Abstract
OBJECTIVES:
Persistent skeletal muscle dysfunction in survivors of critical illness due to acute respiratory failure is common, but biological data elucidating underlying mechanisms are limited. The objective of this study was to elucidate the prevalence of skeletal muscle weakness and fatigue in survivors of critical illness due to COVID-19 and determine if cellular changes associate with persistent skeletal muscle dysfunction.
DESIGN:
A prospective observational study in two phases: 1) survivors of critical COVID-19 participating in physical outcome measures while attending an ICU Recovery Clinic at short-term follow-up and 2) a nested cohort of patients performed comprehensive muscle and physical function assessments with a muscle biopsy; data were compared with non-COVID controls.
SETTING:
ICU Recovery Clinic and clinical laboratory.
PATIENTS/SUBJECTS:
Survivors of critical COVID-19 and non-COVID controls.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
One hundred twenty patients with a median of 56 years old (interquartile range [IQR], 42–65 yr old), 43% female, and 33% individuals of underrepresented race attended follow-up 44 ± 17 days after discharge. Patients had a median Acute Physiology and Chronic Health Evaluation-II score of 24.0 (IQR, 16–29) and 98 patients (82%) required mechanical ventilation with a median duration of 14 days (IQR, 9–21 d). At short-term follow-up significant physical dysfunction was observed with 93% of patients reporting generalized fatigue and performing mean 218 ± 151 meters on 6-minute walk test (45% ± 30% of predicted). Eleven patients from this group agreed to participate in long-term assessment and muscle biopsy occurring a mean 267 ± 98 days after discharge. Muscle tissue from COVID exhibited a greater abundance of M2-like macrophages and satellite cells and lower activity of mitochondrial complex II and complex IV compared with controls.
CONCLUSIONS:
Our findings suggest that aberrant repair and altered mitochondrial activity in skeletal muscle associates with long-term impairments in patients surviving an ICU admission for COVID-19.
Keywords: COVID-19, critical illness, intensive care unit-acquired weakness, mitochondria, muscle, post-intensive care syndrome, recovery
KEY POINTS
Question: Determine the short- and long-term physical impairments and skeletal muscle cellular changes in survivors of critical COVID.
Findings: Survivors of critical COVID have high prevalence of weakness, fatigue, and reduced functional exercise capacity with muscle tissue exhibiting a greater abundance of M2-like macrophages and satellite cells and lower activity of mitochondrial complex II and complex IV.
Meanings: Perturbed muscle recovery associates with fatigue and poor physical function months after ICU admission for COVID-19. Critical care and post-hospital clinicians should be aware of the potential long-term muscle deficits in COVID survivors, but more work is needed to understand the mechanisms underlying these histochemical findings and their relationship to long COVID.
Individuals with COVID-19 have a high occurrence of fatigue and weakness, which often persist into recovery (1, 2). Patients admitted to ICU with COVID-19 are at risk of ICU-acquired weakness (ICUAW) with prevalence ranging from 50% to 65% (1–3). Muscle and physical deficits in survivors of critical COVID have been reported up to 6 months (4) and 12 months (5) after ICU discharge. However, the underlying biological mechanisms driving muscle dysfunction following critical COVID remain relatively unknown. Skeletal muscle might be directly susceptible to COVID because of high expression of angiotensinogen-converting enzyme 2, a binding site for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and “coronavirus-like particles” have been reported in muscle fibers (6, 7). Indeed, 15.4% of postmortem analyses of diaphragm myofibers showed evidence of SARS-CoV-2 (8), but a direct viral infection of peripheral skeletal muscle has not been confirmed since gene expression analyses suggest that only smooth muscle cells are directly impacted by SARS-CoV-2 (9). Nevertheless, skeletal muscle is likely compromised due the impact of high levels of circulating immune cells and inflammatory mediators associated with critical illness and COVID-19 (10). Recent investigations in individuals with long COVID resulting from mild illness demonstrate lower exercise capacity (11) with skeletal muscle changes including metabolic alterations (11, 12) and capillary injury (13, 14). However, changes in skeletal muscle from individuals recovering from critical COVID-19 have not been examined.
Patients with critical illness of COVID-19 (15) and non-COVID (16) etiologies suffer up to 30% of quadriceps muscle wasting in the first 10 days of ICU admission. Muscle wasting likely precedes the development of weakness and physical dysfunction related to post-intensive care syndrome (PICS) (16). Additionally, impairments in muscle excitability and bioenergetic failure are thought to occur in ICU survivors leading to poor functional recovery (17). The specific physiologic mechanisms contributing to muscle dysfunction in the recovery phase of critical illness are largely unknown. Alterations in the number of satellite cells (SCs) with compromised muscle regrowth (18) and lower abundance of mitochondrial biogenesis genes (19) were reported in 11 patients’ 6 months after critical illness of mixed etiologies. Currently, strategies to mitigate long-term impairments in muscle and physical function are equivocal as large rehabilitation randomized controlled trials in the ICU (20–22) and post-ICU (23) fail to demonstrate benefits in functional outcomes. Elucidating the underlying cause of muscle impairments provides evidence to develop targeted strategies to improve outcomes. The objective of this study was to elucidate the prevalence of skeletal muscle weakness and fatigue in survivors of critical illness due to COVID-19 and determine cellular changes associated with persistent skeletal muscle dysfunction.
METHODS
Study Design
A prospective observational study conducted in two phases: 1) a cohort of patients with critical COVID participating in short-term follow-up at an ICU Recovery Clinic (24) and 2) a nested cohort participating in assessments at long-term follow-up.
Patient Population
Adult patients (≥ 18 yr old) surviving an admission to the medical ICU with a diagnosis of COVID-19 pneumonia (polymerase chain reaction confirmed) requiring mechanical ventilation (MV) or high-flow nasal cannula (HFNC) with at least 3-day ICU admission and attended routine follow-up at the ICU Recovery Clinic were eligible (COVID). Patients were excluded when nonambulatory before hospitalization or having an acute or preexisting condition precluding physical assessments. A flow diagram is provided in Supplemental Figure 1 (http://links.lww.com/CCX/B419).
Short-Term Follow-Up Assessment
Patients participated in functional assessment in the ICU Recovery Clinic occurring 1-month post-discharge. A physical therapist performs the Medical Research Council-Sum Score (MRC-SS) (25), Short Physical Performance Battery (SPPB) (26), and the 6-minute walk test (6MWT) (27). A clinical diagnosis of ICUAW at the time of testing was established with cutoff score of less than 48/60 of the MRC-SS; a cutoff of less than or equal to 9/12 on SPPB established physical frailty (28) and clinical frailty scale was scored by the physical therapist (29). Patients’ self-report generalized (nonspecific) fatigue as binary outcome and completed EuroQol 5D five levels (EQ-5D-5L)—Visual Analog Scale (30). A negative composite outcome of requiring at least one emergency department visit or hospital readmission was extracted from electronic medical record (EMR) at 90 and 180 days post-discharge.
Comprehensive Long-Term Follow-Up
A nested group of patients who completed the short-term assessment was recruited to participate in a cross-sectional assessment of muscle and physical function with a muscle biopsy. Patients participated in the following tests:
1] Symptomatology questions were answered in a binary fashion, and subjects completed self-report measures for quality of life (EQ-5D-5L) (30) and Functional Assessment of Chronic Illness Therapy (FACIT)-fatigue subscale (31).
2] Muscular strength was evaluated with three approaches: 1) MRC-SS was repeated as originally described; 2) force production of knee extensors with hand-held dynamometry (HHD) (Lafayette Manual Muscle Test System Model-01165; Lafayette Company, Lafayette, IN) in supine position with knee on a bolster in 20–30° of flexion (32) and; 3) handgrip strength with Jamar Hydraulic Hand Dynamometer with the average of three repetitions of dominant hand used in analyses (33).
3] Lower-extremity muscle power: 1) Power was calculated based on the recorded velocity and distance of a unilateral (right) leg press (HUMAC-360; CSMi, Petaluma, CA) as previously described (34) and 2) chair rise test was performed as a generalized assessment of lower-extremity muscle power.
4] Physical function outcomes: Timed up and go test (TUG) (35), SPPB (26), and 6MWT (27) were assessed.
5] Muscle biopsy: Vastus lateralis muscle biopsies were obtained using the Bergstrom needle technique previously described (36). Approximately 50 mg was prepared for cryosectioning by mounting on cork and freezing in liquid nitrogen-cooled isopentane (37). Remaining tissue was snap-frozen in liquid nitrogen for mitochondrial assays. During the biopsy procedure, we were unable to obtain usable tissue from one patient and not enough tissue for immunohistochemistry from another. Thus, for mitochondrial activity assays (n = 10) and immunohistochemistry (n = 9). All samples were coded for blinding during analyses. Histochemistry, immunohistochemistry, and mitochondrial activity methodologies and a list of kits, antibodies, and working concentrations are provided in Supplemental Table 1 (http://links.lww.com/CCX/B419).
Controls
Subjects (n = 7) of community dwelling adults without history of ICU admission were recruited from local community before COVID-19 pandemic and performed measures described above. Subjects were recruited from advertisements to participate in a study to serve as matched controls for critical illness survivors that was planned in 2019 (ClinicalTrials.gov: ID NCT03717831); however, the COVID pandemic altered the original study, and these subjects were used for comparison in this study. Individuals were median age of 60 years (interquartile range [IQR], 46–62 yr), 29% female gender, median body mass index (BMI) of 31 kg/m2 (IQR, 30–31 kg/m2) ± 3, and median Charlson Comorbidity Index (CCI) of 2 (IQR, 1–2). In addition, we included muscle samples from community-dwelling adults from the University of Kentucky (UK) Center for Muscle Biology (CMB) Biobank (n = 15), which were collected in healthy adults before the COVID pandemic for a different prospective study (median age, 64 [IQR, 59–69], 67% female, and median BMI of 27 [IQR, 26–27]) (38). Total sample size for control was 22 except for two technical errors for SCs and succinate dehydrogenase (SDH) (n = 20); some characteristics from the UK CMB controls are previously published (38). Demographic and physical outcome data from the control cohort are provided in Supplemental Table 2 (http://links.lww.com/CCX/B419).
Demographic and Clinical Variables
Demographic variables were extracted from EMR: age, sex, BMI, and race/ethnicity. Comorbid burden was quantified with the CCI. Clinical data extracted from the EMR: ICU and hospital length of stay (LOS, d), receipt and duration MV (d), receipt of continuous renal replacement therapy, extracorporeal membrane oxygenation, tracheostomy, continuous IV infusion of neuromuscular blocker (cisatracurium), corticosteroid, vasopressor or inotrope, Richmond Agitation-Sedation Scale score, a surrogate marker of sedative state, and Acute Physiology and Chronic Health Evaluation (APACHE)-II scores were quantified in the first 72 hours of ICU admission.
Statistics
Data were assessed using descriptive statistics; normality was assessed by Shapiro-Wilk tests. Independent t test or Mann-Whitney U tests were performed to compare the two cohorts (original vs. nested). Paired t test were performed to examine change in outcomes in the nested group between the two time points. Multivariate regression analyses were performed to explore which demographic and clinical variables are associated with functional outcomes. The a priori selection of candidate variables is described in Supplemental Detailed Methods (http://links.lww.com/CCX/B419). Differences in cell populations and muscle tissue characteristics from COVID compared with controls were assessed using Mann-Whitney U tests. Exploratory correlative testing was performed to determine associations between patient-centered outcomes of muscle strength (HHD), 6MWT, and FACIT with muscle immunohistochemistry features using Spearman rho. Data were analyzed and visualized using GraphPad Prism 8.2 (GraphPad, San Diego, CA) and SAS 9.4 (SAS Institute, Cary, NC) with p value of less than or equal to 0.05 considered significant.
Study Approval
The study was approved by the Institutional Review Board (IRB), at the University of Kentucky and performed in accordance with Helsinki Declarations. Informed consent was waived for patients at the short-term follow-up as data collected were considered routine care (Medical IRB-expedited No. 47751; approved December 14, 2018). Patients in the nested and control groups provided written informed consent (Medical IRB-full No. 46072; approved September 21, 2019). This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (Supplemental Digital Content, http://links.lww.com/CCX/B419).
RESULTS
ICU Recovery COVID Cohort
One hundred twenty patients attended follow-up clinic at 44 ± 17 days after hospital discharge from April 1, 2020, to June 1, 2022. Patients were median 56 years old (IQR, 42–64 yr), 43% female, and 33% of underrepresented race/ethnicity (Table 1). The median APACHE-II score was 24.0 (IQR, 16–29) and 98 patients (82%) required MV with a duration of 14 days (IQR, 9–21 d); the remaining 22 patients received supplemental oxygen via HFNC (Table 1). Patients (n = 115) participated in MRC-SS with median result of 53 U (IQR, 48–56 U) and 27% (n = 31) meeting criteria for ICUAW. The mean time for those patients able to perform the chair rise test (n = 77) was 12 ± 5.5 seconds, whereas 38 (32%) were unable to perform. The mean score on SPPB was 8 (IQR, 5–10) with 71 patients (61%) scoring less than or equal to 9/12 indicating physical frailty. Patients (n = 103) performed a median of 225 meters (IQR, 90–325 meters) on 6MWT equating to 44% (IQR, 21–67%) of predicted distance (Table 1).
TABLE 1.
Demographic and Clinical Data of the Critical COVID Cohort
| Parameter | Total Cohort (n = 120) | Nested Cohort (n = 11) | Group Comparisona, p < 0.05 |
|---|---|---|---|
| Age, yr, median (IQR) | 56 (42–64) | 56 (53–60) | p = 0.84 |
| Sex, female, n (%) | 52 (43) | 5 (45) | p = 0.84 |
| Race/ethnicity, n (%)b | |||
| White/Caucasian | 81 (67.5) | 7 (64) | p = 0.78 |
| Black/African American | 26 (21.7) | 3 (27) | |
| Latino/Hispanic | 10 (8.3) | 1 (9) | |
| Other | 3 (2.5) | 0 (0) | |
| Body mass index, kg/m2, mean ± sd | 34 (29–39) | 35 (34–39) | p = 0.20 |
| Charlson Comorbidity Index, median (IQR) | 3 (1–4) | 2 (1–2.5) | p = 0.41 |
| MV, yes, n (%) | 98 (82) | 8 (73) | p = 0.37 |
| MV duration, d, median (IQR) | 14 (9–21) | 16 (9.5–18) | p = 0.67 |
| Acute Physiology and Chronic Health Evaluation-II score, median (IQR) | 24 (16–29) | 24 (13.5–28) | p = 0.88 |
| Extracorporeal membrane oxygenation, yes, n (%) | 19 (16) | 3 (27) | p = 0.25 |
| Continuous renal replacement therapy, yes, n (%) | 19 (16) | 1 (9) | p = 0.62 |
| Tracheostomy, yes, n (%) | 37 (31) | 2 (18) | p = 0.47 |
| Received inotrope/vasopressor, n (%) | 68 (57) | 7 (63) | p = 0.84 |
| Received neuromuscular blocker, n (%) | 46 (38) | 4 (36) | p = 0.92 |
| Received corticosteroid, n (%) | 99 (83) | 9 (81) | p = 0.84 |
| Richmond Agitation-Sedation Scale, median (IQR) | –3.5 (–4 to –2) | –3.5 (–3.5 to –0.75) | p = 0.92 |
| Discharge disposition, n (%)c | |||
| Secondary facility (acute rehabilitation or long term acute care | 65 (54) | 4 (36) | p = 0.42 |
| Home with services | 23 (19) | 4 (36) | |
| Home without services | 32 (27) | 3 (27) | |
| 90-d negative composite outcome, n (%) | 18 (15) | 1 (9) | p = 0.19 |
| 180-d negative composite outcome, n (%) | 23 (19) | 2 (18) | p = 0.13 |
| Outcomes in ICU Recovery Clinic at short-term follow-up | |||
| Time to testing from hospital discharge, d, median (IQR) | 41 (35–54) | 37 (36–55) | p = 0.75 |
| EuroQol 5D five levels, Visual Analog Scale score, median (IQR) | 75 (63–80) | 74 (70–83) | p = 0.92 |
| Self-reported fatigue, yes, n (%) | 111 (92.5) | 10 (92) | p = 0.37 |
| Medical Research Council-Sum Score, median (IQR) | 53 (48–56)d | 50 (45–53) | p = 0.12 |
| Diagnosis of ICU-acquired weakness, n (%) | 31 (27) | 4 (36) | p = 0.39 |
| Short Physical Performance Battery, total, median (IQR) | 8 (5–10) | 9 (6–10) | p = 0.82 |
| Clinical frailty scale, median (IQR) | 5 (4–6) | 5 (4–6) | p = 0.70 |
| 6MWD, m, median (IQR) | 225 (90–325)e | 250 (102–315) | p = 0.94 |
| Percent achieved of predicted 6MWD, %, median (IQR) | 44 (21–67) | 47 (20–64) | p = 0.41 |
6MWD = 6-min walk distance; IQR = interquartile range, MV = mechanical ventilation.
Mann-Whitney U test or χ2 test to compare two groups.
Comparison of White/Caucasian to historically underrepresented race/ethnicity (Black, Latino, and other).
Comparison of discharge home with and without services vs. discharge to facility.
Five individuals did not participate in Medical Research Council-Sum Score and Short Physical Performance Battery (4-m gait speed and chair rise) testing due to physiotherapist unavailable.
Seventeen patients did not perform the 6-min walk test or various clinical reasons.
Multivariate Regression Models
Hospital LOS, female gender, and CCI were related to performance on MRC-SS, but hospital LOS was the only variable related to a diagnosis of ICUAW at short-term follow-up. Hospital LOS, CCI, and receipt of corticosteroid were associated with performance on 6MWT. Race of underrepresented background, CCI, and hospital LOS were significantly related to having at least one negative outcome (readmission or emergency department visit) 180 days post-hospital discharge occurring in 23 patients (19%) (Supplemental Table 3, http://links.lww.com/CCX/B419).
Muscle Biopsy Cohort
Eleven patients from March 23, 2021, to January 13, 2022 (n = 60 approached, 18%) participated in comprehensive testing occurring a mean 267 ± 98 days after discharge. There were no statistically significant differences in demographic and clinical data between the initial COVID (n = 120) and the nested group (n = 11; Table 1). Short-term outcomes that were repeated at long-term timepoint generally improved in the nested biopsy cohort (Supplemental Table 4, http://links.lww.com/CCX/B419). However, patients in the nested cohort had several significant muscle and physical function deficits compared with controls including a higher prevalence of generalized fatigue, a lower knee extension force production, lower scores on 6MWD, and worse performance on TUG (Table 2).
TABLE 2.
Comprehensive Muscle and Physical Function at Long-Term Assessment
| Parameter | Description of Test | COVID (n = 11) | Controla (n = 7) | Grouped Comparisonb, p < 0.05 |
|---|---|---|---|---|
| Symptoms and self-report measures | ||||
| EuroQol 5D five levels, Visual Analog Scale score, median (IQR) | Subjective report (0–100) higher score representing better quality of life | 90 (77–95) | 98 (95–100) | p = 0.047 |
| Functional Assessment of Chronic Illness Therapy-fatigue, mean ± sdc | Subjective report (0–52), lower score indicating more fatigue | 33 ± 12 | 51 ± 1.7 | p = 0.002 |
| Self-reported fatigue, yes, n (%) | Dichotomous self-report (yes, no) | 7 (63) | 1 (14) | p = 0.007 |
| Self-reported weakness, yes, n (%) | Dichotomous self-report (yes, no) | 6 (54) | 0 (0) | p = 0.017 |
| Self-reported myalgia, yes, n (%) | Dichotomous self-report (yes, no) | 3 (27) | 0 (0) | p = 0.130 |
| Self-reported dyspnea, yes, n (%) | Dichotomous self-report (yes, no) | 3 (27) | 0 (0) | p = 0.130 |
| Self-reported cognitive deficits, yes, n (%) | Dichotomous self-report (yes, no) | 1 (9) | 0 (0) | p = 0.412 |
| Muscle strength and power | ||||
| Handgrip dynamometry, kg, mean ± sd | Higher output in kilograms indicating more muscle strength | 30 ± 7.8 | 37 ± 8.9 | p = 0.071 |
| Knee extension force production, kg, mean ± sd | 26 ± 8.1 | 38 ± 6.7 | p = 0.004 | |
| Knee extension rate of force production, s, mean ± sd | 3.0 ± 0.87 | 2.2 ± 0.4 | p = 0.029 | |
| LE muscle power (2 pounds [lbs]), Watts, mean ± sd | Higher output in Watts indicates better muscle power | 14 ± 3.5 | 17 ± 4.6 | p = 0.193 |
| LE muscle power (21 lbs), Watts, mean ± sd | 127 ± 35 | 162 ± 42 | p = 0.079 | |
| Chair rise test, s, median (IQR) | Lower time indicates better muscle power and strength | 10.3 (9.2–13.1) | 5.6 (5.3–6.3) | p = 0.019 |
| Physical function, frailty, and exercise capacity | ||||
| 4-m habitual gait speed, m/s, median (IQR) | Self-selected gait speed on 4-m track presented in meters per second | 0.92 (0.74–1.06) | 1.1 (1.0–1.2) | p = 0.055 |
| Short Physical Performance Battery, total, median (IQR) | Physical function assessment (0–12), higher score indicating better function | 11 (9–12) | 12 (12–12) | p = 0.200 |
| Clinical frailty scale, mean ± sd | Clinician scored assessment of physical frailty (1 very fit to 9 terminally ill) | 3.6 ± 1.0 | 2.2 ± 0.8 | p = 0.138 |
| 6MWD, m, mean ± sd | Raw distance and percentage achieved on 6-min walk test | 393 ± 84 | 606 ± 103 | p < 0.001 |
| Percent achieved of predicted 6MWD, %, mean ± sd | 74 ± 13 | 104 ± 15 | p < 0.001 | |
| TUG, s, median (IQR) | Lower (faster) times indicate better physical function | 7.8 (6.3–11.6) | 5.8 (5.3–6.9) | p = 0.078 |
| TUG-cognitive, median (IQR) | 9.8 (6.8–13.2) | 5.9 (5.4–7.1) | p = 0.057 | |
6MWD = 6-min walk distance; IQR = interquartile range, LE = lower extremity, TUG = timed up and go test.
Individuals in the control were median age of 60 yr (IQR, 46–62 yr), 29% female gender, median body mass index (BMI) of 31 kg/m2 (IQR, 30–31 kg/m2) ± 3 kg/m2 and median Charlson Comorbidity Index (CCI) 2 (IQR, 1–2). Statistically there were no differences in age, sex, and CCI between the two groups. Patients in COVID did have a higher BMI compared with control (p = 0.005).
Independent t test, Mann-Whitney U test or χ2 test to compare two groups based on distribution of data.
Higher score on Functional Assessment of Chronic Illness Therapy-fatigue subscale represents better quality of life with less subjective reports of fatigue.
Muscle Inflammation and Markers of Regeneration and Repair
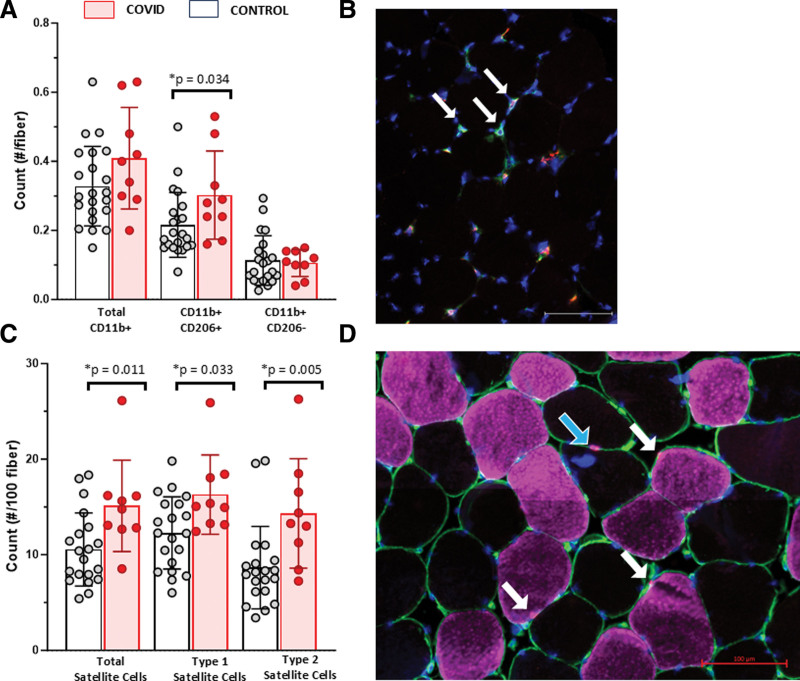
To characterize muscle inflammation and damage, we assessed CD16 (Fc gamma receptor IIIb)+ neutrophils/natural killer cells, CD11b+ macrophage populations (CD206+ or CD206–), regenerating fibers expressing embryonic myosin heavy chain (eMyHC), neural cell adhesion molecule positive (NCAM+) fibers, and SC abundance. We did not detect significant numbers of CD16+ cells. There was no difference in the number of total (CD11b+) or M1-like (CD206–) macrophages in muscle from COVID patients compared with controls, but M2-like (CD206+) macrophages were higher in COVID (Fig. 1, A and B). We observed negligible numbers of eMyHC+ and NCAM+ fibers in COVID samples. Muscles from COVID patients had greater overall as well as fiber type 1 and 2 specific SC abundance (Fig. 1, C and D); representative images are provided in Figure 1; and Supplemental Figure 2 (http://links.lww.com/CCX/B419).
Figure 1.
Greater CD206+ macrophages and satellite cell (SC) abundance in vastus lateralis muscle biopsies from patients recovering from critical COVID-19. Quantification of macrophage populations in muscle from COVID survivors (n = 9, blue) compared with controls (n = 22, white) including total CD11b+ macrophages, CD11b+CD206+ (M2-like) macrophages, and CD11b+CD206– (M1-like) macrophages (A) with representative images of macrophages in muscle from a COVID survivor (B; white arrows indicating CD11b+ CD206+). Quantification of paired box 7 (Pax7)+/4′,6-diamidino-2-phenylindole+ SCs in muscle from COVID survivors (n = 9, blue) compared with controls (n = 22, white) including total SCs, SCs associated with type 1 muscle fibers, and SCs associated with type 2 muscle fibers (C) with representative images of SCs (D; white and blue arrows indicating SC with type 1 and SC with type 2, respectively). Pax7 data from a subset within the control (n = 15) has been previously published (38). Data are expressed as mean ± sd; p values determined by Mann-Whitney U test; *significance p ≤ 0.05. Images acquired at ×200, scale bar = 100 µm.
Muscle Fiber Type and Size
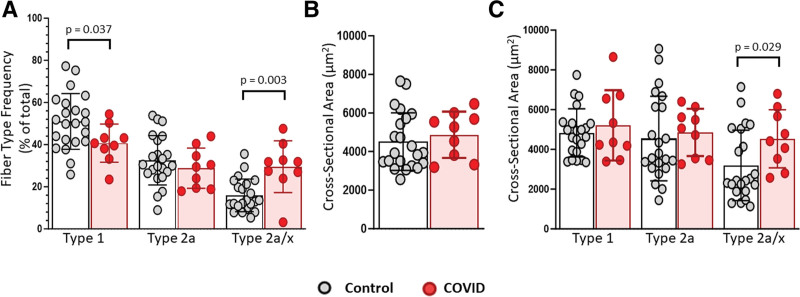
Representative images of myosin heavy chain (MyHC) and laminin are shown in Supplemental Figure 3 (http://links.lww.com/CCX/B419). Patients in COVID group had a lower percentage of type 1 and higher percentage of type 2a/x muscle fibers compared with controls (Fig. 2A). There were no differences in average (Fig. 2B) or type 1 or 2a specific (Fig. 2C) muscle fiber cross-sectional area but COVID had larger type 2a/x fibers compared with controls (Fig. 2C).
Figure 2.
Altered fiber type frequency and fiber size in vastus lateralis muscle from critical COVID. Quantification of fiber type and fiber size within biopsies from COVID patients (n = 9) compared with controls (n = 22). Data from a subset within the community dwelling adults (n = 15) has been previously published (38). A, Bar and dot plots showing frequency of total muscle fibers expressing myosin heavy chain (MyHC) type 1, MyHC type 2a, or both MyHC 2a and MyHC 2x (2a/x hybrids). B, Mean fiber cross-sectional area (CSA) measured using MyoVision (39). C, Fiber type specific (type 1, type 2a, or type 2a/x) CSA. Data are expressed as mean ± sd; p values determined by Mann-Whitney U test; *significance p ≤ 0.05.
Muscle Mitochondrial Enzyme Activity in COVID Patients
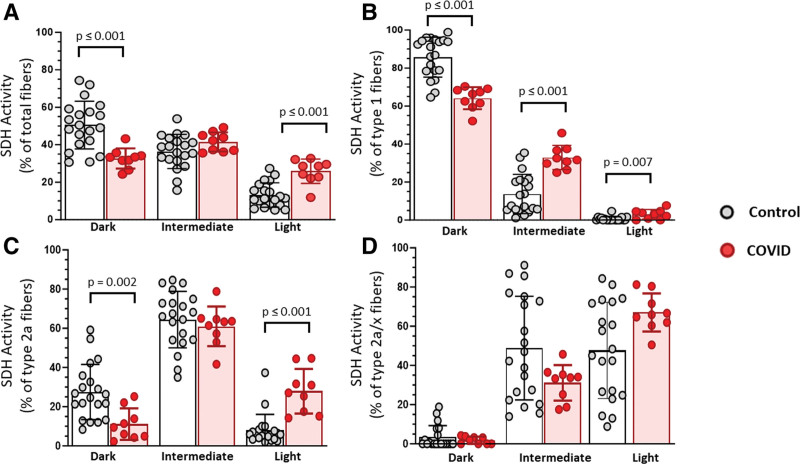
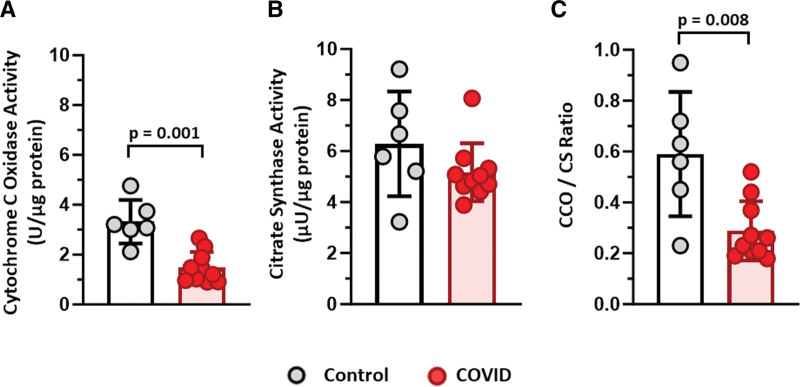
To assess muscle mitochondrial activity, we used SDH histochemistry in conjunction with immunohistochemistry for MyHC expression (Supplemental Fig. 4, http://links.lww.com/CCX/B419). Muscle from COVID exhibited a lower percentage of SDH dark fibers and higher percentage of SDH light fibers compared with controls, indicating lower muscle mitochondrial enzyme activity (Fig. 3A). Also, mitochondrial enzyme activity was lower in both type 1 (Fig. 3B) and 2a (Fig. 3C) fibers. No differences were observed within type 2a/x muscle fibers (Fig. 3D). COVID had lower cytochrome c oxidase (CCO) activity than controls (Fig. 4A), but no differences were observed in citrate synthase (CS) activity (Fig. 4B); the ratio between CCO and CS was significantly lower in muscle from COVID compared with control, indicating lower mitochondrial enzyme activity (Fig. 4C).
Figure 3.
Altered mitochondrial activity across fiber types within COVID muscle compared with controls. A, Quantification of total muscle fibers with dark (high mitochondrial activity), intermediate, or light (low mitochondrial activity) succinate dehydrogenase (SDH) histochemistry. Quantification of SDH histochemistry within individual fiber types: (B) type 1, (C) type 2a, and (D) hybrid type 2a/x. Quantification within vastus lateralis muscle biopsies from COVID patients (n = 9) or controls (n = 20). Data are expressed as mean ± sd; p values determined by Mann-Whitney U test; *significance p ≤ 0.05.
Figure 4.
Muscle from survivors of critical COVID have reduced cytochrome c oxidase (CCO) activity but no change in citrate synthase (CS) compared with controls. A, Quantification of CCO activity per microgram of protein. B, Muscle CS activity per microgram of protein. C, Reduced CCO activity per CS activity. A–C, Measured using muscle homogenates from COVID patients (n = 10) vs. controls (n = 6). Data are expressed as mean ± sd; p values determined by Mann-Whitney U test; *significance p ≤ 0.05.
Details about the selection of biomarkers and their biological significance are provided in Supplemental Table 5 (http://links.lww.com/CCX/B419).
Exploratory Correlative Testing
Significant correlations were observed between total number of SDH light stained fibers with 6MWT (rho = –0.79; p = 0.010) and FACIT-fatigue (rho = –0.76; p = 0.018; Supplemental Fig. 5, http://links.lww.com/CCX/B419); additional correlations are provided in Supplemental Table 6 (http://links.lww.com/CCX/B419).
DISCUSSION
Our findings suggest that metabolic alterations including reduced muscle mitochondrial activity with higher percentage of type 2a/x muscle fibers combined with higher abundances of M2-like macrophages associate with long-term physical impairments after ICU admission for COVID-19. The cellular finding of increased M2-like macrophages which are important for tissue remodeling suggest a perturbated muscle repair occurring several months after hospital discharge. Our data in patients recovering from critical COVID-19 align with recent studies in individuals with long COVID without ICU admission (11–13). Of clinical importance, muscle mitochondrial deficits in our patients associated with fatigue and physical function. Biological data from muscle tissue of critical COVID survivors demonstrate prolonged deficits with the clinical need for long-term assessment and treatment to improve patient-centered outcomes.
Patients surviving critical illness, regardless of etiology, are at high risk of fatigue (5, 40) and deficits in physical function (5, 40, 41). Data from our study are consistent with prior research demonstrating that 20–33% of ICU survivors have ICUAW (42) and poor performance on 6MWT (27) at short-term follow-up. Patients in our study had a high ICU admission acuity level with APACHE-II score of 24 indicating a ~40% risk of in-hospital mortality, which is similar to landmark studies of muscle and physical function recovery in non-COVID ICU survivors (41–43). A high prevalence of fatigue and lower functional scores are also consistent with previous studies of long COVID (11, 12, 14). Patients with long-COVID exhibited exercise intolerance coinciding with a higher proportion of glycolytic type 2x and 2ax muscle fibers and lower oxidative phosphorylation in permeabilized fibers (11, 12). A lower prevalence of type 1 and higher type 2a/x myofibers may also explain the high rates of fatigue reported in our study. Previous work demonstrate that muscle fibers shift from type 1 toward type 2 in metabolic disease (44) and during bedrest (45) explaining the biogenetic inefficiency (type 2 fibers produce lower amounts of adenosine triphosphate [ATP]). Fiber type shifts may also occur in response to targeted exercise training (46) and thus critical COVID survivors may benefit from long-term exercise strategies.
Muscle biopsies in survivors of critical illness due to mixed etiologies, demonstrated reduced SC content, increased collagen deposition, and muscle fiber atrophy (18). Our findings suggest that sustained atrophy does not solely explain functional deficits after critical COVID as mitochondrial alterations were strongly related to fatigue and performance in our patients even despite small sample sizes. Our study design prevents the ability to understand the time course of muscle alterations, but clinicians should recognize that both the acute illness phase and the post-hospital recovery phase likely contribute to long-term muscle deficits. Prolonged immobilization, reduced nutritional support, and an increase in inflammatory mediators in the acute phase of critical illness likely lead to oxidative stress and mitochondrial damage in muscle cells. Post-hospital discharge does not equate to restoration of muscle health as nutrition status (47) and physical activity (48) typically remain well below anticipated norms. Furthermore, previous work in the recovery phase of critical illness demonstrate alterations in mitochondrial biogenesis genes (19). Clinicians in ICU and post-hospital phases should screen for impairments related to PICS as well as long-COVID recognizing risk of prolonged fatigue resulting from muscle mitochondria dysfunction. Muscle mitochondrial health may respond to targeted interventions such as nutritional supplements (49) as well as targeted exercise, but studies are required in ICU and COVID survivors.
Muscle tissue from survivors of critical COVID demonstrated higher abundances of SCs and M2-like macrophages. Perturbations in muscle repair, through prolonged M2 macrophage inflammation might be responsible for the distinct response in critical COVID, such as observed in chronic disease or injury (50). Previous data in patients surviving critical illness reveal high prevalence of inflammation at ICU discharge, but less is known in the recovery phase (51). Inflammation and elevated reactive oxygen species initiate a chain reaction leading to mitochondrial dysfunction and ATP depletion resulting in an inability for cells to restore energy levels with ensuing structural damage (17). Research attention should be placed on understanding the relationship between elevated and prolonged inflammatory state from critical illness with the downstream impact on muscle function. Clinicians should recognize that targeted therapeutic interventions aim at reducing or controlling the time course of inflammation may influence short- and long-term outcomes.
Our study is not without limitations. We selected time points based on the standard of care in the ICU Recovery Clinic with an extended grace time for muscle biopsy. Sample size may limit the strength of our study, especially since critical illness presents several mediators and confounders. However, to maintain rigor in our statistical approach, we used two models for regression analyses with a priori variable selection to enhance clinical interpretability. Still, the regression analyses should be interpreted with caution given the potential for representation and selection biases in critical COVID survivors. Correlative tests are at risk of type I error due to multiple comparisons, and type II error due to small sample sizes in biopsy cohort. Sample sizes undergoing biopsies could be considered small, but comparable to prior studies in this field (18, 19). In addition, consideration must be given to the differences in time to biopsy as other studies have reported inflammatory infiltrates and signs of muscle repair at earlier timepoints. It should be noted that the study did not compare individuals with an ICU admission for non-COVID to those with COVID; therefore, we cannot conclude that the observed deficits are related to COVID-19 alone and not due to any generalized critical illness requiring ICU admission.
CONCLUSIONS
Survivors of critical COVID-19 have a high prevalence of weakness, muscle power deficits, fatigue, and impaired functional exercise capacity. Our data suggest that altered muscle mitochondrial activity associates with long-term physical impairments after an ICU admission for COVID-19.
ACKNOWLEDGMENTS
We thank the patients and subjects for their generosity in participating in research. We would also like to thank the ICU Recovery Clinic team at University of Kentucky for their participation and engagement of clinical research. Finally, we would like to thank the University of Kentucky Center for Muscle Biology for providing muscle biopsy tissues from community dwelling adults for immunohistochemical and histochemical analyses.
Supplementary Material
Footnotes
The project was supported by the Pilot Grant Program of the Office of Research and Scholarship of the College of Health Sciences, University of Kentucky. The project was supported by the National Institutes of Health/National Center for Advancing Translational Sciences through grant number UL1TR001998.
Dr. Mayer was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health K23-AR079583. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute of General Medical Sciences of the National Institute of Health R01AR081002. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All parts of the study were approved by the Institutional Review Board (IRB), Medical IRB at the University of Kentucky, and the study was performed in accordance with the Helsinki Declarations.
Informed consent was waived for patients in the clinical cohort as data collected were considered routine clinical care (MED-expedited No. 47751). Cohorts participating in biopsy provided written informed consent (MED-full No. 46072).
Minimum datasets are provided in the article and Supplemental Methodology File (http://links.lww.com/CCX/B419). Raw data and associated materials may be requested for access by the corresponding author upon reasonable request.
Drs. Mayer, Kosmac, Dupont-Versteegden, and Morris were involved in study conception and design. Drs. Mayer, Kalema, and Montgomery-Yates were involved in clinical assessment. Drs. Kalema and Kern were involved in muscle biopsies. Dr. Mayer, Dr. Ismaeel, Mr. Starck, and Dr. Kosmac were involved in muscle data collection, analysis, and interpretation. Dr. Mayer and Ms. Slone were involved in clinical data analysis and interpretation. Drs. Mayer and Kosmac were involved in drafting article. Drs. Kern, Dupont-Versteegden, and Kosmac were involved in critical revision of the article. All authors reviewed and approved the article before submission. Dr. Morris current appointment is Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL. Dr. Kosmac current appointment is Department of Physical Therapy, Augusta University, Augusta, GA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Ahmed Ismaeel, Email: ahmed.ismaeel@uky.edu.
Anna G. Kalema, Email: anna.kalema@uky.edu.
Ashley A. Montgomery-Yates, Email: ashley.montgomery@uky.edu.
Melissa K. Soper, Email: melissa.soper@uky.edu.
Philip A. Kern, Email: pake222@uky.edu.
Jonathan D. Starck, Email: jdst287@uky.edu.
Stacey A. Slone, Email: stacey.slone@uky.edu.
Peter E. Morris, Email: pmorris@uabmc.edu.
Esther E. Dupont-Versteegden, Email: esther.dupont@uky.edu.
Kate Kosmac, Email: kkosmac@augusta.edu.
REFERENCES
- 1.Medrinal C, Prieur G, Bonnevie T, et al. : Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol 2021; 21:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Aerde N, Van den Berghe G, Wilmer A, et al. ; COVID-19 Consortium: Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med 2020; 46:2083–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Núñez-Seisdedos MN, Lázaro-Navas I, López-González L, et al. : Intensive care unit-acquired weakness and hospital functional mobility outcomes following invasive mechanical ventilation in patients with COVID-19: A single-centre prospective cohort study. J Intensive Care Med 2022; 37:1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, et al. : 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021; 397:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latronico N, Peli E, Calza S, et al. ; LOTO Investigators: Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 2022; 77:300–303 [DOI] [PubMed] [Google Scholar]
- 6.Dodig D, Tarnopolsky MA, Margeta M, et al. : COVID-19-associated critical illness myopathy with direct viral effects. Ann Neurol 2022; 91:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooper JE, Uner M, Priemer DS, et al. : Muscle biopsy findings in a case of SARS-CoV-2-associated muscle injury. J Neuropathol Exp Neurol 2021; 80:377–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Z, de Vries HJ, Vlaar APJ, et al. ; Dutch COVID-19 Diaphragm Investigators: Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med 2021; 181:122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disser NP, De Micheli AJ, Schonk MM, et al. : Musculoskeletal consequences of COVID-19. JBJS 2020; 102:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschman T, Schneider J, Greuel S, et al. : Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol 2021; 78:948–960 [DOI] [PubMed] [Google Scholar]
- 11.Appelman B, Charlton BT, Goulding RP, et al. : Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun 2024; 15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colosio M, Brocca L, Gatti MF, et al. : Structural and functional impairments of skeletal muscle in patients with postacute sequelae of SARS-CoV-2 infection. J Appl Physiol (1985) 2023; 135:902–917 [DOI] [PubMed] [Google Scholar]
- 13.Hejbøl EK, Harbo T, Agergaard J, et al. : Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur J Neurol 2022; 29:2832–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschman T, Wyler E, Baum O, et al. : Post-COVID exercise intolerance is associated with capillary alterations and immune dysregulations in skeletal muscles. Acta Neuropathol Commun 2023; 11:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Andrade-Junior MC, de Salles ICD, de Brito CMM, et al. : Skeletal muscle wasting and function impairment in intensive care patients with severe COVID-19. Front Physiol 2021; 12:640973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, et al. : Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care 2020; 24:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latronico N, Friedrich O: Electrophysiological investigations of peripheral nerves and muscles: A method for looking at cell dysfunction in the critically ill patients. Crit Care 2019; 23:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dos Santos C, Hussain SN, Mathur S, et al. ; MEND ICU Group: Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med 2016; 194:821–830 [DOI] [PubMed] [Google Scholar]
- 19.Walsh CJ, Batt J, Herridge MS, et al. : Transcriptomic analysis reveals abnormal muscle repair and remodeling in survivors of critical illness with sustained weakness. Sci Rep 2016; 6:29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris PE, Berry MJ, Files DC, et al. : Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: A randomized clinical trial. JAMA 2016; 315:2694–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss M, Nordon-Craft A, Malone D, et al. : A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med 2016; 193:1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgson CL, Bailey M, Bellomo R, et al. ; TEAM Study Investigators and the ANZICS Clinical Trials Group: Early active mobilization during mechanical ventilation in the ICU. N Engl J Med 2022; 387:1747–1758 [DOI] [PubMed] [Google Scholar]
- 23.Taito S, Yamauchi K, Tsujimoto Y, et al. : Does enhanced physical rehabilitation following intensive care unit discharge improve outcomes in patients who received mechanical ventilation? A systematic review and meta-analysis. BMJ Open 2019; 9:e026075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer KP, Boustany H, Cassity EP, et al. : ICU recovery clinic attendance, attrition, and patient outcomes: The impact of severity of illness, gender, and rurality. Crit Care Explor 2020; 2:e0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jonghe B, Sharshar T, Lefaucheur JP, et al. ; Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation: Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA 2002; 288:2859–2867 [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, et al. : A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 27.Parry SM, Nalamalapu SR, Nunna K, et al. : Six-minute walk distance after critical illness: A systematic review and meta-analysis. J Intensive Care Med 2021; 36:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritchard J, Kennedy C, Karampatos S, et al. : Measuring frailty in clinical practice: A comparison of physical frailty assessment methods in a geriatric out-patient clinic. BMC Geriatr 2017; 17:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockwood K, Song X, MacKnight C, et al. : A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Needham DM, Sepulveda KA, Dinglas VD, et al. : Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med 2017; 196:1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yellen SB, Cella DF, Webster K, et al. : Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13:63–74 [DOI] [PubMed] [Google Scholar]
- 32.Bohannon RW: Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys Ther 1986; 66:206–209 [DOI] [PubMed] [Google Scholar]
- 33.Parry SM, Berney S, Granger CL, et al. : A new two-tier strength assessment approach to the diagnosis of weakness in intensive care: An observational study. Crit Care 2015; 19:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer KP, Welle MM, Evans CG, et al. : Muscle power is related to physical function in patients surviving acute respiratory failure: A prospective observational study. Am J Med Sci 2021; 361:310–318 [DOI] [PubMed] [Google Scholar]
- 35.Morelli N, Parry SM, Steele A, et al. : Patients surviving critical COVID-19 have impairments in dual-task performance related to post-intensive care syndrome. J Intensive Care Med 2022; 37:890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarnopolsky MA, Pearce E, Smith K, et al. : Suction-modified Bergström muscle biopsy technique: Experience with 13,500 procedures. Muscle Nerve 2011; 43:717–725 [DOI] [PubMed] [Google Scholar]
- 37.Kosmac K, Peck BD, Walton RG, et al. : Immunohistochemical identification of human skeletal muscle macrophages. Bio Protoc 2018; 8:e2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noehren B, Kosmac K, Walton RG, et al. : Alterations in quadriceps muscle cellular and molecular properties in adults with moderate knee osteoarthritis. Osteoarthritis Cartilage 2018; 26:1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen Y, Murach KA, Vechetti IJ, Jr, et al. : MyoVision: Software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 2018; 124:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martillo MA, Dangayach NS, Tabacof L, et al. : Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: Cohort study from a New York City critical care recovery clinic. Crit Care Med 2021; 49:1427–1438 [DOI] [PubMed] [Google Scholar]
- 41.Herridge MS, Tansey CM, Matte A, et al. ; Canadian Critical Care Trials Group: Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364:1293–1304 [DOI] [PubMed] [Google Scholar]
- 42.Fan E, Dowdy DW, Colantuoni E, et al. : Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Crit Care Med 2014; 42:849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herridge MS, Cheung AM, Tansey CM, et al. ; Canadian Critical Care Trials Group: One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003; 348:683–693 [DOI] [PubMed] [Google Scholar]
- 44.Oberbach A, Bossenz Y, Lehmann S, et al. : Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 2006; 29:895–900 [DOI] [PubMed] [Google Scholar]
- 45.Gallagher P, Trappe S, Harber M, et al. : Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand 2005; 185:61–69 [DOI] [PubMed] [Google Scholar]
- 46.Plotkin DL, Roberts MD, Haun CT, et al. : Muscle fiber type transitions with exercise training: Shifting perspectives. Sports (Basel) 2021; 9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jubina LE, Locke A, Fedder KR, et al. : Nutrition in the intensive care unit and early recovery influence functional outcomes for survivors of critical illness: A prospective cohort study. JPEN J Parenter Enteral Nutr 2023; 47:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gandotra S, Lovato J, Case D, et al. : Physical function trajectories in survivors of acute respiratory failure. Ann Am Thorac Soc 2019; 16:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Amico D, Olmer M, Fouassier AM, et al. : Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell 2022; 21:e13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tidball JG: Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 2017; 17:165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffith DM, Vale ME, Campbell C, et al. : Persistent inflammation and recovery after intensive care: A systematic review. J Crit Care 2016; 33:192–199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.