Abstract
Objectives:
Studies have reported bidirectional associations of sleep with daily stressors and negative mood. Yet we know little about how sleep is associated with workers' daily cognitive interference, or the experience of off-task and distracting thoughts. This study examined whether nightly sleep was associated with next-day cognitive interference, and vice versa, during workdays and non-work days.
Design:
Daily telephone interviews.
Setting:
US information technology workplaces.
Participants:
130 middle-aged employees.
Measurements:
On 8 consecutive days, participants reported the frequency of experiencing off-task and distracting thoughts during the day (0 = never to 4 = very often) and multiple sleep characteristics (bedtimes, wake times, sleep duration, sleep quality, and sleep latency). Covariates included sociodemographic characteristics and work hours.
Results:
Multilevel models revealed that, on days following earlier wake times (B = −0.32, P < .01), shorter sleep duration (B = −0.27, P < .01), or poorer sleep quality (B = −0.17, P < .01), participants reported more cognitive interference than usual. That is, waking 19 minutes earlier and sleeping 16 minutes less were associated with one additional point on the cognitive interference scale the next day. With cognitive interference predicting nightly sleep, more same day's cognitive interference was associated with earlier bedtimes (B = −0.19, P < .05) and earlier wake times (B = −0.30, P < .01) than usual. The temporal associations of nightly sleep duration and sleep quality with the following day's cognitive interference were significant on work days, but not on non-work days.
Conclusion:
Our results suggest bidirectional associations between poorer sleep and more cognitive interference, particularly on work days with implications for workday productivity and quality of life.
Keywords: Sleep, Cognitive interference, Daily diary, Recovery, Stress, Workdays
Introduction
Sleep can be both an antecedent and a consequence of daytime experiences. Previous studies report bidirectional associations between sleep and daily stressors and negative mood.1,2 Cognitive interference, the experience of intrusive, unwanted, off-task, and potentially ruminative thoughts, is related to the experience of daily stress.3 The links between sleep and stress and between stress and cognitive interference are well established. However, there is a relative lack of observational research examining the relationship between sleep and cognitive interference, particularly in a relatively healthy sample of workers at midlife. Experimental studies in the laboratory observed effects of acute sleep deprivation on degraded performance in cognitive tests and vigilant attention.4,5 However, we know little about how middle-aged workers—who are particularly vulnerable to insufficient or poor sleep6,7—experience sleep and cognitive interference in their naturalistic daily settings. Even less is known about whether poor sleep is an antecedent of next-day cognitive interference or a consequence of it. The purpose of the present study was to examine the temporal bidirectional associations of daily cognitive interference with nightly sleep variables (sleep duration, sleep quality, sleep latency, and sleep timing), and whether associations differed by type of days (work days vs. non-work days).
Research using the job-stress recovery model8,9 suggests that recovery experiences during non-work time are crucial for employee's cognitive functioning and affective states the next day. Sufficient and good quality sleep may contribute to better recovery of resources for the next day, such as overall energy and feelings of control. Specifically, poor sleep may adversely affect self-regulatory functioning needed for appropriate decision making and cognitive capacity needed for working memory.10 Thus, insufficient or poor sleep may result in undesirable cognitive outcomes, including slowing mental activity, degrading decision-making, increasing mistakes, and cognitive interference in performing job tasks. For example, Mullins et al.11 showed that short sleep duration, poor quality sleep, circadian rhythm disruption, and sleep disorders led to withdrawal or disengagement while at work (ie, cognitive and emotional distraction, and work neglect) through increased daytime sleepiness. Previous research using within-person analyses also found that sleep quality predicted a range of positive and negative affect variables during the subsequent day.12-14 Given that negative affect was also associated with same-day cognitive interference,3 we expect that lack of sleep recovery may predict experiencing more cognitive interference the next day.
Sleep can also be a consequence of daily stressful experiences. Longer self-reported sleep latency (ie, how long takes falling asleep) was predicted by psychosocial stressors (ie, work–family conflict, time inadequacy for child and to engage in exercise) that day, whereas shorter sleep duration and poorer sleep quality predicted greater experiences of those stressors the following day.1 Unlike sleep duration and sleep quality, which may reflect recovery of resources,13 sleep latency may reflect the current day's worries, rumination, and cognitive stress residues.15 Research using ambulatory polysomnography showed that more bedtime worries and concerns predicted longer time from sleep onset to non-REM Stage 3, the deepest stage of sleep.16 Research examining temporal associations between daily stressful experiences and sleep timing variables is limited. Bedtimes and wake times may be prone to both what happened on that day and what is expected on the following day. For example, employees who had a cognitively stressful day may go to bed earlier due to fatigue and subsequently wake up earlier; those who anticipate a stressful day may also wake up earlier. Fig. 1 depicts our research model testing bidirectional associations between daily cognitive interference and nightly sleep variables.
Fig. 1.
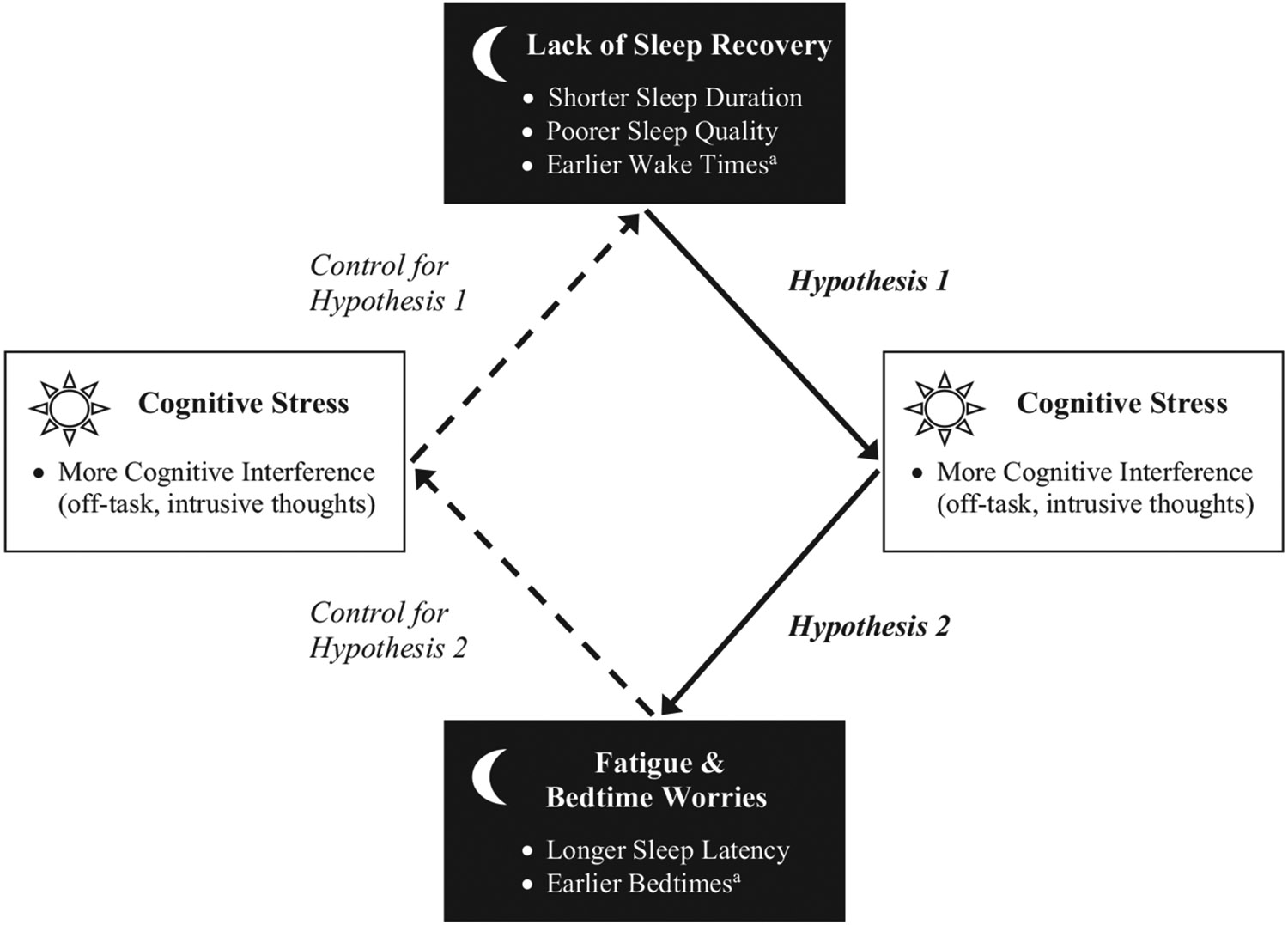
Research model testing bidirectional associations between nightly sleep and daily cognitive interference. Note. The solid arrows with betas indicate hypothesized temporal associations. Hypothesis 1 tests whether lack of sleep recovery predicts more cognitive interference the next day; Hypothesis 2 tests whether experiencing more cognitive interference on a day predicts longer sleep latency and potential earlier bedtimes that indicate fatigue and bedtime worries. Dotted arrows are to control for the opposite direction of the hypothesized direction (eg, more cognitive interference → shorter sleep duration). a Tests with sleep timing variables (wake times and bedtimes) are exploratory.
Sleep schedules are structured around a social clock, particularly for employees whose daily schedule is fixed around work time.17 Typical workers have less opportunities for sleep during work days and try to compensate their “sleep debt” during non-work days.18,19 Similarly, workers may also have more opportunities for cognitive stress on work days than on non-work days.20,21 Cognitive interference may occur when employees need to obtain new information (or engage in a new task) but their working memory resources are not sufficient.22 To our knowledge, no research has examined how the daily associations between sleep and cognitive interference differ between workdays and non-work days. It may be that the predicted associations between sleep and cognitive interference are more apparent on work days than on non-work days.
Using daily diary data from a sample of middle-aged employees, we examined temporal associations of daily cognitive interference with diverse indicators of nightly sleep (ie, sleep duration, sleep quality, sleep latency, and sleep timing) while controlling for relevant covariates. Based on the prior literature, we hypothesized:
Hypothesis 1. Previous night's shorter sleep duration and poorer sleep quality than usual would predict experiencing more cognitive interference than usual the next day.
Hypothesis 2. Same day's more cognitive interference would predict sleep latencies being longer than usual that night.
Given limited research on sleep timing variables, we did not establish a priori hypotheses regarding temporal directionality between sleep timing and cognitive interference (eg, that later bedtimes and earlier wake times would predict more cognitive interference than usual, or that more cognitive interference would predict earlier bedtimes and subsequent earlier wake times than usual), although we speculated bidirectional associations. We also tested whether the expected associations differed on work days versus on non-work days.
Method
Participants
We used a sample of employees who participated in the daily diary sub-study as part of the Work, Family, and Health Study.23 Participants included employees in the information technology (IT) division of a U.S. Fortune 500 company in metropolitan areas. Among 823 employees who completed a baseline interview at the workplace to provide information on demographic and work characteristics, 222 employees who had at least one child aged 9–17 living at home were eligible and invited to participate in a diary study (to recruit the child in a child daily study as well). Of these, 131 (59% of eligible employees) participated in a telephone diary study at baseline. Those who chose to participate were not different from those who were eligible but did not participate in basic demographic characteristics (education, age, number of children living in the household, child gender, marital status) and work variables (tenure at work, schedule control, family-supportive supervisor behaviors, work–family conflict), with the exceptions of child age (those who participated had older children), income (those who participated earned less money), and minority status (those who participated were less likely to be a minority). Details of these comparisons (t tests and χ2 tests) are reported in previous research.24 Of 131 participants, one person who completed only one diary day was excluded from our analyses. Most (85%) completed 8 days, 11% completed 7 days and the rest completed 5–6 days. Thus, the final analytic sample of this study was 130 employees at midlife who had at least one school-age or adolescent-age child in the household.
Procedures
Trained interviewers obtained informed consent and then conducted computer-assisted personal interviews with the employees at the workplace at baseline. The daily diary data collection took place the month following the workplace interviews. Participants were telephoned on eight consecutive evenings and asked about their daily experiences, including sleep and cognitive interference. Call lasted about 20 minutes each, and participants received $150 for their diary study participation.
Daily diary measures
Sleep timing
Participants reported their nightly bedtime (“What time did you go to bed last night?”) and wake time (“What time did you wake up this morning?). We used military hours of bedtime and wake time. We centered bedtimes at midnight (0:00 = 0; 10:00 PM = −2; 2:00 AM = 2) such that higher scores indicate later bedtimes.
Sleep duration
We asked participants, “How many hours and minutes did you sleep last night?” The total time slept the previous night was created by summing the number of hours and minutes (divided by 60) the participant reported sleeping the previous night. We treated this reported sleep hours as sleep duration.
Sleep quality
We used one item adapted from the Pittsburg Sleep Quality Index (PSQI).25 The item reads, “How would you rate (your/last night's) sleep quality overall?” Responses ranged from 1 (very badly) to 4 (very well).
Sleep latency
We asked participants, “How long did it take you to fall asleep?” This item was adapted from the PSQI.25 Responses were coded in minutes.
Cognitive interference
We used the mean of a 9-item daily cognitive interference scale.3,26 The scale is designed to measure the experience of and attempts to control intrusive, unwanted, and potentially ruminative, thoughts. Items from this scale were adapted from existing measures of thought suppression,27 thought control,28 and intrusive and avoidant thinking.29 The items read, “How often did you (1) think about personal worries today, (2) think about something you did not mean to think about today, (3) have trouble concentrating today, (4) have thoughts that kept jumping into your head today, (5) try to avoid certain thoughts today, (6) have thoughts that you could not stop today, (7) try to put problems out of your mind today, (8) do things to distract yourself from your thoughts today, and (9) stay busy just to keep thoughts from entering your mind today?” Responses were coded as 0 = never, 1 = once, 2 = a few times, 3 = often, and 4 = very often. Reliability was calculated at both the between- and within-person (across days) levels,30 and both were adequate (between-person α = .97; within-person α = .78).
Type of day
We created a binary workday variable. If individuals reported they worked on the day of the interview, this variable was coded as work day (=1 vs. non-work day = 0). On average, employees worked on 73% of the diary interview days, resulting in 758 work days and 282 non-work days. Of the work days, 56% occurred at the workplace, 42% occurred at home, and the rest (2%) occurred at some other location.
Covariates
We controlled for sociodemographic and work characteristics because previous research has found them to be related to daily cognitive experiences as well as nightly sleep.32,33 We controlled for age (in years), gender (1 = men, 0 = women), race (1 = non-white, 0 = white), education (1 = less than college graduate, 0 = college graduate or more), and married/partnered status (1 = not married/partnered, 0 = married or living with a partner). We also controlled for work hours in minutes to take into account employees' overall time availability for sleep.7 To better understand the associations between sleep and cognitive interference in the context of daily stressors, we included a total daily stressor frequency in subsets of models. We used 10 items adapted from the Daily Inventory of Stressful Events (DISE)31 that encompass daily home- and work-related stressors (eg, “Did you have demands placed on you at your job that were stressful?”, coded yes[1]/no[0]). The score is the sum of responses.
Data analysis
We used multilevel modeling with lagged effects in SAS 9.4 to take into account the nested data structure.34 1040 total daily observations were nested within 130 employees. Since the use of lagged variables excludes the first day's observation, 910 daily observations were included. Due to missing responses in sleep variables, a total of 869–867 daily observations were used in the analyses (number of observations differed by model with each sleep variable). To test our hypotheses about the temporal association between nightly sleep and daily cognitive interference, variances for key measures were decomposed to within-person (level-1) and between-person (level-2) levels. Within-person variables were centered at the person mean, such that positive values indicate scores higher than the person's own cross-time average (eg, the person's usual cognitive interference level). Between-person variables were centered at the sample mean, such that positive values indicate higher scores than others in the sample. For example, the level-1 model for nightly sleep duration in relation to daily cognitive interference (without covariates) was specified as:
Most cogent for hypothesis H1, indicates whether sleep duration on day (after controlling for the effect of previous night's sleep duration, ) is associated with cognitive interference on day (after controlling for ). For hypothesis H2, indicates whether cognitive interference on day is associated with sleep duration that night (ie, on , after controlling for as well as ). Simultaneously testing both and in a single model allows us to determine whether sleep duration (and also other sleep variables) predict the next day cognitive interference () or whether daily cognitive interference predicts same-night sleep duration ().
We separately modeled each sleep variable. In all models, we used a specific variance classification (ie, a first-order autoregressive variance structure) to account for the fact that consecutive sleep observations might be more highly correlated than non-consecutive observations.1 To test whether the associations between sleep and cognitive interference differed by type of days, we first stratified bidirectional model for each sleep variable by work days and non-work days. Then we conducted follow-up tests with interaction terms in a simplified model including only one temporal direction. We classified the type of days on the basis of when cognitive interference was reported. All continuous variables were centered at sample means.
Results
Descriptive results
Table 1 shows descriptive statistics and correlations of all variables used in this study. Participants' mean age was 45.01 (SD = 6.17); 55% were men; the majority (69%, n = 90) were white, non-Hispanic, 20% (n = 26) were other/mixed races, 9% (n = 12) were Hispanic, and 2% (n = 2) were Black or African American, non-Hispanic; 78% had completed four years of college or more, 20% (n = 26) had some college (1–3 years) or technical school, and 2% (n = 3) were high school graduates; and most (87%) were married or cohabiting. On average, participants had 2 children living in the household (M = 2.11, SD = 1.07, Range = 1–8); all participants had at least one school-aged or adolescent child (ages 6 to 18), 15% had children aged 5 or under, and 6% were living with children older than age 18. Participants worked 8.54 hours (SD = 1.52) per day during the diary week. Most (82%) worked regular daytime schedule; the rest worked variable schedules that changed day-to-day, but not night or evening shift, per se (results are not shown in Table 1).
Table 1.
Descriptive statistics and correlations of all variables
| M or % | (SD) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 45.14 | (6.32) | 0.08 | −0.04 | −0.10 | 0.23 | 0.11 | −0.08 | −0.16 | −0.003 | −0.04 | 0.07 | 0.13 | −0.09 |
| 2. Men (vs. Women) | 55% | −0.17 | −0.19 | −0.25 | 0.13 | 0.06 | −0.01 | −0.17 | −0.14 | 0.07 | −0.13 | −0.04 | ||
| 3. Race, non-White (vs. White) | 31% | 0.17 | 0.04 | 0.05 | 0.01 | −0.01 | 0.05 | −0.03 | 0.08 | −0.07 | 0.05 | |||
| 4. Some college/technical school (vs. College graduates+) | 22% | 0.18 | 0.07 | −0.12 | −0.14 | −0.09 | −0.08 | −0.02 | 0.01 | 0.04 | ||||
| 5. Single (vs. Married/cohabiting) | 13% | −0.11 | 0.05 | −0.04 | −0.21 | −0.04 | 0.16 | 0.26 | 0.04 | |||||
| 6. Work hours | 8.53 | (1.51) | −0.19 | −0.01 | −0.27 | −0.17 | −0.01 | 0.23 | 0.11 | |||||
| 7. Bedtimes | 23:57 | (0:52) | 0.35 | 0.38 | −0.24 | 0.01 | −0.01 | 0.27 | −0.01 | |||||
| 8. Wake times | 6:22 | (1:08) | 0.20 | 0.41 | 0.26 | 0.15 | −0.04 | −0.03 | 0.004 | |||||
| 9. Sleep hours | 6.70 | (0.87) | −0.22 | 0.31 | 0.32 | 0.46 | −0.24 | −0.26 | −0.06 | |||||
| 10. Sleep quality (1 = very badly to 4 = very well) | 3.03 | (0.41) | 0.03 | 0.10 | 0.44 | 0.26 | −0.30 | −0.29 | −0.27 | |||||
| 11. Sleep latency (in minutes) | 19.67 | (11.27) | −0.06 | −0.04 | −0.19 | −0.25 | 0.30 | 0.06 | 0.03 | |||||
| 12. Total stressors (frequency) | 1.02 | (0.78) | 0.09 | −0.13 | −0.18 | −0.15 | 0.05 | 0.29 | 0.37 | |||||
| 13. Cognitive interference (0 = never to 4 = very often) | 1.55 | (0.48) | −0.02 | −0.06 | −0.08 | −0.17 | 0.04 | 0.35 | 0.48 |
Note. N = 130 workers, 1040 daily observations. Unadjusted Means and Standard Deviations were based on person-means across days; Intra-Class Correlations (ICC = between-person level variance / total variance) are reported on the diagonal in italics. Numbers below the diagonal are within-person level correlations and those above the diagonal are between-person level correlations; Correlations in bold were significant at P < .05.
The within-person level correlations between the sleep measures indicated that later bedtimes were associated with later wake times and shorter sleep duration. Later wake times were associated with longer sleep duration and better sleep quality. Longer sleep duration were associated with better sleep quality and shorter sleep latency, with sleep quality also negatively associated with sleep latency. The between-person and within-person level correlations ranged from 0.01 to 0.46, meaning that each of the sleep measures were interrelated, but largely distinct and independent constructs. In terms of the relationship of sleep with cognitive interference, sleep duration and sleep quality were negatively associated with cognitive interference at the within-person level. There was a moderate-level positive correlation between cognitive interference and daily stressors at both the within- and between-person levels (r = 0.35, r = 0.37, respectively), which indicates that our measure of daily cognitive interference shares common variance with daily stressors, but it also has its own unique variance. The Intra-Class Correlations (ICCs; Range = 0.26–0.48) of sleep variables and cognitive interference suggested that high proportions of the variability (52–74%) in these variables were due to day-to-day fluctuations rather than between-person differences, and suggested that it was appropriate to test temporal directionality between them.
Sleep → cognitive interference, or cognitive interference → sleep
Tables 2A and 2B show one bidirectional model testing bidirectional associations between daily cognitive interference and each sleep variable with sleep as an outcome. Beginning with the model predicting bedtimes (Table 2A), previous night's bedtimes were not significantly associated with the next-day cognitive interference. However, in the opposite temporal direction (Table 2B), more same day cognitive interference was associated with going to bed earlier than usual (B = −0.19, SE = 0.09, P < .05). This effect held after controlling for the following significant (P < .05) effects of covariates: Employees who were more educated and worked longer hours reported later bedtimes. Turning to wake times, there were significant bidirectional associations with daily cognitive interference. On days when participants woke up earlier than usual, they reported more cognitive interference than usual (Table 2A; B = −0.32, SE = 0.12, P < .01). Moreover, on the next morning following days with more cognitive interference than usual, employees woke up earlier than usual, by 18 minutes (Table 2B; B = −0.30, SE = 0.11, P < .01). These effects adjusted for previous day's wake times and covariates.
Table 2A.
Results of multilevel models testing the temporal associations of nightly sleep variables with daily cognitive interference
| Daily cognitive interference (Day ) | ||
|---|---|---|
| B | (SE) | |
| Fixed Effects | ||
| Bedtimes (military hours1) | ||
| Between-person, Average bedtimes | 0.15 | (0.17) |
| Within-person (Day ), Previous night's bedtimes | −0.06 | (0.09) |
| Wake times (military hours) | ||
| Between-person, Average wake times | −0.05 | (0.23) |
| Within-person (Day ), Previous night's wake times | −0.32 ** | (0.12) |
| Sleep Duration (in hours) | ||
| Between-person, Average sleep duration | −0.08 | (0.17) |
| Within-person (Day ), Previous night's sleep duration | −0.27 ** | (0.10) |
| Sleep Quality (1 to 5:better) | ||
| Between-person, Average sleep quality | −0.25** | (0.08) |
| Within-person (Day ), Previous night's sleep quality | −0.17 ** | (0.05) |
| Sleep Latency (in minutes) | ||
| Between-person, Average sleep latency | 0.77 | (2.28) |
| Within-person (Day ), Previous night's sleep latency | 1.87 | (1.35) |
Note. 869–867 days from 130 employees; 8 consecutive days' data were nested within each employee. Tables 2A and 2B represent one bidirectional model. Results are presented for each outcome (cognitive interference in 2A and sleep variables in 2B) separately across Tables 2A and 2B for ease of interpretation. As the two competing temporal directions in Table 2A and Table 2B are estimated simultaneously in one model with including each sleep variable on the outcome side, intercepts, the effects of covariates, and random effects are presented only in Table 2B. Significant effects of interest are bolded.
The military hours of bedtimes were centered at midnight (0 = 00:00).
P < .01.
Table 2B.
Results of multilevel models testing the temporal associations of daily cognitive interference with nightly sleep variables
| Nightly sleep (Day ) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bedtimes (military hours2) |
Wake Times (military hours) |
Sleep Duration (in hours) |
Sleep Quality (1 to 5:better) |
Sleep Latency (in minutes) |
||||||
| B | (SE) | B | (SE) | B | (SE) | B | (SE) | B | (SE) | |
| Fixed Effects | ||||||||||
| Intercept | −0.99*** | (0.15) | 6.49*** | (0.20) | 6.95*** | (0.14) | 3.09*** | (0.07) | 16.93*** | (1.94) |
| Cognitive interference (CI; 0 = never to 4 = very often) | ||||||||||
| Between-person, Average CI | 0.15 | (0.17) | −0.05 | (0.23) | −0.08 | (0.17) | −0.25** | (0.08) | 0.77 | (2.28) |
| Within-person lagged (Day ), Same day's CI | −0.19 * | (0.09) | −0.30 ** | (0.11) | −0.10 | (0.10) | −0.01 | (0.05) | 0.31 | (1.26) |
| Covariates | ||||||||||
| Previous night's sleep (Day -1) | −0.03 | (0.04) | −0.12** | (0.04) | −0.29*** | (0.04) | −0.35*** | (0.04) | −0.34*** | (0.03) |
| Age (in years) | −0.03† | (0.01) | −0.03† | (0.02) | 0.01 | (0.01) | −0.004 | (0.01) | 0.01 | (0.17) |
| Gender, Men (vs. women) | 0.09 | (0.16) | −0.06 | (0.22) | −0.35* | (0.16) | −0.11 | (0.08) | 2.46 | (2.12) |
| Race, White (vs. non-white) | 0.04 | (0.17) | 0.07 | (0.23) | 0.15 | (0.16) | 0.01 | (0.08) | 2.47 | (2.22) |
| Education, Less than college (vs. college graduates) | −0.38* | (0.19) | −0.49† | (0.26) | −0.10 | (0.19) | −0.05 | (0.09) | −1.79 | (2.51) |
| Marital status, Single (vs. married/partnered) | 0.48† | (0.25) | 0.10 | (0.34) | −0.85*** | (0.24) | −0.02 | (0.12) | 6.92* | (3.27) |
| Work hours | 0.14** | (0.05) | 0.03 | (0.07) | −0.16** | (0.05) | −0.02 | (0.02) | −0.05 | (0.68) |
| Random Effects | ||||||||||
| Person level variance | 0.49*** | (0.10) | 0.91*** | (0.18) | 0.15 | (0.13) | 0.01 | (0.04) | 45.10* | (23.13) |
| Auto-correlation1 | 0.19* | (0.07) | 0.24*** | (0.05) | 0.44*** | (0.06) | 0.45*** | (0.06) | 0.40*** | (0.06) |
| Residual variance | 1.18*** | (0.08) | 1.89*** | (0.13) | 1.73*** | (0.16) | 0.47*** | (0.05) | 280.8*** | (27.13) |
Note. 869-867 days from 130 employees; 8 consecutive days' data were nested within each employee. Tables 2A and 2B represent one bidirectional model. Results are presented for each outcome (cognitive interference in 2A and sleep variables in 2B) separately across Tables 2A and B for ease of interpretation. As the two competing temporal directions in Table 2A and Table 2B are estimated simultaneously in one model with including each sleep variable on the outcome side, intercepts, the effects of covariates, and random effects are presented only in Table 2B. Significant effects of interest are bolded.
AR(1) function was used to specify a first-order autoregressive variance structure, such that consecutive sleep observations are more highly correlated than non-consecutive observations.
The military hours of bedtimes were centered at midnight (0 = 00:00).
P < .10
P < .05
P < .01
p < .001.
In terms of sleep duration, previous night's sleep duration were significantly associated with the next-day cognitive interference (Table 2A; B = −0.27, SE = 0.10, P < .01). On days following nights with shorter sleep duration than usual, employees reported more cognitive interference than usual. Specifically, about 16 minutes decrease in previous night's sleep was associated with one unit increase in cognitive interference the next day. The other temporal direction, same day's cognitive interference predicting the night's sleep duration, was not significant (Table 2B). The effects of covariates indicated that male (vs. female) and single (vs. married/partnered) employees, and those who worked longer hours reported significantly shorter sleep duration. Results on sleep quality were consistent. In Table 2A, the within-person effect showed that, on days following nights with poorer sleep quality than usual, employees reported more cognitive interference than usual (B = −0.17, SE = 0.05, P < .01). In addition, between-person association was significant, such that employees who had poorer sleep quality than others in the sample, also reported more cognitive interference on average across days (B = −0.25, SE = 0.08, P < .01). Thus, our hypothesis 1 that previous night's shorter sleep duration and poorer sleep quality than usual would predict experiencing more cognitive interference than usual the next day was supported. Lastly, the model with sleep latency revealed no significant associations with cognitive interference at either direction (results did not change by the duration of sleep latency either). Thus, our Hypothesis 2 that more cognitive interference on the same day would predict longer than usual sleep latencies that night was not supported.
We compared changes in −2 log-likelihood (−2LL) statistic to check whether the model testing supported temporal direction fits data better than the model testing unsupported temporal direction. For sleep duration and sleep quality, significant changes in −2LL indicated that the model testing “sleep → cognitive interference” direction was significantly better (P < .01) than the model testing “cognitive interference → sleep” direction. For bedtimes, the model testing “cognitive interference → sleep” direction tended to fit the data better than the other model, although the change in the −2LL was marginal (P = .08). For wake times that exhibited bidirectional associations with cognitive interference, the model testing one direction did not significantly differ from the model testing the other direction (See Table A.1).
For the significant temporal associations between sleep and cognitive interference, we further examined whether the associations were affected by daily stressors (Fig. A.1). Overall, poorer sleep recovery was associated with more stressors the following day and more stressors were associated with more cognitive interference during the same day. More cognitive interference, in turn, was associated with subsequent earlier bedtimes and wake times (detailed results are available upon request).
Differences between work days and non-work days
Next, we examined whether the daily associations between cognitive interference and sleep differed on work days versus non-work days. The association between more same day's cognitive interference and earlier bedtimes was significant on workdays (B = −0.23, SE = 0.11, P < .05) but not on non-work days (B = −0.19, SE = 0.20, P > .05), with no difference in the slopes (ie, betas) between the two types of days. Similarly, the association between earlier wake times and more same day's cognitive interference was also significant on work days (B = −0.19, SE = 0.10, P < .05) but not on non-work days (B = −0.25, SE = 0.29, P > .05), although the slopes did not significantly differ from each other. With regards to sleep duration and sleep quality, significant differences in the slopes between work days and non-work days were found. Fig. 2 shows that previous night's sleep duration was negatively associated with the following day's cognitive interference when the following day was a workday (B = −0.23, SE = 0.10, P < .05); yet the negative association was not found when the following day was a non-work day (B = 0.10, SE = 0.25, P > .05). Moreover, Fig. 3 shows that previous night's poorer sleep quality predicted more cognitive interference on a next workday (B = −0.25, SE = 0.06, P < .001), but not on a non-workday (B = 0.07, SE = 0.12, P > .05). Again, these associations adjusted for sociodemographic covariates, work hours, and previous day's sleep variable of interest. There was no difference between work days and non-work days in the null association between cognitive interference and sleep latency.
Fig. 2.
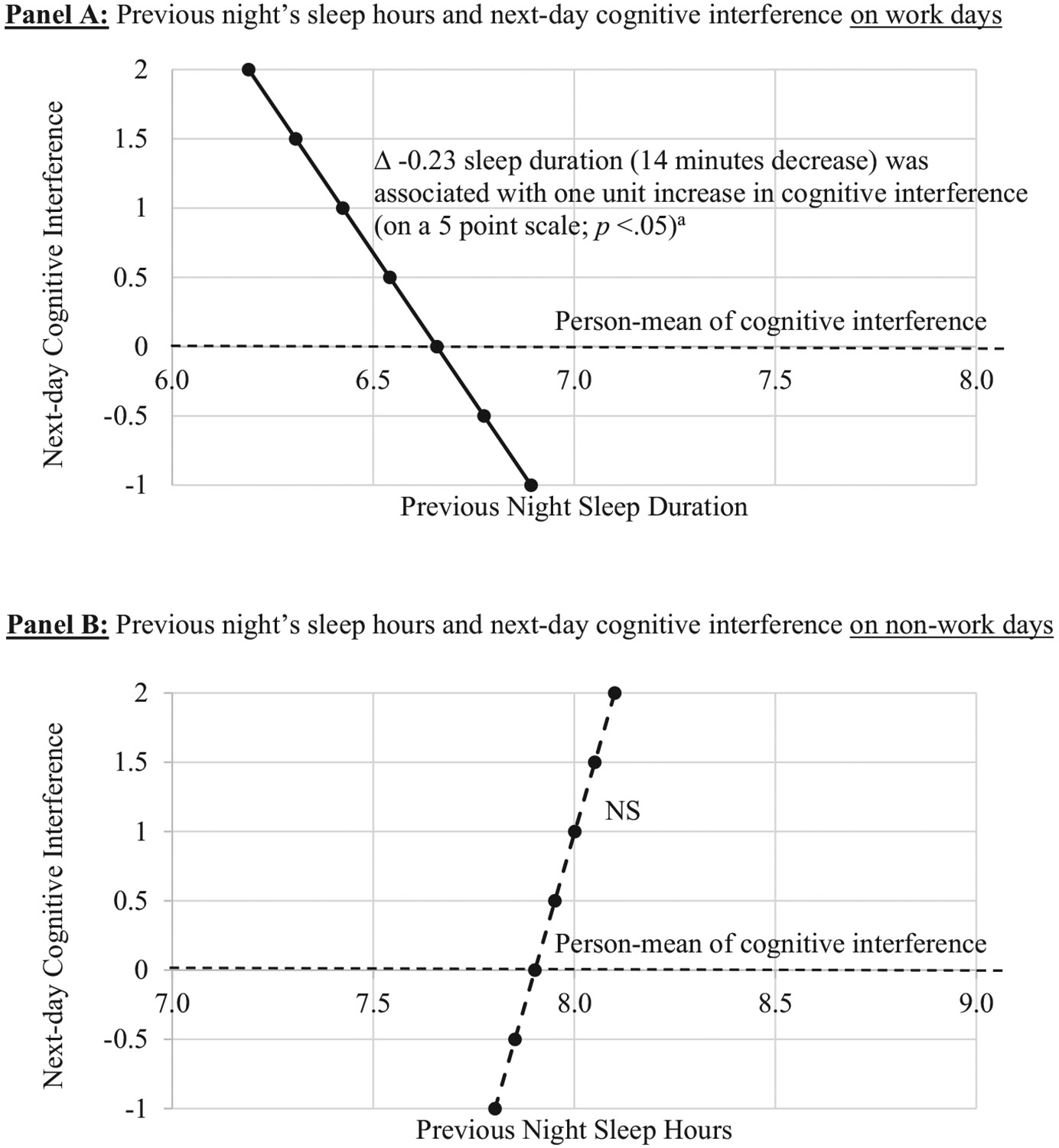
The temporal association between previous night's sleep duration and next-day cognitive interference moderated by type of days. Note. 869-867 days (72% were work days) from 130 employees; 8 consecutive days' data were nested within each employee. In the multilevel model, the effects of same day's and the following day's cognitive interference on nightly sleep duration were simultaneously estimated, and thus the significance of changes in sleep duration with one unit change in cognitive interference was tested. a Using z-scores, this effect can also be expressed as: A 0.07 SD decrease in sleep duration was associated with 1 SD increase in cognitive interference. All analyses adjusted for sociodemographic covariates, work hours, between-person associations, and previous day's sleep duration.
Fig. 3.
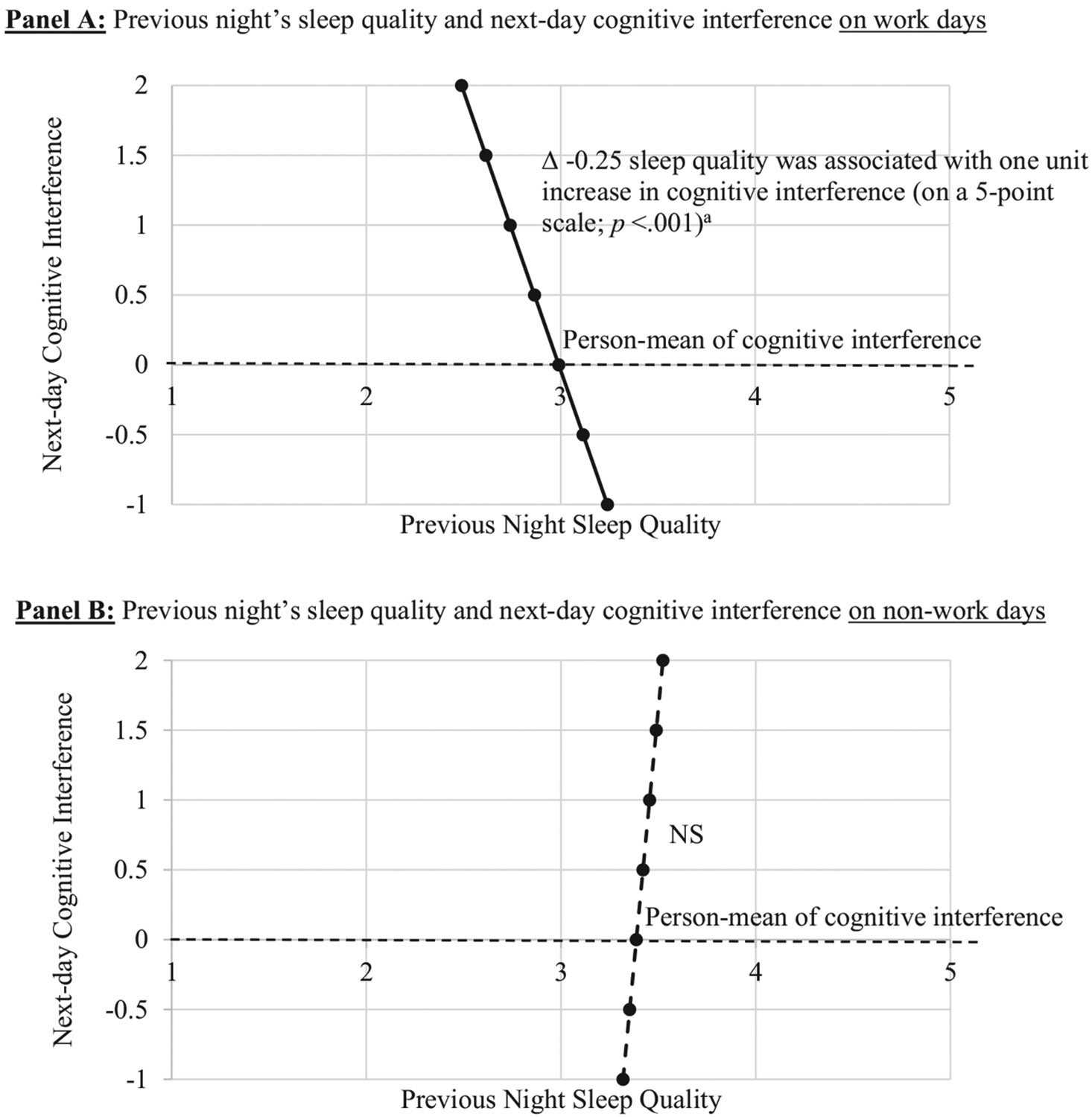
The temporal association between previous night's sleep quality and next-day cognitive interference moderated by type of days. Note. 869-867 days (72% were work days) from 130 employees; 8 consecutive days' data were nested within each employee. In the multilevel model, the effects of same day's and the following day's cognitive interference on nightly sleep quality were simultaneously estimated, and thus the significance of changes in sleep quality with a one unit change in cognitive interference are depicted. a Using z-scores, this effect can also be expressed as: A 0.15 SD decrease in sleep quality was associated with 1 SD increase in cognitive interference. All analyses adjusted for sociodemographic covariates, work hours, between-person associations, and previous day's sleep quality.
The two links (sleep duration or quality → cognitive interference) that were more salient on work days from the stratified bidirectional models were also validated in interaction models with the supported temporal direction only. In additional analyses, we included both workdays and non-work days and tested interaction effect between each sleep variable and workdays predicting next-day cognitive interference. Significant interactions of work days with sleep duration (B = −0.07, SE = 0.03, P < .05) and with sleep quality (B = −0.16, SE = 0.05, P < .01) were found. Consistent with the prior stratified model, shorter sleep duration was associated with the following day's more cognitive interference when the following day was a workday (B = −0.06, SE = 0.02, P < .001), but not when the following day was a non-work day (B = 0.01, SE = 0.02, P > .05). Poorer sleep quality was also associated with the following day's more cognitive interference on a next workday (B = −0.14, SE = 0.03, P < .001), but not on a next non-work day (B = 0.02, SE = 0.04, P > .05).
In supplementary analyses, we explored potential moderation by gender and other daily contextual factors including work location and day-of-week. We first used stratified analyses with both directions and then tested relevant interaction terms for a specific direction. No significant differences by gender were found (results not shown, but available from the authors upon request). In the stratified analyses for work days, we also examined whether the associations differed by the work location. When we compared onsite workdays (56% of work days) vs. working from home days (42%), a significant difference emerged on sleep latency only (B = −8.03, SE = 2.80, P < .01). More cognitive interference was associated with the night's longer sleep latencies after days when employees worked at home (B = 4.30, SE = 2.00, P < .05), but with shorter sleep latencies after days when they worked at the workplace (B = −3.73, SE = 1.87, P < .05). Lastly, a descriptive analysis by day-of-week (from non-nested, unadjusted regression models) indicated that the negative association between previous night's sleep quality and next-day cognitive interference was stronger on the Monday–Tuesday transition than on the Saturday–Sunday transition (B = −0.33, SE = 0.14, P < .05); there was no difference neither with other days of week (vs. Saturday–Sunday) nor with other sleep variables.
Discussion
This study examined temporal associations between daily cognitive interference and nightly sleep variables in a sample of middle-aged employed adults. Guided by the job-stress recovery model8,9 and previous research on the associations between daily stressful experiences and nightly sleep,1,12-14 we expected that poorer sleep recovery previous night would be associated with more cognitive interference the next day. Results revealed that earlier wake times, shorter sleep duration, and poorer sleep quality predicted experiencing more cognitive interference the next day. Significant associations in the opposite direction were also found with sleep timing variables, such that more same day's cognitive interference predicted earlier bedtimes and earlier wake times than usual. Importantly, these associations, particularly the temporal associations of previous night's shorter sleep duration and poorer sleep quality predicting the following day's more cognitive interference, were significant on work days, but not on non-work days. Our findings contribute to understanding how cognitive interference and sleep are associated in employees' daily lives. Sleep can be both an antecedent and consequence of employees' daily cognitive interference, which has implications for future interventions targeting employee sleep and performance at work.
Shorter sleep and poorer sleep than usual may indicate lack of resource recovery13 needed for the next day. Lack of relaxation and energy replenishment may contribute to generating more negative experiences during the following day. Previous research reported that poorer sleep quality was associated with less positive affect and more negative affect the subsequent day.12-14 Our findings extend this line of research by demonstrating that poorer sleep recovery, measured by shorter sleep duration, lower sleep quality, and earlier wake times, predicted more cognitive interference the next day. Specifically, poorer sleep recovery predicted experiencing more stressors the following day and more stressors were associated with more same-day cognitive interference. The associations between sleep and cognitive interference became reduced or non-significant after controlling for daily stressors in the model, which suggests potential mediation by daily stressors. Future research that includes multiple assessments per day could determine the temporal mechanism between sleep, stressors, and cognitive interference within a day. Taken together, poorer sleep recovery may presage more exposure to daily stressors1 and accompanying cognitive stress or interference (ie, off-task, intrusive, and unwanted thoughts), all of which may relate to poorer work performance22 and poorer socially interactive decisions.35
This study also reveals that sleep timing may signal, not only the day's stress and fatigue, but also an anticipation of a stressful day. First, more cognitive interference predicted earlier than usual bedtimes and corresponding earlier wake times. Given that most contemporary employees do not have enough time for sleep,6,7 earlier bedtimes than the employee's usual may mean that more cognitive interference made him/her stressed and tired. Earlier than usual bedtimes may also explain the link between previous day's more cognitive interference and the following morning's earlier than usual wake times, as there was a positive within-person correlation between bedtimes and wake times (Table 1). Unlike our hypothesis and somewhat inconsistent with the finding by Lee et al.,1 which used different types of stressors, we did not find the link between same day's cognitive interference and sleep latency that night. It may be worth to mention that the mean of sleep latency (19.67 minutes) seemed longer than what other studies observed in adult samples.36 In our sample, 24 employees reported 30 minutes or longer sleep latency on average per night. The null association did not change when we excluded those employees with long sleep latency. Our exploratory analyses further showed that the link between daily cognitive interference and the night's sleep latency differed by work location. On days when employees worked at home, more cognitive interference was associated with the night's longer sleep latencies. In contrast, on days when employees worked at the workplace, more cognitive interference during the work day was associated with shorter sleep latencies that night. Currently, we do not have good explanation for this complex finding. Perhaps, cognitive interference experienced while working with other colleagues in office may be qualitatively different (more stressful) from cognitive interference experienced while working from home, making employees fall asleep faster that night. Taken together, employees who experienced more cognitive interference might have gone to bed earlier and fallen asleep earlier, probably due to fatigue driving sleep need, and then might have woken earlier in the morning than usual.
Second, earlier wakes times not only were predicted by more cognitive interference during the prior day, but also predicted more cognitive interference later that day. The link from earlier wake times to more cognitive interference may be due to lack of sleep recovery.8,13,14,37 Earlier wake times predicted by more cognitive interference may also reflect employees' greater anticipation of another stressful day after experiencing more than usual cognitive interference the previous day. In this way, earlier than usual wake times seem to be a sensitive indicator of poorer recovery sleep and anticipation of a stressful day, in employees' daily lives. Note that most employees in our sample worked a daytime work schedule and all of them were office workers in the IT industry. We do not know whether daily variation in morning wake times was driven by variation in work start times (eg, early bird meeting at 8 AM) or not. It is intriguing that both temporal directions were found with employees' wake times and the magnitude of the associations for one direction versus the other was similar (ie, 0.30 or 0.32 hours earlier wake times were associated with one unit increase in cognitive interference). Future research may need to test with more diverse samples of employees to see how daily cognitive interference is associated with sleep timing variables among those who work different work schedules in different settings.
The temporal associations between daily cognitive interference and nightly sleep were observed only on work days, but not on non-work days. As employees' daily schedule is mostly fixed around their work time17 and they may have more opportunities for cognitive interference and less opportunities for sleep during work days,18,19 it is not surprising to find the significant associations between them on days when they worked. Specifically, shorter sleep duration and poorer sleep quality than usual were nightly determinants of the next-workday cognitive interference. Previous studies also reported that whether the day is a workday or not is an important variable that can change employees' stress physiology and the effects of workplace support. For example, employees' cortisol awakening response (CAR) was greater on work days than on non-work days.21 Moreover, a workplace intervention, designed to increase employees' schedule control and supervisor support about work-family issues, significantly improved employees' CAR only on non-work days, but not on work days;20 the authors explained that it may be due to that the employees had more opportunities for recovery on non-work days. The current study adds another evidence that workdays play an important role in the cognitive interference—sleep relationship. Note that workday represents one of the daily contexts that magnify the cognitive interference—sleep relationship in workers' lives. There may be other occupational and daily contextual factors that future research may want to explore more, such as the specific structure of workplace support. For example, one study found that a flextime that allowed workers to easily take time off for personal and family matters was correlated with reduction in the workers' stress and improvement in sleep significantly more than a compressed workweek.38 Future research could further examine whether and how specific aspects of workplace support play roles in the workers' sleep and cognitive performance and productivity at work. In addition, albeit very exploratory and descriptive, we observed that the negative association between previous night's sleep quality and next-day cognitive interference was stronger on the Monday–Tuesday transition than on the Saturday–Sunday transition when days were pooled across participants. Future studies that include more than 1 week's diary data could validate this finding in a more rigorous test after adjusting for person-level differences.
Limitations and future directions
The present study has several strengths, including the use of multiple days of telephone diary interview data and statistical rigor testing two competing directions in a single analytic model. Limitations in this study provide guidance for future research. First, the present study focused on employees purposively selected from an IT firm, and thus our findings may not generalize to employees in other contexts. We were only able to consider two race categories (69% were white) and had limited information regarding education. Future research could target a more diverse sample and capture total years of education. Some occupations involve more physical tasks than cognitive tasks, and for such occupations workplace injury may be a more relevant work outcome related to the workers' poor sleep.39 Moreover, this study used self-reports to measure daily cognitive interference and nightly sleep, which poses a potential risk for common-method bias.40 This concern may be reduced for our within-person level findings that controlled for between-person associations as well as previous night's sleep. Yet, between-person associations may still be inflated. Future research may benefit from incorporating more objective markers of sleep, such as actigraphy. Furthermore, our participants completed the daily diary each evening, which might have caused a recall bias in reporting previous night's sleep relative to the more typical morning sleep diary. Further, information on daytime napping was not collected. Future studies could consider assessing employees' daily sleep patterns more extensively, such as asking previous night's perceived sleep in the morning and measuring napping throughout the day. In addition, future research could test whether findings from this study can be replicated in workers who have clinical sleep problems or those with caregiving responsibilities. Our within-person level findings can rule out potential differences due to unobserved between-person level factors. However, we do not know whether the bidirectional associations between poorer sleep and more cognitive interference found in this study are modified by factors not assessed in this study such as, for example, caregiving stress management, meditation practices, or co-worker support. Lastly, following the emerging line of research that highlights the importance of considering combined influence of multiple sleep parameters on one's health,41 future research could examine whether and how multidimensional sleep health within individuals (eg, a sleep health index) is associated with daily cognitive interference.
Conclusion
This study reports that employees' poorer sleep predicts and is predicted by more cognitive interference especially on work days. Shorter sleep duration, poorer sleep quality, and earlier wake times predicted experiencing more intrusive, off-task thoughts the next work day that may interfere with focusing on job tasks. More cognitive interference on a given day, in turn, also predicted going to bed earlier and waking up earlier than usual potentially due to stress and fatigue. These cyclical associations reflect that employees' sleep is vulnerable to daily cognitive stress and also a contributor to cognitively stressful experiences. Depending on what we measure in participants' sleep, prediction and directionality in relation to daily experiences may differ, and overall this study finds bidirectional associations between sleep and daily cognitive interference. Findings from this study provide empirical evidence for why workplaces need to make more efforts to promote their employees' sleep. Good sleepers may be better performers at work due to greater ability to stay focused and on-task, with fewer errors and interpersonal conflicts.
Funding
This research was conducted as part of the Work, Family and Health Network (www.WorkFamilyHealthNetwork.org), which is funded by a cooperative agreement through the National Institutes of Health and the Centers for Disease Control and Prevention: Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant # U01HD051217, U01HD051218, U01HD051256, U01HD051276), National Institute on Aging (Grant # U01AG027669), Office of Behavioral and Social Sciences Research, and National Institute for Occupational Safety and Health (Grant # U01OH008788, U01HD059773). Grants from the National Heart, Lung, and Blood Institute (Grant #R01HL107240), William T. Grant Foundation, Alfred P. Sloan Foundation, and the Administration for Children and Families have provided additional funding.
Appendix A
Table A.1.
Comparison of fit statistic between models testing one supported temporal direction versus the other unsupported temporal direction.
| Change in −2 log-likelihood (−2LL) |
p-value for the likelihood ratio test (LRT) | |
|---|---|---|
| Sleep → Cognitive Interference Model (vs. Cognitive Interference → Sleep Model) | ||
| Sleep duration | 8.8095 | 0.0076 |
| Sleep quality | 10.9420 | 0.0026 |
| Wake time | 2.9485 | 0.1575 |
| Cognitive Interference → Sleep Model (vs. Sleep → Cognitive Interference Model) | ||
| Bedtime | 4.2090 | 0.0811 |
| Sleep latency | 2.0643 | 0.2535 |
Note. Changes in −2LL indicate whether the model with supported temporal direction (in bold) fits data better than the model with the opposite, unsupported temporal direction.
Fig. A.1.
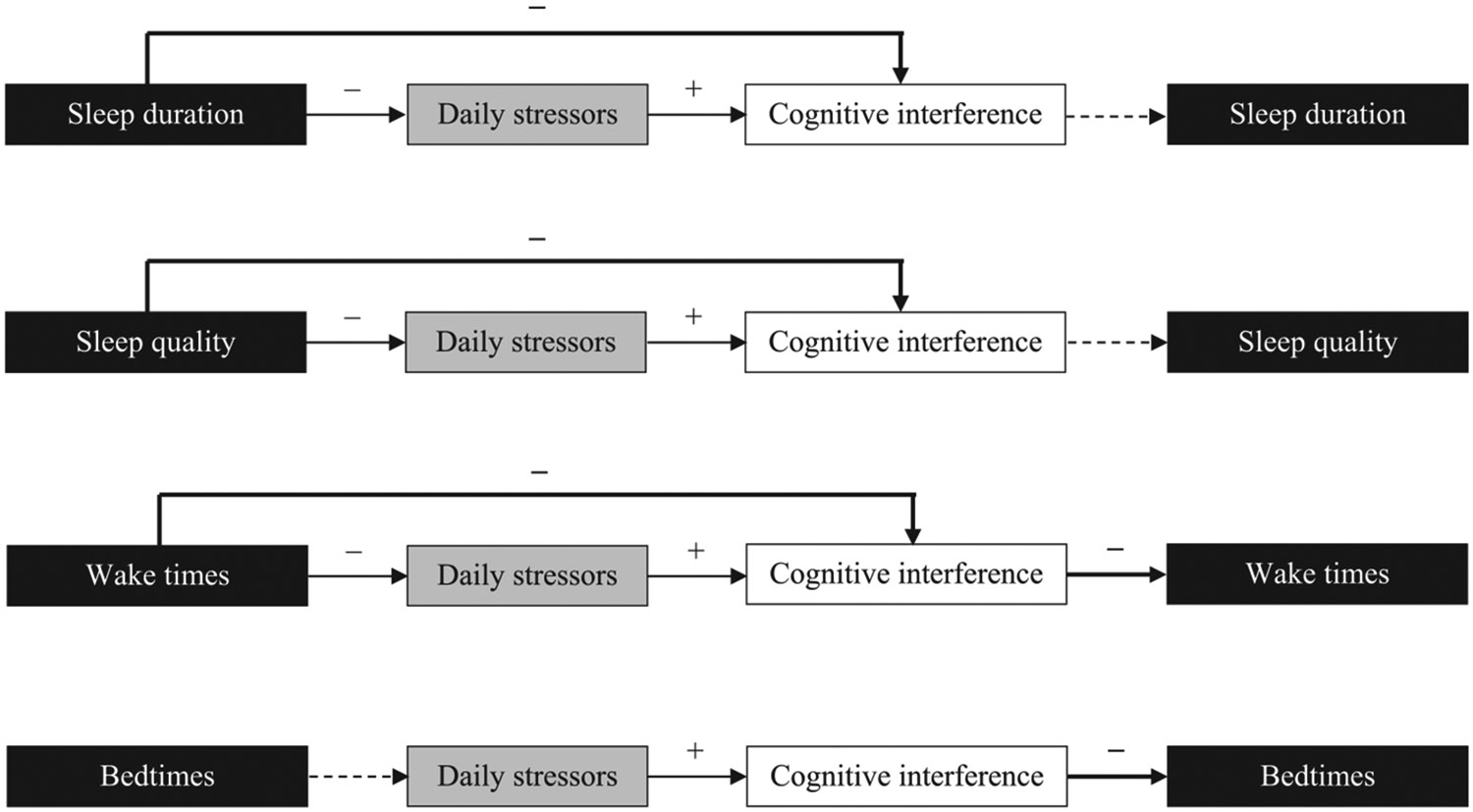
The temporal associations between sleep and cognitive interference in the context of daily stressorsNote. Each path was tested separately after adjusting for all covariates listed in Table 2B. Each path with solid arrow was statistically significant at P < .05; dotted arrows indicate non-significant paths. Bolded arrows indicate our main results on the temporal association between each sleep variable and daily cognitive interference. The associations between sleep and cognitive interference became reduced or non-significant after controlling for daily stressors in the model.
Footnotes
Disclosure statement
The authors have indicated no financial conflicts of interest relevant to the current study. Outside of the current work, Orfeu M. Buxton received two subcontract grants to Penn State from Mobile Sleep Technologies (NSF/STTR #1622766, NIH/NIA SBIR R43AG056250).
References
- 1.Lee S, Crain TL, McHale SM, Berkman L, Almeida DM, Buxton OM. Daily antecedents and consequences of nightly sleep. J Sleep Res. 2016;26(4):498–509. 10.1111/jsr.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, Buxton OM. Bidirectional, temporal associations of sleep with positive events, affect, and stressors in daily life across a week. Ann Behav Med. 2017;51(3):402–415. 10.1007/s12160-016-9864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stawski RS, Mogle J, Sliwinski MJ. Intraindividual coupling of daily stressors and cognitive interference in old age. J Gerontol B Psychol Sci Soc Sci. 2009;66B(S1):i121–i129. 10.1093/geronb/gbr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killgore WDS. Effects of sleep deprivation on cognition. In: Kerkhof GA, Van Dongen HPA, editors. Progress in Brain Research. Elsevier; 2010. p. 105–129. 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 5.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 6.Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geurts SA, Sonnentag S. Recovery as an explanatory mechanism in the relation between acute stress reactions and chronic health impairment. Scand J Work Environ Health. 2006;32(6):482–492. [DOI] [PubMed] [Google Scholar]
- 9.Westman M, Eden D. Effects of a respite from work on burnout: vacation relief and fade-out. J Appl Psychol. 1997;82(4):516–527. [DOI] [PubMed] [Google Scholar]
- 10.Barling J, Barnes C, Carleton E, Wagner D. Work and Sleep: Research Insights for the Workplace. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 11.Mullins HM, Cortina JM, Drake CL. Sleepiness at work: a review and framework of how the physiology of sleepiness impacts the workplace. J Appl Psychol. 2014;99(6):1096–1112. [DOI] [PubMed] [Google Scholar]
- 12.Scott BA, Judge TA. Insomnia, emotions, and job satisfaction: a multilevel study. J Manage. 2006;32(5):622–645. 10.1177/0149206306289762. [DOI] [Google Scholar]
- 13.Sonnentag S, Binnewies C, Mojza EJ. “Did you have a nice evening?” a day-level study on recovery experiences, sleep, and affect. J Appl Psychol. 2008;93(3):674–684. 10.1037/0021-9010.93.3.674. [DOI] [PubMed] [Google Scholar]
- 14.Totterdell P, Reynolds S, Briner RB. Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep. 1994;17(5):466–475. [DOI] [PubMed] [Google Scholar]
- 15.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–243. [DOI] [PubMed] [Google Scholar]
- 16.Åkerstedt T, Kecklund G, Axelsson J. Impaired sleep after bedtime stress and worries. Biol Psychol. 2007;76(3):170–173. 10.1016/j.biopsycho.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Roenneberg T, Wirz-justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 18.Monk TH, Buysse DJ, Rose LR, Hall JA, Kupfer DJ. The sleep of healthy people—a diary study. Chronobiol Int. 2000;17(1):49–60. 10.1081/CBI-100101031. [DOI] [PubMed] [Google Scholar]
- 19.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 20.Almeida DM, Lee S, Walter KN, Lawson KM, Kelly EL, Buxton OM. The effects of a workplace intervention on employees' cortisol awakening response. Community Work Fam. 2018;21(2):151–167. 10.1080/13668803.2018.1428172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. 10.1016/S0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 22.Jett QR, George JM. Work interrupted: a closer look at the role of interruptions in organizational life. Acad Manage Rev. 2003;28(3):494–507. 10.5465/amr.2003.10196791. [DOI] [Google Scholar]
- 23.Bray J, Kelly E, Hammer L, et al. An integrative, multilevel, and transdisciplinary research approach to challenges of Work, family, and health. Methods Rep RTI Press. 2013:1–38. 10.3768/rtipress.2013.mr.0024.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida DM, Davis KD, Lee S, Lawson KM, Walter Kimberly N, Moen P. Supervisor support buffers daily psychological and physiological reactivity to work-to-family conflict. J Marriage Fam. 2015;78(1):165–179. 10.1111/jomf.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Mogle JA, Lovett BJ, Stawski RS, Sliwinski MJ. What's so special about working memory? An examination of the relationships among working memory, secondary memory, and fluid intelligence. Psychol Sci. 2008;19(11):1071–1077. 10.1111/j.1467-9280.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 27.Wegner DM, Zanakos S. Chronic thought suppression. J Pers. 1994;62(4):615–640. [DOI] [PubMed] [Google Scholar]
- 28.Wells A, Davies MI. The thought control questionnaire: a measure of individual differences in the control of unwanted thoughts. Behav Res Ther. 1994;32(8):871–878. 10.1016/0005-7967(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 29.Sarason IG, Sarason BR, Keefe DE, Hayes BE, Shearin EN. Cognitive interference: situational determinants and traitlike characteristics. J Pers Soc Psychol. 1986;51(1):215–226. 10.1037/0022-3514.51.1.215. [DOI] [Google Scholar]
- 30.Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull. 2006;32(7):917–929 http://psp.sagepub.com/content/32/7/917.short, Accessed date: 27 December 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. [DOI] [PubMed] [Google Scholar]
- 32.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med. 2013;79:7–15. 10.1016/j.socscimed.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: relationships to self-reported health and activity life style. J Gerontol Psychol Sci. 1993;48(1):P1–P11. [DOI] [PubMed] [Google Scholar]
- 34.Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis. Newbury Park: Sage; 1992. [Google Scholar]
- 35.Anderson C, Dickinson DL. Bargaining and trust: the effects of 36-h total sleep deprivation on socially interactive decisions. J Sleep Res. 2010;19(1 PART. 1):54–63. 10.1111/j.1365-2869.2009.00767.x. [DOI] [PubMed] [Google Scholar]
- 36.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain. 2006;129(6):1609–1623. 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 37.Demerouti E, Bakker AB, Geurts SAE, Taris TW. Daily recovery from work-related effort during non-work time. Curr Perspect Job Stress Recover Res Occup Stress Well Being. 2009;7:85–123. 10.1108/S1479-3555(2009)0000007006. [DOI] [Google Scholar]
- 38.Haley MR, Miller LA. Correlates of flexible working arrangements, stress, and sleep difficulties in the US workforce: does the flexibility of the flexibility matter? Empir Econ. 2015:1395–1418. 10.1007/s00181-014-0836-4. [DOI] [Google Scholar]
- 39.Nakata A. Effects of long work hours and poor sleep characteristics on workplace injury among full-time male employees of small- and medium-scale businesses. J Sleep Res. 2011;20(4):576–584. 10.1111/j.1365-2869.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 40.Podsakoff PM, MacKenzie SB, Lee J-Y, Podsakoff NP. Common method variance in behavioral research: a critical review of the literature and recommended remedies. J Appl Psychol. 2003;88(5):879–903. 10.1037/0021-9010.88.5.879. [DOI] [PubMed] [Google Scholar]
- 41.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]


