Abstract
The objective of this analysis was to compare clinician‐based and formally calculated risk assessments by REVEAL Lite 2 and COMPERA 2.0 and to characterize parenteral prostacyclin utilization within 90 days of baseline in high‐risk patients. A multisite, double‐blind, retrospective chart review of patients with pulmonary arterial hypertension (PAH) was conducted with an index period of January 2014–March 2017. Patients were categorized into the “any PAH medication” or “prostacyclin‐enriched” cohort based on latest PAH medication initiated within the index period. Clinicians classified the patient's 1‐year mortality risk as “low,” “intermediate,” or “high” based on their clinical assessment. REVEAL Lite 2 and COMPERA 2.0 scores were independently calculated. Risk assessment congruency was evaluated. Parenteral prostacyclin use was evaluated within 90 days of baseline. Thirty‐two clinicians participated and abstracted data for 299 patients with PAH. At baseline, mean patient age was 52 years, 6‐min walk distance was 226 m, and most patients were WHO functional class II or III. Half of the patients (53%) were classified by clinician assessment as intermediate risk, while most were classified as high risk by REVEAL Lite 2 (59%) and intermediate‐high risk by COMPERA 2.0 (52%). Parenteral prostascyclins were underutilized in high‐risk patients, and not initiated in a timely fashion. Clinician‐assessed risk category was incongruent with tool‐based risk assessments in 40%–54% of patients with PAH, suggesting an underestimation of the patient's risk category by clinician gestalt. Additionally, there was a lack of timely prostacyclin initiation for patients with PAH stratified as high‐risk by either tool.
Keywords: COMPERA, prostacyclin, REVEAL Lite 2, risk assessment, treprostinil
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare, progressive, incurable disease that leads to right heart failure and often death. 1 After diagnosis of PAH, the median overall transplant‐free survival is 6.2 years. 2 To aid in guiding treatment decisions that can affect long‐term outcomes, individuals with PAH are stratified by disease characteristics that predict risk of 1‐year mortality. 3 , 4 , 5
Multiparametric tools, such as REVEAL Lite 2 risk calculator, have been developed to aid with risk stratification and include subjective and objective measurements. 6 Another risk tool, COMPERA 2.0, assigns an integer score to cut‐off values for three disease variables, and then stratifies patients into one of four risk groups. 7 These tools allow for a risk‐based, goal‐oriented treatment approach, where achieving or maintaining a low‐risk status is recommended. 4
The uptake of PAH risk assessment tools has been modest in clinical practice, with studies indicating that only about half of healthcare providers report using these tools routinely. 8 , 9 Without implementing a risk assessment tool, clinicians could misjudge the patient's actual risk profile, which can lead to suboptimal management of PAH. 10
Prostacyclin‐class therapy has been a key treatment for PAH due to the hallmark downregulation of the prostacyclin pathway in PAH. 11 Parenteral prostacyclin therapy may modify the disease pathophysiology and improve right heart structure and function, and is recommended as the treatment of choice for patients at high risk of mortality or with rapidly progressing PAH. 3 , 4 , 12 However, previous research has highlighted that parenteral prostacyclin therapy is underutilized, even in patients with more severe disease. 13 , 14
Here, we conducted a multicenter, double‐blind, retrospective chart review of patients with PAH treated in the United States. The objectives of this analysis were (1) to compare clinician‐based risk assessment to calculated REVEAL Lite 2 and COMPERA 2.0 risk assessments and (2) to characterize parenteral prostacyclin utilization within 90 days of baseline visit among patients classified as high risk by REVEAL Lite 2 and COMPERA 2.0.
METHODS
Study design
A multisite, double‐blind, retrospective chart review of patients with PAH treated in the United States was conducted in 2019–2020 by surveying PAH‐treating clinicians who treated at least 15 patients with PAH during the index period of January 2014–March 2017. The New England Independent Review Board approved the research, and a waiver of documentation of informed consent was obtained.
Thirty‐two clinicians were recruited for study participation. Once qualified to participate, clinicians and the assigned staff from each study site were provided with specific written instructions for the study, including patient selection criteria, rules and guidelines for randomly selecting and identifying patient charts, chart data abstraction, and data collection procedures. Each participating investigator completed a practice profile form to gather characteristics of clinicians.
Retrospective chart data were collected by investigators or their designee and abstracted directly from patient medical records via an electronic case report form, and a database was subsequently created. All data transferred to the sponsor/sponsor's representatives was deidentified and complied with the Health Insurance Portability and Privacy Act of 1996. Clinicians were instructed to assign and maintain a synthetic identifier for each patient record enrolled in the study that was to be used only for data validation and queries.
Patient eligibility criteria
Patients were randomly selected if they met the 5th World Symposium on Pulmonary Hypertension diagnostic criteria of PAH (mean pulmonary arterial pressure ≥25 mmHg, pulmonary artery wedge pressure ≤15 mmHg, pulmonary vascular resistance >3 Wood units) 15 ; began at least one FDA‐approved PAH treatment during the index period (January 2014–March 2017); were ≥18 years of age at initial PAH diagnosis; had assessments at baseline and at least one follow‐up visit for: N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) or brain natriuretic peptide (BNP), World Health Organization (WHO) functional class, and 6‐min walk distance (6MWD); and were not enrolled in any PAH‐related interventional clinical trial since the time of baseline assessments. Patients with sickle cell, sarcoidosis, CTEPH, and sleep apnea were excluded from this analysis. Baseline visit was defined as the most recent assessment before latest treatment initiation within the index period (January 2014–March 2017). Diagnosis was defined as the time of the patient's initial PAH diagnosis.
A two‐cohort study design was used to support data collection: (1) “Any PAH medication” cohort: a randomly selected cohort consisting of a sample of approximately 200 patients with PAH who met inclusion and exclusion criteria and started any PAH medication as the latest therapy initiated during the index period (including prostacyclins); (2) “Prostacyclin‐enriched” cohort: consisted of an oversample of approximately 100–125 patients with PAH who met inclusion criteria and were treated with a prostacyclin‐class agent (by any route of administration) as the latest therapy initiated during the index period and which continued for ≥90 days.
Risk assessment methodology
At the time of chart abstraction, clinicians were asked to classify the patient's risk of death in 1 year as “low,” “intermediate,” or “high” at baseline based on their clinical assessment. Subsequently, the REVEAL Lite 2 and COMPERA 2.0 scores were independently calculated by healthcare providers for each patient using data from the baseline visit. REVEAL Lite 2 calculates a patient's risk score based on: WHO functional class, systolic blood pressure, heart rate, 6MWD, BNP or NT‐proBNP, and renal insufficiency (by estimated glomerular filtration rate). Patients were assigned to low‐ (≤5 points), intermediate‐ (6 to 7 points), or high‐risk (≥8 points) groups. 6 COMPERA 2.0 calculates a patient's risk score based on: WHO functional class, 6MWD, and BNP or NT‐proBNP, giving a value of 1 to 4 for each variable based on prespecified cut points. Scores across the four variables were averaged, and patients were assigned to a risk category based on their average score: low risk <1.5, intermediate‐low risk = 1.5 to 2.49, intermediate‐high risk = 2.5 to 3.49, or high risk ≥3.5. 7
For patients classified as high risk by REVEAL Lite 2 or high/intermediate‐high risk for COMPERA 2.0, initiation of prostacyclin therapy within 90 days following baseline visit was then assessed in the two cohorts (“any PAH medication” vs. “prostacyclin‐enriched”) to understand the timing of prostacyclin treatment initiation. PAH‐specific treatments are described at and after baseline. At least 1 day of prostacyclin therapy needed to fall within the first 90 days after baseline, and prostacyclin therapy must have continued for ≥90 days (per cohort definition above).
Data analyses
For each patient, congruency of risk assessment was evaluated between clinician assessment and independent tool calculation. If the same risk category (“low,” “intermediate,” “high”) was assigned by both the clinician and the tool, the risk categories were considered congruent. For COMPERA 2.0 analyses, scores were considered congruent if the clinician assigned a patient “intermediate” risk and the COMPERA 2.0 tool assigned either “intermediate‐low” or “intermediate‐high.” Where applicable, “intermediate” risk assigned by REVEAL Lite 2 was considered equivalent to “intermediate‐low” and “intermediate‐high” risk strata assigned by COMPERA 2.0.
Summary statistics for continuous data are presented as median (interquartile range [IQR]) or mean (standard deviation [SD]), as appropriate. Summary statistics for categorical or ordinal data are presented as frequency and percentage. Where exact dates were not available, the first day of the month was entered (e.g., an event recorded as occurring on an unknown day in September 2016 was entered as 9/1/2016). Analyses were completed with SAS version 9.4 or higher (Cary, NC; SAS Institute, Inc.; 2011).
RESULTS
Clinician characteristics
Thirty‐two clinicians participated in the chart review. Most clinicians (88%) were male, and 78% were between 40 and 59 years of age (Supporting Information: Table S1). Seventeen clinicians (53%) reported a specialty of pulmonology, with the remaining clinicians reporting a specialty of cardiology. Thirteen clinicians (41%) were affiliated with a PAH comprehensive care center and 19 (59%) were affiliated with a PAH regional clinical program.
During the study period (2019–2020), 29 (91%) clinicians reported utilizing guidelines or protocols for the treatment of patients with PAH, with 66% following the 2019 American College of Chest Physicians (ACCP) guidelines and 59% following the 2015 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines.
Patient characteristics
Of the 299 patients with PAH identified in the study, 199 were included in the “any PAH medication” cohort and 100 were included in the “prostacyclin‐enriched” cohort (Figure 1). In the overall study population (N = 299), 66% of patients were female, and most (64%) had idiopathic PAH (Table 1). At baseline, the mean (SD) age was 52 (12) years, 6MWD was 226 (113) m, and most patients were classified as WHO functional class II (32%) or III (58%). The median (IQR) time from diagnosis to baseline was 2.1 (0.4–8.8) months.
Figure 1.
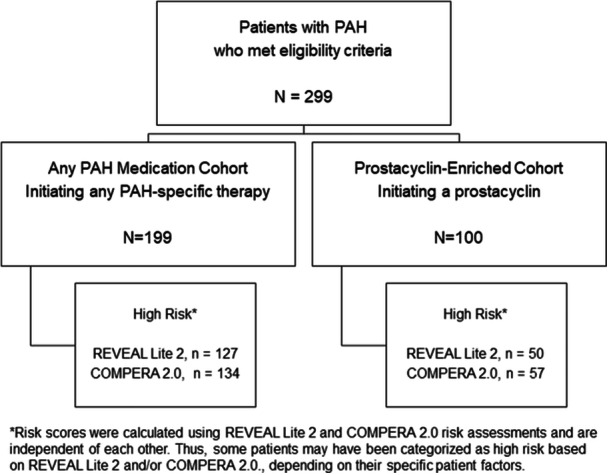
Study flow diagram for retrospective, double‐blinded review of patient records. A total of 299 patients with PAH were included in the study. Of the 299 patients with PAH, approximately one‐third were initiated on prostacyclin and two‐thirds were initiated on any PAH‐specific therapy as the latest treatment between January 2014 and March 2017. Risk scores were calculated using REVEAL Lite 2 and COMPERA 2.0 risk assessments and are independent of each other. Thus, some patients may have been categorized into different risk strata based on REVEAL Lite 2 and/or COMPERA 2.0, depending on their specific patient factors. PAH, pulmonary arterial hypertension.
Table 1.
Baseline and demographic characteristics.
| All patients (N = 299) | Any PAH medication cohort (N = 199) | Prostacyclin‐enriched cohort (N = 100) | |
|---|---|---|---|
| Time from diagnosis to baseline, months; median (Q1, Q3) | 2.1 (0.4, 8.8) | 2.2 (0.4, 8.0) | 2.0 (0.5, 11.4) |
| Age at baseline, years; mean (SD) | 51.6 (12.3) | 51.4 (12.9) | 51.9 (11.0) |
| Female, n (%) | 198 (66.2) | 132 (66.3) | 66 (66.0) |
| Etiology, n (%) | |||
| Idiopathic | 192 (64.2) | 124 (62.3) | 68 (68.0) |
| Heritable/familial associated | 21 (7.0) | 19 (9.6) | 2 (2.0) |
| Connective tissue disease | 56 (18.7) | 41 (20.6) | 15 (15.0) |
| Congenital heart disease | 7 (2.3) | 0 | 7 (7.0) |
| Portal hypertension | 11 (3.7) | 5 (2.5) | 6 (6.0) |
| Drugs/toxin‐induced | 4 (1.3) | 3 (1.5) | 1 (1.0) |
| HIV | 7 (2.3) | 6 (3.0) | 1 (1.0) |
| Schistosomiasis | 1 (0.3) | 1 (0.5) | 0 |
| Baseline NYHA/WHO functional class, n (%) | |||
| I | 8 (2.7) | 6 (3.0) | 2 (2.0) |
| II | 97 (32.4) | 71 (35.7) | 26 (26.0) |
| III | 172 (57.5) | 107 (62.2) | 65 (65.0) |
| IV | 22 (7.4) | 15 (7.5) | 7 (7.0) |
| Baseline 6MWD, in meters, mean (SD) | 225.9 (112.7) | 235.5 (110.1) | 206.8 (116.0) |
| Baseline BNP, n (%) | 179 (59.9) | 114 (57.3) | 65 (65.0) |
| Median, ng/L (IQR) | 299 (110–492) | 330 (125–475) | 226 (72–565) |
| Baseline NT‐proBNP, n (%) | 159 (53.1) | 89 (44.7) | 70 (70.0) |
| Median, ng/L (IQR) | 837 (261–1450) | 933 (360–1342) | 559 (90–1611) |
| Ongoing therapy at baseline, n (%) | |||
| No therapy at baseline | 180 (60.2) | 121 (60.8) | 59 (59.0) |
| PDE‐5 inhibitor, soluble guanylate cyclase stimulator | 101 (33.8) | 65 (32.7) | 36 (36.0) |
| Endothelin receptor antagonist | 89 (29.8) | 58 (29.1) | 31 (31.0) |
| Non‐parenteral prostacyclin | 7 (2.3) | 3 (1.5) | 4 (4.0) |
| Parenteral prostacyclin | 11 (3.7) | 5 (2.5) | 6 (6.0) |
Note: Baseline visit was defined as the most recent assessment before latest treatment initiation within the index period (January 2014–March 2017).
The medication cohorts were defined as: “any PAH medication” = a randomly selected cohort consisting of a sample of ~200 patients with PAH meeting inclusion criteria; “prostacyclin‐enriched” = an oversample (augment) sample of ~100–125 patients with PAH meeting inclusion/exclusion criteria treated with prostacyclin‐class agent as the latest therapy initiated during the index period for ≥90 days, approximately half of which received a parenteral prostacyclin‐class agent.
Abbreviations: 6MWD, 6‐min walk distance; BNP, brain natriuretic peptide; CTEPH, chronic thromboembolic pulmonary hypertension; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PDE, phosphodiesterase; SD, standard deviation; WHO, World Health Organization.
Risk assessment: REVEAL Lite 2
The majority of patients were categorized by clinician assessment as intermediate risk, while the majority were classified as high risk using REVEAL Lite 2. Clinicians classified 21% (62/299), 53% (158/299), and 26% (79/299) of patients to be at low, intermediate, or high risk of death in 1 year, respectively, per their clinical judgment (Figure 2a). Using REVEAL Lite 2, 17% (52/299), 23% (70/299), and 59% (177/299) of patients were classified as low, intermediate, and high risk.
Figure 2.
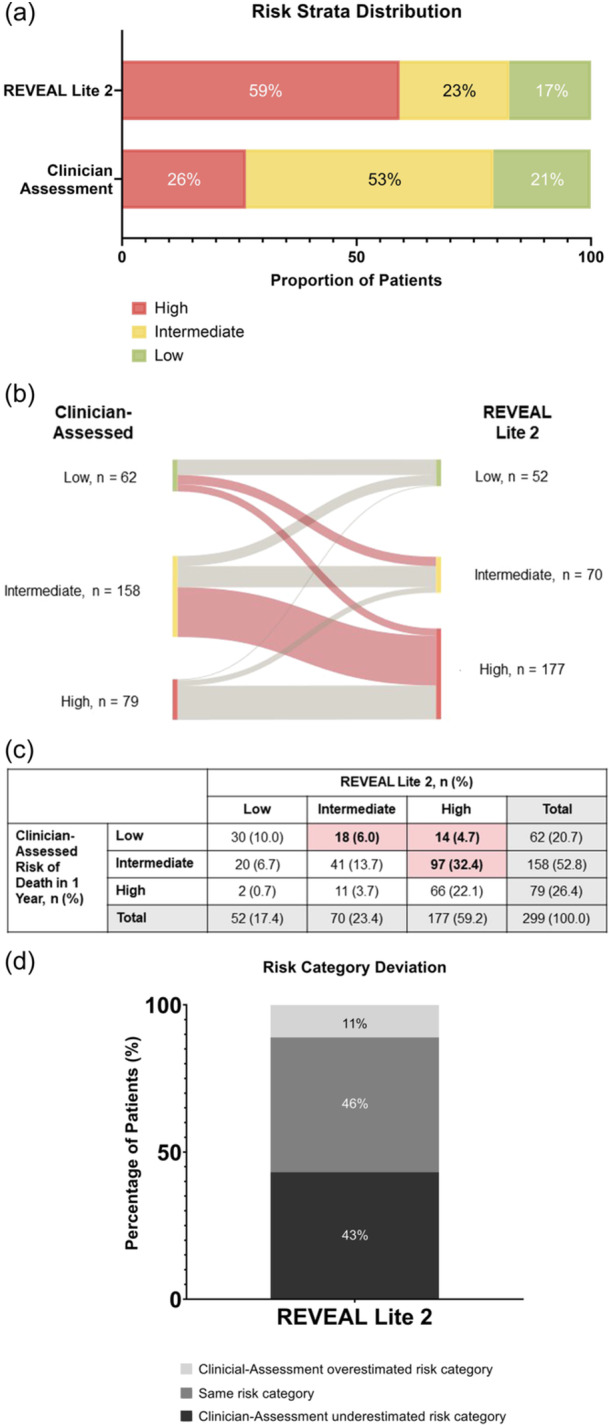
Risk strata distribution using REVEAL Lite 2 versus clinician assessment in total study population (N = 299). (a) The majority of patients were categorized as intermediate risk by clinician assessment. The majority of patients were categorized as high risk by REVEAL Lite 2. (b, c) Red shaded lines and boxes show patients whose risk level was underestimated by clinicians, relative to that calculated by REVEAL Lite 2. Risk was underestimated most commonly in clinician‐assessed intermediate patients. (d) Most risk assessments between clinician gestalt and REVEAL Lite 2 were incongruent at baseline (54.1%). Numbers may not add to 100% due to rounding.
Individual patient assessments were compared across methods to understand the variation between calculated scores and clinician gestalt. With REVEAL Lite 2, over half of the risk assessments at baseline were incongruent with clinician gestalt (54%; Figure 2b,c). Risk was underestimated most commonly in clinician‐assessed intermediate‐risk patients.
Treatment of a high‐risk patient population with prostacyclins using REVEAL Lite 2 to stratify patients
Baseline characteristics were generally similar between the two cohorts of patients who were assessed as intermediate or high risk by REVEAL Lite 2 (Supporting Information: Table S2). The mean (SD) 6MWD was 180 (73) m and 153 (80) m for the “any PAH medication” cohort and the “prostacyclin‐enriched” cohort, respectively. Most patients in each cohort were classified as WHO functional class III (69% and 78%, respectively).
In the cohort initiating any PAH therapy at index, a higher proportion of patients were considered high risk by REVEAL Lite 2 at baseline, compared with the cohort that initiated a prostacyclin therapy at index (64% vs. 50%; Figure 3a). Of the patients deemed high risk by REVEAL Lite 2 in the “prostacyclin‐enriched cohort” who were not receiving parenteral prostacyclin therapy at baseline, 38% of patients initiated a parenteral prostacyclin within 90 days following baseline, as compared to 18% in the “any PAH medication” cohort (Figure 3b,c).
Figure 3.
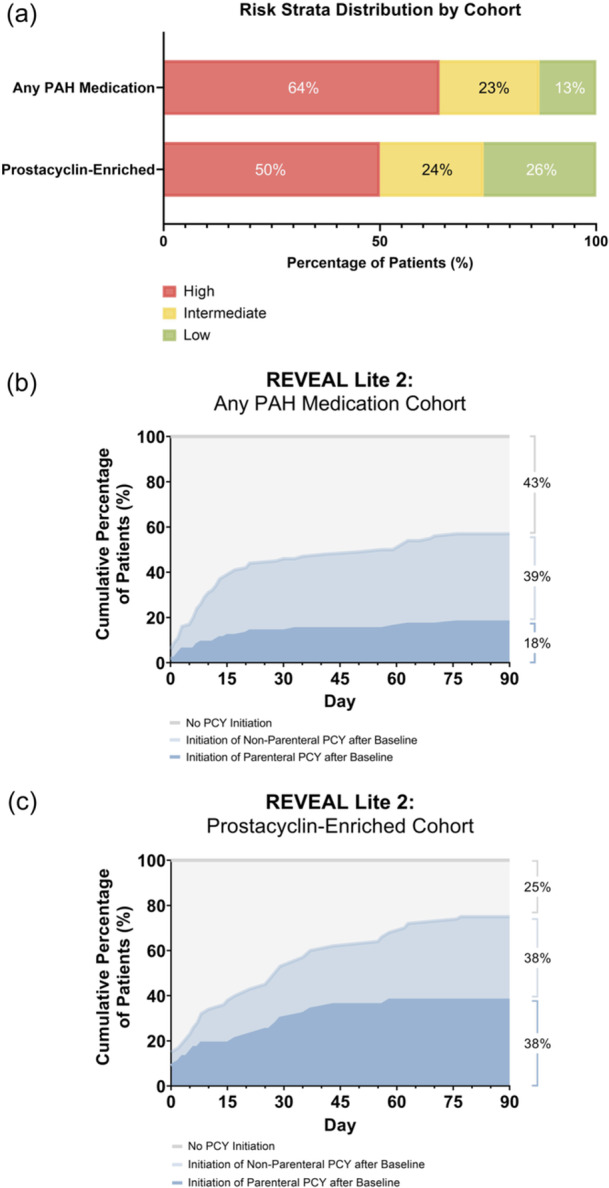
Risk strata distribution using REVEAL Lite 2 by medication cohort. (a) A higher proportion of patients with PAH who were initiated on PAH‐specific therapy were considered high risk, as compared to those initiated on prostacyclin (64% vs. 50%). (b, c) Of the patients deemed high risk by REVEAL Lite 2 who were in the “prostacyclin‐enriched” cohort not on parenteral prostacyclin therapy at baseline, 38% of patients initiated a parenteral prostacyclin within 90 days, as compared to 18% in the “any PAH medication” cohort. Numbers may not add to 100% due to rounding. PAH, pulmonary arterial hypertension; PCY, prostacyclin.
Risk assessment: COMPERA 2.0
The majority of patients were classified by COMPERA 2.0 as high or intermediate‐high risk. As noted above, clinicians classified 21% (62/299), 53% (158/299), and 26% (79/299) of patients to be at low, intermediate, or high risk of death in 1 year, respectively, per their clinical judgment (Figure 4a). Using COMPERA 2.0 risk assessment, 4% (11/299) of patients were classified as low risk, 32% (97/299) as intermediate‐low, 52% (155/299) as intermediate‐high, and 12% (36/299) as high risk (Figure 4a–c).
Figure 4.
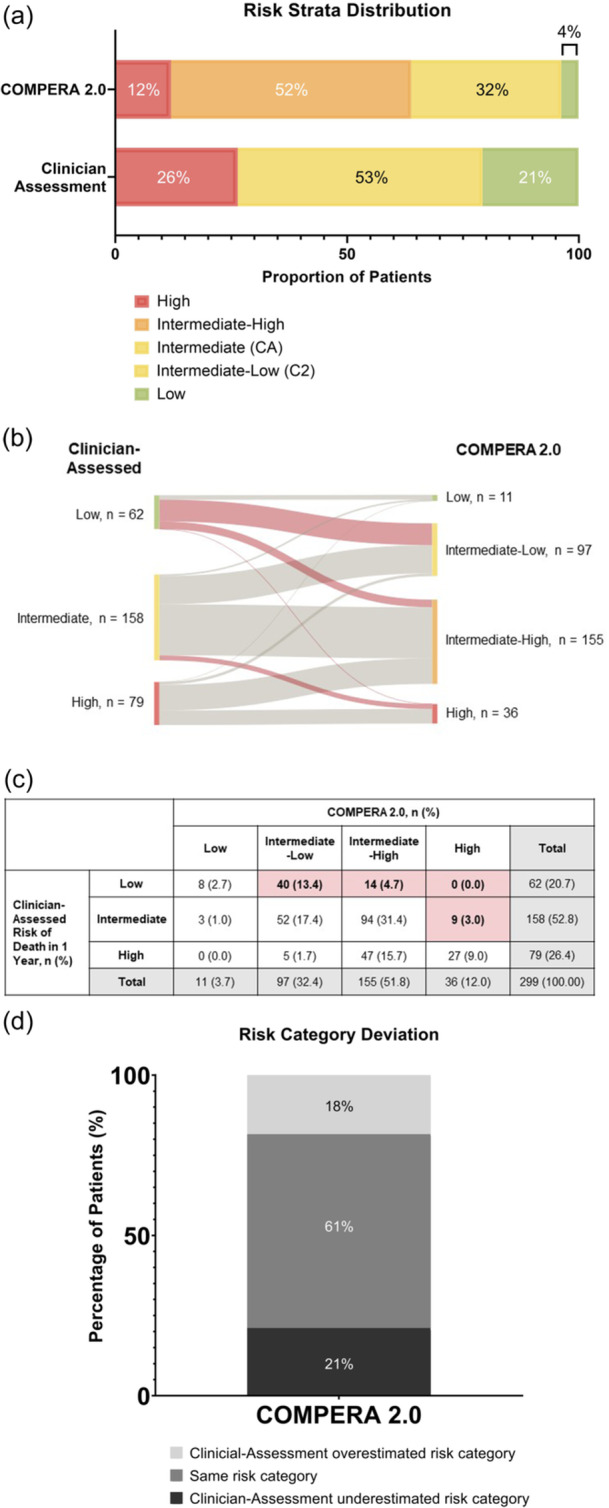
Risk strata using COMPERA 2.0 versus clinician assessment in total study population (N = 299). (a) The majority of patients were categorized as intermediate risk by clinician assessment. The majority of patients were categorized as intermediate‐high or high risk by COMPERA 2.0. (b, c) Red shaded lines and boxes show patients whose risk level was underestimated by clinicians, relative to that calculated by COMPERA 2.0. Risk was underestimated most commonly in clinician‐assessed low patients. (d) Risk assessments between clinician gestalt and COMPERA 2.0 were incongruent at baseline (40%). Numbers may not add to 100% due to rounding.
With COMPERA 2.0, risk assessments at baseline were incongruent with clinician gestalt for 40% of patients (Figure 4d). Risk was most commonly underestimated in clinician‐assessed low‐risk patients.
Treatment of an intermediate‐high and high‐risk patient population with prostacyclins using COMPERA 2.0 to stratify patients
Baseline characteristics were generally similar between the two medication cohorts of patients who were assessed as intermediate‐high or high‐risk by COMPERA 2.0 (Supporting Information: Table S3). More patients in the cohort initiating any PAH therapy at index were considered high or intermediate‐high risk by COMPERA 2.0 at baseline, compared with the cohort that specifically initiated a prostacyclin therapy at index (67% vs. 57%; Figure 5a). Of the patients deemed high risk by COMPERA 2.0 in the “prostacyclin‐enriched cohort” who were not receiving a parenteral prostacyclin therapy at baseline, 37% initiated a parenteral prostacyclin within 90 days, as compared to 18% in the “any PAH medication” cohort (Figure 5b,c).
Figure 5.
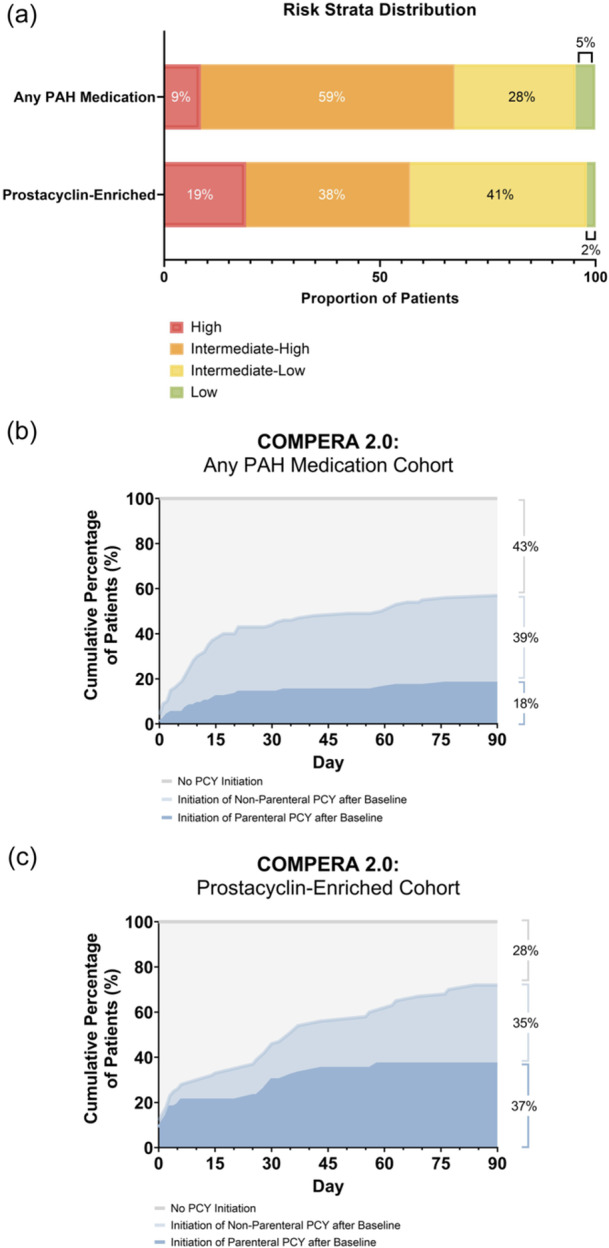
Risk strata distribution using COMPERA 2.0 by medication cohort. (a) A higher proportion of patients with PAH initiated on any PAH therapy were considered high or intermediate‐high risk, as compared to those initiated on prostacyclin (67% vs. 57%). (b, c) Of the patients categorized as high risk by COMPERA 2.0 in the “any PAH medication” cohort who were not on parenteral prostacyclin at baseline, (b) 18% initiated parenteral prostacyclin therapy within 90 days, and (c) 37% of patients in the “prostacyclin‐enriched” cohort not on parenteral prostacyclin therapy at baseline initiated a parenteral prostacyclin within 90 days. Numbers may not add to 100% due to rounding. PAH, pulmonary arterial hypertension; PCY, prostacyclin.
DISCUSSION
Recent updates to diagnosis and management guidelines for PAH have incorporated risk assessment into the treatment of PAH. 4 Patients with PAH can have vast differences in their outcomes, depending on their assigned risk strata. 6 , 16 For example, using REVEAL Lite 2, patients who were deemed intermediate risk were estimated to be 2.3 times as likely to die within 1 year as patients deemed low risk; likewise, patients who were high risk were estimated to be 6.4 times as likely to die within 1 year. 6 Previous guidelines recommended treatment based mainly on functional class, because validated risk scores were not available to guide therapy. 3
Depending on the patient's risk category, different treatment paradigms are recommended. In general, earlier initial treatment in PAH is associated with improved long‐term outcomes for patients. 17 , 18 , 19 Some research has shown that initial triple combination therapy is associated with a higher overall survival rate compared to initial dual therapy or monotherapy, despite greater disease severity at baseline in the patients treated with triple therapy. 20 It is critical to continue implementing serial risk assessments in patients with PAH after the initial diagnosis to identify changes in disease trajectory that may warrant a change in treatment. 21
In this study, the clinician‐assessed risk category was incongruent with risk assessment using a multiparametric tool in 40%–54% of patients with PAH, whether the assessment was performed with the REVEAL Lite 2 or COMPERA 2.0 tool. Most often, this incongruence was due to an underestimation of the patients' risk category by clinician gestalt relative to the tool assessment. This could potentially lead to an undertreatment, and as a consequence disease progression. 21 , 22
The incongruence between clinicians' gestalt and risk assessment tools has been demonstrated in other studies. These studies show a similar trend of approximately half of the patients having an incongruent assessment of risk, with their clinician‐assessed risk often underestimated relative to their calculated risk. 8 , 23 The current study adds to the literature by using the newest risk assessment methods and looking at timely prostacyclin initiation in the high‐risk patient population.
Examining cohorts by medication use at baseline allowed us to consider differential treatment selections in a group of patients representative of the overall PAH population (“any PAH medication” cohort) and elucidate further details in those specifically started on a prostacyclin (“prostacyclin‐enriched” cohort), where parenteral prostacyclin therapy is recommended. In the high‐risk patient population in this study, prostacyclin therapy was underutilized in both cohorts given that current guidelines from the European Society of Cardiology and European Respiratory Society (ESC/ERS) recommend parenteral prostacyclin treatment for intermediate‐high and high‐risk patients. 4 Additionally, there was a lack of timely prostacyclin initiation (within 90 days of baseline) for high‐risk patients. A Delphi panel recently recommended that clinicians should consider treatment escalation in patients at intermediate‐low risk who are on treated triple therapy with a non‐parenteral prostacyclin and have either abnormal or worsening right heart imaging parameters. 24 For patients at intermediate‐high risk, clinicians should consider treatment escalation to a parenteral prostacyclin if right heart imaging shows no improvement or shows worsening. Given retrospective nature of the study, we could not gather information on how clinicians made their treatment decisions. Therefore, it is unknown what factors may have led to the underutilization of prostacyclins in these groups of patients.
There was a higher proportion of patients in the “any PAH medication” cohort who were stratified as high risk by both REVEAL Lite 2 and COMPERA 2.0 than in the “prostacyclin‐enriched” cohort. This might reflect a situation in which patients in the “any PAH medication” cohort, though stratified as higher risk initially, might have been initiated on non‐prostacyclin treatment(s) as a first measure rather than a prostacyclin. One explanation is that the patients treated prostacyclins in the “prostacyclin‐enriched” cohort may have previously been on other PAH medications and thus achieved a slightly lower risk status. However, as they still were not meeting their treatment goals, prostacyclin therapy was initiated.
In this study, most of the surveyed clinicians followed guidelines to treat patients with PAH, with the most common (66%) following the ACCP guidelines, which were published in 2019. According to the published literature, formal risk assessments in PAH are generally underutilized. Only about half of PAH clinicians report using risk assessment tools in regular practice and instead rely on their gestalt to assess clinical status. 9 , 10 Additional real‐world evidence is needed to compare these risk instruments. Additionally, risk assessment tools may be underutilized due to real or perceived barriers to implementing them in clinical practice, such as lack of awareness and knowledge of the tools, time constraints and insufficient integration of technology or electronic medical record. 9 Though multiple risk calculators are available, some offer simplified versions that could make them easier to implement regularly in practice. 6 , 25
Limitations of this study include the use of retrospective medical records, which could be affected by recall and/or selection bias with charts chosen and data abstracted. Of note, patients who met eligibility criteria in the timeframe specified were randomly selected by the surveyed clinician. Patient selection was not prescriptively described. Study results are projectable only to the patients with PAH who initiated treatment during the specified treatment index period, so extrapolation to other time periods is limited to understanding changes in disease management and treatment in these patient populations over time. As clinicians may not routinely capture all risk‐stratification parameters at each measurement period, responses might not be consistent with clinical trial behaviors. It is unknown whether the participating investigators used an objective risk assessment method when providing their clinician assessment. Bias may occur in the retrospective assessment of patient's clinical status, given potential knowledge of future treatment decisions. Further, treatment patterns represent only the practices of cardiologists and pulmonologists agreeing to participate in the study and could vary from non‐responding clinicians. Most of these sites were affiliated with PAH care centers, so practices of community‐based clinicians could also differ. Additionally, guideline‐directed practice has evolved since the index period and treatment regimens may have been selected differently based on previously published guidelines.
In summary, clinician‐assessed risk category was incongruent with a tool‐based risk assessment in 40%–54% of patients with PAH, usually reflecting an underestimation of the patient's risk category by clinician gestalt. Underestimation of the patient's actual risk may result in undertreatment, worsening of signs and symptoms, and progression of PAH. Specifically, this study showed a lack of timely prostacyclin initiation for patients with PAH stratified as high‐risk by two multiparametric tools.
AUTHOR CONTRIBUTIONS
Amresh Raina interpreted data, wrote and edited the original and revised versions of the manuscript; Margaret R. Sketch analyzed the data, wrote and edited the original and revised versions of the manuscript; Benjamin Wu analyzed the data, wrote and edited the original and revised versions of the manuscript; Meredith Broderick analyzed the data, wrote and edited the original and revised versions of the manuscript, and provided project administration; Oksana A. Shlobin interpreted data, wrote and edited the original and revised versions of the manuscript. All authors approved the final version of the manuscript for publication.
CONFLICTS OF INTEREST STATEMENT
Amresh Raina reports no potential conflict of interest with the study. Oksana A. Shlobin has served as a consultant to and on the speaker's bureau for United Therapeutics, Bayer, Merck, and Janssen; and has served as a consultant to Gossamer, Aerami, and Aerovate. Meredith Broderick, Benjamin Wu and Margaret R. Sketch are employees of United Therapeutics Corporation.
ETHICS STATEMENT
The New England Independent Review Board approved the research, and a waiver of documentation of informed consent was obtained for this retrospective chart review.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Medical writing and editorial support were provided by Agnella Izzo Matic, PhD, CMPP (AIM Biomedical, LLC) and Stephanie Hwang, PharmD and sponsored by United Therapeutics Corporation. All authors have approved Dr. Matic to provide these services and submit on their behalf the manuscript (including any declarations), which they have reviewed and approved for submission. This study was sponsored by United Therapeutics Corporation (Research Triangle Park, NC).
Raina A, Sketch MR, Wu B, Broderick M, Shlobin OA. Congruency between clinician‐assessed risk and calculated risk of 1‐year mortality in patients with pulmonary arterial hypertension: a retrospective chart review. Pulm Circ. 2024;14:e12455. 10.1002/pul2.12455
Previous Presentation: A portion of these data was previously presented at the American Thoracic Society 2022 International Conference, May 13–18, San Francisco, CA.
DATA AVAILABILITY STATEMENT
Data underlying the manuscript are available from United Therapeutics with the permission of Medical Data Analytics upon reasonable request.
REFERENCES
- 1. Lai Y‐C, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res. 2014;115:115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hendriks PM, Staal DP, van de Groep LD, van den Toorn LM, Chandoesing PP, Kauling RM, Mager HJ, van den Bosch AE, Post MC, Boomars KA. The evolution of survival of pulmonary arterial hypertension over 15 years. Pulm Circ. 2022;12:e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve‐Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB. Therapy for pulmonary arterial hypertension in adults. Chest. 2019;155:565–586. [DOI] [PubMed] [Google Scholar]
- 4. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. [DOI] [PubMed] [Google Scholar]
- 5. Benza RL, Gomberg‐Maitland M, Farber HW, Vizza CD, Broderick M, Holdstock L, Nelsen AC, Deng C, Rao Y, White RJ. Contemporary risk scores predict clinical worsening in pulmonary arterial hypertension ‐ an analysis of FREEDOM‐EV. J Heart Lung Transplant. 2022;41:1572–1580. [DOI] [PubMed] [Google Scholar]
- 6. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, Elliott CG, Farber HW. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boucly A, Weatherald J, Savale L, de Groote P, Cottin V, Prévot G, Chaouat A, Picard F, Horeau‐Langlard D, Bourdin A, Jutant EM, Beurnier A, Jevnikar M, Jaïs X, Simonneau G, Montani D, Sitbon O, Humbert M. External validation of a refined four‐stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J. 2022;59:2102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15:e0241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: A descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahay S, Balasubramanian V, Memon H, Poms A, Bossone E, Highland K, Kay D, Levine DJ, Mullin CJ, Melendres‐Groves L, Mathai SC, Soto FJ, Shlobin O, Elwing JM. Utilization of risk assessment tools in management of PAH: A PAH provider survey. Pulm Circ. 2022;12:e12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Safdar Z. Treatment of pulmonary arterial hypertension: the role of prostacyclin and prostaglandin analogs. Respir Med. 2011;105:818–827. [DOI] [PubMed] [Google Scholar]
- 12. Vizza CD, Lang IM, Badagliacca R, Benza RL, Rosenkranz S, White RJ, Adir Y, Andreassen AK, Balasubramanian V, Bartolome S, Blanco I, Bourge RC, Carlsen J, Camacho REC, D'Alto M, Farber HW, Frantz RP, Ford HJ, Ghio S, Gomberg‐Maitland M, Humbert M, Naeije R, Orfanos SE, Oudiz RJ, Perrone SV, Shlobin OA, Simon MA, Sitbon O, Torres F, Luc Vachiery J, Wang KY, Yacoub MH, Liu Y, Golden G, Matsubara H. Aggressive afterload lowering to improve the right ventricle: A new target for medical therapy in pulmonary arterial hypertension? Am J Respir Crit Care Med. 2022;205:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burger CD, Pruett JA, Lickert CA, Berger A, Murphy B, Drake W. Prostacyclin use among patients with pulmonary arterial hypertension in the United States: A retrospective analysis of a large health care claims database. Journal of Managed Care & Specialty Pharmacy. 2018;24:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 16. Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, Staehler G, Vizza CD, Gall H, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Park DH, Ewert R, Kaemmerer H, Kabitz HJ, Skowasch D, Behr J, Milger K, Halank M, Wilkens H, Seyfarth HJ, Held M, Dumitrescu D, Tsangaris I, Vonk‐Noordegraaf A, Ulrich S, Klose H, Claussen M, Lange TJ, Rosenkranz S. COMPERA 2.0: A refined four‐stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J. 2022;60:2102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaine S, Sitbon O, Channick RN, Chin KM, Sauter R, Galiè N, Hoeper MM, McLaughlin VV, Preiss R, Rubin LJ, Simonneau G, Tapson V, Ghofrani HA, Lang I. Relationship between time from diagnosis and Morbidity/Mortality in pulmonary arterial hypertension. Chest. 2021;160:277–286. [DOI] [PubMed] [Google Scholar]
- 18. Coghlan JG, Gaine S, Channick R, Chin KM, du Roure C, Gibbs JSR, Hoeper MM, Lang IM, Mathai SC, McLaughlin VV, Mitchell L, Simonneau G, Sitbon O, Tapson VF, Galiè N. Early selexipag initiation and long‐term outcomes: insights from randomised controlled trials in pulmonary arterial hypertension. ERJ Open Res. 2023;9:00456‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naranjo M, Hassoun PM. Time is of the essence in PAH therapy. Chest. 2021;160:25–26. [DOI] [PubMed] [Google Scholar]
- 20. Boucly A, Savale L, Jaïs X, Bauer F, Bergot E, Bertoletti L, Beurnier A, Bourdin A, Bouvaist H, Bulifon S, Chabanne C, Chaouat A, Cottin V, Dauphin C, Degano B, De Groote P, Favrolt N, Feng Y, Horeau‐Langlard D, Jevnikar M, Jutant EM, Liang Z, Magro P, Mauran P, Moceri P, Mornex JF, Palat S, Parent F, Picard F, Pichon J, Poubeau P, Prévot G, Renard S, Reynaud‐Gaubert M, Riou M, Roblot P, Sanchez O, Seferian A, Tromeur C, Weatherald J, Simonneau G, Montani D, Humbert M, Sitbon O. Association between initial treatment strategy and long‐term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;204:842–854. [DOI] [PubMed] [Google Scholar]
- 21. Benza RL, Miller DP, Foreman AJ, Frost AE, Badesch DB, Benton WW, McGoon MD. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: A registry to evaluate early and Long‐Term pulmonary arterial hypertension disease management (REVEAL) analysis. J Heart Lung Transplant. 2015;34:356–361. [DOI] [PubMed] [Google Scholar]
- 22. Farber HW, Miller DP, Meltzer LA, McGoon M. Pulmonary arterial hypertension (PAH)‐specific therapy at time of worsening to functional Class IV in patients from the REVEAL registry. Am J Respir Crit Care Med. 2012;185:A2498. [Google Scholar]
- 23. Simons JE, Mann EB, Pierozynski A. Assessment of risk of disease progression in pulmonary arterial hypertension: insights from an international survey of clinical practice. Adv Ther. 2019;36:2351–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forfia P, Benza R, D'Alto M, De Marco T, Elwing JM, Frantz R, Haddad F, Oudiz R, Preston IR, Rosenkranz S, Ryan J, Schilz R, Shlobin OA, Vachiery JL, Vizza CD, Vonk Noordegraaf A, Sketch MR, Broderick M, McLaughlin V. The heart of the matter: right heart imaging indicators for treatment escalation in pulmonary arterial hypertension. Pulm Circ. 2023;13:e12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson M, Keeley J, Kingman M, McDevitt S, Brewer J, Rogers F, Hill W, Rideman Z, Broderick M. Clinical application of risk assessment in PAH: expert center APRN recommendations. Pulm Circ. 2022;12:e12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data underlying the manuscript are available from United Therapeutics with the permission of Medical Data Analytics upon reasonable request.


