Abstract
OBJECTIVE
Information regarding pregabalin use is limited. We aimed to assess the outcome of the patients who have taken pregabalin at a certain time during their pregnancies.
METHODS
31 patients used pregabalin treatment during pregnancy and 93 control patients were included in the study who applied to hospital between the years 2013–2022. In this multicenter case-controlled study, the outcome of the pregnancies and the health condition of the newborn in the pregabalin and control groups were evaluated.
RESULTS
Preterm delivery rates (5/27 (18.5%) vs. 5/87 (5.7%); OR 0.26, 95% CI 0.07–1.01, p=0.04) and lower birth weight (6/27 (22.2%) vs. 5/81 (6.6%); OR 4.34, 95% CI 1.20–15.65, p=0.016) were found higher in the pregabalin group compared to the control group. However, significant difference was not found between the birth dates of babies in pregabalin and control groups with the log-rank test (p=0.30). Spontaneous abortion rates were not significantly different (2/31 (6.4%) vs. 4/93 (4.3%); OR 1.52, 95% CI 0.26–8.72, p=0.63). Although major malformation rates in pregabalin were higher than those in controls (3/27 (11%) vs. 4/88 (4.5%); OR 0.38, 95% CI 0.07–1.82, p=0.21) the outcomes were statistically insignificant.
CONCLUSION
Preterm delivery rates and lower birth weight were higher in pregabalin group. Also, it should not be ignored that chronic diseases of the pregnant women in pregabalin group may affect the outcomes poorly.
Keywords: Medication, pregabalin, pregnancy, safety
Highlight key points
The teratogenic effects of pregabalin use during pregnancy is limited.
Preterm delivery rates and lower birth weight was associated with pregabalin use.
Increase in congenital malformation was not detected in pregabalin group.
It should be noted that chronic diseases in pregabalin group may affect the pregnancy outcomes.
Pregabalin is a gabapentinoid that acts by inhibiting calcium channels [1]. Binding to the α2δ subunit site of the voltage-dependent calcium channels appears to be responsible for its therapeutic effects [2]. Pregabalin is used for treatment of epilepsy, neuropathic pain, fibromyalgia, and generalized anxiety disorder as an anticonvulsant and anxiolytic agent [3, 4]. Off-label uses of pregabalin include social anxiety disorder, bipolar disorder, insomnia, chronic pain conditions and the prophylaxis of chronic migraine. It was approved by the United States Food and Drug Administration in 2004 for painful diabetic neuropathy and postherpetic neuralgia [5].
The precise molecular mechanisms underlying the potential teratogenic mechanisms of pregabalin are not fully understood and there is limited research on this area. However, there are some factors that may be responsible for potential teratogenicity of pregabalin. Pregabalin mainly exerts its pharmacological effects by binding to the α2δ subunit site of the voltage-dependent calcium channels, this effect may disrupt the levels of neurotransmitters which may regulate fetal brain development. Additionally, pregabalin treatment can alter calcium influx in neurons and other cells and this mechanism also may interfere with normal fetal development [6]. Especially this α2δ subunit is expressed in high amounts in skeletal, cardiac, vascular smooth muscles and in the brain [7]. There is also knowledge that pregabalin may alter the electron transport chain which may cause decrease in ATP generations in neurons [8]. This mechanism may also contribute to the neurological teratogenic effect of pregabalin. The studies evaluating pregnancy outcomes have found cardiac defects such as atrial and ventricular septal defect and neurologic defects which were similar to the findings of preclinical studies [9, 10].
Pregnancy demands a delicate balance between ensuring the health and well-being of the mother while safeguarding the development and safety of the child. As the use of pregabalin increases in clinical practice, questions about its safety and efficacy during pregnancy have arisen. Although pregabalin is commonly used in clinical practice, knowledge about its use during pregnancy is scarce and limited to small case series and individual case reports. Here we present the outcome of pregnancies after the use of pregabalin. In this study, the outcomes of pregnancies with pregabalin exposure were evaluated and compared to the control group. It was aimed to assess the safety of pregabalin and reveal the potential risks to baby which may arise with pregabalin use in pregnancy.
MATERIALS AND METHODS
In this multicenter case-controlled study the outcome of pregnancies in patients who used pregabalin medication during any stage of their pregnancy between 2013–2022 were evaluated and assessed for the teratogenic risk profile of pregabalin exposure during pregnancy. The data of the patients were acquired from the Marmara University Faculty of Medicine. Health information of the patients was acquired from the health recordings and through telephone from the infants’ mothers. Verbal and written consent were acquired from the participants before the assessment of necessary information. The study was conducted in accordance with the WMA Declaration of Helsinki ethical principles. Ethical approval was obtained from Marmara University Faculty of Medicine (date: 03.02.2023, number: 09.2023.424).
Maternal characteristics of the patients (maternal age, tobacco use, alcohol consumption, body mass index (BMI), time of drug exposure and medical conditions) and information on pregabalin and other drug exposure were included in the analysis. Data on pregnancy outcomes (live birth rates, spontaneous abortion rates, medical termination of pregnancies, major birth defects (MBD), preterm deliveries, birthweight of the infants) were also evaluated. Pregnancy outcomes of the pregabalin exposure group were compared with the data of pregnant women who did not use pregabalin (control group). The patients in the control group were selected among patients who applied to pharmacology outpatient clinic and were exposed to drugs in the lower-risk category such as penicillin group antibiotics and paracetamol-like analgesics. Also, patients who underwent radiography examination were included in the control group. The exclusion criteria for both groups were to use a known major teratogenic drug during pregnancy. Randomly selected control group was matched with the pregabalin exposure group at a 3:1 ratio.
Statistical Analysis
Pregnancies resulting in live births, spontaneous abortion rates, medical termination of pregnancies, major birth defects, preterm deliveries, low birth weight among the infants were evaluated using Fisher’s exact test and chi-square test. Continuous data that did not follow normal distribution were evaluated with Mann-Whitney U test. The log-rank test was used for determination of survival rates. The statistical analysis was performed with GraphPad Prism 8.0 program (GraphPad Software, LLC, Boston, MA, 02110 USA).
Results
The analysis comprised 31 patients who had taken pregabalin and 93 patients in the control group, and maternal characteristics are shown in Table 1. The BMI was found to be higher in pregabalin group compared to control group (28.2 (24.4–31.2) vs. 26.4 (22.4–30.0), p=0.0096). The indications of pregabalin use in the patient group were neuropathic pain in 19 patients (61.2%), fibromyalgia in 3 patients (9.7%) and epilepsy in 4 patients (12.9%). First-trimester exposure included 26 patients (81%) in the pregabalin group whereas, 77 patients (82.7%) in the control group. The pregabalin dosage of the patient group varied between 75 mg and 600 mg a day and the most common daily dosage taken by patients was 300 mg. (29.1%).
TABLE 1.
Maternal characteristics
| Characteristics | Pregabalin group (n=31) | Control group (n=93) | p |
|---|---|---|---|
| Maternal age, median (IQR) | 34.1 (31–37) | 32.8 (30–35.5) | 0.17 |
| Tobacco use, (%) | 12.5 | 10.5 | 0.74 |
| Alcohol consumption, n (%) | – | – | – |
| BMI, median (IQR) | 28.2 (24.4–31.2) | 26.4 (22.4–30.0) | 0.0096* |
| Time of exposure | 0.95 | ||
| 1st trimestr, (%) | 83.8 | 82.7 | |
| 2nd trimestr (%) | – | 3.2 | |
| 1st and 2nd trimestr (%) | 3.3 | 3.2 | |
| All trimestrs (%) | 9.6 | 6.4 | |
| Unknown time of exposure (%) | 3.3 | 4.5 | |
| Medical conditions (%) | |||
| Neuropathic pain | 61.2 | 1 | |
| Fibromyalgia | 9.7 | – | |
| Epilepsy | 12.9 | – | |
| Migraine | – | 1 | |
| Psychiatric conditions | 3.2 | 15 | |
| Hypothyroidism | 3.2 | – | |
| Asthma | 3.2 | 2.1 | |
| Restless leg syndrome | 3.2 | – | |
| Drug abuse | 3.2 | – | |
| Trigeminal neuralgia | 3.2 | – |
: P<0.05; BMI: Body mass index; IQR: Interquartel ratio; a: Other than pregabalin.
Pregnancy outcomes are presented in Table 2. MBD rates between pregabalin group and control group were found statistically insignificant (3/27 (11%) vs. 4/88 (4.5%); odd ratio (OR) 0.38, 95% confidence interval (CI) 0.07–1.82, p=0.21). When the MBDs in the pregabalin group were evaluated, one infant was diagnosed with autism spectrum disorder, one infant had choroid plexus cyst in which there was a need for surgical intervention and in another infant thyroid gland agenesis and retinopathy were detected. The MBDs in the control group included ankyloglossia in one newborn that required surgery, a lymphangioma found in the infant’s dorsal region, renal agenesis and proteinuria found in third infant, tonic-clonic epilepsy and mental retardation detected in the fourth infant.
TABLE 2.
Pregnancy outcome
| Pregabalin (n=31) | Controls (n=93) | OR (CI) | p | |
|---|---|---|---|---|
| Pregnancies resulting in live birth, n (%) | 27 (87.1) | 88 (94.6) | 0.38 (0.09–1.5) | 0.22 |
| Spontaneous abortions, (%) | 6.4 | 4.3 | 1.52 (0.26–8.72) | 0.63 |
| Medical termination of pregnancy, (%) | 6.4 | 1.1 | 0.30 (0.04–2.25) | 0.22 |
| Major birth defects, (%) | 11 | 4.5 | 0.38 (0.07–1.82) | 0.21 |
| Preterm deliveries, (%) | 18.5 | 5.7 | 0.26 (0.07–1.01) | 0.04* |
| Gestational age at birth, week, median (IQR) (n=26; 86) | 39 (37–39) | 39 (38–40) | – | 0.53 |
| Birthweight, g, median (IQR) (n= 27; 81) | 3121 (2800–3580) | 3400 (2950–3600) | – | 0.11 |
| Low birth weight, (n=27; 81) (%) | 6/27 (22.2) | 5/81 (6.6) | 4.34 (1.20–15.65) | 0.016* |
: P<0.05; OR: Odds ratio; CI: Confidence interval; IQR: Interquartel ratio; Low birth weight defined as <2500 g. during delivery, Preterm delivery defined as birth <37 weeks
The rate of preterm deliveries was found 18.5% in the pregabalin group and 5.7% in the controls. Preterm delivery can be defined as the birth of the baby before 37 weeks of pregnancy have been completed [11]. The risk for preterm deliveries was found to be increased with pregabalin use according to chi-square analysis (OR 0.26, 95% CI 0.07–1.01, p=0.04). However, a significant difference was not found between the birth times of the pregabalin and the control groups when the results were evaluated with the log-rank test (p=0.30) (Fig. 1). The rate of low birth weight was found to be higher in the pregabalin group than in the control group (6/27 (22.2%) vs. 5/81 (6.6%); OR 4.34, 95% CI 1.20–15.65, p=0.016).
FIGURE 1.
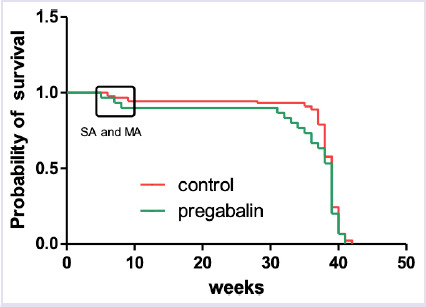
Gestational weeks of birth in both groups. Overall survival of pregnancy over the weeks. Red line indicates control group and green line indicates pregabalin group. The marked place on the graph shows the weeks of spontaneous and medical abortus.
The low birth weight limit was accepted as 2500 g according to the World Health Organization guidelines [12]. Pregnancies resulting in live birth were higher in the control group than in the pregabalin group (87.1% vs. 94.6%); however, the difference between these groups did not have statistical significance (OR 0.38, 95% CI 0.09–1.5, p=0.22).
Spontaneous abortus rates were evaluated between pregabalin and control group. It was found that the rate of spontaneous abortions in pregnant women was higher in the pregabalin group compared to the control group (6.4% vs. 4.3%). However, when the results were evaluated statistical significance was not found between the groups (2/31 (6.4%) vs. 4/93 (4.3%); OR 1.52, 95% CI 0.26–8.72, p=0.63).
Discussion
In this study, we evaluated the rates of major birth defects in pregabalin-exposed pregnants (n=31) compared to control group (n=93). In addition, rates of live births, spontaneous abortions, medical terminations of pregnancies, preterm deliveries, birthweights and gestational age at birth were evaluated.
In pregabalin group the mother of the infant with choroid plexus cyst had epilepsy and received pregabalin 150 mg daily throughout her pregnancy. Also, this pregnant woman was prescribed phenytoin in addition to pregabalin. Phenytoin is a known teratogen that may cause anomalies in the nervous system [13, 14]. This may be a contributing factor to the central nervous system anomaly in the child. The other infant was diagnosed with autism spectrum disorder and the mother of the baby had used pregabalin in the first trimester of her pregnancy. This patient had uncontrolled hypocalcemia throughout her pregnancy and received calcium supplements, which may have also affected the outcome of her pregnancy. Pregabalin was administered at a daily dose of 375 mg to the mother of another infant with abnormalities during the first trimester for peripheral neuropathy. This baby had retinopathy and thyroid gland agenesis. Throughout her pregnancy, she also took various non-steroidal anti-inflammatory drugs. The women whose babies had teratogenic outcomes were evaluated. It was detected that these pregnant women have different diseases that affect their health status, the use of different drugs other than pregabalin, and the fact that some of these drugs were known teratogenic drugs make it difficult to reach a definitive conclusion about the teratogenicity of pregabalin.
There have been reports that pregabalin may cause abnormal pregnancy outcomes [9, 13]. A potential association between pregabalin use and major birth defects was suggested but a definitive conclusion could not be obtained. In a study evaluating outcomes of pregabalin use in 30 pregnant patients, there were 7 miscarriages. Among 13 infants who had been exposed to pregabalin in the first trimester, ventricular septal defect was detected in one of the infants [10]. A database study that evaluated 477 pregnancy outcomes in which pregabalin was used during the first trimester found 5.9% malformations in pregabalin-exposed infants compared to 3.3% in non-exposed infants [14]. This difference was not found to be statistically significant, and it was concluded that pregabalin exposure was not linked with malformations. Neurodevelopment abnormalities after antiepileptic drug usage were evaluated in another study and it was stated that pregabalin exposure was not associated with neurodevelopment abnormalities [15]. In our study, the rate of major birth defects in the pregabalin group was statistically insignificant compared to control group. These findings support the studies which had found no significant increase in major congenital anomalies with pregabalin exposure.
Spontaneous abortion rates were found similar between two groups and our study indicates that pregabalin does not increase the risk of spontaneous abortus rates in pregabalin exposed group when compared with control group. In a study which evaluated the spontaneous abortus rates among the pregnancies exposed to pregabalin, spontaneous abortus rates were found statistically insignificant compared to unexposed group [10]. Winterfeld et al. [9] found higher rates of spontaneous abortus rates in pregabalin group compared to controls (15.1% vs. 8.5%) but the difference was statistically insignificant, the study concluded that pregabalin did not increase the spontaneous abortus rates. Research on the effects of pregabalin on spontaneous abortion is limited, and there is no strong consensus on the exact effects of pregabalin for spontaneous abortion. According to results of the conducted studies, similar to our results, pregabalin exposure during pregnancy does not cause a significant risk increase in spontaneous abortion rates.
Preterm delivery rates were found to be higher in pregabalin group compared to controls in our study using chi-square analysis. The preterm delivery rates were statistically insignificant with the log-rank test; however, we assume that the outcome of the log-rank test may be due to the small number of patients in our study. There is limited research and scant information regarding pregabalin’s impact on preterm deliveries. In a study evaluating the risk of premature birth in pregnant women using pregabalin, it was shown that the babies of pregnant women using pregabalin were born, on average, 1 day earlier than the reference group [16]. However, these results were not considered significant. In another study the preterm birth rate with pregabalin use was found 9.2% vs. 7.2% compared to controls and these results were statistically insignificant [9]. The studies that evaluated the effect of pregabalin on preterm deliveries found no significant relationship which is different from our findings. Although the results of our study are remarkable, chronic diseases in pregnant women using pregabalin may have affected the outcomes.
Pregabalin exposure was also associated with an increase in the risk of low birth weight in our study. However, there is a lack of evidence about the link between pregabalin use during pregnancy and low birth weight in infants and no definitive conclusion is made on this issue. In a report published by Toft et al. [17], the use of pregabalin monotherapy compared with unexposed had the similar prevalence ratio for low birth weight (95% CI, 1.06 (0.90–1.24)). In another study evaluating the effects of pregabalin on low birth weight the results compared to controls were found to be statistically insignificant (3,300 g. (3,000–3690) vs. 3,350 g. (3,030–3,660); p=not significant) [9]. Also in a study evaluating the teratogenic effects of pregabalin, it was not associated with an increase in low birth weight in infants [18]. Pregabalin exposure could be a risk for low birth weight according to our results contrary to previous studies but underlying maternal diseases may also have a role in this outcome.
One of the limitations of this trial was the heterogeneity of the features between the control and pregabalin groups. Disease-matched control group could be more useful in evaluating neonatal outcomes. Additionally, the information on neonatal outcomes was acquired from the medical records as well as through phone calls with the mother of the infants. Even if mothers were extensively questioned about the health conditions of the babies, there was a possibility of providing incomplete information due to memory errors, communication issues, and lack of knowledge. We also had limited data about folate use during pregnancy because folate use is recommended especially in pregnants with epilepsy and pregnants who use high-risk drugs such as antiepileptics and pregabalin which increase the risk of neural tube defects in the infants. Unfortunately, we had limited data about folate use of the patients in the health records so we couldn’t rule out the effects of folate use in the data of our patients.
Conclusion
In this study, it was found that pregabalin exposure in pregnancy was not associated with an increase in major birth defect rates. Low birth weight and preterm delivery rates were found significantly higher with pregabalin medication but it should not be ignored that concomitant drug use and chronic diseases of pregnant women may affect the outcomes poorly. More comprehensive studies are needed to further evaluate the use of pregabalin during pregnancy.
Acknowledgements
We thank Dr. Mustafa Sevim for statistical analysis and interpretation of the data.
Footnotes
Cite this article as: Kaskal M, Kuru B, Erkoseoglu I, Yilmaz H, Karadas B, Goren MZ. Evaluation of the teratogenic effects of pregabalin usage during pregnancy: A multicenter case-control study. North Clin Istanb 2024;11(5):460–465.
Ethics Committee Approval
The Marmara University Clinical Research Ethics Committee granted approval for this study (date: 03.02.2023, number: 09.2023.424).
Authorship Contributions
Concept – MK, MZG; Design – MK, BK, IE, MK, MZG, BK, MZG; Supervision – MK, MZG; Fundings – MK, BK, IE, HY, BK, MZG; Materials – MK, BK, IE, HY, BK, MZG; Data collection and/or processing – MK, BK, IE, HY, BK, MZG; Analysis and/or interpretation – MK, BK, IE, HY, BK, MZG; Literature review – MK, BK, IE, HY, BK, MZG; Writing – MK, BK, IE, HY, BK, MZG; Critical review – MK, BK, IE, HY, BK, MZG.
Conflict of Interest
No conflict of interest was declared by the authors.
Use of AI for Writing Assistance
Not declared.
Financial Disclosure
The authors declared that this study has received no financial support.
Peer-review
Externally peer-reviewed.
References
- 1.Uchitel OD, Di Guilmi MN, Urbano FJ, Gonzalez-Inchauspe C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels (Austin) 2010;4:490–6. doi: 10.4161/chan.4.6.12864. [DOI] [PubMed] [Google Scholar]
- 2.Calandre EP, Rico-Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother. 2016;16:1263–77. doi: 10.1080/14737175.2016.1202764. [DOI] [PubMed] [Google Scholar]
- 3.Onakpoya IJ, Thomas ET, Lee JJ, Goldacre B, Heneghan CJ. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9:e023600. doi: 10.1136/bmjopen-2018-023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frampton JE. Pregabalin: a review of its use in adults with generalized anxiety disorder. CNS Drugs. 2014;28:835–54. doi: 10.1007/s40263-014-0192-0. [DOI] [PubMed] [Google Scholar]
- 5.Verma V, Singh N, Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol. 2014;12:44–56. doi: 10.2174/1570159X1201140117162802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsanie WF, Alhomrani M, Gaber A, Habeeballah H, Alkhatabi HA, Felimban RI, et al. The effects of prenatal exposure to pregabalin on the development of ventral midbrain dopaminergic neurons. Cells. 2022;11:852. doi: 10.3390/cells11050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi I, Taylor CP. Pregabalin action at a model synapse: binding to presynaptic calcium channel alpha2-delta subunit reduces neurotransmission in mice. Eur J Pharmacol. 2006;553:82–8. doi: 10.1016/j.ejphar.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Sałat K, Librowski T, Nawiesniak B, Gluch-Lutwin M. Evaluation of analgesic, antioxidant, cytotoxic and metabolic effects of pregabalin for the use in neuropathic pain. Neurol Res. 2013;35:948–58. doi: 10.1179/1743132813Y.0000000236. [DOI] [PubMed] [Google Scholar]
- 9.Winterfeld U, Merlob P, Baud D, Rousson V, Panchaud A, Rothuizen LE, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology. 2016;86:2251–7. doi: 10.1212/WNL.0000000000002767. [DOI] [PubMed] [Google Scholar]
- 10.Mostacci B, Poluzzi E, D’Alessandro R, Cocchi G, Tinuper P. ESPEA Study Group. Adverse pregnancy outcomes in women exposed to gabapentin and pregabalin: data from a population-based study. J Neurol Neurosurg Psychiatry. 2018;89:223–4. doi: 10.1136/jnnp-2017-316143. [DOI] [PubMed] [Google Scholar]
- 11.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Global nutrition targets 2025: low birth weight policy brief. Available at: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.5. Accessed Sep 4, 2024. [Google Scholar]
- 13.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261:579–88. doi: 10.1007/s00415-013-7239-x. [DOI] [PubMed] [Google Scholar]
- 14.Patorno E, Bateman BT, Huybrechts KF, MacDonald SC, Cohen JM, Desai RJ, et al. Pregabalin use early in pregnancy and the risk of major congenital malformations. Neurology. 2017;88:2020–5. doi: 10.1212/WNL.0000000000003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blotière PO, Miranda S, Weill A, Mikaeloff Y, Peyre H, Ramus F, et al. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open. 2020;10:e034829. doi: 10.1136/bmjopen-2019-034829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margulis AV, Hernandez-Diaz S, McElrath T, Rothman KJ, Plana E, Almqvist C, et al. Relation of in-utero exposure to antiepileptic drugs to pregnancy duration and size at birth. PLoS One. 2019;14:e0214180. doi: 10.1371/journal.pone.0214180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toft G, Ehrenstein V, Assomaning K. A population-based cohort study of pregabalin to characterize pregnancy outcomes. Available at: https://catalogues.ema.europa.eu/node/2360/administrative-details. Accessed Sep 4 2024.
- 18.Dudukina E, Szépligeti SK, Karlsson P, Asomaning K, Daltveit AK, Hakkarainen K. Prenatal exposure to pregabalin, birth outcomes and neurodevelopment-a population-based cohort study in four Nordic countries. Drug Saf. 2023;46:661–75. doi: 10.1007/s40264-023-01307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


