Abstract
Purpose
One major consequence of lung transplantation is the development of oropharyngeal dysphagia. This systematic review aims to appraise and synthesize the available evidence of the use of instrumental assessments to outline the characteristics of post‐lung transplant dysphagia.
Methods
Following the identification of appropriate search terms for the question, a literature search was conducted in PubMed, Scopus, and the Health and Medical Collection of Proquest Research Library and included records between inception and September 14, 2023. Search strategies included the use of text words and subject headings (e.g., MeSH and Index terms) related to (1) dysphagia or swallowing (swallow*, deglutition disorder*), (2) lung transplant (lung transplant*, post‐operative, post‐lung), and (3) complications (adverse effects, *complications, treatment outcome).
Results
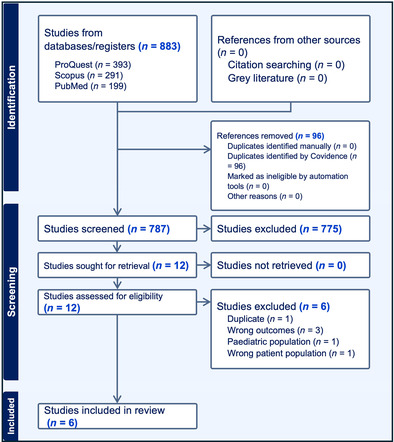
The literature search strategy yielded a total of 883 studies from the electronic database search, with no additional records identified through other sources. After the removal of duplicates (n = 96), a total of 787 studies were screened through title and abstracts which eliminated 775 studies. Six studies were ultimately included in the systematic review. The selected articles included patients who underwent lung transplantation and all but one study utilized a retrospective design. A lack of transparency regarding instrumental evaluation protocols (videofluoroscopic [VFSS] and Flexible Endoscopic Evaluation of Swallowing [FEES]) including the number and bolus types used during the instrumental evaluations appeared as a theme in the studies included. The Penetration‐Aspiration Scale (PAS) was systematically utilized to measure dysphagia safety outcome. Handling of the PAS scale was not consistent across studies, however penetration or aspiration ranged from 52.4% up to 100%. Additionally, silent aspiration rates ranged from 14.2% to 61.9%.
Conclusions
This review sought to describe the post‐operative swallowing function and its physiological parameters following lung transplantation. We examined the results reported and the methods utilized in obtaining these results in the existing literature. Limited reporting practices for physiological parameters were found, however the airway invasion was reported in all studies with variation in degrees of swallowing safety related deficits, with PAS being the most widely used scale to describe airway invasion depth and response. Future studies exploring dysphagia outcomes post‐lung transplant should comment on the altered physiological mechanisms of the swallow to further expand on the physiological deficits observed following transplantation in this group and allow for treatment planning.
Level of evidence
Level 1.
Keywords: dysphagia, lung transplant, oropharynx
One major consequence of lung transplantation is the development of oropharyngeal dysphagia. This systematic review aims to appraise and synthesize the available evidence using instrumental assessments to outline the characteristics of post‐lung transplant dysphagia.
1. INTRODUCTION
Lung disease affects 26 million people in the United States, with an annual mortality rate of 156,979 people. 1 , 2 More recent data estimates as of January 2024 were reported by the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients, revealing 3026 lung transplants (LTs) occurred in the US alone in 2023. 3 LTs are considered a critical and life‐saving treatment for those with end‐stage lung disease, which may develop from a number of respiratory diseases. 4 , 5 Postoperative LT complications that have been known to include primary graft dysfunction, chronic allograft dysfunction, pleural complications, and nerve injury. 6 , 7 These complications often result from damage to the neuroanatomic pathways during surgery, which may result in altered sensation, motor programming, coordination, and airway protection. 8 , 9
During LTs, the phrenic nerve and the recurrent laryngeal branch (RLN) of the vagus nerve are exposed and susceptible to damage. 6 , 10 , 11 Following repeat or prolongated endotracheal intubations, the superior laryngeal branch (SLN) of the vagus nerve and RLN are susceptible to compression injury. 12 The RLN provides motor control to essential intrinsic laryngeal muscles, which allow for closure and protection of the airway, expiratory force generation, and vocal fold adduction, in addition to sensory innervation to the larynx below the level of the true vocal folds. 13 , 14 Damage to this nerve reduces respiratory capacity, which may compromise one's ability to clear or dislodge aspirate material from the airway. Perhaps even more concerning for this immunocompromised patient group are the potential for damage to the vagus nerve to lead to silent aspiration. 9 , 15 Instrumental evaluations are considered the gold standard assessment methods (Videofluoroscopic Swallow Studies [VFSS] and Fiberoptic Endoscopic Evaluation of Swallowing [FEES]) for identifying dysphagia as they allow for direct visualization of the swallow mechanism to determine impairments in function and physiology that resulting in safety concerns (e.g., silent aspiration) and efficiency concerns (e.g., post‐swallow residue). 16 , 17 In human LT recipients, sensory recovery of the cough reflex has been noted at 12 months or more following transplantation, which emphasizes the need to conduct repeated instrumental assessments that allow for direct visualization of the swallow. 18
A previous systematic review by Black et al. 15 explored the frequency and characteristics for the development of oropharyngeal dysphagia and laryngeal dysfunction after heart and/or LTs. The review explored the incidence and characteristics of voice and swallowing function following these procedures along with the risk factors leading to these complications. Despite the review reporting these findings, no comment was made on the mechanistic parameters of the swallow that may lead to the presentation of dysphagia in this patient population. The goal of this systematic review is to scrutinize the available evidence regarding the pathophysiological presentation of dysphagia following LT alone in studies that utilized instrumental evaluations of swallowing. Questions we aim to explore include:
What pathophysiological parameters are reported and how do they characterize the swallow of this patient population?
Which validated outcome measures are used to quantify pathophysiology post‐LT dysphagia?
2. METHODS
The development and methodology of this systematic review study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 19
2.1. Search strategy
A reference and instruction librarian at The George Washington University Health Sciences Library assisted by providing guidance in building and conducting a comprehensive systematic search of the available literature in three online databases (PubMed, Scopus, and the Health and Medical Collection of Proquest Research Library) and included records between inception and September 14, 2023. Search strategies included the use of truncated text words (root word followed by an asterisk) and subject headings (e.g., MeSH and Index terms) related to (1) dysphagia or swallowing (swallow*, deglutition disorder*), (2) lung transplant (lung transplant*, post‐operative, post‐lung), and (3) complications (adverse effects, *complications, treatment outcome). See Appendix S1 for full search strategies for all databases.
2.2. Eligibility criteria
The systematic review focus was to identify studies that met the following criteria: (1) Population: involved adult patients (≥18 years of age) who underwent either single or double LT procedures, (2) Exposure: had an instrumental swallowing assessment completed which involved either a VFSS or a FEES (3) Outcome: discussed swallow physiology related to oral or pharyngeal dysphagia. As such, studies that were: (1) animal studies, (2) involved a pediatric population, (3) did not assess oropharyngeal swallowing, (4) did not assess swallowing using either VFSS or FEES, and (5) reported solely on esophageal dysphagia were excluded. Additionally, we excluded studies that were case studies and case series in their design, tutorials, educational reports, other systematic reviews, book chapters, and gray literature (conference abstracts, proceedings, and dissertations).
2.3. Study selection
The search was then inputted into the Covidence, a web‐based collaboration software platform and systematic review management system. 20 Any duplicate studies from the initial search yield were removed using the software. Dyads of members of the study team then independently reviewed titles and abstracts, excluding studies that did not meet the inclusion criteria of the systematic review. Percent agreement were calculated for each dyad in the study team at the title and abstract screening level Any disagreements or discrepancies in judgments led to the study being retained for full text review to ensure accuracy of inclusion of the appropriate studies. The full texts of all studies that were included was then reviewed independently and in duplicate to determine whether any further studies were to be excluded. A reason for rejection was provided for any article that was excluded at the full text review. Cohen's Kappa was calculated to evaluate inter‐rater agreement between both raters at the full text review level. Any conflicts in rating following full text review of studies were ultimately resolved by a third reviewer.
2.4. Data extraction process
Data extraction was completed independently and in duplicate by two of the three members of the review team for the articles that met the inclusion criteria. Data was extracted directly into Covidence and included the following information: (1) study design, (2) patient demographics (sex; preliminary diagnoses; sample size; age range; type of transplant), (3) type of instrumental swallowing assessment conducted (VFSS, FEES, both VFSS and FEES, mix of clinical bedside and instrumental assessment), and (4) swallowing data reported/outcome measure (penetration/aspiration status, residue, any other physiological parameters reported). Further scrutiny of the details of instrumental assessment were then extracted including: type of assessment (e.g., FEES or VFSS) and number of patients who underwent instrumental assessment, protocol of assessment (number of boluses presented, consistency of bolus, volume, order of presentation, etc.), evaluation outcomes (presence of airway invasion, degree of invasion, etc.), analysis procedure used (rating of swallows, number of raters, etc.), post hoc review of instrumental assessment (recording and review of imaging for confirmation of findings as opposed to real‐time review), definitions for aspiration and penetration (definitions authors used to categorize aspiration and penetration), and their reported distributions (number of patients presenting with airway invasion compared to total number of patients included in the study).
2.5. Risk of bias (quality) assessment
To evaluate the quality of the extracted studies, two reviewers independently assessed the design and reporting methods of the included articles and explored their risk of bias. Various tools were used to assess and record the risk of bias using multiple means of assessing bias. First, we used Cochrane Collaboration's Tool for Assessing Risk of Bias. 21 The criteria assessed using the tool were bias in the domains of selection, performance, detection, attrition, and reporting. An overall bias rating was also provided for each study. We then used a swallowing disorders specific research tool to reexamine the risk of bias in the reporting rigor and transparency of swallowing related information using the Framework for Rigor and Transparency in Research on Swallowing (FRONTIERS). 22 This framework is used for dysphagia‐related research and asks vital questions about study design and reporting in a number of domains, however only the universally applicable questions and the instrumental assessment domains (videofluoroscopic swallow study and flexible endoscopic evaluation of swallowing) were used. 23
3. RESULTS
3.1. Study selection
The literature search strategy yielded a total of 883 studies from the electronic database search, with no additional records identified through other sources. After the removal of duplicates (n = 96), a total of 787 studies were screened through title and abstracts which eliminated 775 studies. Percentage agreement at the title and abstract review level was 94.3%. The remaining 12 studies underwent full text review by two raters and an additional 6 studies were excluded. At the full text review level, Cohen's Kappa was calculated to be 0.71 indicating substantial agreement between raters. 24 Exclusion at the full text level was for the following reasons: (1) did not assess oropharyngeal swallowing (n = 4), (2) involved a pediatric population (n = 1), and (3) used the same patient group with no new physiological outcome data reported (n = 1). 25 Ultimately a total of six articles met the eligibility criteria and were included. Details regarding study screening and selection for inclusion can be found in the PRISMA flow diagram presented in Figure 1.
FIGURE 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources.
3.2. Study characteristics
Table 1 provides a summary of the six studies included in the systematic review, 26 , 27 , 28 , 29 , 30 , 31 with the authors and titles of the studies, study types, and patient population specific data (sample size, transplant type, underlying diagnosis leading to transplantation, and age). Additionally, all but one study utilized a retrospective design. The highest number of participants for any single study included was 297. The majority of studies included a higher proportion of patients that underwent bilateral/double lung transplantation compared to single lung transplants.
TABLE 1.
Overview of study populations.
| Study | Title | Underlying diagnosis leading to lung transplantation | Lung transplant type | Study type | N (Males) | Age (years) | |||
|---|---|---|---|---|---|---|---|---|---|
| SLTx | DLTx | Other | Mean | SD | |||||
| Atkins et al. 26 | Assessing oropharyngeal dysphagia after lung transplantation: altered swallowing mechanisms and increased morbidity | COPD | NR | NR | NA | Retrospective | 149 (76) | 49.0 | NR |
| Bronchiectasis | |||||||||
| IPF | |||||||||
| PPH | |||||||||
| Other | |||||||||
| Baumann et al. 27 | Postoperative swallowing assessment after lung transplantation | Not reported | 40 | 247 | DLTx with other cardiothoracic surgery (10) | Retrospective | 297 (165) | 56.2 | NR |
| Dallal‐York et al. 28 | Incidence, risk factors, and sequelae of dysphagia mediated aspiration following lung transplantation | Restrictive lung disease | 12 | 193 | NA | Retrospective | 205 (104) | 59.8 | 12.4 |
| Obstructive lung disease | |||||||||
| Cystic fibrosis or immunodeficiency disorder | |||||||||
| Pulmonary vascular disease | |||||||||
| Other | |||||||||
| Dallal‐York et al. 29 | A prospective examination of swallow and cough dysfunction after lung transplantation. | Restrictive lung disease | 4 | 41 | NA | Prospective | 45 (33) | 60.1 | 10.8 |
| Coronavirus disease 2019 | |||||||||
| Obstructive lung disease | |||||||||
| Cystic fibrosis of immunodeficiency disorder | |||||||||
| Congenital malformation | |||||||||
| Miles et al. 30 | Dysphagia and medicine regimes in patients following lung transplant surgery: a retrospective review. | COPD | 0 | 101 | NA | Retrospective | 101 (48) | 50.4 | 12.9 |
| Cystic fibrosis | |||||||||
| Interstitial lung | |||||||||
| Disease/IPF | |||||||||
| Bronchiectasis | |||||||||
| Pulmonary | |||||||||
| hypertension | |||||||||
| Scleroderma | |||||||||
| Other | |||||||||
| Reedy et al. 31 | Characterizing swallowing impairment in a post‐lung transplant population. | Cystic fibrosis | 2 | 40 | NA | Retrospective | 42 (22) | 58.4 | 9.94 |
| COPD | |||||||||
| Idiopathic pulmonary fibrosis | |||||||||
| Interstitial lung disease | |||||||||
| Sarcoidosis | |||||||||
Abbreviations: COPD, chronic obstructive pulmonary disease; DLTx, double lung transplant; IPF, interstitial pulmonary fibrosis; M, mean; NR, not reported; PPH, primary pulmonary hypertension; SD, standard deviation; SLTx, single lung transplant.
3.3. Swallowing biomechanics
Instrumental assessments to describe swallowing physiology included videofluorosocopic swallowing studies alone in two studies, flexible endoscopic evaluation of swallowing alone in two studies, and a mix of VFSS and FEES in the remaining three studies. As can be noted in Table 2, administration protocol information provided for the instrumental evaluations that were reported in only two studies. 29 , 31 The remainder of the studies failed to report administration protocols (e.g., number of trials presented and bolus consistencies, volumes, and presentation methods: including contrast material, type of barium sulfate and/or food dye along with concentrations and brand), image acquisition rates (VFSS), or noted a lack of standardization in the process of rating described relative to time of the study (e.g., real‐time or post‐hoc).
TABLE 2.
Details of instrumental assessment.
| Study | Title | Instrumental assessment (N) | Protocol | Instrumental swallow evaluation outcomes reported | Analysis procedure | Post hoc review procedure | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N/total | % | N/PenOrAsp | % | |||||||
| Atkins et al. 26 | Assessing oropharyngeal dysphagia after lung transplantation: altered swallowing mechanisms and increased morbidity |
VFSS (12.1% of Group 1; N = 18 a ) FEES (87.9% of Group 1; N = 131 a ) Group 1 = instrumental assessment; Group 2 = no instrumental assessment |
NR | Normal swallowing a | 44/149 | 29.53 | NR | NR | ||
| Laryngeal penetration or frank tracheal aspiration | 105/149 | 70.50 | ||||||||
| Tracheal aspiration | 67/149 | 44.96 | 67/105 | 63.80 | ||||||
| Silent aspiration | 52/149 | 34.89 | 52/67 | 77.61 | ||||||
| Patients with sensory response to aspirant a | 15/149 | 10.06 | 15/67 | 22.38 | ||||||
| Baumann et al. 27 | Postoperative swallowing assessment after lung transplantation |
FEES (N = 10) VFSS a (N = 287) |
Lack of standardization in timing and completion of exams | Normal swallow a | 99/297 | 33.33 | Completed VFSS as standard of care by primary SLP and that data was extracted from EMR | NR | ||
| Deep laryngeal penetration or aspiration | 198/297 | 66.66 | ||||||||
| Silent deep laryngeal penetration and aspiration a | 184/297 | 61.95 | 184/198 | 92.92 | ||||||
| Dallal‐York et al. 28 | Incidence, risk factors, and sequelae of dysphagia mediated aspiration following lung transplantation. | VFSS (N = 205) | NR | Safe Swallow | 41/205 | 20.00 | Completed VFSS as standard of care with primary SLP and that data was extracted from EMR | NR | ||
| Penetration | 82/205 | 40.00 | ||||||||
| Aspiration | 82/205 | 40.00 | ||||||||
| Dallal‐York et al. 29 | A prospective examination of swallow and cough dysfunction after lung transplantation. | FEES (N = 45) |
Gatorade trials: 5 mL ×2; 10 mL ×2; comfortable cup sip ×2 teaspoon pudding ×2; 0.25 portions of saltine cracker 2×; all mixed with white food dye |
Safe Swallow | 0/45 | 0 | Two blinded raters (SLP and research assistant) independently rated PAS for each swallow during the exam (worst PAS used per swallow) | NR | ||
| Penetration | 27/45 | 60.00 | ||||||||
| Did not eject material above the vocal folds | 19/45 | 42.22 | ||||||||
| Did not clear material on the VF | 8/45 | 17.78 | ||||||||
| Aspiration | 18/45 | 40.00 | ||||||||
| Material was ejected from the trachea despite effort | 1/45 | 5.55 | ||||||||
| Material not ejected from the trachea despite effort | 4/45 | 8.89 | ||||||||
| Silent aspirators | 13/45 | 28.89 | ||||||||
| Miles et al. 30 | Dysphagia and medicine regimes in patients following lung transplant surgery: A retrospective review. | FEES (N = 65) | NR | Normal swallowing a | 16/65 a | 24.61 | Completed FEES as standard of care by primary treating SLP clinician; NZSS; PAS; DOSS; FOIS | A second experienced SLP marked 20% of all FEES recordings for reliability | ||
| Aspiration (PAS 4–8) | 49/65 | 75.38 | ||||||||
| Aspirated silently | 31/65 | 47.69 | 31/49 | 63.26 | ||||||
| Reedy et al. 31 | Characterizing swallowing impairment in a post‐lung transplant population. | VFSS (N = 42) | MBSImp base protocol (12 swallows) | Normal swallowing (PAS typical range 1–2) a | 20/42 a | 47.61 | Completed VFSS as standard of care by treating clinician (six MBSImp trained clinicians) and that data (MBSImp; PAS; IDDSI; FOIS) was extracted from EMR | NR | ||
| Penetration (PAS = 3 only) | 13/42 | 30.95 | 13/22 | 59.09 | ||||||
| Aspiration | 8/42 | 19.04 | 8/22 | 36.36 | ||||||
| Silent aspiration | 6/42 | 14.2 | 6/22 | 27.27 | ||||||
Abbreviations: DOSS, dysphagia outcome and severity scale; EMR, electronic medical record; FEES, fiberoptic endoscopic evaluation of swallowing; FOIS, functional oral intake scale; IDDSI, International Dysphagia Diet Standardization Initiative; MBSImp, The Modified Barium Swallow Impairment Profile; n, sample size; NR, not reported; NZSS, New Zealand Secretion Scale; PAS, penetration aspiration scale; SLP, speech‐language pathologist; VF, vocal fold; VFSS, Videofluoroscopic Swallow Study.
Inference made based on other reported numbers in the article.
3.4. Swallowing outcomes
Analysis procedures of the instrumental assessments lacked rigor and transparency in their reporting, with many studies reporting results grading airway safety alone using descriptors or the 8‐point Penetration Aspiration Score scale 32 without clearly indicating how a final score was selected (worst score or most occurring score). A categorical grouping of “normal” versus “penetration” versus “aspiration” or a dichotomized grouping of “safe versus unsafe” or “normal versus abnormal” was attempted, along with a further classification of sensory response in the abnormal swallows to indicate presence or absence of sensory response to aspirant. Despite similar methodologies in terms of number of groups, the definitions for which scores fell into the corresponding dichotomized categories differed across studies (Table 3). For example, one study 30 grouped PAS scores from 4 to 8 into the aspirator category while scores of 5&8 fell into the silent aspiration category which varied significantly from other studies where scores of 1–5 were considered non‐aspirators 28 , 29 and only PAS 8 fell into the silent aspiration category. Finally, it was impossible to determine the exact meaning of some descriptors used in the remaining studies. 26 , 27 For example, “gross aspiration” and “deep laryngeal penetration” do not demarcate the exact boundary in which a selection would be made which hinders the assigning of a PAS score that would allow for comparisons with other studies.
TABLE 3.
Penetration‐aspiration definitions.
| Study | Safe/normal swallowing | Penetration or aspiration grouping | Aspiration | Silent aspiration | ||
|---|---|---|---|---|---|---|
| Definition | Definition | Reported (Y/N) | Definition | Reported (Y/N) | Definition | |
| Atkins et al. 26 | Normal swallowing | Laryngeal penetration or frank tracheal aspiration | Y | Gross aspiration alone | Y | Silent episodes without protective mechanism such as reflex cough |
| Baumann et al. 27 | Normal | Deep laryngeal penetration or aspiration | Y | Absent sensory response | N | NR |
| Dallal‐York et al. 28 | Safe (PAS 1–2) | Unsafe (PAS 3–8) | Y | Aspirators (PAS 6–8) | N | NR |
| Dallal‐York et al. 29 | NR | Aspirators (PAS: 6–8) | Y | Non‐silent aspirators (PAS 6–7) | Y | Silent aspirators (PAS 8) |
| Miles et al. 30 | NR | NR | Y | Aspiration (PAS 4–8) | Y | Silent aspiration (PAS 5 and 8) |
| Reedy et al. 31 | Typical (PAS 1–2) | Atypical (PAS 3–8) | Y | NR | Y | Silent aspiration (PAS 8) |
Abbreviations: NR, not reported; PAS, penetration‐aspiration scale.
Ultimately, despite not using the PAS scale to describe airway invasion (penetration or aspiration), studies did indicate penetration and/or aspiration status and mostly specified when silent aspiration occurred. As can be seen in Table 4, varying degrees of airway safety concerns following LT were found for either penetration or aspiration, ranging from 52.4% 31 to 100%. 28 Penetration alone was reported in three studies, where it ranged from 33.3% 31 to 60%. 29 Aspiration alone was found to range between 19% 31 and 75%. 30
TABLE 4.
Swallowing safety distributions.
| Study | Penetration or aspiration | Penetration alone | Aspiration alone | Silent aspiration | ||||
|---|---|---|---|---|---|---|---|---|
| PenOrASP/total cohort | % | Pen/total cohort | % | Asp/total cohort | % | Silent Asp/total cohort | % | |
| Atkins et al. 26 | 105/149 | 70.5 | NR | NR | 67/149 | 44.9 | 52/149 | 34.8 |
| Baumann et al. 27 | 198/297 | 66.6 | NR | NR | NR | NR | 184/297 | 61.9 |
| Dallal‐York et al. 28 | ||||||||
| 1. Underwent pre‐ and post‐operative VFSS | 142/170 a | 83.5 a | 66/170 | 38.8 | PAS 6–8: 76/170 | 44.7 | PAS 8: 36/170 | 21.1 |
| 2. Underwent postoperative VFSS only | 164/205 a | 80.0 a | 82/205 | 40.0 | PAS 6–8: 82/205 | 40.0 | NR | NR |
| Dallal‐York et al. 29 | 45/45 a | 100 a | 27/45 | 60.0 | PAS 6–8: 18/45 | 40.0 | PAS 8: 13/45 | 28.8 a |
| Miles et al. 30 | NR | NR | NR | NR | PAS 4–8: 49/65 | 75.0 | PAS 5&8: 31/65 | 47.0 |
| Reedy et al. 31 | 22/42 | 52.4 | 14/42 a | 33.3 a | PAS 6–8: 8/42 | 19.0 | PAS 8: 6/42 | 14.2 a |
Abbreviation: NR, not reported.
Inference/recalculation made based on other reported numbers in the article.
3.5. Swallowing efficiency and other parameters
Only one study included information about swallowing efficiency in their descriptions of swallowing physiology. 31 In their study, Reedy and colleagues focused on safety as the primary outcome, however, they reported that 59.52% (25/42 patients) presented with abnormal post‐swallow residue scores (MBSImp™ scores of 2 or more) 33 for component 16 (pharyngeal residue). Additionally, they were the only study that provided information regarding other swallowing physiology and biomechanics that may be contributing to the unsafe swallowing presentation outside of the global safety measurement itself. They note significant associations between unsafe airway protection (PAS scores of 3 or higher) and impairments in laryngeal elevation, epiglottic inversion, laryngeal vestibule closure, and the pharyngeal stripping wave. An additional physiological mechanism that is discussed is esophageal pathophysiology (abnormal esophageal clearance from obstruction or reflux/retrograde flow) which presented in more than half of their sample (57.1%) and may contribute to post‐prandial aspiration, a well‐documented risk in this patient group.
3.6. Quality assessment
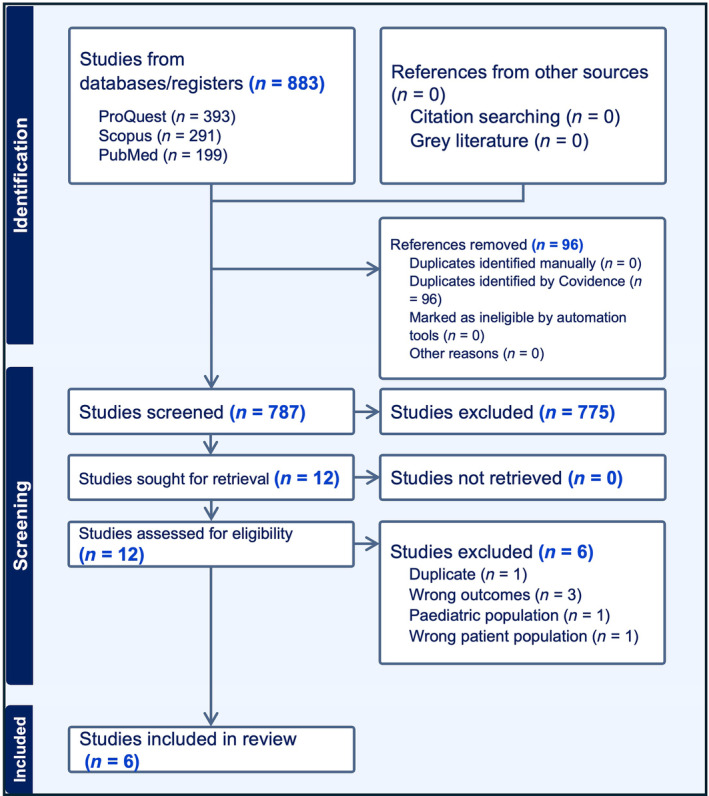
Using the Cochrane Risk of Bias Tool (Table 5), we found that all of the included studies were susceptible to bias in the domains of selection and performance. In terms of detection bias, only one study reported blinding of raters, 29 whereas the remaining studies lacked sufficient information to make a clear decision or were deemed susceptible to bias. The overall of the included studies thus demonstrates a pattern of high bias in most of the studies included in the review (Figure 2).
TABLE 5.
Cochrane risk of bias tool.
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Overall |
|---|---|---|---|---|---|---|
| Atkins et al. 26 | + | + | ? | − | + | High |
| Baumann et al. 27 | + | + | + | − | + | High |
| Dallal‐York et al. 28 | + | + | + | − | − | High |
| Dallal‐York et al. 29 | + | + | − | − | − | Unclear |
| Miles et al. 30 | + | + | + | − | + | High |
| Reedy et al. 31 | + | + | ? | − | − | Unclear |
Note: “+” Yes to susceptibility of bias; “−” not susceptible to bias; “?” unsure/could not determine appropriate rating.
FIGURE 2.
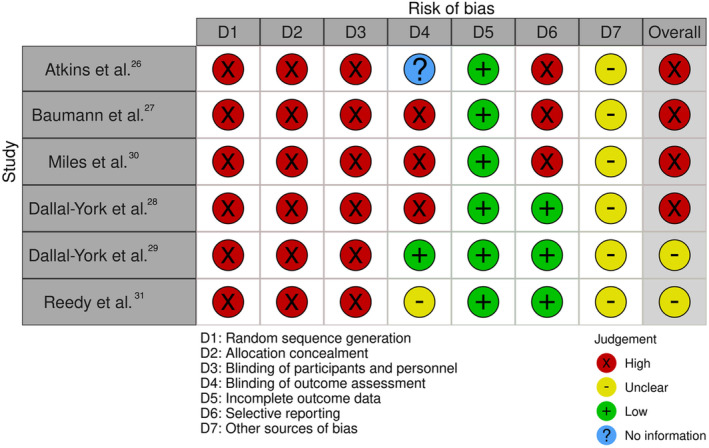
Cochrane tool for risk of bias.
The FRONTIERS tool 22 was utilized to determine rigor and transparency of the studies included in this review. Most of the studies were retrospective in nature and failed to adequately blind/duplicate rating of instrumental evaluations (Figure 3). Additionally, all but one study failed to transparently report the full assessment protocol (including bolus consistencies, volumes, and order or presentation) used to probe for impairments in the swallowing mechanism. This critical appraisal tool demonstrates huge gaps in the reporting practices of the studies published in the post‐LT dysphagia literature, which increases their risk of bias.
FIGURE 3.
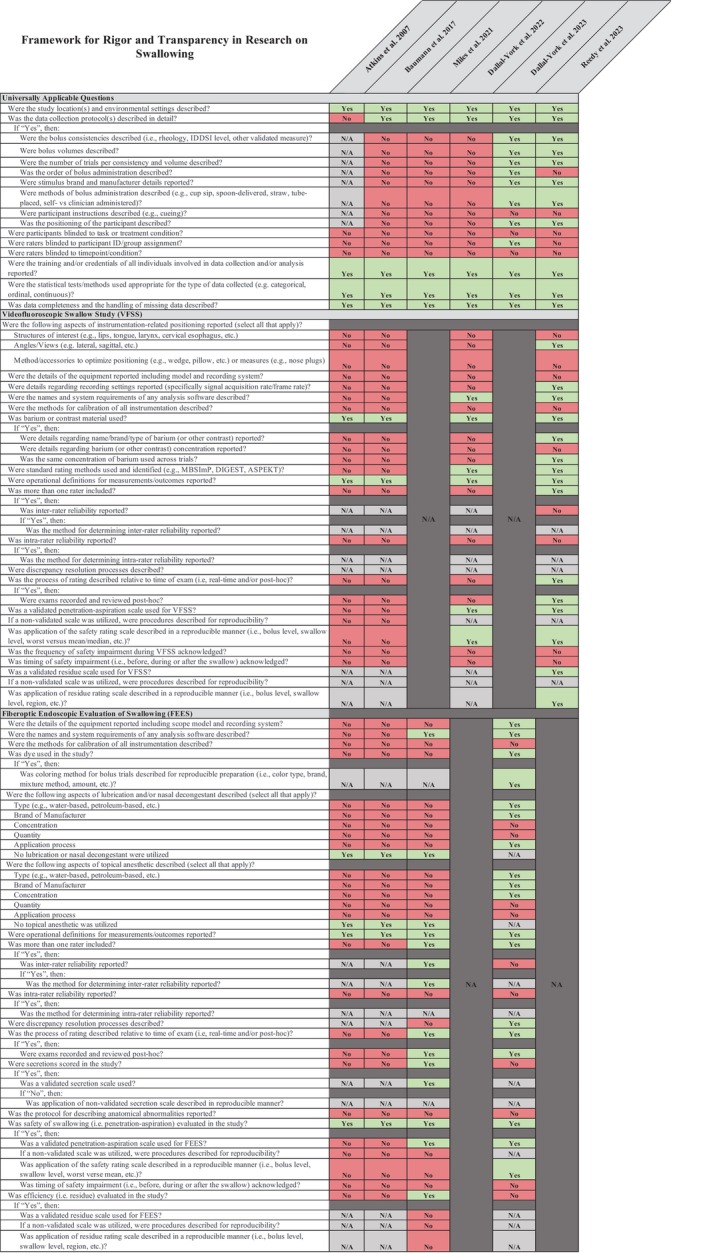
Framework for rigor and transparency in research on swallowing.
4. DISCUSSION
This review sought to describe the post‐operative swallowing (patho)physiology and its parameters following LTs. We provided scrutiny of the results reported and the methods utilized in obtaining these results in the existing literature. Due to the retrospective nature of the majority of the studies included in the review, a lack of transparency regarding instrumental evaluation protocols including the number and bolus types used during the instrumental evaluations appeared as a theme in the studies included.
Variations in approaches to summarizing patient performance were evident in all studies despite the use of the Penetration‐Aspiration Scale in four of the six studies identified. The differences in summarizing the data may be the reason why proportions of patients varied in each category considered. For example, when considering patients who presented with penetration alone, one study identified 60% of patients as penetrators. 29 This value is almost double the proportion of penetrators reported by Reedy et al., 31 which was 33%. Similarly, the large percentage of aspirators reported in Miles et al. 30 for aspiration was defined as PAS scores of 4–8, compared to the other studies where the more common classification was used (PAS 6–8).
The choice of VFSS or FEES as the preferred standard of care assessment instrument may have led to the differences in results reported. For example, it is well documented that the FEES exam involves a period of whiteout at the height of the swallow due to the blocking of the endoscope's light which may have impacted accuracy of detecting airway invasion during the swallow. Perhaps even more importantly, the live interpretation examinations in some studies may have also impacted the difference in incidence that was reported in the studies in this review, as some there is evidence in the literature to suggest that reliability of PAS score rating was higher when recorded and reviewed post‐hoc as opposed to live interpretations. 34 On the contrary, no studies utilizing VFSS indicated live interpretation of results.
The results point to wide differences in the presentation of patients following LT which can likely be attributed to the different underlying indications for LTs where dysphagia may have already been present at baseline. In patient groups with chronic obstructive lung disease for example, the well documented literature on respiratory‐swallow discoordination or reduced sensation to the pharynx and larynx may predispose individuals to an undiagnosed pre‐operative dysphagia. 35 , 36 , 37 In the studies that met criteria for our review, only one study 28 explored pre‐existing dysphagia prior to LT surgery and found 17% of patients presenting with penetration or aspiration (penetration: 10%; aspiration: 7%) at baseline.
Finally, we noted that the two most recent studies exploring swallowing outcomes in patients following LT had the smallest sample sizes. We hypothesize that a shift in the current reporting standards in the dysphagia literature has guided the more systematic and rigorous methodologies in these studies which may have ultimately led to the inclusion of less patients.
5. LIMITATIONS OF THE REVIEW
Despite this systematic review following PRISMA guidelines for best practices in terms of database searching, inclusion/exclusion criteria, data extraction, and reporting of findings, there are several limitations that should be addressed. First, as we aimed to provide a comprehensive and unbiased synthesis of the available evidence, we choose not to include gray literature and case studies in the analyses due to generalizability, quality, and validity concerns. Second, we only examined studies that reported oropharyngeal swallowing outcomes using instrumental assessments. As esophageal dysphagia is a well‐documented post‐operative occurrence, we acknowledge the impact of post‐prandial aspiration but did not systematically search for it due to the different physiological presentation than prandial aspiration. 38 , 39 , 40 , 41 , 42
6. CONCLUSION
This systematic review aimed to explore the current available literature with regards to dysphagia outcomes following lung transplant as measured using instrumental assessments. The studies included in this review were heterogenous in their swallowing assessment protocols, analysis procedures and practices and in the alignment with regards to their dichotomized grouping of patients. The degree of silent aspiration found across the articles varied significantly, which was surprising due to the aligned definitions used to describe silent aspiration. Future studies exploring dysphagia outcomes post‐lung transplant should comment on the altered physiological mechanisms of the swallows in order to support treatment planning targets for this group.
FUNDING INFORMATION
This research did not receive any grants from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting information.
ACKNOWLEDGMENTS
The authors wish to thank Bailey Marks, former GWU Swallowing Lab RA, for her assistance in abstract review and Paul Levett, Reference and Instruction Librarian at the Himmelfarb Health Sciences Library—George Washington University, for his assistance in the development and translation of the search strategy.
Smaoui S, Cummins E, Mena M, Scott S, Tobar‐Fredes R. The pathophysiology of dysphagia post‐lung transplant: A systematic review. Laryngoscope Investigative Otolaryngology. 2024;9(5):e70022. doi: 10.1002/lio2.70022
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article and its Supplemental Material files. Supporting data can be found in the references and DOI links to articles referenced in the study.
REFERENCES
- 1. Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021;70:1‐114. [PubMed] [Google Scholar]
- 2. International Thoracic Organ Transplant (TTX) Registry Data Slides . Special report: death/retransplant among primary, deceased donor transplant recipients. [Internet]. 2023. Available from: https://ishltregistries.org/registries/slides.asp
- 3. National Data . Organ Procurement & Transplantation Network [Internet]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#
- 4. Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159‐171. [DOI] [PubMed] [Google Scholar]
- 5. Fuller J, Fisher AJ. An update on lung transplantation. Breathe. 2013;9:188‐200. [Google Scholar]
- 6. Garrido G, Dhillon GS. Medical course and complications after lung transplantation. In: Sher, Y., Maldonado, J. (eds) Psychosocial Care of End‐Stage Organ Disease and Transplant Patients. Springer, Cham; 2019. 10.1007/978-3-319-94914-7_26 [DOI] [Google Scholar]
- 7. Machuzak M, Santacruz JF, Gildea T, Murthy SC. Airway complications after lung transplantation. Thorac Surg Clin. 2015;25:55‐75. [DOI] [PubMed] [Google Scholar]
- 8. Black RJ, Bogaardt H, McCabe P, Glanville AR, MacDonald P, Madill C. Clinical predictors for oropharyngeal dysphagia and laryngeal dysfunction after lung and heart transplantation. Int J Lang Commun Disord. 2019;54:894‐901. [DOI] [PubMed] [Google Scholar]
- 9. Duarte AG, Myers AC. Cough reflex in lung transplant recipients. Lung. 2012;190:23‐27. [DOI] [PubMed] [Google Scholar]
- 10. Yuan H, Silberstein SD. Vagus nerve and Vagus nerve stimulation, a comprehensive review: part I. Headache. 2016;56:71‐78. [DOI] [PubMed] [Google Scholar]
- 11. de la Torre M, Fernández R, Fieira E, et al. Postoperative surgical complications after lung transplantation. Rev Port Pneumol. 2015;21:36‐40. [DOI] [PubMed] [Google Scholar]
- 12. Macht M, Wimbish T, Bodine C, Moss M. ICU‐acquired swallowing disorders. Crit Care Med. 2013;41:2396‐2405. [DOI] [PubMed] [Google Scholar]
- 13. Allen E, Minutello K, Murcek BW. Anatomy, head and neck, larynx recurrent laryngeal nerve. StatPearls [Internet]. Treasure Island (FL), StatPearls Publishing; 2024. Available from. http://www.ncbi.nlm.nih.gov/books/NBK470179/ [PubMed] [Google Scholar]
- 14. Mattsson P, Hydman J, Svensson M. Recovery of laryngeal function after intraoperative injury to the recurrent laryngeal nerve. Gland Surg. 2015;4:27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Black R, McCabe P, Glanville A, Bogaardt H, MacDonald P, Madill C. Oropharyngeal dysphagia and laryngeal dysfunction after lung and heart transplantation: a systematic review. Disabil Rehabil. 2020;42:2083‐2092. [DOI] [PubMed] [Google Scholar]
- 16. Giraldo‐Cadavid LF, Leal‐Leaño LR, Leon‐Basantes GA, et al. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. Laryngoscope. 2017;127:2002‐2010. [DOI] [PubMed] [Google Scholar]
- 17. Yang S, Park J‐W, Min K, et al. Clinical practice guidelines for oropharyngeal dysphagia. Ann Rehabil Med. 2023;47:S1‐S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duarte AG, Terminella L, Smith JT, Myers AC, Campbell G, Lick S. Restoration of cough reflex in lung transplant recipients. Chest. 2008;134:310‐316. [DOI] [PubMed] [Google Scholar]
- 19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg Lond Engl. 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 20. Covidence Systematic Review Software. Veritas Health Innovation; www.covidence.org [Google Scholar]
- 21. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Framework for rigor and transparency in research on swallowing. The Frontiers Framework [Internet]. 2023. Available from: https://www.frontiersframework.com/ [DOI] [PubMed]
- 23. Rogus‐Pulia N, Affoo R, Namasivayam‐MacDonald A, Noad B, Steele C. Framework for rigor and transparency in research on swallowing: an overview. Am J Speech Lang Pathol. 2024;33:2130‐2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360‐363. [PubMed] [Google Scholar]
- 25. Atkins BZ, Petersen RP, Daneshmand MA, Turek JW, Lin SS, Davis RD. Impact of oropharyngeal dysphagia on long‐term outcomes of lung transplantation. Ann Thorac Surg. 2010;90:1622‐1628. [DOI] [PubMed] [Google Scholar]
- 26. Atkins BZ, Trachtenberg MS, Prince‐Petersen R, et al. Assessing oropharyngeal dysphagia after lung transplantation: altered swallowing mechanisms and increased morbidity. J Heart Lung Transplant. 2007;26:1144‐1148. [DOI] [PubMed] [Google Scholar]
- 27. Baumann B, Byers S, Wasserman‐Wincko T, et al. Postoperative swallowing assessment after lung transplantation. Ann Thorac Surg. 2017;104:308‐312. [DOI] [PubMed] [Google Scholar]
- 28. Dallal‐York J, Segalewitz T, Croft K, et al. Incidence, risk factors, and sequelae of dysphagia mediated aspiration following lung transplantation. J Heart Lung Transplant. 2022;41:1095‐1103. [DOI] [PubMed] [Google Scholar]
- 29. Dallal‐York J, Croft K, Anderson A, et al. A prospective examination of swallow and cough dysfunction after lung transplantation. Neurogastroenterol Motil. 2023;35:e14458. [DOI] [PubMed] [Google Scholar]
- 30. Miles A, Barua S, McLellan N, Brkic L. Dysphagia and medicine regimes in patients following lung transplant surgery: a retrospective review. Int J Speech Lang Pathol. 2021;23:339‐348. [DOI] [PubMed] [Google Scholar]
- 31. Reedy EL, Simpson AN, O'Rourke AK, Bonilha HS. Characterizing swallowing impairment in a post‐lung transplant population. Am J Speech Lang Pathol. 2023;32:1236‐1251. [DOI] [PubMed] [Google Scholar]
- 32. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration‐aspiration scale. Dysphagia. 1996;11:93‐98. [DOI] [PubMed] [Google Scholar]
- 33. Martin‐Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia. 2008;23:392‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hey C, Pluschinski P, Pajunk R, et al. Penetration‐aspiration: is their detection in FEES® reliable without video recording? Dysphagia. 2015;30:418‐422. [DOI] [PubMed] [Google Scholar]
- 35. Good‐Fratturelli MD, Curlee RF, Holle JL. Prevalence and nature of dysphagia in VA patients with COPD referred for videofluoroscopic swallow examination. J Commun Disord. 2000;33:93‐110. [DOI] [PubMed] [Google Scholar]
- 36. Mancopes R, Peladeau‐Pigeon M, Barrett E, et al. Quantitative videofluoroscopic analysis of swallowing physiology and function in individuals with chronic obstructive pulmonary disease. J Speech Lang Hear Res. 2020;63:3643‐3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steidl E, Ribeiro CS, Gonçalves BF, Fernandes N, Antunes V, Mancopes R. Relationship between dysphagia and exacerbations in chronic obstructive pulmonary disease: a literature review. Int Arch Otorhinolaryngol. 2015;19:74‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastro‐oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707‐713. [DOI] [PubMed] [Google Scholar]
- 39. Castor JM, Wood RK, Muir AJ, Palmer SM, Shimpi RA. Gastroesophageal reflux and altered motility in lung transplant rejection. Neurogastroenterol Motil. 2010;22:841‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fisichella PM, Davis CS, Kovacs EJ. A review of the role of GERD‐induced aspiration after lung transplantation. Surg Endosc. 2012;26:1201‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mertens V, Dupont L, Sifrim D. Relevance of GERD in lung transplant patients. Curr Gastroenterol Rep. 2010;12:160‐166. [DOI] [PubMed] [Google Scholar]
- 42. de León R, San JA. Lung transplantation and esophageal dysfunction. Rev Esp Enferm Dig. 2018;110:339‐341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplemental Material files. Supporting data can be found in the references and DOI links to articles referenced in the study.


