Abstract
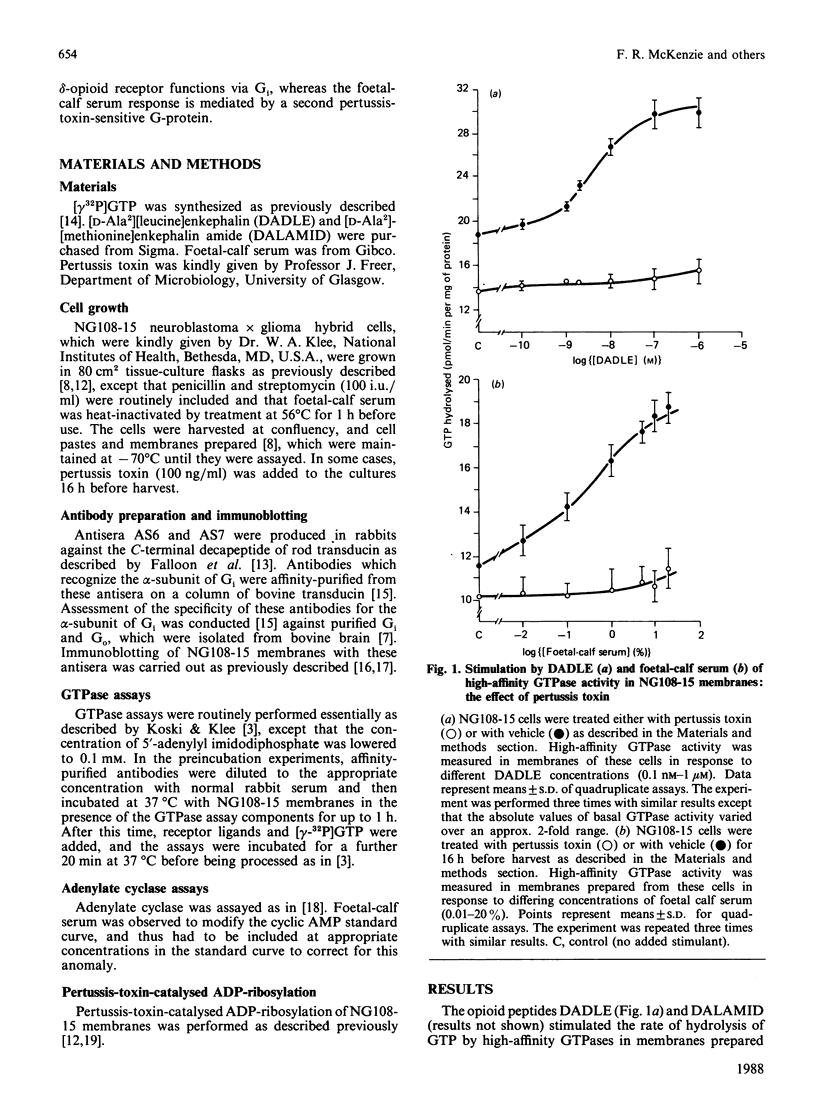
We investigated the mechanisms of receptor-mediated stimulation of high-affinity GTPase activity in response to opioid peptides and to foetal-calf serum in membranes of the neuroblastoma X glioma hybrid cell line NG108-15. Increases in GTPase activity in response to both of these ligands was abolished by prior exposure of the cells to pertussis toxin. Pertussis toxin in the presence of [32P]NAD+ catalysed incorporation of radioactivity into a broad band of approx. 40 kDa in membranes prepared from untreated, but not from pertussis-toxin-pretreated, cells. Additivity studies indicated that the responses to opioid peptides and to foetal-calf serum were mediated by separate guanine-nucleotide-binding proteins (G-proteins). Whereas opioid peptides produced an inhibition of adenylate cyclase in membranes of untreated cells, foetal-calf serum did not. Affinity-purified antibodies which recognize the C-terminus of the inhibitory G-protein identified a 40 kDa polypeptide in membranes of NG108-15 cells. These antibodies attenuated opioid-stimulated high-affinity GTPase activity, but did not markedly affect the response to foetal-calf serum. We conclude that receptors for the opioid peptides function via the inhibitory G-protein (Gi), whereas foetal-calf serum activates a second pertussis-toxin-sensitive G-protein, which has a C-terminal sequence significantly different from that of Gi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Ui M., Ogasawara N. Prevention of the agonist binding to gamma-aminobutyric acid B receptors by guanine nucleotides and islet-activating protein, pertussis toxin, in bovine cerebral cortex. Possible coupling of the toxin-sensitive GTP-binding proteins to receptors. J Biol Chem. 1985 Oct 15;260(23):12653–12658. [PubMed] [Google Scholar]
- Bourne H. R., Masters S. B., Sullivan K. A. Mammalian G proteins: structure and function. Biochem Soc Trans. 1987 Feb;15(1):35–38. doi: 10.1042/bst0150035. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Asano T., Pedersen S. E., Ross E. M. Reconstitution of catecholamine-stimulated guanosinetriphosphatase activity. Biochemistry. 1983 Sep 13;22(19):4357–4362. doi: 10.1021/bi00288a002. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Regan J. W., Nakata H., Codina J., Benovic J. L., Gierschik P., Somers R. L., Spiegel A. M., Birnbaumer L., Lefkowitz R. J. Functional reconstitution of the alpha 2-adrenergic receptor with guanine nucleotide regulatory proteins in phospholipid vesicles. J Biol Chem. 1986 Mar 15;261(8):3901–3909. [PubMed] [Google Scholar]
- Didsbury J. R., Ho Y. S., Snyderman R. Human Gi protein alpha-subunit: deduction of amino acid structure from a cloned cDNA. FEBS Lett. 1987 Jan 26;211(2):160–164. doi: 10.1016/0014-5793(87)81428-x. [DOI] [PubMed] [Google Scholar]
- Falloon J., Malech H., Milligan G., Unson C., Kahn R., Goldsmith P., Spiegel A. Detection of the major pertussis toxin substrate of human leukocytes with antisera raised against synthetic peptides. FEBS Lett. 1986 Dec 15;209(2):352–356. doi: 10.1016/0014-5793(86)81141-3. [DOI] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Milligan G., Pines M., Goldsmith P., Codina J., Klee W., Spiegel A. Use of specific antibodies to quantitate the guanine nucleotide-binding protein Go in brain. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2258–2262. doi: 10.1073/pnas.83.7.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierschik P., Morrow B., Milligan G., Rubin C., Spiegel A. Changes in the guanine nucleotide-binding proteins, Gi and Go, during differentiation of 3T3-L1 cells. FEBS Lett. 1986 Apr 7;199(1):103–106. doi: 10.1016/0014-5793(86)81233-9. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Gawler D., O'Hagan S., Wilson A. Thrombin, unlike vasopressin, appears to stimulate two distinct guanine nucleotide regulatory proteins in human platelets. Biochem J. 1986 Aug 15;238(1):109–113. doi: 10.1042/bj2380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff R. M., Axton J. M., Neer E. J. Physical and immunological characterization of a guanine nucleotide-binding protein purified from bovine cerebral cortex. J Biol Chem. 1985 Sep 5;260(19):10864–10871. [PubMed] [Google Scholar]
- Klee W. A., Koski G., Tocque B., Simonds W. F. On the mechanism of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:153–159. [PubMed] [Google Scholar]
- Koski G., Klee W. A. Opiates inhibit adenylate cyclase by stimulating GTP hydrolysis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4185–4189. doi: 10.1073/pnas.78.7.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law P. Y., Hom D. S., Loh H. H. Multiple affinity states of opiate receptor in neuroblastoma x glioma NG108-15 hybrid cells. Opiate agonist association rate is a function of receptor occupancy. J Biol Chem. 1985 Mar 25;260(6):3561–3569. [PubMed] [Google Scholar]
- Masters S. B., Stroud R. M., Bourne H. R. Family of G protein alpha chains: amphipathic analysis and predicted structure of functional domains. Protein Eng. 1986 Oct-Nov;1(1):47–54. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michel T., Winslow J. W., Smith J. A., Seidman J. G., Neer E. J. Molecular cloning and characterization of cDNA encoding the GTP-binding protein alpha i and identification of a related protein, alpha h. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7663–7667. doi: 10.1073/pnas.83.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Foetal-calf serum stimulates a pertussis-toxin-sensitive high-affinity GTPase activity in rat glioma C6 BU1 cells. Biochem J. 1987 Jul 15;245(2):501–505. doi: 10.1042/bj2450501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Gierschik P., Spiegel A. M., Klee W. A. The GTP-binding regulatory proteins of neuroblastoma x glioma, NG108-15, and glioma, C6, cells. Immunochemical evidence of a pertussis toxin substrate that is neither Ni nor No. FEBS Lett. 1986 Jan 20;195(1-2):225–230. doi: 10.1016/0014-5793(86)80165-x. [DOI] [PubMed] [Google Scholar]
- Milligan G., Gierschik P., Spiegel A. M. The use of specific antibodies to identify and quantify guanine nucleotide-binding proteins. Biochem Soc Trans. 1987 Feb;15(1):42–45. doi: 10.1042/bst0150042. [DOI] [PubMed] [Google Scholar]
- Milligan G. Guanine nucleotide regulation of the pertussis and cholera toxin substrates of rat glioma C6 BU1 cells. Biochim Biophys Acta. 1987 Jul 6;929(2):197–202. doi: 10.1016/0167-4889(87)90176-5. [DOI] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Milligan G., Simonds W. F., Streaty R. A., Tocque B., Klee W. A. Functional control of the delta-opiate receptor by the inhibitory guanine nucleotide-binding protein. Biochem Soc Trans. 1985 Dec;13(6):1110–1113. doi: 10.1042/bst0131110. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Pines M., Gierschik P., Milligan G., Klee W., Spiegel A. Antibodies against the carboxyl-terminal 5-kDa peptide of the alpha subunit of transducin crossreact with the 40-kDa but not the 39-kDa guanine nucleotide binding protein from brain. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4095–4099. doi: 10.1073/pnas.82.12.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof D. J., Applebury M. L., Sternweis P. C. Relationships within the family of GTP-binding proteins isolated from bovine central nervous system. J Biol Chem. 1985 Dec 25;260(30):16242–16249. [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Yano K., Higashida H., Inoue R., Nozawa Y. Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem. 1984 Aug 25;259(16):10201–10207. [PubMed] [Google Scholar]




