Abstract
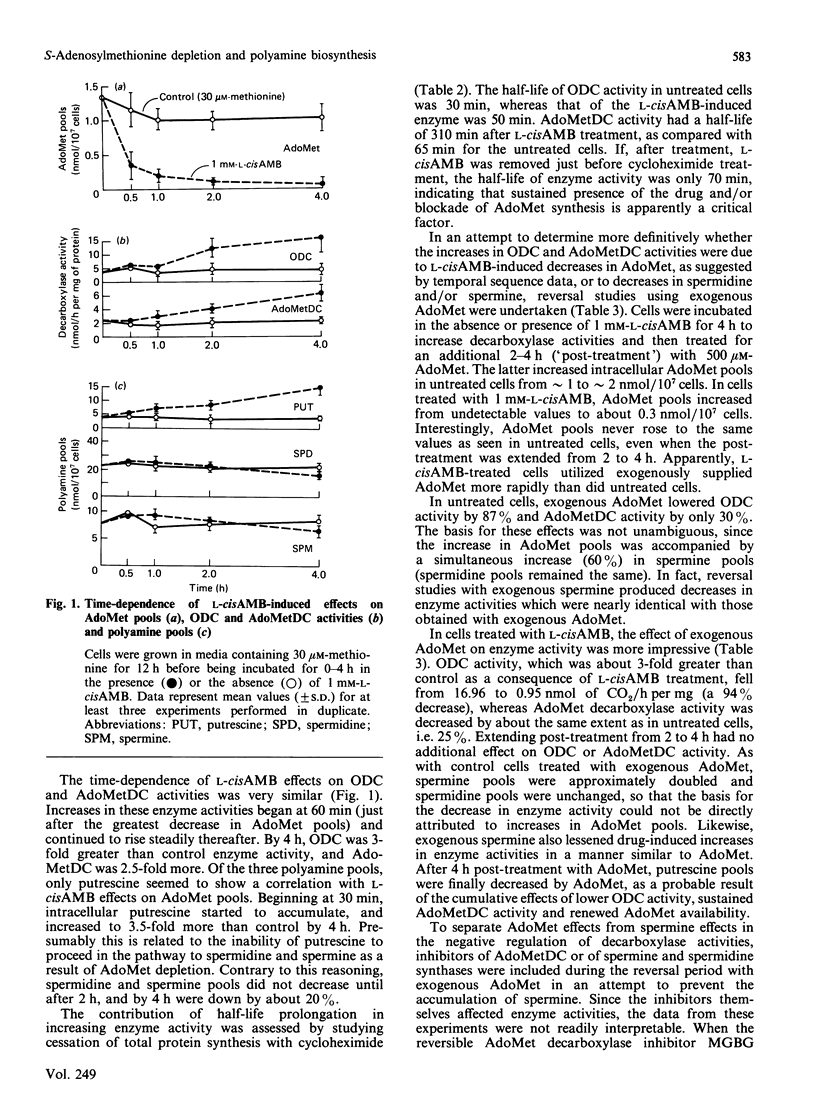
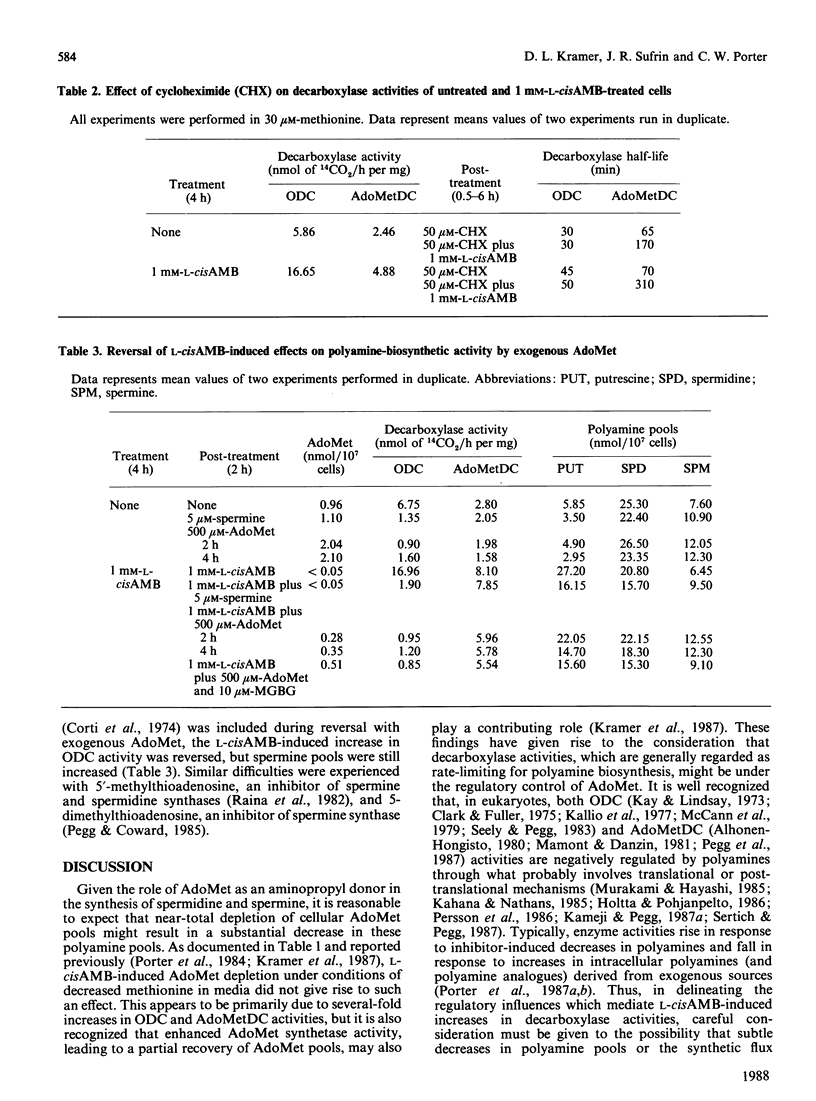
The methionine-analogue inhibitor of S-adenosylmethionine (AdoMet) synthetase, L-2-amino-4-methoxy-cis-but-3-enoic acid (L-cisAMB), was used to study the early effects of AdoMet depletion on polyamine biosynthesis. In the presence of decreased methionine (30 microM) in the medium, treatment of cultured L1210 cells with 1 mM-L-cisAMB resulted in a near-total (95%) depletion of cellular AdoMet pools by 4 h. This was accompanied by a 3-fold increase in ornithine decarboxylase (ODC) activity, a 2.5-fold increase in AdoMet decarboxylase (AdoMetDC) activity and a 20% decrease in spermidine and spermine pools. The increase in enzyme activities seemed to be partially due to prolongation of enzyme activity half-life, since that of ODC was extended from 30 to 50 min and that of AdoMetDC from 65 to 310 min. By temporal sequence characterization (0-4 h), the onset of elevations of enzyme activity (0.5-1 h) seemed to be causally related to an earlier (0-0.5 h) decline in AdoMet pools, as opposed to the 20% decrease in spermidine and spermine pools, which occurred much later (2-4 h); the latter are known to regulate decarboxylase activities negatively. Drug-induced elevations in ODC and, to a lesser extent, AdoMetDC activities were reversed by later treatment with exogenous AdoMet. However, because the latter also increased spermine pools (which could not be prevented with various enzyme inhibitors), the reversal of elevations in enzyme activities could not be directly linked to AdoMet. Although not definitive, the data raise the interesting possibility that, in addition to being negatively regulated by polyamines, ODC and AdoMetDC activities may also be subject to negative control by cellular AdoMet (or an AdoMet metabolite). The net effect of either or both of these influences would be to conserve polyamine-biosynthetic activity in the face of declining AdoMet supplies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Corti A., Dave C., Williams-Ashman H. G., Mihich E., Schenone A. Specific inhibition of the enzymic decarboxylation of S-adenosylmethionine by methylglyoxal bis(guanylhydrazone) and related substances. Biochem J. 1974 May;139(2):351–357. doi: 10.1042/bj1390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Tanaka M., Nagai J., Ikeda T., Pegg A. E. Inhibition of aminopropyltransferases by S-adenosylmethionine. Effect of simultaneous administration of S-adenosylmethionine and methylglyoxal bis(guanylhydrazone) on polyamine concentrations in regenerating rat liver. FEBS Lett. 1980 Feb 11;110(2):323–326. doi: 10.1016/0014-5793(80)80102-5. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Pohjanpelto P. Control of ornithine decarboxylase in Chinese hamster ovary cells by polyamines. Translational inhibition of synthesis and acceleration of degradation of the enzyme by putrescine, spermidine, and spermine. J Biol Chem. 1986 Jul 15;261(20):9502–9508. [PubMed] [Google Scholar]
- Iizasa T., Carson D. A. Differential regulation of polyamine synthesis and transmethylation reactions in methylthioadenosine phosphorylase deficient mammalian cells. Biochim Biophys Acta. 1985 Mar 21;844(3):280–287. doi: 10.1016/0167-4889(85)90128-4. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Kallio A., Pösö H., Scalabrino G., Jänne J. Regulation of ornithine decarboxylase by diamines in regenerating rat liver. FEBS Lett. 1977 Feb 1;73(2):229–234. doi: 10.1016/0014-5793(77)80987-3. [DOI] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Effect of putrescine on the synthesis of S-adenosylmethionine decarboxylase. Biochem J. 1987 Apr 1;243(1):285–288. doi: 10.1042/bj2430285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D. L., Sufrin J. R., Porter C. W. Relative effects of S-adenosylmethionine depletion on nucleic acid methylation and polyamine biosynthesis. Biochem J. 1987 Oct 15;247(2):259–265. doi: 10.1042/bj2470259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardini J. B., Sufrin J. R. Chemotherapeutic potential of methionine analogue inhibitors of tumor-derived methionine adenosyltransferases. Biochem Pharmacol. 1983 Feb 1;32(3):489–495. doi: 10.1016/0006-2952(83)90528-2. [DOI] [PubMed] [Google Scholar]
- Lombardini J. B., Talalay P. Formation, functions and regulatory importance of S-adenosyl-L-methionine. Adv Enzyme Regul. 1970;9:349–384. doi: 10.1016/s0065-2571(71)80054-7. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Seiler N., Siat M., Joder-Ohlenbusch A. M., Knödgen B. Metabolism of acetyl derivatives of polyamines in cultured polyamine-deficient rat hepatoma cells. Med Biol. 1981 Dec;59(5-6):347–353. [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Hayashi S. Role of antizyme in degradation of ornithine decarboxylase in HTC cells. Biochem J. 1985 Mar 15;226(3):893–896. doi: 10.1042/bj2260893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Coward J. K. Growth of mammalian cells in the absence of the accumulation of spermine. Biochem Biophys Res Commun. 1985 Nov 27;133(1):82–89. doi: 10.1016/0006-291x(85)91844-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Matsui I., Seely J. E., Pritchard M. L., Pösö H. Formation of putrescine in rat liver. Med Biol. 1981 Dec;59(5-6):327–333. [PubMed] [Google Scholar]
- Pegg A. E., Pösö H. S-adenosylmethionine decarboxylase (rat liver). Methods Enzymol. 1983;94:234–239. doi: 10.1016/s0076-6879(83)94041-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Pajunen A. Increase in S-adenosylmethionine decarboxylase in SV-3T3 cells treated with S-methyl-5'-methylthioadenosine. Biochem J. 1987 May 15;244(1):49–54. doi: 10.1042/bj2440049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Translational regulation of ornithine decarboxylase by polyamines. FEBS Lett. 1986 Sep 15;205(2):175–178. doi: 10.1016/0014-5793(86)80892-4. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Berger F. G., Pegg A. E., Ganis B., Bergeron R. J. Regulation of ornithine decarboxylase activity by spermidine and the spermidine analogue N1N8-bis(ethyl)spermidine. Biochem J. 1987 Mar 1;242(2):433–440. doi: 10.1042/bj2420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Cavanaugh P. F., Jr, Stolowich N., Ganis B., Kelly E., Bergeron R. J. Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res. 1985 May;45(5):2050–2057. [PubMed] [Google Scholar]
- Porter C. W., McManis J., Casero R. A., Bergeron R. J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987 Jun 1;47(11):2821–2825. [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986 Jul-Aug;6(4):525–542. [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R., Keith D. D. Growth inhibition by methionine analog inhibitors of S-adenosylmethionine biosynthesis in the absence of polyamine depletion. Biochem Biophys Res Commun. 1984 Jul 18;122(1):350–357. doi: 10.1016/0006-291x(84)90482-0. [DOI] [PubMed] [Google Scholar]
- Raina A., Tuomi K., Pajula R. L. Inhibition of the synthesis of polyamines and macromolecules by 5'-methylthioadenosine and 5'-alkylthiotubercidins in BHK21 cells. Biochem J. 1982 Jun 15;204(3):697–703. doi: 10.1042/bj2040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes S. E., Lea P. J., Miflin B. J. S-adenosylmethionine--a novel regulator of aspartate kinase. Nature. 1980 Sep 25;287(5780):357–359. doi: 10.1038/287357a0. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- Sertich G. J., Pegg A. E. Polyamine administration reduces ornithine decarboxylase activity without affecting its mRNA content. Biochem Biophys Res Commun. 1987 Mar 13;143(2):424–430. doi: 10.1016/0006-291x(87)91371-4. [DOI] [PubMed] [Google Scholar]
- Sufrin J. R., Lombardini J. B. Differences in the active-site region of tumor versus normal isozymes of mammalian ATP:L-methionine S-adenosyltransferase. Mol Pharmacol. 1982 Nov;22(3):752–759. [PubMed] [Google Scholar]
- Sufrin J. R., Lombardini J. B., Keith D. D. L-2-Amino-4-methoxy-cis-but-3-enoic acid, a potent inhibitor of the enzymatic synthesis of S-adenosylmethionine. Biochem Biophys Res Commun. 1982 May 31;106(2):251–255. doi: 10.1016/0006-291x(82)91102-0. [DOI] [PubMed] [Google Scholar]
- Tisdale M. J. Effect of methionine deprivation on S-adenosylmethionine decarboxylase of tumour cells. Biochim Biophys Acta. 1981 Jul 17;675(3-4):366–372. doi: 10.1016/0304-4165(81)90027-1. [DOI] [PubMed] [Google Scholar]
- Zappia V., Galletti P., Porcelli M., Manna C., Ragione F. D. High-performance liquid chromatographic separation of natural adenosyl-sulphur compounds. J Chromatogr. 1980 Mar 7;189(3):399–405. doi: 10.1016/s0021-9673(00)80319-2. [DOI] [PubMed] [Google Scholar]



