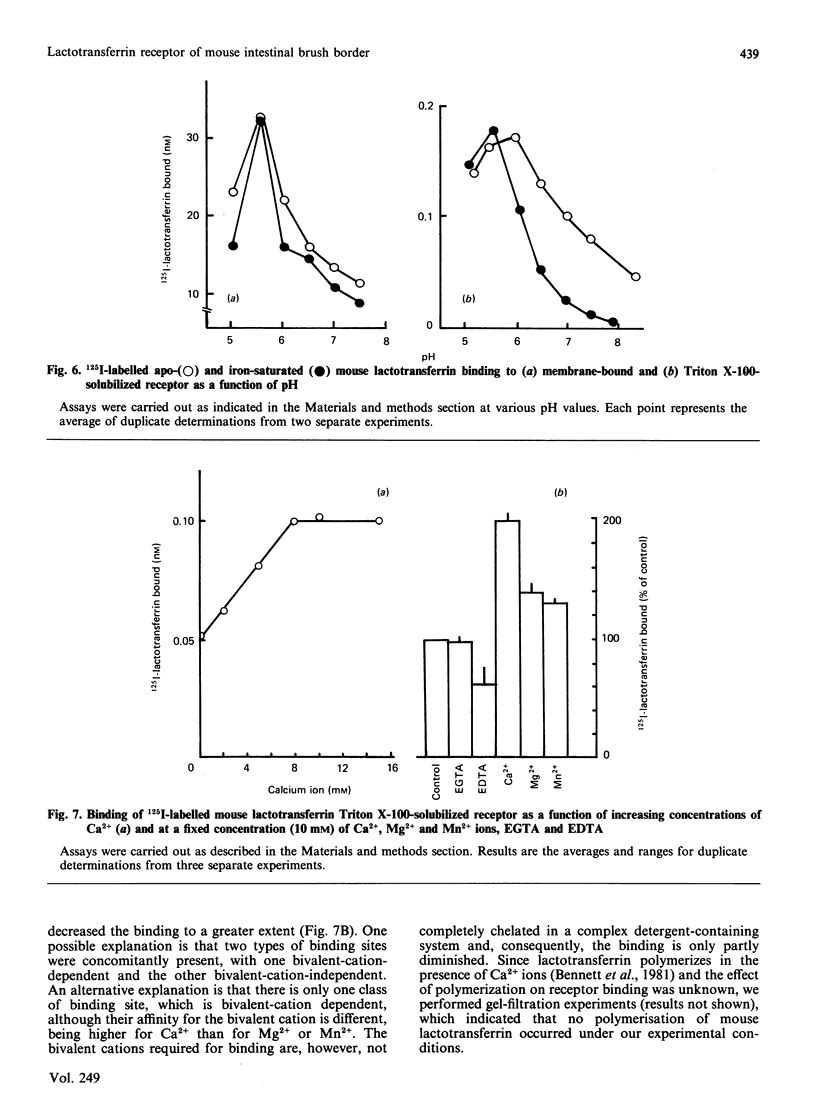
A specific lactotransferrin receptor was identified in the mouse small-intestinal brush-border membrane and the binding features were investigated in homologous and heterologous systems. The receptor was found to be specific for lactotransferrins isolated from milk of various species, but the affinity was higher toward the homologous ligand (Ka = 3.5 x 10(6) M-1 compared with 2.6 x 10(6) M-1 for both human and bovine lactotransferrins). However, the number of binding sites (n) was the same for the three lactotransferrins, namely 0.53 x 10(12)/micrograms of membrane protein. The binding of mouse lactotransferrin to its receptor was found to be pH-dependent, with an optimal binding at pH 5.5, and seemed unlikely to be carbohydrate-mediated. The receptor was demonstrated to be devoid of any affinity for human and mouse serotransferrins or for a 'serotransferrin-like' protein isolated from mouse milk. The receptor was solubilized with 1% Triton X-100 with good yield. The solubilized receptor was found to retain lactotransferrin-binding activity and sensitivity to pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D., Flanagan P. R., Cluett J., Valberg L. S. Transferrin receptors in the human gastrointestinal tract. Relationship to body iron stores. Gastroenterology. 1986 Oct;91(4):861–869. doi: 10.1016/0016-5085(86)90687-6. [DOI] [PubMed] [Google Scholar]
- Benajiba A., Maroux S. Purification and characterization of an aminopeptidase A from hog intestinal brush-border membrane. Eur J Biochem. 1980 Jun;107(2):381–388. doi: 10.1111/j.1432-1033.1980.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Bagby G. C., Davis J. Calcium-dependent polymerization of lactoferrin. Biochem Biophys Res Commun. 1981 Jul 16;101(1):88–95. doi: 10.1016/s0006-291x(81)80014-9. [DOI] [PubMed] [Google Scholar]
- Campbell E. J. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6941–6945. doi: 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéron A., Mazurier J., Fournet B. Fractionnement chromatographique et études sur la microhétérogénété de la lactotransferrine de vache préparée par un procédé original. C R Acad Sci Hebd Seances Acad Sci D. 1977 Feb 14;284(7):585–588. [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Regoeczi E., Sweeney G. D., Krestynski F. Interaction of human lactoferrin with the rat liver. Am J Physiol. 1985 Apr;248(4 Pt 1):G463–G469. doi: 10.1152/ajpgi.1985.248.4.G463. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Leclercq Y., Sawatzki G., Wieruszeski J. M., Montreuil J., Spik G. Primary structure of the glycans from mouse serum and milk transferrins. Biochem J. 1987 Nov 1;247(3):571–578. doi: 10.1042/bj2470571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M. L., Schneider W., Haberich F. J., Blair J. A. Direct measurement by pH-microelectrode of the pH microclimate in rat proximal jejunum. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):39–48. doi: 10.1098/rspb.1975.0150. [DOI] [PubMed] [Google Scholar]
- MONTREUIL J., MULLET S. [Isolation of lactosiderophilin from human milk]. C R Hebd Seances Acad Sci. 1960 Feb 29;250:1736–1737. [PubMed] [Google Scholar]
- MONTREUIL J., TONNELAT J., MULLET S. [Preparation and properties of lactosiderophilin (lactotransferrin) of human milk]. Biochim Biophys Acta. 1960 Dec 18;45:413–421. doi: 10.1016/0006-3002(60)91478-5. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Montreuil J., Spik G. Visualization of lactotransferrin brush-border receptors by ligand-blotting. Biochim Biophys Acta. 1985 Dec 19;821(3):453–460. doi: 10.1016/0005-2736(85)90050-1. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Spik G. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim Biophys Acta. 1980 May 7;629(2):399–408. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Bailly P. Tumoricidal activation of murine alveolar macrophages by muramyldipeptide substituted mannosylated serum albumin. Biochem Biophys Res Commun. 1984 Jun 15;121(2):579–584. doi: 10.1016/0006-291x(84)90221-3. [DOI] [PubMed] [Google Scholar]
- Murer H., Ammann E., Biber J., Hopfer U. The surface membrane of the small intestinal epithelial cell. I. Localization of adenyl cyclase. Biochim Biophys Acta. 1976 May 21;433(3):509–519. doi: 10.1016/0005-2736(76)90277-7. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Barton J. C., Conrad M. E. Ultrastructural localization of transferrin, transferrin receptor, and iron-binding sites on human placental and duodenal microvilli. Br J Haematol. 1985 May;60(1):81–89. doi: 10.1111/j.1365-2141.1985.tb07388.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sawatzki G., Anselstetter V., Kubanek B. Isolation of mouse transferrin using salting-out chromatography on Sepharose CL-6B. Biochim Biophys Acta. 1981 Jan 30;667(1):132–138. doi: 10.1016/0005-2795(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Schümann K., Osterloh K., Forth W. Independence of in vitro iron absorption from mucosal transferrin content in rat jejunal and ileal segments. Blut. 1986 Nov;53(5):391–400. doi: 10.1007/BF00321101. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Lee Y. C., Schlesinger P. H., Stahl P. D. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Brunet B., Mazurier-Dehaine C., Fontaine G., Montreuil J. Characterization and properties of the human and bovine lactotransferrins extracted from the faeces of newborn infants. Acta Paediatr Scand. 1982 Nov;71(6):979–985. doi: 10.1111/j.1651-2227.1982.tb09560.x. [DOI] [PubMed] [Google Scholar]
- Spik G., Strecker G., Fournet B., Bouquelet S., Montreuil J., Dorland L., van Halbeek H., Vliegenthart J. F. Primary structure of the glycans from human lactotransferrin. Eur J Biochem. 1982 Jan;121(2):413–419. doi: 10.1111/j.1432-1033.1982.tb05803.x. [DOI] [PubMed] [Google Scholar]
- van Halbeek H., Dorland L., Vliegenthart J. F., Spik G., Cheron A., Mohtreuil J. Structure determination of two oligomannoside-type glycopeptides obtained from bovine lactotransferrin, by 500 MHz 1H-NMR spectroscopy. Biochim Biophys Acta. 1981 Jul;675(2):293–296. doi: 10.1016/0304-4165(81)90240-3. [DOI] [PubMed] [Google Scholar]


