Abstract
Superabsorbent hydrogels made from agro waste materials have the potential to promote sustainable agriculture and environmental sustainability. These hydrogels not only help reduce water consumption and increase crop yields but also contribute to minimizing waste and lowering greenhouse gas emissions. Recent research on superabsorbent hydrogels derived from agro wastes has focused on the preparation of hydrogels based on natural polymers isolated from agro wastes, such as cellulose, hemicellulose, and lignin. This review provides an in-depth examination of hydrogels developed from raw agro waste materials and natural polymers extracted from agro wastes, highlighting that these studies start with raw wastes as the main materials. The utilization strategies for specific types of agro wastes are comprehensively described. This review outlines different methods utilized in the production of these hydrogels, including physical cross-linking techniques such as dissolution-regeneration and freeze–thawing, as well as chemical cross-linking methods involving various cross-linking agents and graft polymerization techniques such as free radical polymerization, microwave-assisted polymerization, and γ radiation graft polymerization. Specifically, this review explores the applications of agro waste-based superabsorbent hydrogels in enhancing soil properties such as water retention and slow-release of fertilizers for sustainable agriculture.
Keywords: agricultural waste, physical cross-linking, chemical cross-linking, slow-release fertilizer, soil conditioner, plant growth
1. Introduction
Sustainable development is a crucial global objective, and agriculture plays a vital role in reaching this goal as the world’s population continues to grow, leading to greater demand for food production and clean water.1,2 Agriculture consumes more than 70% of freshwater resources, and climate change is reducing water availability and adversely affecting crop yields, particularly in drought-prone arid and semiarid regions.3,4 Sustainable agriculture can be achieved by adopting practices that conserve natural resources and protect the environment, such as using farming techniques to increase water-use efficiency, reduce chemical use and utilize renewable resources.5−8
1.1. Superabsorbent Hydrogels Derived from Agro Waste Materials
Superabsorbent hydrogels (SAHs) offer a promising solution for sustainable agriculture.9,10 These hydrogels are materials that can absorb and retain hundreds of times their own weight in water and are then gradually released into plants over time. Using these hydrogels in agriculture could reduce the reliance on irrigation and ensure that crops receive a steady supply of water even during periods of drought. Agro waste materials such as wheat straw,11 tree branches,12 rice husk,13 cotton stalks,14 corn stover,15 sugar cane bagasse,16,17 and soybean residue18,19 possess properties such as high cellulose, hemicellulose, and lignin contents, which make them ideal for hydrogel preparation. The biodegradability and high availability of these materials also contribute to their suitability for hydrogel synthesis, providing a sustainable and eco-friendly option for agricultural applications.
SAHs derived from agro waste materials have the potential to simultaneously contribute to sustainable agriculture and environmental sustainability.20 First, the use of these hydrogels could enable more sustainable agricultural practices by reducing water usage and increasing crop yields, whereas the use of agro waste materials as a source for hydrogel production could reduce waste and promote a circular economy.21 Second, the use of AW-SAHs can reduce the need for petroleum-based materials commonly used in hydrogel production, which can minimize greenhouse gas emissions and thus have a positive impact on the environment.22
In recent years, several studies and reviews have focused on the preparation of agricultural waste-based polymers and hydrogels, highlighting their potential in sustainable agriculture.23−40 Moreover, these hydrogels have shown promise in improving soil moisture retention and nutrient delivery, which are critical for enhancing crop growth and resilience. Interest in these materials for applications such as wastewater treatment, energy storage, and biomedical fields is also growing due to their biocompatibility and environmental benefits.41−64
1.2. Scope of This Review
Many recent reports have focused on the isolation, extraction methods, and processing of cellulose, hemicellulose, and lignin from various natural resources.25,65−69 Cellulose, hemicellulose and lignin can be extracted from lignocellulosic biomass using a variety of methods, such as acid hydrolysis, alkali extraction, and enzymatic hydrolysis.65,70,71
The process of extracting cellulose, hemicellulose and lignin involves isolating these components from agricultural waste material and using them as building blocks for hydrogel synthesis.72,73 For instance, microcrystalline cellulose and lignin extracted from rice straw have been used as raw materials for the synthesis of hydrogels, employing methods such as dilute acid pretreatment and alkaline hydrolysis.74 Some studies have developed efficient separation methods, such as the use of freeze–thawing, which provides physical channels for the effective penetration of p-toluenesulfonic acid, thereby improving the separation efficiency and selectivity of bagasse lignin extraction.16
It is widely reported that superabsorbent hydrogels can be synthesized from biopolymers extracted from agro waste materials, such as corncob lignin,75 millet straw cellulose,76 wheat straw cellulose,77 bagasse cellulose,17 and cotton stalk cellulose.78 These hydrogels have been used in various fields, including agriculture and dye/metal pollutant removal. These studies show that natural polymers extracted from agro waste materials can be effectively converted into valuable hydrogels using different synthetic techniques.
However, the extraction of polymers faces environmental challenges, as the use of acids and bases is generally not considered eco-friendly in these studies. Furthermore, current reviews have not comprehensively covered the utilization strategies of agro waste materials and synthetic techniques for directly using raw waste materials, which can help avoid harsh conditions and be more environmentally friendly.
When utilizing raw waste materials, the process usually involves converting agro waste materials into suitable forms for use in hydrogel synthesis. For instance, fresh okara, a byproduct of soybean curd production, was homogenized and then used directly for hydrogel synthesis.79
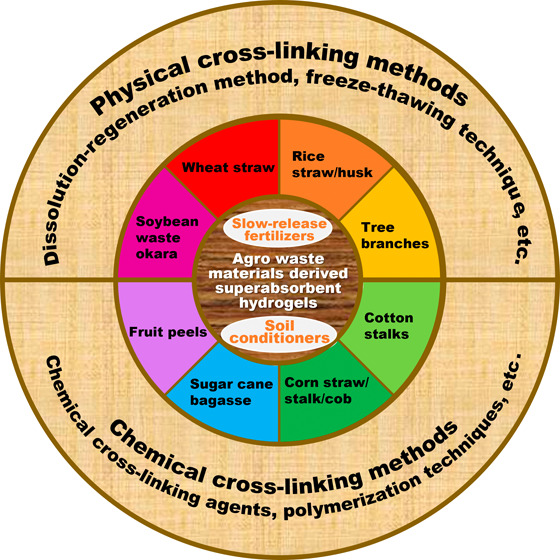
This review summarizes recent scientific reports on synthetic techniques for developing SAHs derived from raw agro waste materials and natural polymers extracted from agro wastes, emphasizing that these studies began with raw wastes as the foundational materials. The utilization strategies for specific agro waste types are comprehensively described. Additionally, this review addresses the specific applications of AW-SAHs in sustainable agriculture as soil conditioners and slow-release fertilizers (Figure 1), providing insights into their production methods, properties, and potential benefits.
Figure 1.
Schematic diagram of the development and application of AW-SAHs in sustainable agriculture.
2. Agro Waste Materials: Chemical Properties and Utilization Strategies
Agro waste materials usually refer to the residues or byproducts generated during agricultural operations, such as growing crops, processing and consuming agricultural products.80 Some examples of agro waste materials include agricultural crop residues (e.g., wheat straw, rice straw/husk, tree branches, dry leaves, cotton stalks, corn straw/stover/cob, sugar cane bagasse),11−17,74,81 and food wastes/byproducts (waste vegetables, fruit peels, and soybean wastes).82−84 The abundantly available agro residues or byproducts are inexpensive carbon-based materials that can be used as feedstocks for the production of renewable products.85−87
2.1. Chemical Properties
Understanding the chemical properties of agro waste materials is crucial for developing strategies to modify and convert them into value-added products. The composition and chemical structures of agro waste materials have been extensively studied, and a number of related research papers and review articles have been published on this topic.33,88,89 Most agro waste materials are cellulosic and lignocellulosic biomasses, which are mainly composed of three types of natural polymers: cellulose, hemicellulose and lignin.20,90
Cellulose is the most abundant linear polysaccharide that provides structural support to plants.91 It consists of a long polymer chain of hundreds to thousands of glucose monosaccharide units joined by β-acetal linkages. Cellulose contains three hydroxyl groups per glucose monosaccharide unit, and the abundance of hydroxyl groups makes cellulose a good candidate for modification via various physical/chemical reactions.92,93
Hemicellulose consists of a group of polysaccharides that are commonly branched, including xylans, xyloglucans, and mannans.67 The composition and structure of hemicellulose from different biomass sources are different, and the hemicellulose extracted from different parts of the same raw material or via different extraction methods is also different. Hemicellulose contains large amounts of active hydroxyl and carboxyl groups for physical/chemical reactions.68
Lignin is a highly branched cross-linked macromolecule with a high molecular weight.25 The basic phenylpropane units of lignin (mainly p-hydroxy phenol, guaiacyl, and syringyl) are bonded together by carbon–carbon bonds or ether linkages to form a complex matrix.94 Lignin is rich in aromatic subunits, so it is relatively hydrophobic. This aromatic polymer can also be modified into useful functional materials by appropriate reactions, such as oxidation reactions on the aromatic rings of lignin and reactions with hydroxyl (alcohol and phenolic) groups.89,95,96
Here, we classify these agro waste types on the basis of their characteristics and generation processes, and delve into their chemical properties to understand their potential for hydrogel synthesis and applications in sustainable agriculture. Additionally, we discuss how to deal with these specific waste types for effective waste utilization.
2.2. Utilization Strategies for Specific Agro Waste Types
2.2.1. Wheat Straw, Rice Straw, and Rice Husk
Wheat straw, rice straw, and rice husk are agricultural residues remaining after wheat and rice are harvested, respectively. The common practice of in situ burning of these residues creates numerous ecological, environmental, health, and economic issues.97 However, these residues are highly nutritious biomasses composed of high concentrations of cellulose, hemicellulose, and lignin.98 For example, the contents of cellulose, hemicellulose and lignin in rice straw are reported to be approximately 40%, 18%, and 5.5%, respectively.99,100 Huang et al.101 estimated the lignocellulosic composition of the rice straw to be approximately 42.2% cellulose, 24.3% hemicellulose, and 20.8% lignin. The composition of the rice hull was found to be approximately 32.9% cellulose, 23.6% hemicellulose, and 26.2% lignin.
Researchers have investigated various utilization strategies for wheat straw, rice straw, and rice husk. One study explored the use of wheat straw to create hydrogels for soil conditioning. In this study, wheat straw was chopped and subjected to ball milling for five minutes at a speed of 20 rpm, which reduced it to a median particle size of 43 μm. This processed straw was then used to prepare the hydrogel.102 Similarly, another study involved washing wheat straw, drying it at 70 °C, and then crushing and sieving it to prepare it for further use.103
In another study, rice husk was examined both in its natural form and after chemical treatment to remove lignin and hemicelluloses. The chemical treatment involved soaking the rice husk in sodium hydroxide for 24 h, rinsing it with distilled water until a neutral pH was achieved, and then drying it. The dried husk, both untreated and treated, was then crushed and sieved. Researchers have assessed the water absorption properties of composites made with both treated and untreated rice husk.104
Further research focused on isolating cellulose from rice straw using chemical pulping and bleaching methods. The chemical composition of the isolated fibers was analyzed, and the cellulose, lignin, and ash contents were determined according to TAPPI standards.105
Additionally, rice husk has been utilized to produce ash.106 The husks were washed thoroughly with distilled water to remove any adhered materials, dried and crushed. The crushed husks were calcined before being used to synthesize the hydrogel composites.107
2.2.2. Tree Branches
Pruning activities in orchards and landscape maintenance produce tree branches as byproducts. Over the past few decades, the amount of urban green waste has increased with the expansion of urban green spaces globally. Research has focused on assessing the potential of this waste as a biomass resource.108,109 Tree branches, which are rich in lignocellulosic fibers, are suitable for structural applications such as particleboard and paper production.110 Additionally, researchers are exploring ways to transform these wastes into eco-friendly fillers for biocomposites.111
A study reported that mulberry branches contain approximately 37% cellulose, 27% hemicellulose, and 20% lignin. In this research, the branches were first cleaned, cut, and dried. They were then smashed and sieved to obtain particles between 100 and 200 mesh. These processed particles were used to create a slow-release urea fertilizer. The cellulose content was determined using the Kurschner-Hoffer method, whereas the hemicellulose and lignin contents were measured according to TAPPI standards.112 The same research team applied this method to produce a urea-loaded superabsorbent composite from mulberry branches.113
In another study, peach branches were crushed, sifted to 80 mesh, stirred, heated, bleached, and treated with sodium hydroxide to remove impurities.12 The solids were then washed and dried to create nanofiber reinforcers for the hydrogel.
In a more eco-friendly approach, Xu et al. developed a method to coat waste sea buckthorn branches, which consist mainly of crude fiber (64.7–78.96%), with a dopamine solution.114 They created a superabsorbent composite by grafting poly(acrylic acid) (PAA) onto dopamine-coated branches. This method enhances the use of renewable sea buckthorn branches, simplifies the process, avoids environmental pollution, and reduces production costs.
2.2.3. Corn Straw, Stalk, and Cob
Corn residues, including straw, stalks, and the central part of the maize ear (the cob), are left in fields after harvest. These materials are significant sources of cellulose, hemicellulose, and lignin. Corn straw contains approximately 25%-40% cellulose, 20%-30% hemicellulose, and 10%-20% lignin.115 Corn stalks are composed of 35%-50% cellulose, 20%-30% hemicellulose, and 10%-20% lignin.116,117 The composition of the entire corn cob is approximately 30%-45% cellulose, 25%-35% hemicellulose, and 15%-20% lignin.86,118,119 A different study focused on corncob residues from furfural production reported that these residues contained 42.0% cellulose and 43.7% lignin (comprising both acid-soluble and acid-insoluble lignin).120
Researchers typically either grind residues into fine powders or extract natural polymers before further processing and utilization. For instance, corn stalks are initially crushed using a disintegrator and then ground to a particle size of 100 mesh to produce a corn stalk-composite superabsorbent.121 Similarly, in another study, corncobs were dried, ground into fine powders, and sieved through 100 mesh sieves for hydrogel synthesis.122
In another study, chemically treated corn straw and raw corn straw were compared. The chemical treatment process can be briefly described as follows: corn straw was ground and sieved. The straw was treated with NaOH solution, oxidized, bleached with H2O2, dried, and ground to produce cellulose. This cellulose was further processed with NaOH, neutralized, filtered, and dried to obtain alkaline fibers. SEM images revealed that, initially, the corn straw fibers were tightly packed with closed micropores. After NaOH and H2O2 treatment, the fibers become loose with increased surface area and visible micropores, reducing crystallinity.123
2.2.4. Fruit Peels
Fruit peels, a common agricultural residue, are often discarded as waste but possess valuable properties that make them suitable for sustainable applications.124 These peels are generated as byproducts during the processing of fruits for various products, such as juices, jams, and salads. They are notable sources of cellulose, a key component in many industrial applications. For instance, pineapple peels, which are left after the fruit is processed, contain 20–25% cellulose42 alongside hemicellulose, lignin, and pectin.125 Lemon peels, which are generally discarded and allowed to biodegrade, also contain more than 20% cellulose.44 Banana peels are particularly rich in cellulose and hemicelluloses, with approximately 50% of their composition being cellulose.126,127
Cellulose extraction is a prevalent method for transforming fruit peels into valuable materials, especially for applications in hydrogel synthesis. This process utilizes various fruit peels, such as banana and pineapple peels, which are rich in cellulose. For instance, cellulose nanofibers can be isolated from banana peels through a combination of chemical treatments, including alkaline treatment, bleaching, and acid hydrolysis, followed by mechanical processing.126 Similarly, cellulose from pineapple peels undergoes a detailed extraction process involving washing, pulping, delignification, and hemicellulose removal to yield a purified product.
Fruit peels are also reported to be incorporated into polymer gels in their natural form after simple processing. For example, an orange peel was washed with water and a dilute acid solution and then dried, smashed and sieved to obtain particles with a size of 260–600 μm.128 The inclusion of orange peels enhances the mechanical properties of materials without affecting their loading or release performance. Furthermore, in a recent study, orange peel was added to agar-based hydrogels to potentially increase plant disease resistance.30 Various pretreatments of orange peels were tested to determine the best method for combination with agar. The orange peel particles were either acid hydrolyzed, treated with ultrasound, or simply added to hot water before being mixed into the agar solution.
2.2.5. Sugar Cane Bagasse
Sugar cane bagasse is the fibrous residue remaining after sugar cane stalks are crushed to extract juice. It is abundant in cellulose (30–45%), hemicellulose (20–35%), and lignin (15–35%).129,130 For example, sugar cane bagasse obtained from Tamil Nadu Pulp and Paper Mills, India, contains approximately 43% cellulose, 30% xylan (a type of hemicellulose), and 20% lignin.131
Like other agricultural residues, sugar cane bagasse can be used for hydrogel synthesis either in its natural form after simple processing or in the form of natural polymers after extraction.132−134 For instance, in one study, sugar cane bagasse was dried and ground into a fine powder, which was sieved through 250 mesh sieves for hydrogel synthesis.135 Different physical, chemical, and biological methods are employed to extract natural polymers from sugar cane bagasse.129,130,136 In one approach, cellulose was effectively purified from sugar cane bagasse using gamma irradiation. This process decreased the crystallinity of the cellulose. A composite of irradiated cellulose fibers and polyacrylamide hydrogel was subsequently created.137
3. Synthesis of Agro Waste-Based Superabsorbent Hydrogels (AW-SAHs)
Superabsorbent hydrogels (SAHs) are a class of hygroscopic materials capable of absorbing hundreds of times their own weight in water or aqueous solutions.138−143 With the growing emphasis on environmental sustainability, there is a new trend to reduce the reliance on nonrenewable synthetic petroleum-based materials and instead look for more environmentally friendly biobased materials to develop superabsorbent hydrogels.144,145 In recent years, great efforts have been made to develop innovative agro waste-based hydrogels.74,92,146
There are two main synthetic methods that can be used to produce SAHs from agro waste materials: physical methods and chemical methods.27,28,147,148 Since agro waste materials are mostly lignocellulosic biomass, the main components of cellulose, hemicellulose and lignin are rich in active hydroxyl and carboxyl groups, which can form physically cross-linked hydrogels through physical interactions such as hydrogen bonds and ionic bonds or chemically cross-linked hydrogels through covalent linkages.149 Both the raw waste materials and the extracted components (cellulose, hemicellulose and lignin) can be used to synthesize AW-SAHs.
3.1. Physical Cross-Linking Methods
Physical cross-linking methods for synthesizing AW-SAHs involve physical interactions, such as hydrogen bonding, ionic bonding, and hydrophobic forces, to form networks within the hydrogel.28,150 Some of the common physical cross-linking methods used include dissolution-regeneration methods and freeze–thawing techniques.44,150 Some recent studies related to AW-SAH synthesis via physical cross-linking are summarized in Table 1. The table underscores the prevalent focus on extracting primary polymer components (i.e., cellulose, hemicelluloses, and lignin) from lignocellulosic biomass, which often necessitates harsh separation and multiple purification procedures. Conversely, relatively few studies have explored the direct use of raw waste. While the direct use of waste raw materials can circumvent these rigorous processes, they often exhibit lower reactivity and may contain impurities, posing challenges for hydrogel formation.66
Table 1. Summary of Recent Studies Related to AW-SAH Synthesis via Physical Cross-Linking.
| Physically cross-linked hydrogels | Precursors derived from agro waste materials | Interactions | Synthetic techniques | Authors | Year |
|---|---|---|---|---|---|
| Coffee pulp cellulose/alginate/pectin/calcium chloride hydrogels | Coffee pulp cellulose | Hydrogen bonding and ionic bonding | Ion induced gelation | Sommano et al.151 | 2023 |
| Sisal-based hydrogels | Lignocellulosic sisal fibers | Hydrogen bonding | Dissolution-regeneration method using LiCl/DMAc | Queiroz et al.152 | 2021 |
| Cellulose/xylan/lignin hydrogels; Sorghum hydrogels; Poplar hydrogels | Microcrystalline cellulose, Kraft lignin, and xylan from Beechwood; Biomass sorghum; Poplar wood | Hydrogen bonding | Dissolution-regeneration method using ionic liquids | Kalinoski et al.153 | 2019 |
| Lemon peel/cellulose hydrogels | Lemon peel (LP) and its isolated microcrystalline cellulose | Hydrogen bonding | Dissolution-regeneration method using ionic liquid | Dai et al.44 | 2021 |
| Cellulose hydrogels | Cellulose | Hydrogen bonding and physical entanglement between cellulose chains | Dissolution-regeneration method using ionic liquid | Satani et al.154 | 2020 |
| Lignocellulose hydrogels | Lignocellulose from poplar wood chips | Entanglement of molecular chains and hydrogen bonding | Dissolution-regeneration method using NMMO | Zhang et al.155 | 2019 |
| Cellulose hydrogels | Cellulose from rice straw | Hydrogen bonding | Dissolution-regeneration method using LiCl/DMAc | Lai et al.146 | 2022 |
| PVA/lignin hydrogels | Lignin | Hydrogen bonding | Freeze–thawing | Morales et al.156 | 2020 |
| Lignin/PVA hydrogels | Lignins extracted from almond and walnut shells | Hydrogen bonding | Freeze–thawing | Morales et al.150 | 2022 |
| PVA/cellulose hydrogels | Cellulose | Hydrogen bonding | Freeze–thawing | Butylina et al.157 | 2016 |
| Carboxymethyl cellulose/regenerated nanocellulose/PVA/Fe3O4hydrogels | Carboxymethyl cellulose from pineapple peel | Hydrogen bonding | Freeze–thawing | Dai et al.158 | 2019 |
| Okara cellulose nanofibers/carrageenan composite hydrogels | Cellulose nanofibers from okara | Hydrogen bond and electrostatic interaction | A process involving hydration, sonication, heating, and coagulation | Wu et al.159 | 2024 |
| Lignosulfonate/polyvinylpyrrolidone hydrogels | Lignin | Hydrogen bonding and hydrophobic interactions | pH induced sol–gel transformation | Cao et al.160 | 2022 |
| Carboxymethyl cellulose hydrogels | Carboxymethyl cellulose from biomass waste of oil palm empty fruit bunch | Electrostatic interactions | Self-assembly | Tuan Mohamood et al.161 | 2021 |
| Chitosan-alkali lignin hydrogels | Alkali lignin, byproduct of the paper producing industries | Electrostatic interactions | Self-assembly | Ravishankar et al.162 | 2019 |
For instance, in one study, a cellulosic composite hydrogel was crafted using cellulose extracted from coffee pulp biomass.151 The process involved suspending coffee pulp powder in a HCl/KCl buffer solution and centrifuging it to isolate the alcohol insoluble fraction (AIF). Subsequently, insoluble dietary fiber was extracted from AIFs using ammonium oxalate. The resulting soluble fractions were combined and dried to obtain coffee pulp pectins. Further purification was achieved by removing lignin from the remaining fraction through treatment with hydrogen peroxide and sodium borohydride. After washing and drying, the resulting precipitate yielded cellulose from the coffee pulp.
A study attempted to use lignocellulosic raw materials as a whole, starting with the deconstruction of sisal lignocellulosic fibers in lithium chloride/dimethylacetamide (LiCl/DMAc) and using the resulting solution as the hydrogel precursor.152 Physically cross-linked hydrogels were prepared from dissolving biomass sorghum, poplar wood or cellulose/xylan/lignin using ionic liquids and regenerated in ethanol/water followed by pure water.153 Another study used raw lemon peel (LP) together with its isolated microcrystalline cellulose to fabricate hydrogels by a dissolution-regeneration method. The hydrogel preparation process used the ionic liquid 1-butyl-3-methylimidazolium chloride (BmimCl) and water as the solvent and regeneration solvent, respectively. The isolated microcrystalline cellulose was used as the structural backbone to strengthen the hydrogel network, and the polymer components in the LP were used as structural modifiers.44
Ionic liquids can dissolve lignocellulosic biomass by breaking down hydrogen bonds between its components, which can then be reformed into physical hydrogels in antisolvents (such as water and ethanol) by disrupting the hydrogen bonds between the lignocellulosic biomass and the ionic liquid. A physically cross-linked cellulose hydrogel was prepared via a dissolution–regeneration method in which an ionic liquid was mixed with dimethyl sulfoxide (DMSO) via the following process. Cellulose with a concentration of 6–20 wt% was completely dissolved in a 1-butyl-3-methylimidazolium acetate ([BMIm][OAc])/DMSO mixed solution, and then the prepared cellulose solution was placed in a mold and immersed in DI water to replace the mixed solvent to form a hydrogel.154
The mechanism of the dissolution-regeneration method using other solvents, such as LiCl/DMAc and N-methylmorpholine-N-oxide (NMMO), is similar to that using ionic liquids. By dissolving lignocellulose in an NMMO solvent system and controlling the lignin content, lignocellulose hydrogels with tailorable microscopic morphologies and physical properties can be fabricated.155 It has also been reported that cellulose extracted from rice straw can be dissolved in LiCl/DMAc and converted into a physically cross-linked hydrogel via phase inversion in ethanol. Physical cross-linking was achieved through noncovalent interactions of hydroxyl groups via complex hydrogen bonding. Compared with other alkaline concentrations, the 6% NaOH treatment resulted in higher purity cellulose, and the regenerated hydrogels presented greater tensile strength due to enhanced hydrogen bonding interactions.146
Freeze–thawing is another commonly used technique for the synthesis of physical hydrogels. In one study, freeze–thawing was chosen as the cross-linking method to prepare physically cross-linked PVA-lignin hydrogels.156 To find the optimal lignin and PVA concentrations and improve synthesis efficiency, researchers have tested six different synthesis pathways with various cross-linking and curing methods. In another study, two types of lignins extracted from almond and walnut shells were synthesized into lignin/poly(vinyl alcohol) physical hydrogels. The properties of the resulting physical lignin hydrogels were found to be influenced by the type and source of lignin due to their different hydrogen bonding interactions with the matrix polymer.150 Freeze–thawing techniques have also been used to prepare hydrogels based on poly(vinyl alcohol) (PVA) and cellulose nanocrystals157 and hydrogels based on pineapple peel cellulose and PVA.158
Other physical cross-linking methods, such as hydration, sonication, heating, and coagulation, have recently been reported.159 In a study by Wu et al., composite hydrogels made from okara cellulose nanofibers (CNFs) and carrageenan were fabricated using this methodology. This technique, notable for its efficiency, allows simple and rapid fabrication without the need for harsh chemicals. Moreover, researchers have explored the effects of chemically modifying okara CNFs via TEMPO oxidation. The results revealed that TEMPO oxidation led to notable enhancements in various properties of the composite CNF-carrageenan hydrogels, primarily attributed to increased interactions, including ion bridging and/or hydrogen bonding among the ionic (-COO– and -OSO3–) and polar (-OH) groups on the okara cellulose nanofibers and carrageenan molecules subsequent to TEMPO oxidation.
Hydrogen bonding is one of the most common interactions in the formation of physically cross-linked hydrogels, but other noncovalent interactions such as electrostatic interactions and hydrophobic interactions also contribute to hydrogel formation, sometimes through multiple interactions simultaneously. For example, a lignosulfonate (LS)/polyvinylpyrrolidone (PVP) complex (LPC) adhesive hydrogel with a noncovalent network structure was developed through a simple and ultrafast pH-induced gelation process. The LPC hydrogel demonstrated self-healing ability and strong adhesion to various substrates due to multiple noncovalent interactions, including hydrogen bonding and hydrophobic interactions.160
Another method for preparing physically cross-linked hydrogels involves electrostatic interactions. For instance, electrostatic forces exist between the ionic charges of carboxymethyl cellulose polymer chains and the multivalent cation Ca2+.161 In another case, solid-state NMR confirmed the interaction between the chitosan polycation and alkali lignin, whereas pH, solubility, and electrokinetic studies further validated the ionic cross-linking nature of these two constituents.162
3.2. Chemical Cross-Linking Methods
The chemical cross-linking methods used to synthesize AW-SAHs usually involve the formation of covalent cross-linking bonds within the hydrogels. The covalent cross-linking can be formed by direct cross-linking of agro wastes or by graft polymerization of monomers to agro wastes with cross-linkers. Chemical cross-linking agents, such as glutaraldehyde or epichlorohydrin, can form covalent bonds between polymer chains to create a stable three-dimensional hydrogel network.163 Graft polymerization involves creating covalent cross-linking bonds between monomers to form polymer chains, whereas cross-linkers act as bridges between polymer chains to form a three-dimensional network structure.27
3.2.1. Direct Cross-Linking of Agro Wastes with Cross-Linkers
Several chemical cross-linking agents can be used for AW-SAH synthesis, including epichlorohydrin, glutaraldehyde, formaldehyde, and isocyanate.163 The cross-linking agents for AW-SAH synthesis should have reactive functional groups and high reactivity toward the lignocellulosic biomass polymers that can react with the polymer chains to ensure efficient cross-linking. The choice of cross-linking agent depends on factors such as the specific agro waste material being used, the desired properties of the SAH, and the intended application of the final products. Table 2 summarizes some recent studies that used cross-linkers to synthesize chemically cross-linked AW-SAHs.
Table 2. Summary of Recent Studies Related to AW-SAH Synthesis Using Chemical Cross-Linkers.
| Chemically cross-linked hydrogels | Precursors derived from agro waste materials | Chemical cross-linkers | Authors | Year |
|---|---|---|---|---|
| Cellulose nanofibers/carboxymethyl cellulose hydrogels | Cellulose from pine wood scraps | Epichlorohydrin | Jha et al.39 | 2024 |
| Cellulose/Zeolite hydrogels | Cellulose from rice husk | Epichlorohydrin | Lunardi et al.46 | 2024 |
| Cellulose hydrogel | Cellulose from jute sticks | Epichlorohydrin | Ahmed et al.165 | 2023 |
| Lignin/PVA hydrogel | Lignin from poplar wood | Epichlorohydrin; PEG/ECH; Epoxide-terminated PEG | Shen et al.166 | 2016 |
| Cellulose hydrogel | Cellulose from poplar wood | Epichlorohydrin | Shen et al.167 | 2016 |
| Cellulose hydrogel | Cellulose from rice straw | Epichlorohydrin | Kadry et al.168 | 2023 |
| PVA/lignin hydrogels | Lignin from corncob | Epichlorohydrin | Wu et al.169 | 2019 |
| Hydroxyethylcellulose/PVA/lignin hydrogels | Lignin from corncob | Epichlorohydrin | Huang et al.75 | 2019 |
| PVA/GA/cellulose hydrogels | Cellulose from sugar cane bagasse | Glutaraldehyde | Ban et al.134 | 2022 |
| PVA/GA/okara hydrogels | Soybean meal (okara) | Glutaraldehyde | Songsrirote et al.170 | 2017 |
| Microcrystalline cellulose/lignin hydrogels | Microcrystalline cellulose (MCC) and lignin from rice straw | Glutaraldehyde/epichlorohydrin | Kaur et al.74 | 2023 |
| Nanocellulose/wood ash-reinforced starch-chitosan hydrogels | Cellulose from sugar cane bagasse | Citric acid | Iqbal et al.35 | 2024 |
| PVA/CMC-HEC/whey-based hydrogels | Cellulose derivatives (carboxymethylcellulose sodium (CMCNa) and hydroxyethylcellulose (HEC)) | Citric acid | Fabian et al.171 | 2024 |
| Chitosan/cellulose/poly(acrylic acid) hydrogels | Cellulose | Thiourea formaldehyde resin | Essawy et al.172 | 2016 |
| Sisal-based hydrogels | Lignocellulosic sisal fibers | Polymeric methylene diphenyl diisocyanate | Queiroz et al.173 | 2023 |
| Liquefied corn stover/Chicken feather protein/poly(acrylic acid) hydrogels | Liquefied corn stover | Isocyanate | Yang et al.174 | 2013 |
Epichlorohydrin (ECH) has an epoxy group (C-O-C) that can react with the hydroxyl (-OH) groups of lignocellulosic biomass polymers to form cross-links.46,164,165 The reaction occurs through an opening of the epoxy ring, resulting in a covalent bond between the epoxy carbon and the hydroxyl oxygen. In a recent study, a urea-loaded cellulose nanofiber/carboxymethyl cellulose hydrogel was crafted through an eco-conscious method using pine wood scraps.39 This hydrogel, which was cross-linked with epichlorohydrin and enriched with carboxymethyl cellulose, demonstrated increased water absorption. Its efficacy as a gradual-release fertilizer for nurturing plant growth has been successfully confirmed. Shen et al. used ECH to cross-link lignin or cellulose extracted from wood biomass to form hydrogels, but the lignin-based hydrogels obtained via direct reaction with ECH were poor in terms of water absorption, whereas the hydrogels obtained by cross-linking with epoxy-terminated polyethylene glycol (ETPEG) lignin exhibited improved water absorption.166,167 Epichlorohydrin can also be utilized in conjunction with polymerization processes. Hydrogels featuring cellulose and cellulose/poly(acrylic acid) and boasting superabsorbent properties were prepared from cellulose extracted from rice straw. These cellulose-based hydrogels, which exhibit improved swelling ratios and water retention capacities, were synthesized through solution polymerization and chemical cross-linking. Potassium persulfate acted as the initiator, whereas ECH served as the cross-linker.168
In one study, Lignin-PVA SAHs were synthesized by increasing the lignin concentration within a conventional PVA hydrogel matrix using ECH as a cross-linker. This study employed alkali lignin as the primary raw material, integrating it with PVA as the matrix template and employing ECH as the cross-linker for the synthesis of Lignin-PVA hydrogels. Notably, the swelling ratio of these hydrogels increased markedly, increasing from 92 g/g to 456 g/g as the lignin concentration increased from 0% to 5%.169 The same research team also developed hydrogels with excellent swelling properties (swelling ratio of 1220 g/g in alkaline aqueous solution) by using ECH to cross-link PVA, hydroxyethylcellulose, and lignin. The hydrogels exhibited greater swelling when the starting lignin molecules had a medium molecular weight and higher phenolic hydroxyl content. The authors demonstrated that hydrogels can absorb many positively charged dyes, which is expected to have promising applications in both agriculture and dye pollutant removal.75
Glutaraldehyde (GA) has two aldehyde functional groups (-CHO) that can react with hydroxyl groups in lignocellulosic biomass polymers through a cross-linking reaction.134 The aldehyde groups can undergo nucleophilic addition reactions with the hydroxyl groups in cellulose, hemicellulose, and lignin to form covalent bonds, making the polymers cross-linked into hydrogels.
GA was mixed with PVA to produce PVA/GA/okara-based hydrogels for plant growth applications. As the reaction of PVA with GA occurs through the formation of acetal bridges between the pendant hydroxyl groups of the PVA chains in the presence of GA, higher concentrations of GA lead to fewer hydroxyl groups in the hydrophilic part of the hydrogel, resulting in reduced water swelling. However, when the concentration of GA (5.0 mM) was too low, the gel could not maintain its shape after swelling, and 10.0 mM GA was selected for this study.170
In a recent study, glutaraldehyde and epichlorohydrin were used together as cross-linking agents, and polyvinyl alcohol was used as a matrix template to cross-link microcrystalline cellulose (MCC) and lignin extracted from rice straw into chemically cross-linked hydrogels.74 Citric acid, a nontoxic alternative, has also been employed as a cross-linker.35 When exposed to high temperatures, citric acid reacts with the hydroxyl groups on adjacent polymer chains, forming cross-links.171 Other cross-linking agents, including thiourea formaldehyde resin,172 polymeric methylene diphenyl diisocyanate,173 and isocyanate,174 have also been reported to cross-link lignocellulosic biomass to form hydrogels, and some of these hydrogels have been used for the controlled release of fertilizers.
A promising alternative strategy for preparing chemically cross-linked AW-SAHs involves converting lignocellulosic biomass into functionalized cross-linkers. Hydroxyethylcellulose (HEC) from processed pineapple peel residue was oxidized to form aldehyde groups, which then reacted with the amino groups of carboxymethyl chitosan to form a hydrogel.42 This approach might be more sustainable than the use of traditional cross-linkers such as glutaraldehyde, which can have negative impacts on human health and the environment.
3.2.2. Graft Polymerization of Monomers with Cross-Linkers
Free radical polymerization is a key synthetic route for producing SAHs derived from agro waste materials, where a free-radical initiator or radiation initiates the graft polymerization reactions of monomers with cross-linkers, to convert lignocellulosic biomass into a three-dimensional hydrogel network. Various chemical induction systems can be used to generate free radicals to initiate polymerization.175 For example, thermal initiators generate free radicals via thermal decomposition,176 photoinitiators generate free radicals when exposed to light (usually UV or visible light),78,177 and redox initiators generate free radicals via decomposition of an oxidizing agent (usually a peroxide).17 Polymerization techniques commonly used in the synthesis of AW-SAHs include γ radiation graft copolymerization and microwave-assisted polymerization, in which microwave radiation can accelerate chemical reactions.
Vinyl monomers such as acrylic acid (AA) and acrylamide (AAm) are commonly used for copolymerization with lignocellulosic biomass to form SAHs via free radical graft copolymerization. Bifunctional cross-linking agents such as N,N′-methylenebis(acrylamide) (MBA), which contain two vinyl groups and a methylene bridge, can easily undergo free radical polymerization with these vinyl monomers to form a 3D hydrogel network. Generally, the polymerization mechanism for preparing a lignocellulosic biomass hydrogel grafted with acrylic acid and acrylamide involves three steps:28,148,178,179 (1) Initiation: In this step, an initiator undergoes homolytic cleavage or redox reactions to form free radicals. Some of the hydroxyl groups (-OH) of lignocellulosic biomass polymers are converted into macroradicals in contact with the produced free radicals. The macroradicals are formed by the abstraction of a hydrogen atom from the -OH group by a free radical, resulting in the formation of a new -O• radical on the lignocellulosic biomass polymer. Free radicals and macroradicals initiate the polymerization reaction. (2) Propagation: Free radicals and macroradicals attack vinyl monomers to form copolymer chains. (3) Termination: A 3D hydrogel network is formed in the presence of cross-linkers. Termination occurs when free radicals and macroradicals are consumed, either by reacting with each other or with other species in the system.
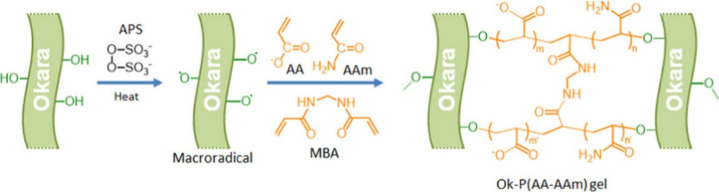
An example is shown in Figure 2 to illustrate the process of preparing a biomass okara-based hydrogel grafted with acrylic acid and acrylamide via free radical graft copolymerization.79 First, an ammonium persulfate (APS) initiator is added to okara, and heat causes the initiator to form free radicals, which react with the -OH in okara to form macroradicals. The macroradicals then react with the monomers AA and AAm, leading to the formation of polymer chains.19 In this process, macroradicals act as initiators of graft copolymerization so that the polymer chains are grafted onto the okara. The cross-linking agent MBA reacts with the polymer chain to form a 3D network structure to obtain the final Okara-P(AA-AAm) hydrogel. The carboxyl or amide groups in the AA and AAm monomers can provide hydrogen bonding sites to interact with water molecules and improve the water absorption capacity of the prepared AW-SAHs.
Figure 2.
Synthetic route for the preparation of biomass-based Okara-P(AA-AAm) superabsorbent hydrogels.79
This free radical graft copolymerization technique has also been widely used to develop SAHs derived from other agro waste materials. Bagasse-based superabsorbents, including sugar cane bagasse/P(AA-co-AAm),180 sugar cane bagasse-PAA,181 sugar cane bagasse-g-PAAm/attapulgite,135 and bagasse cellulose/P(AA-co-AAm)/nano-CaCO3,17 were prepared using a similar initiator/monomer/cross-linker system, in which attapulgite or nano-CaCO3 was used as a second cross-linked network in addition to MBA to further increase the gel strength. Other superabsorbents prepared using this polymerization technique include rice straw cellulose-g-PAA,105 rice husk ash-P(AA-co-AAm),182 pineapple peel carboxymethyl cellulose-g-poly(AA-co-AAm)/graphene oxide,183 pineapple peel carboxymethyl cellulose-g-poly(AA-co-AAm)/carclazyte,184 passion fruit peel pectin-PAA,125 and raw corn straw-PAA.115
Microwave-assisted polymerization is another technique that can be used to initiate free-radical polymerization to synthesize AW-SAHs. This method can use various types of initiators, such as thermal or redox initiators. The basic mechanism of microwave-assisted polymerization is similar to that of conventional free radical graft polymerization in that both processes use free radicals to initiate and propagate polymerization. The main difference between the two methods is the method used to initiate the polymerization reaction. In conventional free radical graft polymerization, the polymerization reaction is typically initiated via heat, light (usually UV or visible light), or a redox system. In microwave-assisted polymerization, the polymerization reaction is initiated via microwave energy. An example is shown in Figure 3 to illustrate the process of preparing a corncob-based hydrogel for slow-release nitrogen fertilizer (SRF) via microwave irradiation.122 The microwave energy caused the initiator potassium persulfate (KPS) to decompose into free radicals, which reacted with the -OH in the corncob to form macroradicals. The macroradicals then initiated the monomers AA and acrylamide (produced by AA reacting with parts of urea), leading to polymer chain propagation and the generation of grafted copolymers. In addition, owing to the presence of the cross-linking agent MBA, a cross-linked 3D network structure can be formed.
Figure 3.
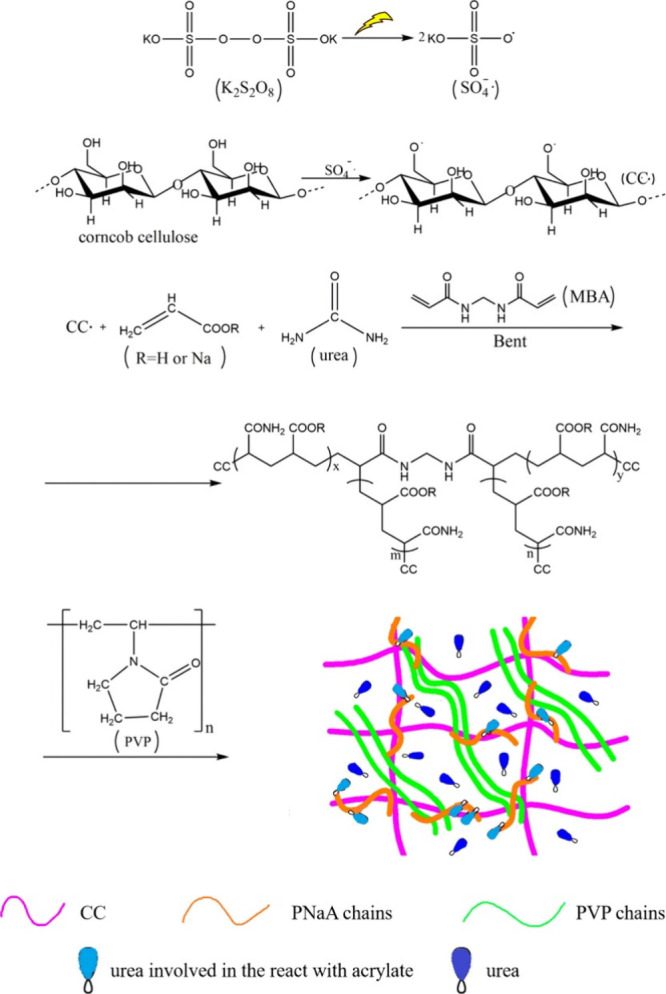
Preparation of a corncob-based hydrogel for slow-release nitrogen fertilizers (SRFs) via microwave irradiation.122
In microwave-assisted polymerization, the reaction mixture is exposed to microwave radiation, which generates heat and accelerates the reaction rate, thereby achieving advantages such as a faster reaction rate, shorter reaction time, and higher yield. The polymerization of the corncob-g-PAA/bentonite/PVP/urea (CC-g-PAA/Bent/PVP/urea) hydrogel shown in Figure 3 was completed by irradiation of the reaction mixture for 4.5 min. Wen et al. also prepared cotton stalk-based SRFs via microwave irradiation for 3–3.5 min.14,185 A recent study reported the etherification of cotton-derived microcellulose via microwave irradiation, followed by the preparation of hydrogels via microwave-assisted radical polymerization, and the polymerization was completed under 400 W of microwave irradiation for 2 min.186 PVA/GA/okara-based hydrogels were prepared in a previous study via microwave-assisted heating. Compared with conventional hotplate heating, the hydrogel could be fabricated with 100% replacement of starch by okara via microwave irradiation, while at least 50% starch was required in the latter.170 Another study revealed that a carboxymethyl cellulose-g-2-acrylamido-2-methylpropanesulfonic acid hydrogel synthesized through a microwave-assisted technique has a higher gel content than that obtained through conventional methods.187 Even when the reaction time in the conventional water bath heating method was extended to 180 min, the gel content was still lower than the optimal time of 14.60 min in the microwave-assisted method. Thus, the microwave-assisted method offers advantages over the conventional method in terms of faster hydrogel formation and a higher yield of the product.
γ Radiation graft copolymerization uses γ radiation to initiate the grafting of monomers onto lignocellulosic biomass. Like other free radical polymerization reactions, this mechanism involves three steps: initiation, propagation, and termination. An example is shown in Figure 4 to illustrate the synthesis of hydrogel composites composed of guar gum-pectin/PAAm/ZnO via gamma irradiation.188 In this study, guar gum and pectin were irradiated with γ radiation, which led to the formation of macroradicals that initiated the polymerization of the AAm monomer to form a 3D hydrogel network. This polymerization technique has also been used to develop hydrogels such as carboxymethyl cellulose/PAAm189 garlic straw-derived composite hydrogels190 and agro waste/PAA superabsorbents based on agricultural residues, including rice straw, soft coconut shells, palm fruit bunches, water hyacinth and sugar cane.191
Figure 4.
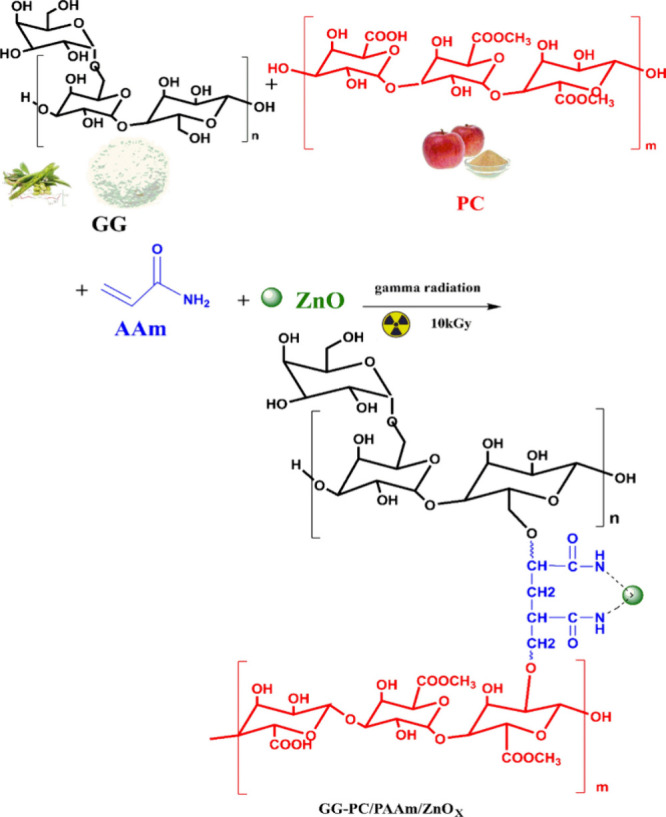
Synthesis of hydrogel composites composed of guar gum-pectin/PAAm/ZnO via gamma irradiation.188
4. Application of AW-SAHs in Sustainable Agriculture
SAHs are widely used in agriculture, mainly for the management of water and nutrients, the two key factors affecting crop production.33,148 In early years, many studies explored the application of conventional petroleum-based SAHs in agriculture, but their practical application in agriculture was limited because of their high cost and environmental concerns associated with their production and disposal.192 In contrast, the advantages of using waste-derived SAHs in agriculture are more obvious, and the application of AW-SAHs in agriculture is gradually increasing. Since these hydrogels are made from abundant agricultural waste materials, they not only reduce waste disposal problems but also make the production of AW-SAHs more sustainable and cost-effective.21,193
4.1. AW-SAH-Based Slow-Release Fertilizer (SRF)
One of the main uses of SAHs in agriculture is as carriers for slow-release fertilizers (SRFs).194 After fertilizers are incorporated into the hydrogel matrix, they can be slowly released over time, providing plants with a sustainable source of nutrients. The use of slow-release fertilizer hydrogel (SRFH) prevents fertilizer leaching and runoff, reduces the fertilization frequency, and improves plant growth.195
There are several methods for the preparation of AW-SAH based SRFs, among which the following three methods are commonly reported in the literature.196,197 (1) Hydrogel/fertilizer solution mixing method: In this method, the dried hydrogel is immersed in the fertilizer solution, and the swollen hydrogel is then dried to form the SRFH. (2) In situ polymerization method: This method involves the direct polymerization of monomers in the presence of fertilizer to form SRFH. (3) Coating method: In this method, the solid-form fertilizer is coated with a layer of hydrogel, which acts as a slow-release matrix, and the coated fertilizer is dried to form the SRFH.
Compared with the in situ polymerization method, the advantage of the hydrogel/fertilizer solution mixing method offers better control over SRFH formation, as it loads the fertilizer after the hydrogel is prepared, avoiding the side reaction of adding all the materials together. It is usually conducted in four steps: hydrogel synthesis, hydrogel drying, fertilizer loading and SRFH drying. AW-SAHs, which include wheat straw-g-poly(acrylic acid),198 carboxymethyl cellulose-g-poly(acrylamide)/montmorillonite,199 sugar cane bagasse-g-poly(acrylamide)/attapulgite,135 cellulose nanofibers/metal–organic frameworks (MOFs)/sodium alginate,200 and carboxymethylcellulose/graphite oxide,201 have been used to load urea via this method. Another study also used cellulose-based hydrogels to load KNO3 fertilizer by immersing the dried hydrogel in KNO3 aqueous solution, followed by swelling and drying to prepare SRFH.202
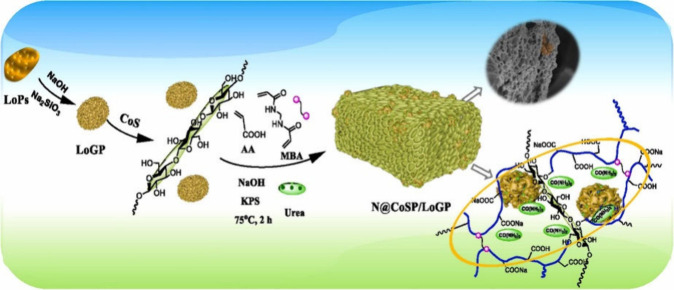
The advantage of the in situ polymerization method is its simplicity, as it requires fewer steps and is more cost-effective due to reduced drying costs. Figure 5 shows an example of the synthesis of corn stalk fiber/loess-based geopolymer (CoSP/LoGP)-based SRFs (N@ CoSP/LoGP) via an in situ polymerization method.203 Urea was mixed with all the raw materials for polymerization, including the CoS fiber/LoGP, monomer AA, cross-linker MBA, and initiator KPS, to form the SRFH. The AW-SAH-based SRFs demonstrated a remarkable improvement in nitrogen utilization efficiency, promoting corn growth with the sustained release of 60.6% of nutrients within 45 days. Urea was also incorporated as a source of nitrogen via in situ polymerization in a carboxylated cellulose nanocrystal-reinforced sodium alginate (SA)-g-P(AA-co-AAm) hydrogel204 and a leftover rice-g-PAA/montmorillonite AW-SAH.205 In addition to urea, the method has also been applied to load nitrogen (N), phosphorus (P), and potassium (K) (NPK) fertilizer compounds into a maize bran-g-P(AA-co-AAm)/montmorillonite hydrogel206 and a sulfonated-carboxymethyl cellulose (SCMC)-PAA/PVP/silica hydrogel.207
Figure 5.
Procedure for the synthesis of corn stalk fiber/geopolymer-based SRFs via an in situ polymerization method.203
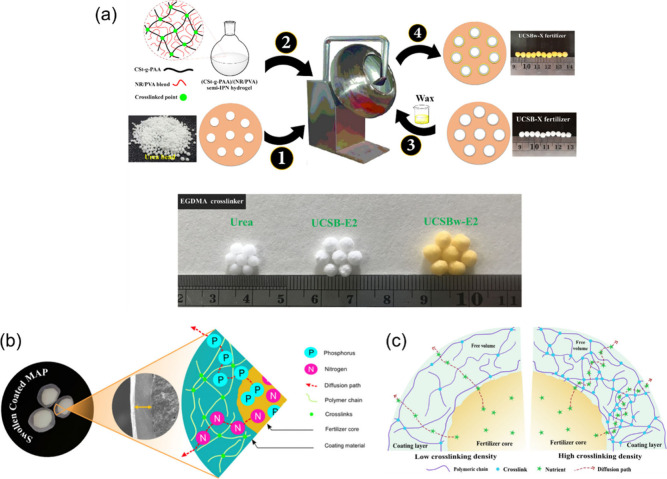
The coating method allows for precise control over the release rate of nutrients, which can be adjusted by altering the physical/chemical properties (such as hydrophilicity/hydrophobicity and cross-linking density) of the coating materials and the thickness of the coating layer. An example is shown in Figure 6a to illustrate the fabrication routes of the urea coating process via the coating method.208 Solid urea prills were coated with cassava starch (CSt)-g-PAA/natural rubber/PVA semi-IPN hydrogel by gradually injecting the hydrogel solution onto the surface of urea at 70 °C within 1 min, followed by curing at the same temperature for 10 min. Any cracks or flaws on the coating surface were sealed with a wax solution to produce the final coated SRF product, as shown. Kassem et al. developed an all-cellulose hydrogel formulation using CMC/hydroxyethyl cellulose/regenerated cellulose, which was applied as a coating material for monoammonium phosphate fertilizer (MAP) in a rotating pan to produce coated SRFs varying in thickness and degree of cross-linking (Figure 6b-c).209 This study revealed that coating thickness and cross-linking conditions can affect the properties of coated products. Increasing the coating thickness and highly cross-linked networks resulted in a higher hardness and slower release rates (12 h in water and 19–20 days in soil). Thicker coatings also resulted in better water holding and retention capacities. It was also found that cross-linking influenced the degradation of the coating material in the soil, with a higher cross-linking density resulting in slower decomposition (63.15% loss after 24 days). Other coating methods, such as using AW-SAHs as an inner coating or outer coating in double-coated multifunctional SRFs,174,210,211 and wrapping waste mulberry branch-based SAHs in the “egg-shell” structure of sodium alginate/Ca2+ have also been reported.112
Figure 6.
(a) Fabrication routes of the urea coating process via the coating method and digital photographs of the coated SRFs,208 (b) schematic diagram of swollen coated monoammonium phosphate fertilizer (MAP) granules209 and (c) schematic illustration of the effect of the degree of cross-linking of the coating on the diffusion of nutrients.209
4.2. Characteristics of AW-SAHs for Soil Conditioning
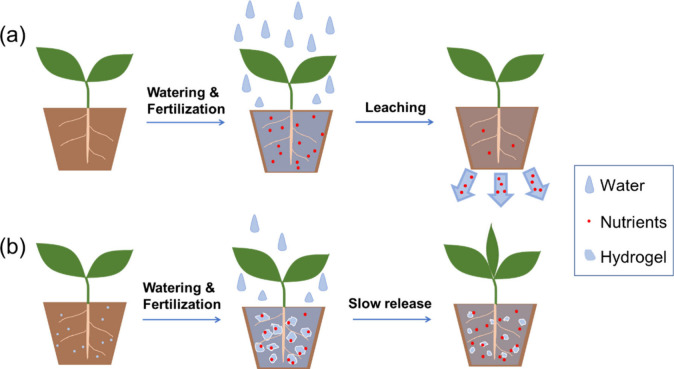
Another major use of AW-SAHs in sustainable agriculture is as a soil conditioner to increase the water holding capacity of the soil.102 These hydrogels can absorb and retain large amounts of water and nutrients, which are then slowly released to plants over time to increase plant growth and yield.148Figure 7 illustrates the phenomenon of water leaching and nutrient loss caused by the repeated watering of plants growing in bare soil, while water and nutrients are retained in the soil supplemented with SAHs and then slowly released for plant growth. In bare soil, water and fertilizers need to be applied frequently, as excess water drains out of the soil, leaching nutrients along with it. This leads to nutrient loss and the inefficient use of water resources. Conversely, when SAHs are added to the soil, they absorb and retain water, creating a reservoir that can slowly release water to plant roots over time. This reduces the frequency of watering and fertilization needed, which in turn reduces water leaching and nutrient loss.
Figure 7.
Illustration of (a) water leaching and nutrient loss caused by repeated watering and fertilization of plants growing in bare soil and (b) water and nutrient retention and slow release for plants growing in soil supplemented with superabsorbent hydrogels.
AW-SAHs are usually characterized by their swelling capacity, water holding and retention in soil, mechanical properties, and biodegradability, among other properties, to evaluate their suitability for application as a soil conditioner.212 Research in the field often focuses on producing AW-SAHs with high swelling capacity, good water holding and retention capacities in soil, good mechanical properties, the incorporation of relatively high levels of low-cost waste.104,213 Researchers can tune the properties of AW-SAHs by strategies such as changing the chemical composition and functional groups of the hydrogel, optimizing the synthesis parameters (such as the concentration and feed ratio of the raw materials) and reaction conditions, and incorporating nanomaterials or other fillers.214
The swelling behavior of SAHs can be characterized by measuring their swelling capacity and swelling kinetics.215 The water swelling capacity is the amount of water absorbed by the hydrogel per unit weight, and it can be determined by immersing a known weight of dry hydrogel in water and measuring the weight gain after equilibrium is reached.84 Swelling kinetics are the rate at which a hydrogel swells and can be determined by measuring the weight of the hydrogel at various time intervals after immersion in water.83 The swelling properties can be improved by adjusting the polymerization parameters, including the monomer concentration, initiator concentration, feed ratio of the raw materials and cross-linking density.216 Several techniques, such as the incorporation of nanoclay fillers217 and plasma modification,15 were also found to be useful for improving the swelling properties of SAHs.
The water-holding capacity or water-retention performance effectiveness of AW-SAHs in soil is very important to prove that AW-SAHs can be used as a soil conditioner for improving soil quality.102,197 To improve the water holding and retention properties of AW-SAHs in soil, various strategies can be employed, such as changing the hydrogel composition and cross-linking density.218 One common approach is to introduce hydrophilic functional groups or monomers such as AA and AAm into the hydrogel network, which can increase the affinity of the hydrogel for water.219 Our group introduced AA and AAm into the biomass of okara to form okara-P(AA-AAm) and okara-PAA hydrogel networks.79,84 The hydrogels were demonstrated to enhance vegetable growth by improving the water holding and retention properties of soils. The water retention capacity of the same material in different soils also varies. Compared with the nutrient-rich pot mixture, the okara-PAA hydrogel supplement improved the water holding and retention capacity of the sand and mixed soils more significantly.84 Optimization of the hydrogel synthesis parameters and reaction conditions can also lead to improved water holding and retention properties.
Improving the mechanical strength of SAHs can usually improve their water-holding and water-retention properties in soil.220,221 The mechanical strength of a hydrogel refers to its ability to resist deformation or breakage under an applied force. A hydrogel with greater mechanical strength will be more resistant to compression and maintain its structure for a longer time, which can lead to improved water holding and retention properties. The mechanical strength of a hydrogel can be improved by strategies such as increasing the cross-linking density of the hydrogel, blending hydrogels with polymers that can strengthen interactions, and incorporating fillers. For example, the addition of kaolin was found to improve the mechanical properties of soybean residue-g-poly(acrylic acid)/polyethylene glycol 2000/kaolin/urea hydrogels by forming a dense and rigid network.177 Wei et al. used peach branch-cellulose nanofibers as reinforcing agents to enhance the mechanical performance and adsorption capacity of hydrogels.12
Compared with conventional petroleum-based hydrogels, which can persist in the environment for a long time, AW-SAHs generally have better biodegradability because they are made from biodegradable biomass materials that can easily decompose in the environment.145 The use of AW-SAHs in agriculture can therefore reduce the accumulation of nondegradable materials in the environment and promote sustainable agricultural practices.
4.3. Application of AW-SAHs for Enhanced Plant Growth
The use of AW-SAHs or AW-SAH-based SRFs in soil has shown promising results in promoting plant growth. By increasing the water holding capacity and nutrient retention of soil, AW-SAHs can increase plant growth, especially under water-limited or drought conditions.79 Several studies have reported improvements in plant growth, yield, and water use efficiency when crops such as leafy vegetables,222 beans,223 cucumbers,224 corn,11 wheat,76 and maize225 are grown in soil supplemented with AW-SAHs. However, the effectiveness of AW-SAHs as soil supplements may vary depending on factors such as the application rate, soil type, and crop species.
For example, the effects of different concentrations of okara-P(AA-AAm) hydrogel on the initial growth of the Asian leafy vegetable choy sum were studied by our group.79 It was found that the initial growth of the choy sum seedlings was not adversely affected by the addition of 0–2% hydrogel to the potting mix. The study revealed that seeds grown in a water-saturated pot with 2% hydrogel had a 100% survival rate after 12 days without the addition of water after sowing, whereas no plants survived beyond 9 days without the hydrogel. Compared with those mixed with 1% or less okara-P(AA-AAm), those mixed with 2% okara-P(AA-AAm) hydrogel presented better drought tolerance. Using the hydrogel as a soil supplement for plant growth led to more than 80% growth enhancement in terms of shoot fresh weight and leaf area under water-limited conditions. The addition of the okara-P(AA-AAm) hydrogel significantly improved the growth and yield of choy sum, reduced leaching and watering frequency by half, increased irrigation water-use efficiency (>80%), and prevented excessive nutrient leaching.9 Okara-based SAHs can also be applied under field conditions to keep plants hydrated for longer periods of time, potentially reducing the need for frequent watering.222 A field study revealed that the okara-based hydrogel amendment retained more soil nutrients, and the use of the 0.2% (w/v) hydrogel increased choy sum and pak choi vegetable growth by 35–60% and increased water use efficiency by 30–60%. These findings suggest that okara-based SAHs have potential for plant growth applications, particularly in situations with limited availability of irrigation water.
In addition, the efficacy of AW-SAHs can vary depending on the soil type. In one study, an okara-PAA hydrogel was evaluated in three different types of soils, namely, lawn sand, mixed soil, and potting mix.84 While the hydrogel enhanced the survival of choy sum seedlings in mixed soil, there was only a slight difference in the survival of seedlings grown in pots supplemented with or without the hydrogel. Thus, compared with other soil types, supplementation with the okara-PAA hydrogel is more beneficial for choy sum in mixed soil under drought conditions. Calcagnile et al.202 conducted a study on tomatoes and chicory using a poly(lactic acid)/cellulose-based superabsorbent hydrogel in two different soil types: red soil (a sandy soil) and white soil, which is composed of a certain amount of clay. The results showed that the hydrogel material improved the water retention capacity of the soil, particularly in red soil. Furthermore, increasing the amount of hydrogel in the system led to higher fruit yields than those in the control case.
The application of AW-SAHs for improving plant growth has been reported in a wide variety of crop species. In pot experiments with wheatgrass, the use of zinc-loaded carboxymethyl cellulose/carrageenan hydrogels had positive effects on plant growth. Compared with those in the control group, the height of the treated plants increased, the number of germinated seeds increased, and the total fresh and dry weights increased.226 The addition of wheat straw cellulose-based hydrogels to the soil improved the soil water holding capacity, resulting in improved corn germination and seedling growth, as evidenced by significant increases in the germination index and overall plant growth.11 Similarly, a stalk fiber-based SRFH greatly improved soil fertility, promoting corn germination and growth.203 The application of millet straw-based SRFH in soil for wheat growth resulted in a significant delay in phosphorus and nitrogen release rates, promoting the growth indices and yields of wheat.76 The application of rice straw-based hydrogels significantly improved the growth of monocot (wheat) and dicot (moong bean) plants by increasing their height, total biomass, and leaf area and prolonging their survival time, and these plants performed well under stress conditions compared with the control.74 In a field experiment, the effects of rice straw-based hydrogels combined with agricultural fertilizer and effective microorganisms on fava bean production and their ability to alleviate salinity stress were investigated. The analysis revealed that all amendments had significant effects on bean growth parameters, in terms of seed yield, seed nutrient content, irrigation water use efficiency, and economic water productivity.223
Thus, AW-SAHs offer substantial agronomic benefits by increasing soil water and nutrient retention, which is particularly valuable under drought or water-limited conditions. These hydrogels improve soil moisture retention, reduce the frequency of irrigation and increase water-use efficiency. By acting as water and nutrient reservoirs, AW-SAHs ensure a steady supply of essential nutrients to plants, minimizing nutrient leaching and promoting better plant growth and higher yields. Crops such as corn, wheat, and leafy vegetables have shown significant growth improvements and yield increases when grown in soil supplemented with AW-SAHs. The enhanced nutrient and water retention capabilities of these hydrogels also lead to more efficient nutrient management, reducing the need for external fertilizers and irrigation inputs, lowering production costs, and mitigating environmental impacts by decreasing water and nutrient runoff. Furthermore, AW-SAHs support sustainable agriculture by enhancing the soil structure, increasing the organic matter content, and fostering beneficial microbial activity, contributing to long-term soil fertility and productivity. The use of agricultural waste-derived hydrogels aligns with circular economy principles by recycling waste materials into valuable agricultural inputs, further enhancing farming sustainability and resilience.
5. Challenges, Opportunities, and Perspectives
The integration of AW-SAHs into sustainable agriculture presents both significant challenges and promising opportunities. Derived from agro waste materials rich in natural biopolymers such as cellulose, hemicellulose, and lignin, AW-SAHs utilize various physical and chemical cross-linking methods essential for their synthesis. Physical methods such as dissolution-regeneration and freeze–thawing establish networks through hydrogen bonding, ionic bonding, and hydrophobic forces. Chemical cross-linking, involving agents such as epichlorohydrin and glutaraldehyde, and advanced polymerization techniques such as free radical graft polymerization, offer enhanced functionality but introduce complexities related to reaction conditions and environmental safety.
Despite their potential, AW-SAHs face several challenges. The variability in the quality and properties of agro waste materials can affect the consistency and performance of the hydrogels. The economic feasibility of large-scale production and application is a major concern, as well as addressing environmental issues related to the biodegradability and potential toxicity of some hydrogel components.
On the positive side, AW-SAHs offer significant opportunities for enhancing sustainable agriculture. They improve soil moisture and nutrient retention, leading to more resilient cropping systems, especially in arid and semiarid regions. The use of waste materials for hydrogel production aligns with circular economy principles, reducing agricultural waste and promoting resource efficiency. Advances in materials science and biotechnology could further increase the efficiency and environmental friendliness of these hydrogels.
Moving forward, it is crucial to standardize production processes for AW-SAHs to ensure consistency and quality. Research should also focus on understanding the long-term effects of AW-SAHs on soil health and crop productivity. Integrating AW-SAHs with other sustainable practices, such as precision agriculture and organic farming, could create more holistic and resilient farming systems. Collaboration among researchers, industry stakeholders, and policymakers will be essential to overcoming current barriers and realizing the full potential of AW-SAHs in promoting sustainable agriculture.
AW-SAHs have several innovative applications beyond their primary use as slow-release fertilizers and soil amendments. With respect to seed coating, SAHs enhance germination and seedling establishment by protecting seeds from environmental stresses and pathogens while delivering essential nutrients and growth promoters directly to the seeds.227 This technique has shown promise in improving crop yields and reducing the need for external inputs. However, reports on coatings with AW-SAH are still limited compared with those with petroleum-based polymers, or alginate and starch.228−231 Additionally, AW-SAHs can be integrated into controlled-release pesticide systems, enhancing pest management and reducing chemical leaching, although precise formulations are needed to avoid unintended environmental impacts.232,233 In hydroponic and greenhouse cultivation, AW-SAHs could be developed into soilless substrates to increase water and nutrient delivery, potentially improving plant growth and resource efficiency.40,234,235 Adapting these hydrogels to various growth conditions and scaling up their application remains a challenge.
While these applications offer substantial benefits, addressing challenges such as cost-effectiveness, scalability, and compatibility with diverse agricultural systems is essential. Ongoing research and technological advancements will be pivotal in overcoming these obstacles and maximizing the potential of AW-SAHs in advancing sustainable agricultural practices.
Acknowledgments
This work was supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme, the Intra-CREATE Thematic Grant on Science of Sustainable Cities – Food (grant no. NRF2020-THE003-0005) and Singapore-China Joint Flagship Project on Water and Environment (grant no. A-8001631-00-00). The work was also cosupported by the Science and Technology Project of Jiangsu Province (grant no. BZ2022056) and the Chongqing Natural Science Foundation (grant no. CSTB2022NSCQ-MSX1473), through NUS (Suzhou) Research Institute and NUS (Chongqing) Research Institute, respectively.
The authors declare no competing financial interest.
References
- Bhattacharya R.; Bose D. Energy and Water: COVID-19 Impacts and Implications for Interconnected Sustainable Development Goals. Environmental Progress & Sustainable Energy 2023, 42 (1), e14018 10.1002/ep.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezoni R. A.; Seto K. C.; Puppim de Oliveira J. A.. What Can Cities Do to Enhance Water-Energy-Food Nexus as a Sustainable Development Strategy? In Water-Energy-Food Nexus and Climate Change in Cities; Lazaro L. L. B., Giatti L. L., Valente de Macedo L. S., Puppim de Oliveira J. A. Eds.; Springer International Publishing, 2022; pp 39–57. [Google Scholar]
- Roy P.; Pal S. C.; Chakrabortty R.; Chowdhuri I.; Saha A.; Shit M. Climate Change and Groundwater Overdraft Impacts on Agricultural Drought in India: Vulnerability Assessment, Food Security Measures and Policy Recommendation. Science of The Total Environment 2022, 849, 157850 10.1016/j.scitotenv.2022.157850. [DOI] [PubMed] [Google Scholar]
- Rakotovao N. H.; Razafimbelo T. M.; Razakamanarivo H. R.; Hewson J. H.; Ramifehiarivo N.; Andriamananjara A.; Ndour Y. B.; Sall S.; Sabir M.; Aholoukpe H.; et al. Agricultural Soil and Water Conservation Issues in East Africa. In Global Degradation of Soil and Water Resources: Regional Assessment and Strategies; Li R., Napier T. L., El-Swaify S. A., Sabir M., Rienzi E., Eds.; Springer Nature; Singapore, 2022; pp 29–48. [Google Scholar]
- Muhie S. H. Novel Approaches and Practices to Sustainable Agriculture. Journal of Agriculture and Food Research 2022, 10, 100446 10.1016/j.jafr.2022.100446. [DOI] [Google Scholar]
- Sahmat S. S.; Rafii M. Y.; Oladosu Y.; Jusoh M.; Hakiman M.; Mohidin H. A Systematic Review of the Potential of a Dynamic Hydrogel as a Substrate for Sustainable Agriculture. Horticulturae 2022, 8 (11), 1026. 10.3390/horticulturae8111026. [DOI] [Google Scholar]
- Xi L.; Zhang M.; Zhang L.; Lew T. T. S.; Lam Y. M. Novel Materials for Urban Farming. Adv. Mater. 2022, 34 (25), 2105009 10.1002/adma.202105009. [DOI] [PubMed] [Google Scholar]
- Zou H.; Yang X.; Zhu J.; Wang F.; Zeng Z.; Xiang C.; Huang D.; Li J.; Wang R. Solar-driven scalable hygroscopic gel for recycling water from passive plant transpiration and soil evaporation. Nature Water 2024, 2 (7), 663–673. 10.1038/s44221-024-00265-y. [DOI] [Google Scholar]
- Tan W. K.; Zhu J.; Lim J. Y.; Gao Z.; Loh C. S.; Li J.; Ong C. N. Use of Okara-derived Hydrogel for Enhancing Growth of Plants by Minimizing Leaching and Locking Nutrients and Water in Growing Substrate. Ecological Engineering 2021, 159, 106122 10.1016/j.ecoleng.2020.106122. [DOI] [Google Scholar]
- Oladosu Y.; Rafii M. Y.; Arolu F.; Chukwu S. C.; Salisu M. A.; Fagbohun I. K.; Muftaudeen T. K.; Swaray S.; Haliru B. S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8 (7), 605. 10.3390/horticulturae8070605. [DOI] [Google Scholar]
- Sasmal P. K.; Patra S. Effect in Growth of Corn Plant from Cellulose-Based Hydrogel Derived from Wheat Straw. Journal of The Institution of Engineers (India): Series E 2022, 103 (1), 41–46. 10.1007/s40034-020-00180-3. [DOI] [Google Scholar]
- Wei W.; Luo Q.; Liu Y.; Qu R.; Sun D.; Gao F.; Li B.; Wu M. Feasibility of Preparing Nanofiber Reinforcer of Gelatin Hydrogel from Waste Peach Branches. Biomass Conversion and Biorefinery 2023, 13, 5831. 10.1007/s13399-021-01598-4. [DOI] [Google Scholar]
- Rashad M.; Kenawy E.-R.; Hosny A.; Hafez M.; Elbana M. An environmental friendly superabsorbent composite based on rice husk as soil amendment to improve plant growth and water productivity under deficit irrigation conditions. Journal of Plant Nutrition 2021, 44 (7), 1010–1022. 10.1080/01904167.2020.1849293. [DOI] [Google Scholar]
- Wen P.; Wu Z.; He Y.; Ye B.-C.; Han Y.; Wang J.; Guan X. Microwave-Assisted Synthesis of a Semi-interpenetrating Polymer Network Slow-Release Nitrogen Fertilizer with Water Absorbency from Cotton Stalks. ACS Sustainable Chemistry & Engineering 2016, 4 (12), 6572–6579. 10.1021/acssuschemeng.6b01466. [DOI] [Google Scholar]
- Liang L.; Guo Y.; Wang H.; Liao Z.; Zhang J.; Wei L.; Hou K. Study on Plasma-modified Corn Stover-Humic Acid-based Superabsorbent Resin. J. Appl. Polym. Sci. 2023, 140 (5), e53390 10.1002/app.53390. [DOI] [Google Scholar]
- Zeng H.; Liu B.; Li J.; Li M.; Peng M.; Qin C.; Liang C.; Huang C.; Li X.; Yao S. Efficient Separation of Bagasse Lignin by Freeze–Thaw-assisted P-Toluenesulfonic Acid Pretreatment. Bioresour. Technol. 2022, 351, 126951 10.1016/j.biortech.2022.126951. [DOI] [PubMed] [Google Scholar]
- Xie X.; Ma L.; Chen Y.; Luo X.; Long M.; Ji H.; Chen J. Bagasse Cellulose Composite Superabsorbent Material with Double-Crosslinking Network Using Chemical Modified Nano-CaCO3 Reinforcing Strategy. Nanomaterials 2022, 12 (9), 1459. 10.3390/nano12091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.; McClements D. J.; He M.; Li Y.; Teng F. The Measurement of Molecular Interactions, Structure and Physical Properties of Okara Cellulose Composite Hydrogels using Different Analytical Methods. Journal of the Science of Food and Agriculture 2022, 102 (10), 4162–4170. 10.1002/jsfa.11765. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Song X.; Tan W. K.; Wen Y.; Gao Z.; Ong C. N.; Loh C. S.; Swarup S.; Li J. Chemical Modification of Biomass Okara Using Poly(acrylic acid) through Free Radical Graft Polymerization. J. Agric. Food Chem. 2020, 68 (46), 13241–13246. 10.1021/acs.jafc.0c01818. [DOI] [PubMed] [Google Scholar]
- Demirbas A. Heavy Metal Adsorption onto Agro-based Waste Materials: A Review. Journal of Hazardous Materials 2008, 157 (2), 220–229. 10.1016/j.jhazmat.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Miljković V.; Gajić I.; Nikolić L. Waste Materials as a Resource for Production of CMC Superabsorbent Hydrogel for Sustainable Agriculture. Polymers 2021, 13 (23), 4115. 10.3390/polym13234115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janaswamy S.; Yadav M. P.; Hoque M.; Bhattarai S.; Ahmed S. Cellulosic Fraction from Agricultural Biomass as a Viable Alternative for Plastics and Plastic Products. Industrial Crops and Products 2022, 179, 114692 10.1016/j.indcrop.2022.114692. [DOI] [Google Scholar]
- Karlinskii B. Y.; Ananikov V. P. Recent Advances in the Development of Green Furan Ring-containing Polymeric Materials based on Renewable Plant Biomass. Chemical Society Reviews 2023, 52 (2), 836–862. 10.1039/D2CS00773H. [DOI] [PubMed] [Google Scholar]
- Nguyen Thi H. Y.; Kim S.; Duy Nguyen B. T.; Lim D.; Kumar S.; Lee H.; Szekely G.; Kim J. F. Closing the Sustainable Life Cycle Loop of Membrane Technology via a Cellulose Biomass Platform. ACS Sustainable Chemistry & Engineering 2022, 10 (7), 2532–2544. 10.1021/acssuschemeng.1c08554. [DOI] [Google Scholar]
- Sternberg J.; Sequerth O.; Pilla S. Green Chemistry Design in Polymers Derived from Lignin: Review and Perspective. Prog. Polym. Sci. 2021, 113, 101344 10.1016/j.progpolymsci.2020.101344. [DOI] [Google Scholar]
- Rai P.; Mehrotra S.; Priya S.; Gnansounou E.; Sharma S. K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739 10.1016/j.biortech.2021.124739. [DOI] [PubMed] [Google Scholar]
- Zainal S. H.; Mohd N. H.; Suhaili N.; Anuar F. H.; Lazim A. M.; Othaman R. Preparation of Cellulose-based Hydrogel: A Review. Journal of Materials Research and Technology 2021, 10, 935–952. 10.1016/j.jmrt.2020.12.012. [DOI] [Google Scholar]
- Kaur J.; Mankoo R. K.; Dudeja I.; Kapil S. Recent Approaches to the Synthesis of Hydrogels from Lignocellulosic Biomass: A Review. Cellulose Chemistry and Technology 2022, 56 (7–8), 891–906. 10.35812/CelluloseChemTechnol.2022.56.80. [DOI] [Google Scholar]
- Mansouri H.; Ait Said H.; Noukrati H.; Oukarroum A.; Ben youcef H.; Perreault F. Advances in Controlled Release Fertilizers: Cost-Effective Coating Techniques and Smart Stimuli-Responsive Hydrogels. Advanced Sustainable Systems 2023, 7 (9), 2300149 10.1002/adsu.202300149. [DOI] [Google Scholar]
- Merino D.; Mansilla A. Y.; Salcedo M. F.; Athanassiou A. Upcycling Orange Peel Agricultural Waste for the Preparation of Green Hydrogels as Active Soil Conditioners. ACS Sustainable Chemistry & Engineering 2023, 11 (29), 10917–10928. 10.1021/acssuschemeng.3c02992. [DOI] [Google Scholar]
- Jayanthi B.; Vinoth S.; Hariharan M.; Raja R. K.; Kamaraj C.; Narayanan M. Valorization of agro-industry wastes for nanocellulose fabrication and its multifunctional applications. Biocatalysis and Agricultural Biotechnology 2024, 57, 103124 10.1016/j.bcab.2024.103124. [DOI] [Google Scholar]
- Skrzypczak D.; Izydorczyk G.; Taf R.; Moustakas K.; Chojnacka K. Cellulose-based fertilizers for sustainable agriculture: Effective methods for increasing crop yield and soil health. Industrial Crops and Products 2023, 205, 117500 10.1016/j.indcrop.2023.117500. [DOI] [Google Scholar]
- Li S.; Chen G. Agricultural Waste-derived Superabsorbent Hydrogels: Preparation, Performance, and Socioeconomic Impacts. Journal of Cleaner Production 2020, 251, 119669 10.1016/j.jclepro.2019.119669. [DOI] [Google Scholar]
- Patra S. K.; Poddar R.; Brestic M.; Acharjee P. U.; Bhattacharya P.; Sengupta S.; Pal P.; Bam N.; Biswas B.; Barek V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. International Journal of Polymer Science 2022, 2022, 4914836 10.1155/2022/4914836. [DOI] [Google Scholar]
- Iqbal D. N.; Tariq Z.; Philips B.; Sadiqa A.; Ahmad M.; Al-Ahmary K. M.; Ali I.; Ahmed M. Nanocellulose/wood ash-reinforced starch–chitosan hydrogel composites for soil conditioning and their impact on pea plant growth. RSC advances 2024, 14 (13), 8652–8664. 10.1039/D3RA08725E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Abidi N.; Lucia L.; Chabi S.; Denny C. T.; Parajuli P.; Rumi S. S. Cellulose/nanocellulose superabsorbent hydrogels as a sustainable platform for materials applications: A mini-review and perspective. Carbohydr. Polym. 2023, 299, 120140 10.1016/j.carbpol.2022.120140. [DOI] [PubMed] [Google Scholar]
- do Nascimento D. M.; Nunes Y. L.; de Almeida J. S.; Leitão R. C.; Feitosa J. P. A.; Dufresne A.; Rosa M. d. F. Development of an integrated process to produce CNFs and lignin and its potential applications for agrochemical delivery. Cellulose 2021, 28 (17), 10891–10904. 10.1007/s10570-021-04200-2. [DOI] [Google Scholar]
- Rodrigues Sousa H.; Sá Lima I.; Matheus Lima Neris L.; Santos Silva A.; Maria Silva Santos Nascimento A.; Pereira de Araújo F.; Felippe Ratke R.; Anteveli Osajima J.; Loiola Edvan R.; Kauany da Silva Azevedo C.; et al. Innovative hydrogels made from babassu mesocarp for technological application in agriculture. J. Mol. Liq. 2023, 376, 121463 10.1016/j.molliq.2023.121463. [DOI] [Google Scholar]
- E P.; Jha A.; Sarkar S.; Maji P. K. A urea-loaded hydrogel comprising of cellulose nanofibers and carboxymethyl cellulose: An effective slow-release fertilizer. Journal of Cleaner Production 2024, 434, 140215 10.1016/j.jclepro.2023.140215. [DOI] [Google Scholar]
- Zhang Z.; Zhu J.; Song X.; Wen Y.; Zhu C.; Li J. Biomass-based single- and double-network hydrogels derived from cellulose microfiber and chitosan for potential application as plant growing substrate. Carbohydr. Polym. 2023, 319, 121170 10.1016/j.carbpol.2023.121170. [DOI] [PubMed] [Google Scholar]
- Gu P.; Liu W.; Hou Q.; Ni Y. Lignocellulose-derived Hydrogel/aerogel-based Flexible Quasi-solid-state Supercapacitors with High-performance: A Review. Journal of Materials Chemistry A 2021, 9 (25), 14233–14264. 10.1039/D1TA02281D. [DOI] [Google Scholar]
- Yin H.; Song P.; Chen X.; Xiao M.; Tang L.; Huang H. Smart pH-Sensitive Hydrogel Based on the Pineapple Peel-Oxidized Hydroxyethyl Cellulose and the Hericium erinaceus Residue Carboxymethyl Chitosan for Use in Drug Delivery. Biomacromolecules 2022, 23 (1), 253–264. 10.1021/acs.biomac.1c01239. [DOI] [PubMed] [Google Scholar]
- El-Ramady H.; Brevik E. C.; Bayoumi Y.; Shalaby T. A.; El-Mahrouk M. E.; Taha N.; Elbasiouny H.; Elbehiry F.; Amer M.; Abdalla N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-Waste Nexus. Sustainability 2022, 14 (23), 15717. 10.3390/su142315717. [DOI] [Google Scholar]
- Dai H.; Chen Y.; Ma L.; Zhang Y.; Cui B. Direct Regeneration of Hydrogels Based on Lemon Peel and its Isolated Microcrystalline Cellulose: Characterization and Application for Methylene Blue Adsorption. International Journal of Biological Macromolecules 2021, 191, 129–138. 10.1016/j.ijbiomac.2021.09.063. [DOI] [PubMed] [Google Scholar]
- Mohammadinejad R.; Maleki H.; Larrañeta E.; Fajardo A. R.; Nik A. B.; Shavandi A.; Sheikhi A.; Ghorbanpour M.; Farokhi M.; Govindh P.; et al. Status and Future Scope of Plant-based Green Hydrogels in Biomedical Engineering. Applied Materials Today 2019, 16, 213–246. 10.1016/j.apmt.2019.04.010. [DOI] [Google Scholar]
- Lunardi V. B.; Santoso S. P.; Angkawijaya A. E.; Cheng K.-C.; Tran-Nguyen P. L.; Go A. W.; Nakamura Y.; Lin S.-P.; Hsu H.-Y.; Yuliana M.; et al. Synthesis of cellulose hydrogel by utilizing agricultural waste and zeolite for adsorption of copper metal ions. Industrial Crops and Products 2024, 210, 118179 10.1016/j.indcrop.2024.118179. [DOI] [Google Scholar]
- Liu M.; Zhu J.; Song X.; Wen Y.; Li J. Smart Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) and Chitosan through Polyelectrolyte Complexation and Its Controlled Release Properties. Gels 2022, 8 (7), 441. 10.3390/gels8070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Wen Y. T.; Song X.; Zhu J. L.; Li J. A smart thermoresponsive adsorption system for efficient copper ion removal based on alginate-g-poly(N-isopropylacrylamide) graft copolymer. Carbohydr. Polym. 2019, 219, 280–289. 10.1016/j.carbpol.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Liu M.; Song X.; Wen Y. T.; Zhu J. L.; Li J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9 (41), 35673–35682. 10.1021/acsami.7b12849. [DOI] [PubMed] [Google Scholar]
- Song X.; Mensah N. N.; Wen Y. T.; Zhu J. L.; Zhang Z. X.; Tan W. S.; Chen X. W.; Li J. beta-Cyclodextrin-Polyacrylamide Hydrogel for Removal of Organic Micropollutants from Water. Molecules 2021, 26 (16), 5031. 10.3390/molecules26165031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.; Zhu J.; Guo L.; Yu J.; Li J.; Li F. Synthesis and characterization of a β-cyclodextrin-MOF-based porous hydrogel for efficient adsorption of Au3+, Ag+, and Pb2+ ions. Separation and Purification Technology 2024, 348, 127664 10.1016/j.seppur.2024.127664. [DOI] [Google Scholar]
- Jia J.; Wu D.; Yu J.; Gao T.; Li J.; Guo L.; Li F. β-Cyclodextrin-based adsorbents integrated with N-doped TiO2 photocatalysts for boosting dye elimination of industrial wastewater. Surfaces and Interfaces 2024, 45, 103901 10.1016/j.surfin.2024.103901. [DOI] [Google Scholar]
- Zheng D.; Xu X.; Zhu J.; Bai B.; Wang Q.; Shi W.; Li J. Humidity capture and solar-driven water collection behaviors of alginate-g-PNIPAm-based hydrogel. Journal of Environmental Chemical Engineering 2023, 11 (1), 109247 10.1016/j.jece.2022.109247. [DOI] [Google Scholar]
- Chen T.; Wen Y.; Song X.; Zhang Z.; Zhu J.; Tian X.; Zeng S.; Li J. Rationally designed β-cyclodextrin-crosslinked polyacrylamide hydrogels for cell spheroid formation and 3D tumor model construction. Carbohydr. Polym. 2024, 339, 122253 10.1016/j.carbpol.2024.122253. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Wang J.; Zheng W.; Zhu J.; Song X.; Chen T.; Zhang M.; Huang Z.; Li J. A supramolecular colloidal system based on folate-conjugated β-cyclodextrin polymer and indocyanine green for enhanced tumor-targeted cell imaging in 2D culture and 3D tumor spheroids. J. Colloid Interface Sci. 2024, 667, 259–268. 10.1016/j.jcis.2024.04.072. [DOI] [PubMed] [Google Scholar]
- Wang F.; Ma R.; Zhu J.; Zhan J.; Li J.; Tian Y. Physicochemical properties, in vitro digestibility, and pH-dependent release behavior of starch–steviol glycoside composite hydrogels. Food Chem. 2024, 434, 137420 10.1016/j.foodchem.2023.137420. [DOI] [PubMed] [Google Scholar]
- Ooi Y. J.; Wen Y.; Zhu J.; Song X.; Li J. Codelivery of Doxorubicin and p53 Gene by β-Cyclodextrin-Based Supramolecular Nanoparticles Formed via Host–Guest Complexation and Electrostatic Interaction. Biomacromolecules 2024, 25 (5), 2980–2989. 10.1021/acs.biomac.4c00123. [DOI] [PubMed] [Google Scholar]
- Li J.; Osada Y.; Cooper-White J.. Functional Hydrogels as Biomaterials; Springer: Berlin, 2018 10.1007/978-3-662-57511-6. [DOI] [Google Scholar]
- Chua S. T.; Song X.; Li J.. Hydrogels for Stem Cell Encapsulation: Toward Cellular Therapy for Diabetes. In Functional Hydrogels as Biomaterials; Li J., Osada Y., Cooper-White J., Eds.; Springer: Berlin, 2018; pp 113–127. [Google Scholar]
- Song X.; Li J.. Recent Advances in Polymer-Cyclodextrin Inclusion Complex-Based Supramolecular Hydrogel for Biomedical Applications. In Functional Hydrogels as Biomaterials; Li J., Osada Y., Cooper-White J., Eds.; Springer: Berlin, 2018; pp 141–163. [Google Scholar]
- Wen Y.; Mensah N. N.; Song X.; Zhu J.; Tan W. S.; Chen X.; Li J. A hydrogel with supramolecular surface functionalization for cancer cell capture and multicellular spheroid growth and release. Chemical Communications 2022, 58 (5), 681–684. 10.1039/D1CC05846K. [DOI] [PubMed] [Google Scholar]
- Soh W. W. M.; Zhu J.; Song X.; Jain D.; Yim E. K. F.; Li J. Detachment of bovine corneal endothelial cell sheets by cooling-induced surface hydration of poly[(R)-3-hydroxybutyrate]-based thermoresponsive copolymer coating. Journal of Materials Chemistry B 2022, 10 (41), 8407–8418. 10.1039/D2TB01926D. [DOI] [PubMed] [Google Scholar]
- Soh W. W. M.; Teoh R. Y. P.; Zhu J.; Xun Y.; Wee C. Y.; Ding J.; Thian E. S.; Li J. Facile Construction of a Two-in-One Injectable Micelleplex-Loaded Thermogel System for the Prolonged Delivery of Plasmid DNA. Biomacromolecules 2022, 23, 3477–3492. 10.1021/acs.biomac.2c00648. [DOI] [PubMed] [Google Scholar]
- Liu X.; Yin H.; Song X.; Zhang Z.; Li J. Lignin-Based Nonviral Gene Carriers Functionalized by Poly[2-(Dimethylamino)ethyl Methacrylate]: Effect of Grafting Degree and Cationic Chain Length on Transfection Efficiency. Biomolecules 2022, 12 (1), 102. 10.3390/biom12010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan D.; Jaiswal A. K.; Jaiswal S. Emerging Technologies for the Production of Nanocellulose from Lignocellulosic Biomass. Carbohydr. Polym. 2022, 285, 119258 10.1016/j.carbpol.2022.119258. [DOI] [PubMed] [Google Scholar]
- Noremylia M. B.; Hassan M. Z.; Ismail Z. Recent Advancement in Isolation, Processing, Characterization and Applications of Emerging Nanocellulose: A Review. International Journal of Biological Macromolecules 2022, 206, 954–976. 10.1016/j.ijbiomac.2022.03.064. [DOI] [PubMed] [Google Scholar]
- Huang L.-Z.; Ma M.-G.; Ji X.-X.; Choi S.-E.; Si C.. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Frontiers in Bioengineering and Biotechnology 2021, 9, 10.3389/fbioe.2021.690773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Liu H.; Dong C.; An Y.; Liu J.; Li J.; Li X.; Si C.; Zhang M. Synthesis and Characterization of Temperature/pH Dual Sensitive Hemicellulose-based Hydrogels from Eucalyptus APMP Waste Liquor. Carbohydr. Polym. 2020, 247, 116717 10.1016/j.carbpol.2020.116717. [DOI] [PubMed] [Google Scholar]
- Phiri R.; Rangappa S. M.; Siengchin S.; Marinkovic D. Agro-waste natural fiber sample preparation techniques for bio-composites development: Methodological insights. Facta Universitatis, Series: Mechanical Engineering 2023, 21 (4), 631–656. 10.22190/FUME230905046P. [DOI] [Google Scholar]
- Zhou Z.; Ouyang D.; Liu D.; Zhao X. Oxidative Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis: Progress and Challenges. Bioresour. Technol. 2023, 367, 128208 10.1016/j.biortech.2022.128208. [DOI] [PubMed] [Google Scholar]
- Padhi S.; Singh A.; Routray W. Nanocellulose from agro-waste: a comprehensive review of extraction methods and applications. Reviews in Environmental Science and Bio/Technology 2023, 22 (1), 1–27. 10.1007/s11157-023-09643-6. [DOI] [Google Scholar]
- Sugiarto S.; Pong R. R.; Tan Y. C.; Leow Y.; Sathasivam T.; Zhu Q.; Loh X. J.; Kai D. Advances in Sustainable Polymeric Materials from Lignocellulosic Biomass. Materials Today Chemistry 2022, 26, 101022 10.1016/j.mtchem.2022.101022. [DOI] [Google Scholar]
- Ahmed Yassine B.; Bezbiz M.; Belachemi L.; Moreau C.; Garnier C.; Jonchere C.; Ben youcef H.; Cathala B.; Kaddami H. Preparation of superabsorbent composite(s) based on dialdehyde cellulose extracted from banana fiber waste. Carbohydr. Polym. 2024, 343, 122504 10.1016/j.carbpol.2024.122504. [DOI] [PubMed] [Google Scholar]
- Kaur J.; Mankoo R. K.; Chahal G. K. Synthesis of Rice Straw Biopolymers based Hydrogels and Their Use as Media for Growth of Monocot (Wheat) and Dicot (Moong Bean) Plants. Chemical Papers 2023, 77, 2539. 10.1007/s11696-022-02644-9. [DOI] [Google Scholar]
- Huang S.; Wu L.; Li T.; Xu D.; Lin X.; Wu C. Facile Preparation of Biomass Lignin-based Hydroxyethyl Cellulose Super-absorbent Hydrogel for Dye Pollutant Removal. International Journal of Biological Macromolecules 2019, 137, 939–947. 10.1016/j.ijbiomac.2019.06.234. [DOI] [PubMed] [Google Scholar]
- Wang W.; Yang S.; Liu H.; Yang Z.; Zhang A. A Novel Multifunctional Fertilizer Derived from Wasted Straw: Synthesis, Characteristics and Agriculture Applications. Industrial Crops and Products 2022, 176, 114308 10.1016/j.indcrop.2021.114308. [DOI] [Google Scholar]
- Li X.; Li Q.; Xu X.; Su Y.; Yue Q.; Gao B. Characterization, Swelling and Slow-release Properties of a New Controlled Release Fertilizer Based on Wheat Straw Cellulose Hydrogel. Journal of the Taiwan Institute of Chemical Engineers 2016, 60, 564–572. 10.1016/j.jtice.2015.10.027. [DOI] [Google Scholar]
- Sawut A.; Yimit M.; Sun W.; Nurulla I. Photopolymerisation and Characterization of Maleylatedcellulose-g-Poly(acrylic acid) Superabsorbent Polymer. Carbohydr. Polym. 2014, 101, 231–239. 10.1016/j.carbpol.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Tan W. K.; Song X.; Gao Z.; Wen Y.; Ong C. N.; Loh C. S.; Swarup S.; Li J. Converting Okara to Superabsorbent Hydrogels as Soil Supplements for Enhancing the Growth of Choy Sum (Brassica sp.) under Water-Limited Conditions. ACS Sustainable Chemistry & Engineering 2020, 8 (25), 9425–9433. 10.1021/acssuschemeng.0c02181. [DOI] [Google Scholar]
- Cheng H. N.; Gross R. A.. Sustainability and Green Polymer Chemistry—An Overview. In Sustainability & Green Polymer Chemistry Volume 1: Green Products and Processes; ACS Symposium Series, Vol. 1372; American Chemical Society, 2020; pp 1–11. [Google Scholar]
- Wu F.; Zhang Y.; Liu L.; Yao J. Synthesis and Characterization of a Novel Cellulose-g-Poly(acrylic acid-co-acrylamide) Superabsorbent Composite Based on Flax Yarn Waste. Carbohydr. Polym. 2012, 87 (4), 2519–2525. 10.1016/j.carbpol.2011.11.028. [DOI] [Google Scholar]
- Zhang W.; Liu Q.; Guo L.; Wang P.; Liu S.; Chen J.; Lei Z. White Cabbage (Brassica oleracea L.) Waste, as Biowaste for the Preparation of Novel Superabsorbent Polymer Gel. Journal of Environmental Chemical Engineering 2021, 9 (6), 106689 10.1016/j.jece.2021.106689. [DOI] [Google Scholar]
- Olad A.; Doustdar F.; Gharekhani H. Fabrication and Characterization of a Starch-based Superabsorbent Hydrogel Composite Reinforced with Cellulose Nanocrystals from Potato Peel Waste. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020, 601, 124962 10.1016/j.colsurfa.2020.124962. [DOI] [Google Scholar]
- Zhu J.; Tan W. K.; Song X.; Zhang Z.; Gao Z.; Wen Y.; Ong C. N.; Swarup S.; Li J. Synthesis and Characterization of Okara-Poly(acrylic acid) Superabsorbent Hydrogels for Enhancing Vegetable Growth through Improving Water Holding and Retention Properties of Soils. ACS Food Science & Technology 2023, 3 (4), 553–561. 10.1021/acsfoodscitech.2c00158. [DOI] [Google Scholar]
- Murova O. An Overview of Sustainable Agricultural Waste Practices of West Texas. Natural Resources 2022, 13 (4), 91–104. 10.4236/nr.2022.134007. [DOI] [Google Scholar]
- Sharma H.; Yadav A.; Rajendran N.; Abinandan S.; Baskar G.; Krishnamurthi T.. Techno-Economic Process Parameter Studies for Hydrogel Composite Production from Corncob Biomass and its Application as Fertilizer Releasing Agent. Chemical Papers 2023, 10.1007/s11696-023-02701-x. [DOI] [Google Scholar]
- Phiri R.; Mavinkere Rangappa S.; Siengchin S. Agro-waste for renewable and sustainable green production: A review. Journal of Cleaner Production 2024, 434, 139989 10.1016/j.jclepro.2023.139989. [DOI] [Google Scholar]
- Shamsul N. S.; Kamarudin S. K.; Rahman N. A. Study on the Physical and Chemical Composition of Agro Wastes for the Production of 5-Hydroxymethylfurfural. Bioresour. Technol. 2018, 247, 821–828. 10.1016/j.biortech.2017.09.140. [DOI] [PubMed] [Google Scholar]
- Agustiany E. A.; Rasyidur Ridho M.; Rahmi M. D. N.; Madyaratri E. W.; Falah F.; Lubis M. A. R.; Solihat N. N.; Syamani F. A.; Karungamye P.; Sohail A.; et al. Recent Developments in Lignin Modification and its Application in Lignin-based Green Composites: A Review. Polymer Composites 2022, 43 (8), 4848–4865. 10.1002/pc.26824. [DOI] [Google Scholar]
- Zhang J.; Choi Y. S.; Yoo C. G.; Kim T. H.; Brown R. C.; Shanks B. H. Cellulose–Hemicellulose and Cellulose–Lignin Interactions during Fast Pyrolysis. ACS Sustainable Chemistry & Engineering 2015, 3 (2), 293–301. 10.1021/sc500664h. [DOI] [Google Scholar]
- Bhaladhare S.; Das D. Cellulose: A Fascinating Biopolymer for Hydrogel Synthesis. Journal of Materials Chemistry B 2022, 10 (12), 1923–1945. 10.1039/D1TB02848K. [DOI] [PubMed] [Google Scholar]
- Wong L. C.; Leh C. P.; Goh C. F. Designing Cellulose Hydrogels from Non-woody Biomass. Carbohydr. Polym. 2021, 264, 118036 10.1016/j.carbpol.2021.118036. [DOI] [PubMed] [Google Scholar]
- Kundu R.; Mahada P.; Chhirang B.; Das B. Cellulose Hydrogels: Green and Sustainable Soft Biomaterials. Current Research in Green and Sustainable Chemistry 2022, 5, 100252 10.1016/j.crgsc.2021.100252. [DOI] [Google Scholar]
- Gujjala L. K. S.; Kim J.; Won W. Technical Lignin to Hydrogels: An Eclectic Review on Suitability, Synthesis, Applications, Challenges and Future Prospects. Journal of Cleaner Production 2022, 363, 132585 10.1016/j.jclepro.2022.132585. [DOI] [Google Scholar]
- Fernandes M. R. C.; Huang X.; Abbenhuis H. C. L.; Hensen E. J. M. Lignin Oxidation with an Organic Peroxide and Subsequent Aromatic Ring Opening. International Journal of Biological Macromolecules 2019, 123, 1044–1051. 10.1016/j.ijbiomac.2018.11.105. [DOI] [PubMed] [Google Scholar]
- Rico-García D.; Ruiz-Rubio L.; Pérez-Alvarez L.; Hernández-Olmos S. L.; Guerrero-Ramírez G. L.; Vilas-Vilela J. L. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12 (1), 81. 10.3390/polym12010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G.; Arya S. K. A review on management of rice straw by use of cleaner technologies: Abundant opportunities and expectations for Indian farming. Journal of Cleaner Production 2021, 291, 125278 10.1016/j.jclepro.2020.125278. [DOI] [Google Scholar]
- Goodman B. A. Utilization of waste straw and husks from rice production: A review. Journal of Bioresources and Bioproducts 2020, 5 (3), 143–162. 10.1016/j.jobab.2020.07.001. [DOI] [Google Scholar]
- Kaur P. P.; Arneja J. S.; Singh J. Enzymic Hydrolysis of Rice Straw by Crude Cellulase from Trichoderma Reesei. Bioresour. Technol. 1998, 66 (3), 267–269. 10.1016/S0960-8524(97)00138-7. [DOI] [Google Scholar]
- Prasad S.; Singh A.; Joshi H. C. Ethanol as an Alternative Fuel from Agricultural, Industrial and Urban Residues. Resources, Conservation and Recycling 2007, 50 (1), 1–39. 10.1016/j.resconrec.2006.05.007. [DOI] [Google Scholar]
- Huang Y.-F.; Lo S.-L.. 19 - Utilization of rice hull and straw. In Rice (Fourth Edition), Bao J., Ed.; AACC International Press, 2019; pp 627–661. [Google Scholar]
- Heise K.; Kirsten M.; Schneider Y.; Jaros D.; Keller H.; Rohm H.; Kalbitz K.; Fischer S. From Agricultural Byproducts to Value-Added Materials: Wheat Straw-Based Hydrogels as Soil Conditioners?. ACS Sustainable Chemistry & Engineering 2019, 7 (9), 8604–8612. 10.1021/acssuschemeng.9b00378. [DOI] [Google Scholar]
- Li X.; Li Q.; Su Y.; Yue Q.; Gao B.; Su Y. A novel wheat straw cellulose-based semi-IPNs superabsorbent with integration of water-retaining and controlled-release fertilizers. Journal of the Taiwan Institute of Chemical Engineers 2015, 55, 170–179. 10.1016/j.jtice.2015.04.022. [DOI] [Google Scholar]
- Kenawy E.-R.; Seggiani M.; Hosny A.; Rashad M.; Cinelli P.; Saad-Allah K. M.; El-Sharnouby M.; Shendy S.; Azaam M. M. Superabsorbent Composites Based on Rice Husk for Agricultural Applications: Swelling Behavior, Biodegradability in Soil and Drought Alleviation. Journal of Saudi Chemical Society 2021, 25 (6), 101254 10.1016/j.jscs.2021.101254. [DOI] [Google Scholar]
- Ibrahim M. M.; Abd-Eladl M.; Abou-Baker N. H.. Lignocellulosic Biomass for the Preparation of Cellulose-based Hydrogel and its Use for Optimizing Water Resources in Agriculture. J. Appl. Polym. Sci. 2015, 132 ( (42), ) 10.1002/app.42652. [DOI] [Google Scholar]
- Moayedi H.; Aghel B.; Abdullahi M. a. M.; Nguyen H.; Safuan A Rashid A. Applications of rice husk ash as green and sustainable biomass. Journal of Cleaner Production 2019, 237, 117851 10.1016/j.jclepro.2019.117851. [DOI] [Google Scholar]
- de Vasconcelos M. C.; Gomes R. F.; Sousa A. A. L.; Moreira F. J. C.; Rodrigues F. H. A.; Fajardo A. R.; Neto L. G. P. Superabsorbent Hydrogel Composite Based on Starch/Rice Husk Ash as a Soil Conditioner in Melon (Cucumis melo L.) Seedling Culture. Journal of Polymers and the Environment 2020, 28 (1), 131–140. 10.1007/s10924-019-01593-x. [DOI] [Google Scholar]
- Ma J.; Ma N. L.; Zhang D.; Wu N.; Liu X.; Meng L.; Ma D.; Gao X.; Chu Z.; Zhang P.; Li M. Zero waste multistage utilization of Ginkgo biloba branches. Chemosphere 2022, 292, 133345 10.1016/j.chemosphere.2021.133345. [DOI] [PubMed] [Google Scholar]
- Li M.; Li F.; Zhou J.; Yuan Q.; Hu N. Fallen leaves are superior to tree pruning as bulking agents in aerobic composting disposing kitchen waste. Bioresour. Technol. 2022, 346, 126374 10.1016/j.biortech.2021.126374. [DOI] [PubMed] [Google Scholar]
- Lee S. H.; Lum W. C.; Boon J. G.; Kristak L.; Antov P.; Pędzik M.; Rogoziński T.; Taghiyari H. R.; Lubis M. A. R.; Fatriasari W.; et al. Particleboard from agricultural biomass and recycled wood waste: a review. Journal of Materials Research and Technology 2022, 20, 4630–4658. 10.1016/j.jmrt.2022.08.166. [DOI] [Google Scholar]
- Viretto A.; Gontard N.; Angellier-Coussy H. Urban parks and gardens green waste: A valuable resource for the production of fillers for biocomposites applications. Waste Management 2021, 120, 538–548. 10.1016/j.wasman.2020.10.018. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liang X.; Yang X.; Liu H.; Yao J. An Eco-Friendly Slow-Release Urea Fertilizer Based on Waste Mulberry Branches for Potential Agriculture and Horticulture Applications. ACS Sustainable Chemistry & Engineering 2014, 2 (7), 1871–1878. 10.1021/sc500204z. [DOI] [Google Scholar]
- Liang X.; Zhang Y.; Liu L.; Yao J.. Synthesis and urea-loading of an eco-friendly superabsorbent composite based on mulberry branches. BioResources 2012, 8( (1), ), 130–144. [Google Scholar]
- Xu X.; Bai B.; Ding C.; Wang H.; Suo Y. Synthesis and Properties of an Ecofriendly Superabsorbent Composite by Grafting the Poly(acrylic acid) onto the Surface of Dopamine-Coated Sea Buckthorn Branches. Ind. Eng. Chem. Res. 2015, 54 (13), 3268–3278. 10.1021/acs.iecr.5b00092. [DOI] [Google Scholar]
- Jin S.; Chen J.; Mao J.; Yue G.; Han Y.; Yu X. A Novel Superabsorbent from Raw Corn Straw and Poly(acrylic acid). Polymer Composites 2017, 38 (7), 1353–1362. 10.1002/pc.23701. [DOI] [Google Scholar]
- Zhang Y.; Liu Z.; Liu H.; Hui L.; Wang H.; Liu H. Characterization of liquefied products from corn stalk and its biomass components by polyhydric alcohols with phosphoric acid. Carbohydr. Polym. 2019, 215, 170–178. 10.1016/j.carbpol.2019.03.096. [DOI] [PubMed] [Google Scholar]
- Liang J.; Li Z.; Dai S.; Tian G.; Wang Z. Production of hemicelluloses sugars, cellulose pulp, and lignosulfonate surfactant using corn stalk by prehydrolysis and alkaline sulfite cooking. Industrial Crops and Products 2023, 192, 115880 10.1016/j.indcrop.2022.115880. [DOI] [Google Scholar]
- Gandam P. K.; Chinta M. L.; Gandham A. P.; Pabbathi N. P. P.; Konakanchi S.; Bhavanam A.; Atchuta S. R.; Baadhe R. R.; Bhatia R. K. A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives. Fermentation 2022, 8 (12), 704. 10.3390/fermentation8120704. [DOI] [Google Scholar]
- Bhatia S. K.; Jagtap S. S.; Bedekar A. A.; Bhatia R. K.; Patel A. K.; Pant D.; Rajesh Banu J.; Rao C. V.; Kim Y.-G.; Yang Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724 10.1016/j.biortech.2019.122724. [DOI] [PubMed] [Google Scholar]
- Zheng T.; Yang L.; Li J.; Cao M.; Shu L.; Yang L.; Zhang X.-F.; Yao J. Lignocellulose hydrogels fabricated from corncob residues through a green solvent system. International Journal of Biological Macromolecules 2022, 217, 428–434. 10.1016/j.ijbiomac.2022.07.077. [DOI] [PubMed] [Google Scholar]
- Wan T.; Xiong L.; Huang R.; Zhao Q.; Tan X.; Qin L.; Hu J. Structure and properties of corn stalk-composite superabsorbent. Polymer Bulletin 2014, 71 (2), 371–383. 10.1007/s00289-013-1066-1. [DOI] [Google Scholar]
- Wen P.; Han Y.; Wu Z.; He Y.; Ye B.-C.; Wang J. Rapid Synthesis of a Corncob-based Semi-Interpenetrating Polymer Network Slow-Release Nitrogen Fertilizer by Microwave Irradiation to Control Water and Nutrient Losses. Arabian Journal of Chemistry 2017, 10 (7), 922–934. 10.1016/j.arabjc.2017.03.002. [DOI] [Google Scholar]
- Cheng W.-M.; Hu X.-M.; Wang D.-M.; Liu G.-H. Preparation and Characteristics of Corn Straw-Co-AMPS-Co-AA Superabsorbent Hydrogel. Polymers 2015, 7 (11), 2431–2445. 10.3390/polym7111522. [DOI] [Google Scholar]
- Suri S.; Singh A.; Nema P. K. Current applications of citrus fruit processing waste: A scientific outlook. Applied Food Research 2022, 2 (1), 100050 10.1016/j.afres.2022.100050. [DOI] [Google Scholar]
- Carmo I. A. D.; de Almeida C. G.; Brandão H. M.; Cappa de Oliveira L. F.; Luis Gonçalves Dias de Souza N. Experimental Planning Applied to the Synthesis of Superabsorbent Polymer by Acrylic Acid Graft in Pectin Extracted from Passion Fruit Peel. Materials Research Express 2019, 6 (9), 095328 10.1088/2053-1591/ab332f. [DOI] [Google Scholar]
- Tibolla H.; Pelissari F. M.; Martins J. T.; Vicente A. A.; Menegalli F. C. Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: Characterization and cytotoxicity assessment. Food Hydrocolloids 2018, 75, 192–201. 10.1016/j.foodhyd.2017.08.027. [DOI] [Google Scholar]
- Lohmousavi S. M.; Abad H. H. S.; Noormohammadi G.; Delkhosh B. Synthesis and characterization of a novel controlled release nitrogen-phosphorus fertilizer hybrid nanocomposite based on banana peel cellulose and layered double hydroxides nanosheets. Arabian Journal of Chemistry 2020, 13 (9), 6977–6985. 10.1016/j.arabjc.2020.06.042. [DOI] [Google Scholar]
- Flores-Rojas A. I.; Díaz-Flores P. E.; Medellín-Castillo N. A.; Ovando-Medina V. M.; Rodríguez-Ortiz J. C. Biomaterials Based on Chitosan/Orange Peel as a Controlled Release Matrix for KNO3: Synthesis, Characterization and their Performance Evaluation. Iranian Polymer Journal 2020, 29 (11), 1007–1017. 10.1007/s13726-020-00858-w. [DOI] [Google Scholar]
- Alokika; Anu; Kumar A.; Kumar V.; Singh B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. International Journal of Biological Macromolecules 2021, 169, 564–582. 10.1016/j.ijbiomac.2020.12.175. [DOI] [PubMed] [Google Scholar]
- Yao S.; Nie S.; Yuan Y.; Wang S.; Qin C. Efficient extraction of bagasse hemicelluloses and characterization of solid remainder. Bioresour. Technol. 2015, 185, 21–27. 10.1016/j.biortech.2015.02.052. [DOI] [PubMed] [Google Scholar]
- Singh R.; Varma A. J.; Seeta Laxman R.; Rao M. Hydrolysis of Cellulose Derived from Steam Exploded Bagasse by Penicillium Cellulases: Comparison with Commercial Cellulase. Bioresour. Technol. 2009, 100 (24), 6679–6681. 10.1016/j.biortech.2009.07.060. [DOI] [PubMed] [Google Scholar]
- Mondal M. I. H.; Haque M. O.; Ahmed F.; Pervez M. N.; Naddeo V.; Ahmed M. B. Super-Adsorptive Biodegradable Hydrogel from Simply Treated Sugarcane Bagasse. Gels 2022, 8 (3), 177. 10.3390/gels8030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Cai P.; Pan Y. Dual-responsive bagasse cellulose/PVA hydrogels for sustained release of plant growth regulator. Industrial Crops and Products 2023, 195, 116432 10.1016/j.indcrop.2023.116432. [DOI] [Google Scholar]
- Ban M. T.; Mahadin N.; Abd Karim K. J. Synthesis of hydrogel from sugarcane bagasse extracted cellulose for swelling properties study. Materials Today: Proceedings 2022, 50, 2567–2575. 10.1016/j.matpr.2021.08.342. [DOI] [Google Scholar]
- Kenawy E.-R.; Seggiani M.; Cinelli P.; Elnaby H. M. H.; Azaam M. M. Swelling Capacity of Sugarcane Bagasse-g-Poly(acrylamide)/Attapulgite Superabsorbent Composites and their Application as Slow Release Fertilizer. Eur. Polym. J. 2020, 133, 109769 10.1016/j.eurpolymj.2020.109769. [DOI] [Google Scholar]
- Lu P.; Yang Y.; Liu R.; Liu X.; Ma J.; Wu M.; Wang S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831 10.1016/j.carbpol.2020.116831. [DOI] [PubMed] [Google Scholar]
- Chanklinhorm P.; Rattanawongwiboon T.; Ummartyotin S. Development of Cellulose from Sugarcane Bagasse and Polyacrylamide-Based Hydrogel Composites by Gamma Irradiation Technique: A Study of Controlled-Release Behavior of Urea. Journal of Polymers and the Environment 2022, 30 (6), 2631–2641. 10.1007/s10924-021-02362-5. [DOI] [Google Scholar]
- Azeem B.; KuShaari K.; Man Z. B.; Basit A.; Thanh T. H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Controlled Release 2014, 181, 11–21. 10.1016/j.jconrel.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Farrell C.; Ang X. Q.; Rayner J. P. Water-retention additives increase plant available water in green roof substrates. Ecological Engineering 2013, 52, 112–118. 10.1016/j.ecoleng.2012.12.098. [DOI] [Google Scholar]
- Li X.; He J.-Z.; Liu Y.-R.; Zheng Y.-M. Effects of super absorbent polymers on soil microbial properties and Chinese cabbage (Brassica chinensis) growth. Journal of soils and sediments 2013, 13 (4), 711–719. 10.1007/s11368-013-0657-7. [DOI] [Google Scholar]
- Ahmed E. M. Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research 2015, 6 (2), 105–121. 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam D.; Kaliappa S. Superabsorbent Polymers: A State-of-art Review on Their Classification, Synthesis, Physicochemical Properties, and Applications. Reviews in Chemical Engineering 2023, 39 (1), 127. 10.1515/revce-2020-0102. [DOI] [Google Scholar]
- Zhang Z.; Fu H.; Li Z.; Huang J.; Xu Z.; Lai Y.; Qian X.; Zhang S. Hydrogel Materials for Sustainable Water Resources Harvesting & Treatment: Synthesis, Mechanism and Applications. Chemical Engineering Journal 2022, 439, 135756 10.1016/j.cej.2022.135756. [DOI] [Google Scholar]
- Bashari A.; Rouhani Shirvan A.; Shakeri M. Cellulose-based hydrogels for personal care products. Polymers for Advanced Technologies 2018, 29, 2853. 10.1002/pat.4290. [DOI] [Google Scholar]
- Chen J.; Wu J.; Raffa P.; Picchioni F.; Koning C. E. Superabsorbent Polymers: From long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog. Polym. Sci. 2022, 125, 101475 10.1016/j.progpolymsci.2021.101475. [DOI] [Google Scholar]
- Lai V. W. X.; Jamaluddin J.; Mat Nasir N. A. F.; Baharulrazi N.; Majid R. A.; Lai J. C.; Mohd Bohari S. P.; Chua L. S.; Hasham R.; Rahmat Z.; Adrus N. Extraction of Cellulose from Rice Straw for Regeneration of Hydrogels. Environmental Quality Management 2022, 32 (1), 333–341. 10.1002/tqem.21825. [DOI] [Google Scholar]
- Rizwan M.; Rubina Gilani S.; Iqbal Durani A.; Naseem S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. Journal of Advanced Research 2021, 33, 15–40. 10.1016/j.jare.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Wang J.; Chen H.; Cheng D. Environmentally Friendly Hydrogel: A Review of Classification, Preparation and Application in Agriculture. Science of The Total Environment 2022, 846, 157303 10.1016/j.scitotenv.2022.157303. [DOI] [PubMed] [Google Scholar]
- Guilherme M. R.; Aouada F. A.; Fajardo A. R.; Martins A. F.; Paulino A. T.; Davi M. F. T.; Rubira A. F.; Muniz E. C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. 10.1016/j.eurpolymj.2015.04.017. [DOI] [Google Scholar]
- Morales A.; Labidi J.; Gullón P. Impact of the Lignin Type and Source on the Characteristics of Physical Lignin Hydrogels. Sustainable Materials and Technologies 2022, 31, e00369 10.1016/j.susmat.2021.e00369. [DOI] [Google Scholar]
- Sommano S. R.; Jantrawut P.; Sangta J.; Chanabodeechalermrung B.; Sunanta P.; Bakshani C.; Willats W. Utilization of coffee pulp for the production of sustainable cellulosic composite and plant-based hydrogel as a potential human wound dressing. Food Structure 2023, 37, 100347 10.1016/j.foostr.2023.100347. [DOI] [Google Scholar]
- Queiroz B. G.; Ciol H.; Inada N. M.; Frollini E. Hydrogel from All in All Lignocellulosic Sisal Fibers Macromolecular Components. International Journal of Biological Macromolecules 2021, 181, 978–989. 10.1016/j.ijbiomac.2021.04.088. [DOI] [PubMed] [Google Scholar]
- Kalinoski R. M.; Shi J. Hydrogels Derived from Lignocellulosic Compounds: Evaluation of the Compositional, Structural, Mechanical and Antimicrobial Properties. Industrial crops and products 2019, 128, 323–330. 10.1016/j.indcrop.2018.11.002. [DOI] [Google Scholar]
- Satani H.; Kuwata M.; Shimizu A. Simple and Environmentally Friendly Preparation of Cellulose Hydrogels Using an Ionic Liquid. Carbohydr. Res. 2020, 494, 108054 10.1016/j.carres.2020.108054. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Lu H.; Yu J.; Fan Y.; Ma J.; Wang Z. Contribution of Lignin to the Microstructure and Physical Performance of Three-dimensional Lignocellulose Hydrogels. Cellulose 2019, 26 (4), 2375–2388. 10.1007/s10570-019-02251-0. [DOI] [Google Scholar]
- Morales A.; Labidi J.; Gullón P. Assessment of green approaches for the synthesis of physically crosslinked lignin hydrogels. Journal of Industrial and Engineering Chemistry 2020, 81, 475–487. 10.1016/j.jiec.2019.09.037. [DOI] [Google Scholar]
- Butylina S.; Geng S.; Oksman K. Properties of as-prepared and Freeze-dried Hydrogels Made from Poly(vinyl alcohol) and Cellulose Nanocrystals Using Freeze-thaw Technique. Eur. Polym. J. 2016, 81, 386–396. 10.1016/j.eurpolymj.2016.06.028. [DOI] [Google Scholar]
- Dai H.; Zhang H.; Ma L.; Zhou H.; Yu Y.; Guo T.; Zhang Y.; Huang H. Green pH/Magnetic Sensitive Hydrogels Based on Pineapple Peel Cellulose and Polyvinyl Alcohol: Synthesis, Characterization and Naringin Prolonged Release. Carbohydr. Polym. 2019, 209, 51–61. 10.1016/j.carbpol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Wu C.; McClements D. J.; Ma B.; Lai Z.; Wu F.; Liu X.; Wang P. Composite hydrogels formed from okara cellulose nanofibers and carrageenan: Fabrication and characterization. International Journal of Biological Macromolecules 2024, 258, 129079 10.1016/j.ijbiomac.2023.129079. [DOI] [PubMed] [Google Scholar]
- Cao J.; Zhao Y.; Jin S.; Li J.; Wu P.; Luo Z. Flexible Lignin-based Hydrogels with Self-healing and Adhesive Ability Driven by Noncovalent Interactions. Chemical Engineering Journal 2022, 429, 132252 10.1016/j.cej.2021.132252. [DOI] [Google Scholar]
- Tuan Mohamood N. F. A.-Z.; Abdul Halim A. H.; Zainuddin N. Carboxymethyl Cellulose Hydrogel from Biomass Waste of Oil Palm Empty Fruit Bunch Using Calcium Chloride as Crosslinking Agent. Polymers 2021, 13 (23), 4056. 10.3390/polym13234056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar K.; Venkatesan M.; Desingh R. P.; Mahalingam A.; Sadhasivam B.; Subramaniyam R.; Dhamodharan R. Biocompatible Hydrogels of Chitosan-Alkali Lignin for Potential Wound Healing Applications. Materials Science and Engineering: C 2019, 102, 447–457. 10.1016/j.msec.2019.04.038. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Peng K.; Mitragotri S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv. Mater. 2021, 33 (25), 2006362 10.1002/adma.202006362. [DOI] [PubMed] [Google Scholar]
- Ma L.; Zhu Y.; Huang Y.; Zhang L.; Wang Z. Strong Water-resistant, UV-blocking Cellulose/Glucomannan/Lignin Composite Films Inspired by Natural LCC Bonds. Carbohydr. Polym. 2022, 281, 119083 10.1016/j.carbpol.2021.119083. [DOI] [PubMed] [Google Scholar]
- Ahmed M. S.; Maniruzzaman M.; Al-Mamun M. R.; Ali M. A.; Badal M. M. R.; Aziz M. A.; Jafar Mazumder M. A.; Hakeem A. S.; Yousuf M. A. Jute Stick-Derived Cellulose-Based Hydrogel: Synthesis, Characterization, and Methylene Blue Removal from Aqueous Solution. ACS Omega 2023, 8 (50), 47856–47873. 10.1021/acsomega.3c06349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.; Berton P.; Shamshina J. L.; Rogers R. D. Preparation and Comparison of Bulk and Membrane Hydrogels Based on Kraft- and Ionic-liquid-isolated Lignins. Green Chemistry 2016, 18 (20), 5607–5620. 10.1039/C6GC01339B. [DOI] [Google Scholar]
- Shen X.; Shamshina J. L.; Berton P.; Bandomir J.; Wang H.; Gurau G.; Rogers R. D. Comparison of Hydrogels Prepared with Ionic-Liquid-Isolated vs Commercial Chitin and Cellulose. ACS Sustainable Chemistry & Engineering 2016, 4 (2), 471–480. 10.1021/acssuschemeng.5b01400. [DOI] [Google Scholar]
- Kadry G.; El-Gawad H. A. Rice straw derived cellulose-based hydrogels synthesis and applications as water reservoir system. International Journal of Biological Macromolecules 2023, 253, 127058 10.1016/j.ijbiomac.2023.127058. [DOI] [PubMed] [Google Scholar]
- Wu L.; Huang S.; Zheng J.; Qiu Z.; Lin X.; Qin Y. Synthesis and Characterization of Biomass Lignin-based PVA Super-absorbent Hydrogel. International Journal of Biological Macromolecules 2019, 140, 538–545. 10.1016/j.ijbiomac.2019.08.142. [DOI] [PubMed] [Google Scholar]
- Songsrirote K.; Naiviriya T.; Rungwipoosana T.; Gutrasaeng C. The Study of Properties and Nutrient Determination of Hydrogel Made of Soybean Meal (Okara) Using Microwave-assisted Heating. Materials Today: Proceedings 2017, 4 (5), 6519–6527. 10.1016/j.matpr.2017.06.162. [DOI] [Google Scholar]
- Fabian D. R. C.; Durpekova S.; Dusankova M.; Hanusova D.; Bergerova E. D.; Sedlacik M.; Skoda D.; Sedlarik V. Renewable whey-based hydrogel with polysaccharides and polyvinyl alcohol as a soil amendment for sustainable agricultural application. International Journal of Biological Macromolecules 2024, 259, 129056 10.1016/j.ijbiomac.2023.129056. [DOI] [PubMed] [Google Scholar]
- Essawy H. A.; Ghazy M. B. M.; El-Hai F. A.; Mohamed M. F. Superabsorbent Hydrogels via Graft Polymerization of Acrylic Acid from Chitosan-Cellulose Hybrid and their Potential in Controlled Release of Soil Nutrients. International Journal of Biological Macromolecules 2016, 89, 144–151. 10.1016/j.ijbiomac.2016.04.071. [DOI] [PubMed] [Google Scholar]
- Queiroz B. G.; Ciol H.; Inada N. M.; Frollini E. Cross-linked Bio-based Hydrogels Generated from Solutions Derived from the Deconstruction of Sisal Fibers. J. Mol. Liq. 2023, 369, 120876 10.1016/j.molliq.2022.120876. [DOI] [Google Scholar]
- Yang Y.; Tong Z.; Geng Y.; Li Y.; Zhang M. Biobased Polymer Composites Derived from Corn Stover and Feather Meals as Double-Coating Materials for Controlled-Release and Water-Retention Urea Fertilizers. J. Agric. Food Chem. 2013, 61 (34), 8166–8174. 10.1021/jf402519t. [DOI] [PubMed] [Google Scholar]
- Gong S.; Wu X.; Liao Q.; Deng S.; Hou J.; Tang K.; Xiong Y.; Li Z.; Tang H. Novel Multifunctional Unimolecular Initiators Built on Natural Indole Featuring Fast Photobleaching and Visible Light/Thermal Double Polymerization. Green Chemistry 2023, 25, 2730. 10.1039/D2GC04718G. [DOI] [Google Scholar]
- Neidinger P.; Davis J.; Voll D.; Jaatinen E. A.; Walden S. L.; Unterreiner A. N.; Barner-Kowollik C. Near Infrared Light Induced Radical Polymerization in Water. Angewandte Chemie International Edition 2022, 61 (42), e202209177 10.1002/anie.202209177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.; Wang L.; Kang H.; Dan Y.; Tian D.; Zheng Y. UV-light Irradiation Preparation of Soybean Residue-based Hydrogel Composite from Inorganic/Organic Hybrids for Degradable Slow-Release N-Fertilizer. Res. Chem. Intermed. 2020, 46 (2), 1437–1451. 10.1007/s11164-019-04043-4. [DOI] [Google Scholar]
- Etemadi Baloch F.; Afzali D.; Fathirad F. Design of Acrylic Acid/Nanoclay Grafted Polysaccharide Hydrogels as Superabsorbent for Controlled Release of Chlorpyrifos. Applied Clay Science 2021, 211, 106194 10.1016/j.clay.2021.106194. [DOI] [Google Scholar]
- Zhu J.; Li J.. Superabsorbent Hydrogels Derived from Okara as Soil Supplements for Enhancing Vegetable Growth and Production. In Sustainable Green Chemistry in Polymer Research. Volume 1. Biocatalysis and Biobased Materials, ACS Symposium Series, Vol. 1450; American Chemical Society, 2023; pp 187–204. [Google Scholar]
- Ren J.; Kong W.; Sun R. Preparation of Sugarcane Bagasse/Poly (acrylic acid-co-acrylamide) Hydrogels and their Application. BioResources 2014, 9 (2), 3290–3303. 10.15376/biores.9.2.3290-3303. [DOI] [Google Scholar]
- Neamjan N.; Wiwattananukul R.; Hiangrat K.; Matkaran K.; Na-iam Y.; Sakulsombat M.; Sriroth K. Preparation of Superabsorbent Polymer from Sugarcane Bagasse via Extrusion Process. Sugar Tech 2019, 21 (2), 296–300. 10.1007/s12355-018-0670-7. [DOI] [Google Scholar]
- Cândido J. d. S.; Pereira A. G. B.; Fajardo A. R.; Ricardo N. M. P. S.; Feitosa J. P. A.; Muniz E. C.; Rodrigues F. H. A. Poly(acrylamide-co-acrylate)/Rice Husk Ash Hydrogel Composites. II. Temperature Effect on Rice Husk Ash Obtention. Composites Part B: Engineering 2013, 51, 246–253. 10.1016/j.compositesb.2013.03.027. [DOI] [Google Scholar]
- Dai H.; Zhang Y.; Ma L.; Zhang H.; Huang H. Synthesis and Response of Pineapple Peel Carboxymethyl Cellulose-g-Poly (acrylic acid-co-acrylamide)/Graphene Oxide Hydrogels. Carbohydr. Polym. 2019, 215, 366–376. 10.1016/j.carbpol.2019.03.090. [DOI] [PubMed] [Google Scholar]
- Dai H.; Huang H. Enhanced Swelling and Responsive Properties of Pineapple Peel Carboxymethyl Cellulose-g-poly(acrylic acid-co-acrylamide) Superabsorbent Hydrogel by the Introduction of Carclazyte. J. Agric. Food Chem. 2017, 65 (3), 565–574. 10.1021/acs.jafc.6b04899. [DOI] [PubMed] [Google Scholar]
- Wen P.; Wu Z.; Han Y.; Cravotto G.; Wang J.; Ye B.-C. Microwave-Assisted Synthesis of a Novel Biochar-Based Slow-Release Nitrogen Fertilizer with Enhanced Water-Retention Capacity. ACS Sustainable Chemistry & Engineering 2017, 5 (8), 7374–7382. 10.1021/acssuschemeng.7b01721. [DOI] [Google Scholar]
- Tamaddon F.; Ahmadi-AhmadAbadi E. Microwave-Assisted Fabrication of a pH/Salt Responsive Hydrogel from the Micro-CMC, In Situ Polymerized Acrylamide, and Nanoγ-Fe2O3-SO3H Cross-Linked by a Phenyl Bisamide Linker for Pb2+ and Hg2+ Removal. Journal of Polymers and the Environment 2023, 31 (1), 406–422. 10.1007/s10924-022-02628-6. [DOI] [Google Scholar]
- Panchan N.; Niamnuy C.; Dittanet P.; Devahastin S. Optimization of Synthesis Condition for Carboxymethyl Cellulose-based Hydrogel from Rice Straw by Microwave-Assisted Method and its Application in Heavy Metal Ions Removal. J. Chem. Technol. Biotechnol. 2018, 93 (2), 413–425. 10.1002/jctb.5370. [DOI] [Google Scholar]
- Sayed A.; Mohamed M. M.; Abdel-raouf M. E.-S.; Mahmoud G. A. Radiation Synthesis of Green Nanoarchitectonics of Guar Gum-Pectin/Polyacrylamide/Zinc Oxide Superabsorbent Hydrogel for Sustainable Agriculture. Journal of Inorganic and Organometallic Polymers and Materials 2022, 32 (12), 4589–4600. 10.1007/s10904-022-02465-z. [DOI] [Google Scholar]
- Maziad N. A.; Abou El Fadl F. I.; El-Kelesh N. A.; El-Hamouly S. H.; Zeid I. F.; Gayed H. M. Radiation Synthesis and Characterization of Super Absorbent Hydrogels for Controlled Release of Some Agrochemicals. Journal of Radioanalytical and Nuclear Chemistry 2016, 307 (1), 513–521. 10.1007/s10967-015-4201-7. [DOI] [Google Scholar]
- Elshahawy M. F.; Ahmed N. A.; Mahmoud G. A. Garlic straw-derived composite hydrogel via electron beam irradiation for adsorptive removal of methylene blue. Mater. Chem. Phys. 2024, 321, 129487 10.1016/j.matchemphys.2024.129487. [DOI] [Google Scholar]
- Wongsawaeng D.; Jumpee C. Superabsorbentmaterials from Grafting of Acrylic Acid onto Thai Agricultural Residues by Gamma Irradiation. J. Nucl. Sci. Technol. 2016, 53, 1723. 10.1080/00223131.2016.1152924. [DOI] [Google Scholar]
- Kazanskii K. S.; Dubrovskii S. A.. Chemistry and Physics of “Agricultural” Hydrogels. In Polyelectrolytes Hydrogels Chromatographic Materials; Springer: Berlin, 1992; pp 97–133. [Google Scholar]
- Kaur P.; Bohidar H. B.; Nisbet D. R.; Pfeffer F. M.; Rifai A.; Williams R.; Agrawal R. Waste to High-value Products: The Performance and Potential of Carboxymethylcellulose Hydrogels via the Circular Economy. Cellulose 2023, 30 (5), 2713–2730. 10.1007/s10570-023-05068-0. [DOI] [Google Scholar]
- Chen J.; Lü S.; Zhang Z.; Zhao X.; Li X.; Ning P.; Liu M. Environmentally Friendly Fertilizers: A Review of Materials Used and their Effects on the Environment. Science of The Total Environment 2018, 613–614, 829–839. 10.1016/j.scitotenv.2017.09.186. [DOI] [PubMed] [Google Scholar]
- Fertahi S.; Ilsouk M.; Zeroual Y.; Oukarroum A.; Barakat A. Recent Rrends in Organic Coating Based on Biopolymers and Biomass for Controlled and Slow Release Fertilizers. J. Controlled Release 2021, 330, 341–361. 10.1016/j.jconrel.2020.12.026. [DOI] [PubMed] [Google Scholar]
- El-Aziz M. E. A.; Salama D. M.; Morsi S. M. M.; Youssef A. M.; El-Sakhawy M. Development of Polymer Composites and Encapsulation Technology for Slow-release Fertilizers. Reviews in Chemical Engineering 2022, 38 (5), 603–616. 10.1515/revce-2020-0044. [DOI] [Google Scholar]
- Ramli R. A. Slow Release Fertilizer Hydrogels: A Review. Polymer Chemistry 2019, 10 (45), 6073–6090. 10.1039/C9PY01036J. [DOI] [Google Scholar]
- Liang R.; Yuan H.; Xi G.; Zhou Q. Synthesis of Wheat Straw-g-Poly(acrylic acid) Superabsorbent Composites and Release of Urea from it. Carbohydr. Polym. 2009, 77 (2), 181–187. 10.1016/j.carbpol.2008.12.018. [DOI] [Google Scholar]
- Kenawy E.-R.; Azaam M. M.; El-nshar E. M. Preparation of Carboxymethyl Cellulose-g-Poly (acrylamide)/Montmorillonite Superabsorbent Composite as a Slow-release Urea Fertilizer. Polymers for Advanced Technologies 2018, 29 (7), 2072–2079. 10.1002/pat.4315. [DOI] [Google Scholar]
- Guo L.; Wang Y.; Wang M.; Shaghaleh H.; Hamoud Y. A.; Xu X.; Liu H. Synthesis of Bio-based MIL-100(Fe)@CNF-SA Composite Hydrogel and its Application in Slow-release N-fertilizer. Journal of Cleaner Production 2021, 324, 129274 10.1016/j.jclepro.2021.129274. [DOI] [Google Scholar]
- Kim B.; Kim T.-H.; Lee B. Optimal Synthesis of Carboxymethylcellulose-based Composite Superabsorbents. Korean Journal of Chemical Engineering 2021, 38 (1), 215–225. 10.1007/s11814-020-0681-4. [DOI] [Google Scholar]
- Calcagnile P.; Sibillano T.; Giannini C.; Sannino A.; Demitri C. Biodegradable Poly(lactic acid)/Cellulose-based Superabsorbent Hydrogel Composite Material as Water and Fertilizer Reservoir in Agricultural Applications. J. Appl. Polym. Sci. 2019, 136 (21), 47546. 10.1002/app.47546. [DOI] [Google Scholar]
- Yang H.; Dai F.; Chen H.; He Y.; Wang Z.; Wang R. Fabrication of Stalk Fiber/Geopolymers-based Slow-Release Fertilizer with Agricultural Waste and Loess for Promoting Plant Growth. Journal of Environmental Chemical Engineering 2023, 11 (2), 109481 10.1016/j.jece.2023.109481. [DOI] [Google Scholar]
- El Idrissi A.; El Gharrak A.; Achagri G.; Essamlali Y.; Amadine O.; Akil A.; Sair S.; Zahouily M. Synthesis of Urea-containing Sodium Alginate-g-Poly(Acrylic Acid-co-Acrylamide) Superabsorbent-Fertilizer Hydrogel Reinforced with Carboxylated Cellulose Nanocrystals for Efficient Water and Nitrogen Utilization. Journal of Environmental Chemical Engineering 2022, 10 (5), 108282 10.1016/j.jece.2022.108282. [DOI] [Google Scholar]
- Zhou T.; Wang Y.; Huang S.; Zhao Y. Synthesis Composite Hydrogels from Inorganic-Organic Hybrids Based on Leftover Rice for Environment-Friendly Controlled-Release Urea Fertilizers. Science of The Total Environment 2018, 615, 422–430. 10.1016/j.scitotenv.2017.09.084. [DOI] [PubMed] [Google Scholar]
- Olad A.; Gharekhani H.; Mirmohseni A.; Bybordi A. Superabsorbent Nanocomposite Based on Maize Bran with Integration of Water-Retaining and Slow-Release NPK Fertilizer. Advances in Polymer Technology 2018, 37 (6), 1682–1694. 10.1002/adv.21825. [DOI] [Google Scholar]
- Olad A.; Zebhi H.; Salari D.; Mirmohseni A.; Reyhani Tabar A. Slow-release NPK Fertilizer Encapsulated by Carboxymethyl Cellulose-based Nanocomposite with the Function of Water Retention in Soil. Materials Science and Engineering: C 2018, 90, 333–340. 10.1016/j.msec.2018.04.083. [DOI] [PubMed] [Google Scholar]
- Jungsinyatam P.; Suwanakood P.; Saengsuwan S. Multicomponent Biodegradable Hydrogels Based on Natural Biopolymers as Environmentally Coating Membrane for Slow-Release Fertilizers: Effect of Crosslinker Type. Science of The Total Environment 2022, 843, 157050 10.1016/j.scitotenv.2022.157050. [DOI] [PubMed] [Google Scholar]
- Kassem I.; Ablouh E.-H.; El Bouchtaoui F.-Z.; Kassab Z.; Hannache H.; Sehaqui H.; El Achaby M. Biodegradable All-cellulose Composite Hydrogel as Eco-friendly and Efficient Coating Material for Slow-release MAP Fertilizer. Progress in Organic Coatings 2022, 162, 106575 10.1016/j.porgcoat.2021.106575. [DOI] [Google Scholar]
- Xie L.; Liu M.; Ni B.; Wang Y. New Environment-Friendly Use of Wheat Straw in Slow-Release Fertilizer Formulations with the Function of Superabsorbent. Ind. Eng. Chem. Res. 2012, 51 (10), 3855–3862. 10.1021/ie2016043. [DOI] [Google Scholar]
- Zhang M.; Yang J. Preparation and Characterization of Multifunctional Slow Release Fertilizer Coated with Cellulose Derivatives. International Journal of Polymeric Materials and Polymeric Biomaterials 2021, 70 (11), 774–781. 10.1080/00914037.2020.1765352. [DOI] [Google Scholar]
- Saha A.; Sekharan S.; Manna U. Superabsorbent Hydrogel (SAH) as a Soil Amendment for Drought Management: A Review. Soil and Tillage Research 2020, 204, 104736 10.1016/j.still.2020.104736. [DOI] [Google Scholar]
- Hu Z.-Y.; Chen G.; Yi S.-H.; Wang Y.; Liu Q.; Wang R. Multifunctional Porous Hydrogel with Nutrient Controlled-release and Excellent Biodegradation. Journal of Environmental Chemical Engineering 2021, 9 (5), 106146 10.1016/j.jece.2021.106146. [DOI] [Google Scholar]
- Das D.; Bhattacharjee S.; Bhaladhare S. Preparation of Cellulose Hydrogels and Hydrogel Nanocomposites Reinforced by Crystalline Cellulose Nanofibers (CNFs) as a Water Reservoir for Agriculture Use. ACS Applied Polymer Materials 2023, 5, 2895. 10.1021/acsapm.3c00109. [DOI] [Google Scholar]
- Sorokin A.; Sukhanov P.; Popov V.; Kannykin S.; Lavlinskaya M. A New Approach to Increasing the Equilibrium Swelling Ratio of the Composite Superabsorbents Based on Carboxymethyl Cellulose Sodium Salt. Cellulose 2022, 29 (1), 159–173. 10.1007/s10570-021-04326-3. [DOI] [Google Scholar]
- Chandrika K. S. V. P.; Singh A.; Rathore A.; Kumar A. Novel Cross Linked Guar Gum-g-Poly(acrylate) Porous Superabsorbent Hydrogels: Characterization and Swelling Behaviour in Different Environments. Carbohydr. Polym. 2016, 149, 175–185. 10.1016/j.carbpol.2016.04.077. [DOI] [PubMed] [Google Scholar]
- Pandey P.; Giriraj; De N. Halloysite Nanoclay Polymer Composite: Synthesis, Characterization and Effect on Water Retention Behaviour of Soil. Chemical Science International Journal 2018, 23 (3), 1–11. 10.9734/CSJI/2018/42613. [DOI] [Google Scholar]
- Chen M.; Ni Z.; Shen Y.; Xiang G.; Xu L. Reinforced Swelling and Water-Retention Properties of Super-absorbent Hydrogel Fabricated by a Dual Stretchable Single Network Tactic. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020, 602, 125133 10.1016/j.colsurfa.2020.125133. [DOI] [Google Scholar]
- Wang W.; Yang S.; Zhang A.; Yang Z. Synthesis of a Slow-release Fertilizer Composite Derived from Waste Straw that Improves Water Retention and Agricultural Yield. Science of The Total Environment 2021, 768, 144978 10.1016/j.scitotenv.2021.144978. [DOI] [PubMed] [Google Scholar]
- Chang L.; Xu L.; Liu Y.; Qiu D. Superabsorbent Polymers used for Agricultural Wate Retention. Polymer Testing 2021, 94, 107021 10.1016/j.polymertesting.2020.107021. [DOI] [Google Scholar]
- Zhang C.; García Meza J. V.; Zhou K.; Liu J.; Song S.; Zhang M.; Meng D.; Chen J.; Xia L.; Xiheng H. Superabsorbent Polymer Used for Saline-alkali Soil Water Retention. Journal of the Taiwan Institute of Chemical Engineers 2023, 145, 104830 10.1016/j.jtice.2023.104830. [DOI] [Google Scholar]
- Zhu J.; Suhaimi F.; Lim J. Y.; Gao Z.; Swarup S.; Loh C. S.; Li J.; Ong C. N.; Tan W. K. A Field Study on Using Soybean Waste-derived Superabsorbent Hydrogel to Enhance Growth of Vegetables. Science of The Total Environment 2022, 851, 158141 10.1016/j.scitotenv.2022.158141. [DOI] [PubMed] [Google Scholar]
- Abou-Baker N. H.; Ouis M.; Abd-Eladl M.; Ibrahim M. M. Transformation of Lignocellulosic Biomass to Cellulose-Based Hydrogel and Agriglass to Improve Beans Yield. Waste and Biomass Valorization 2020, 11 (7), 3537–3551. 10.1007/s12649-019-00699-6. [DOI] [Google Scholar]
- Bauli C. R.; Lima G. F.; de Souza A. G.; Ferreira R. R.; Rosa D. S. Eco-friendly Carboxymethyl Cellulose Hydrogels Filled with Nanocellulose or Nanoclays for Agriculture Applications as Soil Conditioning and Nutrient Carrier and their Impact on Cucumber Growing. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 623, 126771 10.1016/j.colsurfa.2021.126771. [DOI] [Google Scholar]
- Mazloom N.; Khorassani R.; Zohury G. H.; Emami H.; Whalen J. Lignin-based Hydrogel Alleviates Drought Stress in Maize. Environmental and Experimental Botany 2020, 175, 104055 10.1016/j.envexpbot.2020.104055. [DOI] [Google Scholar]
- Akalin G. O.; Pulat M. Controlled Release Behavior of Zinc-Loaded Carboxymethyl Cellulose and Carrageenan Hydrogels and their Effects on Wheatgrass Growth. Journal of Polymer Research 2020, 27 (1), 6. 10.1007/s10965-019-1950-y. [DOI] [Google Scholar]
- Sohail M.; Pirzada T.; Opperman C. H.; Khan S. A. Recent advances in seed coating technologies: transitioning toward sustainable agriculture. Green Chemistry 2022, 24 (16), 6052–6085. 10.1039/D2GC02389J. [DOI] [Google Scholar]
- Kumari P.; Kumari N.; Mohan C.; Chinglenthoiba C.; Amesho K. T. T. Environmentally benign approach to formulate nanoclay/starch hydrogel for controlled release of zinc and its application in seed coating of Oryza Sativa plant. International Journal of Biological Macromolecules 2024, 257, 128278 10.1016/j.ijbiomac.2023.128278. [DOI] [PubMed] [Google Scholar]
- Hassanisaadi M.; Kennedy J. F.; Rabiei A.; Riseh R. S.; Taheri A. Nature’s coatings: Sodium alginate as a novel coating in safeguarding plants from frost damages. International Journal of Biological Macromolecules 2024, 267, 131203 10.1016/j.ijbiomac.2024.131203. [DOI] [PubMed] [Google Scholar]
- Pathak V.; Ambrose R. P. K. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage. J. Appl. Polym. Sci. 2020, 137 (14), 48523. 10.1002/app.48523. [DOI] [Google Scholar]
- Zvinavashe A. T.; Laurent J.; Mhada M.; Sun H.; Fouda H. M. E.; Kim D.; Mouhib S.; Kouisni L.; Marelli B. Programmable design of seed coating function induces water-stress tolerance in semi-arid regions. Nature Food 2021, 2 (7), 485–493. 10.1038/s43016-021-00315-8. [DOI] [PubMed] [Google Scholar]
- Zheng L.; Seidi F.; Liu Y.; Wu W.; Xiao H. Polymer-based and stimulus-responsive carriers for controlled release of agrochemicals. Eur. Polym. J. 2022, 177, 111432 10.1016/j.eurpolymj.2022.111432. [DOI] [Google Scholar]
- Singh A.; Dhiman N.; Kar A. K.; Singh D.; Purohit M. P.; Ghosh D.; Patnaik S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. Journal of Hazardous Materials 2020, 385, 121525 10.1016/j.jhazmat.2019.121525. [DOI] [PubMed] [Google Scholar]
- Ma L.; Chai C.; Wu W.; Qi P.; Liu X.; Hao J. Hydrogels as the plant culture substrates: A review. Carbohydr. Polym. 2023, 305, 120544 10.1016/j.carbpol.2023.120544. [DOI] [PubMed] [Google Scholar]
- Kalossaka L. M.; Sena G.; Barter L. M. C.; Myant C. Review: 3D printing hydrogels for the fabrication of soilless cultivation substrates. Applied Materials Today 2021, 24, 101088 10.1016/j.apmt.2021.101088. [DOI] [Google Scholar]