Abstract
Muscle strength is essential for autonomy in daily activities and performance in sports activities. Yet, conventional strength training is challenging during recovery from pathological conditions. This study investigates a novel combined intervention employing Focal Muscle Vibration (FMV) and Action Observation (AO) to enhance muscle strength. Twenty-seven healthy volunteers (18 females and 9 males, aged 22 to 42 years) were enrolled for an intervention-control study comparing 2 groups: the intervention group received AO treatment with FMV on the right leg, and the control group underwent only FMV on the right leg. This design allowed the comparison of four conditions: FMV+AO (intervention group, right leg), AO alone (intervention group, left leg), FMV alone (control group, right leg), and no-treatment NT (control group, left leg). The treatment, conducted five times a week (Mon-Fri) for two weeks, involved a 20-minute session of FMV on the right quadriceps, coupled, for the intervention group, with the observation of a gym training video. The assessments of Maximum Voluntary Contraction (MVC), and Fatigue Coefficient (FC) expressed at knee extension bilaterally were measured at the beginning (T0), after the first week (T1), at the end of treatment (T2), and one-week post-intervention for the follow-up (T3). The FMV+AO group demonstrated a significant improvement in MVC over time, reaching statistical significance at T2 and maintaining the gain at T3. In contrast, all the other conditions demonstrated milder MVC increases without statistical significance. FC did not differ significantly in any condition. The combination of FMV and AO optimized muscle strengthening, offering insights for targeted treatments in various settings.
Key Words: muscle weakness, rehabilitation, vibration, action observation, muscle strengthening
Muscle strength, the capacity of muscles to generate force against resistance, is a pivotal physiological factor influencing autonomy in daily activities. It is fundamental for basic human movements, from reaching and maintaining anti-gravity positions to walking and jumping.1 It is governed by a complex interplay of factors such as muscle size, fiber type composition, neural activation, and training status.2
Diminished muscle strength, a common concern in aging populations, and various pathological states, can detrimentally impact gait control, posture, and overall performance, leading to premature fatigue.3 In this condition, interventions aimed at increasing muscular strength, are useful for recovery in clinical and sports fields.1,2 While a few sessions of traditional strength training programs are effective in increasing muscle section area and strength in healthy subjects,4,5,6 their application is restricted by premature fatigue, and muscle deconditioning due to immobility, neural damage, or pain in pathological conditions. Therefore, alternative strategies are sought to precede or complement these programs, facilitating initial strength gains.
Usually, the employed strategies focus, either on the peripheral or central components involved in force generation. Within this framework, two distinct approaches can be recognized: a Bottom-Up and a Top-Down approach.7 The former targets the peripheral execution through intervention or stimulation of a muscle, leading to improvement in force generation and motor control. The Top-Down approach seeks to enhance specific neurocognitive domains and cortical reorganizations, leading to improvement of motor planning, coordination, and overall movement proficiency.8 Within all the peripheral interventions, Focal Muscle Vibration (FMV) has emerged as a promising technique, finding application in both physiological and clinical settings. By applying mechanical vibrations to a muscle and manipulating parameters such as frequency, amplitude, and intensity, FMV can elicit diverse effects.9 Indeed, FMV offers benefits including strength augmentation, contracture alleviation, pain management, edema reduction, spasticity amelioration, enhanced proprioception, and improved postural stability.10
On the other hand, the Top-Down approach includes interventions, like Action Observation Therapy (AOT), primarily used for motor skill learning. AOT involves observing an action, presented through a video or performed by another individual, followed by immediate imitation. This process induces reorganization in the primary motor cortex, contributing to the formation of motor memory traces and facilitating motor learning.11 Furthermore, AOT is used to intensify the effect of rehabilitation and to reduce recovery time after injuries and periods of immobilization.12 Even if, each phase of this treatment (observation and execution) can yield beneficial effects on cortical reorganization, the sole observation of the action without subsequent reproduction has shown a lower, albeit still present, benefit.13
Given the potential benefits of both peripheral and central interventions, a combined approach could synergistically optimize interventions. Our primary hypothesis is that the combination of FMV with the observation of strength training exercises will result in improved muscle strength. Additionally, we aim to explore the impact of this combined intervention on muscle fatigue.
Materials and Methods
Participants
The study involved 27 healthy volunteers (18 females and 9 males), aged 22 to 42 years. Participants were recruited among the staff working in the Unit of Neurorehabilitation of the University Hospital of Pisa. Inclusion criteria were: age range 18-50 years; no history of musculoskeletal or neurological diseases; capacity to understand and express informed consent. Exclusion criteria were: athletes or individuals used to intensive sports training. Participants refrained from other physical activities during the study. Informed consent was obtained in accordance with the Helsinki Declaration, and the study received approval from the local Ethics Committee.
Study design
Participants were randomly assigned to an intervention group, watching an AO video during FMV treatment, or a control group without AO. Both groups received FMV on the right femoral quadriceps. This design resulted in four conditions: FMV+AO (intervention group, right leg), AO alone (intervention group, left leg), FMV alone (control group, right leg), and no-treatment NT (control group, left leg) (Figure 1A). The treatment session of 20 minutes per day had a frequency of five days a week (Monday – Friday) for two consecutive weeks. Assessments for the bilateral knee extension Maximal isometric Voluntary Contraction (MVC) and the Fatigue Coefficient (FC) were conducted at baseline (T0), after the first week of treatment (T1), at the end of treatment (T2), and one week later (T3) (Figure 1B).
Intervention
Focal Muscle Vibration (FMV)
The FMV treatment consisted of a 20-minute session of muscle vibration administered by a pneumatic vibrator powered by compressed air (Vibra 3.0; A Circle s.p.a. Company, San Pietro in Casale, Bologna, Italy). Four hemispherical cap-transducers were placed on the femoral quadriceps: the largest transducer (with a diameter of 2.5 cm) was centrally positioned, 4 cm above the superior border of the patella; the remaining three transducers (each with a diameter of 1 cm) were situated along an imaginary line originating from the greater trochanter, proceeding from lateral to medial, intersecting three quadriceps heads (Figure 1C). Throughout the treatment, participants maintained a seated resting position. The selected vibration frequency was 100 Hz, applied at an intensity of 120 mBar.
Action Observation (AO)
The AO consisted of a video of a 20-minute gym training session designed to strengthen the quadriceps. The video featured two training circuits, each consisting of six rounds of exercises and pauses, alternating every 30 seconds. A 90-second break separated the two circuits. In the second circuit, exercises were performed at a slower pace, with a close-up zoom focusing on limbs, knee, and hip joint movements. At the end of the second circuit, the video displayed a soccer player performing a high kick of a ball, emphasizing knee extension in this specific task. Participants were instructed to closely observe the contraction of the quadriceps muscles throughout the video, focusing and imagining as they were executing the movement themself. Both male and female subjects were equally represented in the video while performing the exercises.
Outcome measurements
Maximal strength and muscle fatigue were examined using the isokinetic dynamometer “PrimusRS Multi-Joint System Dynamometer” (BTE Technologies, Hanover MD, USA). During the dynamometric assessment, subjects were seated in a standardized comfortable position with straps securing their thighs and pelvis to ensure greater stability. The dynamometer recorded the force exerted in leg extension with the knee at the fixed angle of 120°, with the full extension being considered at 180°. The evaluation tool comprised an adaptable rod, a pivot point connecting to the dynamometer, and a resistance point. The dynamometer’s pivot point was positioned at the juncture of the lateral femoral condyle and the tibial plateau, while the resistance point was located between the middle and lower thirds of the tibia, approximately 4 centimeters above the lateral malleolus (Figure 1D). For maximal strength, participants were instructed to exert the MVC during knee extension for 3 seconds. Each subject performed three consecutive repetitions for each limb with a 15-second pause between each trial.
The same setup was used to assess fatigue. Participants were instructed to maintain the isometric MVC for 60 seconds. The investigator provided participants with verbal encouragement and informed them of the remaining time at intervals, including a countdown for the last 10 seconds. The force was probed at 100 Hz frequency. The participants repeated the evaluation three times with a 30-second pause between each trial. To assess the muscular resistance to fatigue, the Fatigue Coefficient (FC) has been calculated from force-time curves (see Data Analysis section).
All muscular tests were conducted bilaterally. Only results with a Coefficient of Variation (CV) of less than 7% were considered reliable; otherwise, participants were asked to repeat the single trial after a longer pause (40-80 seconds).
Data Analysis
Each MVC evaluation triplicate was averaged. Similarly, for each FC estimation, each force-time curve triplicate was averaged. Statistical analysis of MVC comparison in time within a treatment group was done in a logarithmic domain as data failed the normality test but passed the log-normality test (see below); all estimated parameters were further recalculated to the linear (non-logarithmic) scale.
Figure 1.
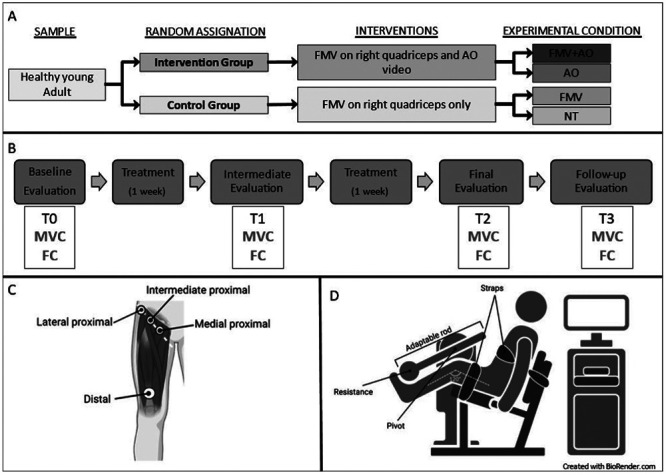
Study design (A), timeline (B), and setting (C, D). 1A - Study design: The participants were randomly assigned to one of the two groups, the Intervention or Control group. Participants in the Intervention Group underwent Focal Muscle Vibration (FMV) treatment on the right leg while watching a quadriceps training video (Action observation - AO - described in the text). Considering the single leg, this group generated two experimental conditions: the right leg underwent FMV and AO treatment (FMV+AO), while the left leg received only the AO treatment. The Control group underwent only FMV treatment, generating the other two conditions: FMV for the right leg, and No-treatment (NT) for the left leg. 1B - Timeline: All the participants were evaluated at the baseline (T0) for their Maximum Voluntary Contraction (MCV) and Fatigue coefficient (FC). After the evaluation, the participants underwent two weeks of treatment according to their group. The patients were re-evaluated for MVC and FC for an intermediate assessment after one week of treatment (T1), at the end of the treatment (T2), and after a week of interruption (T3), to assess if the induced modifications lasted over time. 1C Localization on the right leg of the cup transducers for FMV. 1D Schematic representation of the setting for dynamometric evaluation.
For the FC estimation, we excluded the initial 10 seconds of each 60-second long force-time curve, where, participants are reaching the peak of their MVC, and the final 10 seconds where some participants had trouble maintaining consistent strength control, resulting in sudden drops or significant oscillations in strength values.
Although the Literature suggests that muscle tension decreases in time exponentially towards a Plateau,14,15 in about half of our results we failed to estimate a positive value of the Plateau (Figure 2A, Table 1 – parameter P). Omitting the factor representing the Plateau in the estimation resulted in a large discrepancy in the time constant values between fits with and without the Plateau (Figure 2B, Table 1). Thus, we used a simplified fatigue estimation using a linear model fit, defining the FC as a ratio between the negative value of the slope and the y-intercept (Figure 2, Table 1), as this approximation will be more robust to deviations from the more widely accepted models. The analysis and comparisons of different models were done with Microsoft Excel for Microsoft 365 (Microsoft, Redmond, WA, USA), GraphPad Prism version 9.5.1 for Windows (GraphPad Software, Boston, MA, USA), and Wolfram Mathematica version 11.2 for Windows (Wolfram Research, Inc., Champaign, IL, USA).
Table 1.
Model Parameters Fitted for FC Estimation for two representative subjects under FMV+AO treatment at T0 as presented in Figure 2.
| Fit parameters | R2 | FC | ||||||
|---|---|---|---|---|---|---|---|---|
| Fitted equation 1⁄τ | a | b | P | –a/b | (1/s) | |||
| (1/s) | (N/s) | (N) | (N) | (1/s) | ||||
| Estimation 1 (Figure 2a) | ||||||||
| Exponential fit with plateau | f (t )=be-t^+P | 0.00929 | - | 196.4 | 10-11 | - | 0.936 | |
| 0.00929 | ||||||||
| Exponential fit | f (t)=b·e-t'x | 0.00928 | - | 196.4 | - | - | 0.936 | |
| 0.00928 | ||||||||
| Linear fit | f(t)=at+b | - | -1.4 | 191.5 | - | 0.00731 | 0.944 | |
| 0.00731 | ||||||||
| Estimation 2 (Figure 2b) | ||||||||
| Exponential fit with plateau | f(t)=be-at+P | 0.02822 | - | 165.6 | 142.9 | - | 0.953 | |
| 0.02822 | ||||||||
| Exponential fit | f(t)=be-at | 0.00961 | - | 288.7 | - | - | 0.944 | |
| 0.00961 | ||||||||
| Linear fit | f(t)=at+b | - | -2.069 | 279.8 | - | 0.00739 | 0.934 | |
| 0.00739 | ||||||||
Figure 2.
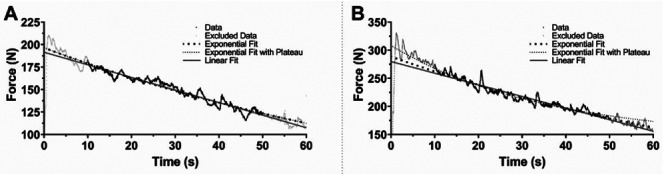
Force-time curves plot. Representative plots for force-time curves obtained from two representative subjects under FMV+AO treatment at T0 for FC Estimation. Data used for fitting is marked with black dots, and data excluded from estimation (the first and the last 10 s of the measurement as explained in the main text) is marked with gray dots. Big black dotted line, small black dotted line and solid gray line represent Exponential fit, Exponential Fit with Plateau, and Linear Fit to the data, respectively. 2A. shows a curve that we fail to estimate a Plateau region, in such a case both kind of Exponential fits showed similar values for FC estimation (see Table 1). 2B shows a curve that we were able to fit Exponential Curve with Plateau, yet comparing both estimated FC from both Exponential fit it shows 3 a fold difference (see Table 1). Thus, as our data shows, a fail to estimate a Plateau level from the data may lead to a couple fold of FC estimation error. We did a linear regression on our data and represented FC as the negative ratio of slope and Y-interception of the fit. The FC values estimated using this model closely align with those obtained through Exponential fits, and R2 values fall within a comparable range as other fitting methods, demonstrating a strong fit for the data (see Table 1).
For the MVC treatment comparison, the data was normalized in the following way. For each treatment, for each subject, we calculated a geometric mean for all time point values. Next, each data time point, for each treatment, for each subject was divided by the respective geometric means preserving the ratiometric relations between timepoints values and normalizing for the distribution of the initial MVC in the population. Subsequently, for each treatment, we averaged the resulting values within each T0, and then divided all data points within each treatment group by the respective average, normalizing the average value of each T0 to 1, and preserving the ratiometric information within the data.
Statistical analyses were done using GraphPad Prism version 9.5.1 for Windows (GraphPad Software, Boston, MA, USA). To test the statistical difference of Gender composition within groups we used Fischer’s exact test. Numerical data was tested for normality and lognormality with the Shapiro-Wilk normality test. For each data analysis segment to pass the normality (or lognormality) test, all the data sets in the analysis segment had to pass the normality (or lognormality) test. After checking the normality, the appropriate statistical test was applied to evaluate the statistical differences between groups and timepoints.
For a two-group comparison failing the normality test, we used the Mann-Whitney test. For a non-matching multiplegroup comparison failing the normality test, we used the Kruskal-Wallis test. For a non-matching multiple-group comparison passing the normality test, we used the Brown-Forsythe and Welch ANOVA test. For a matching multiplegroup comparison failing the normality test, we used the Friedman test with Dunn’s multiple comparison test. For a matching multiple-group comparison passing the normality test, and for comparing the effectiveness of treatments we used the Repeated Measures one-way ANOVA test with Geisser-Greenhouse correction, with Tukey multiple comparison test with individual variances computed for each comparison. Datasets failing the normality test were indicated in the figures.
To calculate the Number Needed to Treat (NTT) we compared the FMV+AO group (intervention) with the FMV group (control). As a negative outcome, we assumed an increase lower than 20% of MVC.
Results
Anthropometric measures, including gender, age, and body mass index (BMI), were examined for statistical differences between the two groups, and no significant disparities were found. Additionally, comparisons of MVCs and FCs among the four conditions at baseline revealed no statistically significant differences (Table 2).
While there was a slight overall gain in the MVC over time in all experimental conditions, only the FMV+AO group exhibited a statistically significant difference. In particular, the group FMV+AO showed a continuous improvement in MVC over the timepoints, reaching a statistical significance at T2 and maintaining a significant gain also at T3 (p-value: 0.0344 and 0.0239, respectively). Condition AO, FMV, and NT showed a milder increase in the MVC value that did not reach statistical significance (Figure 3A-D). Based on our sample, the NTT is 4.
Table 2.
Baseline characteristics between groups.
| Intervention group | Control group | p-value | ||||
|---|---|---|---|---|---|---|
| N° of participants | 14 | 13 | ||||
| Age | 30.7 (±4.05) | 29.85 (±4.02) | 0.76 | |||
| Gender F/M | 10/4 | 8/5 | 0.69 | |||
| BMI | 23,37 (±2,76) | 23,11 (±2,74) | 0.72 | |||
| Right Leg (FMV+AO) | Left Leg (AO) | Right Leg (FMV) | Left Leg (NT) | |||
| MVC T0 (N) | 345.8 (±124.5) | 335.6 (±75.1) | 326.2 (±78.75) | 298.5 (±97.91) | 0.87 | |
| FC T0 | 0.005 (±0.003) | 0.005 (±0.002) | 0.003 (±0.003) | 0.004 (±0.001) | 0.13 | |
Continuous numeric data have been summarized by average and standard deviation in brackets. Categorical data (Gender) has been represented as proportion. Age and BMI in the two groups comparison were analyzed with the Mann-Whitney test; Gender was analyzed with Fisher’s exact test. The Kruskal-Wallis test was done to compare the initial MVC and the Brown-Forsythe and Wech ANOVA test to compare the initial FC.
The statistical analyses were done in the logarithmic domain, as described in the Materials and Methods section. Results are summarized in Table 3.
The FC showed a slight increase over the timepoints, depicting a faster decrease in strength in all the conditions, without a statistical significance at any timepoints (Figure 4A-D).
Table 3.
Comparison of MVC by timepoints.
| T0 | T1 | T2 | T3 | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0-T1 | T1-T2 | T2-T3 | T0-T2 | T0-T3 | |||||
| FMV+AO | 329.1 | 380.5 | 408.7 | 407.1 | |||||
| (-88.35, +120.78) | (-79.95, +101.21) | (-97.42, +127.92) | (-97.85, +132.31) | 0.0832 | 0.2346 | 0.9994 | 0.0344 | 0.0239 | |
| AO | 316.2 | 393.4 | 380.1 | 399.2 | |||||
| (-96.77, +139.44) | (-74.08, +91.27) | (-78.19, +98.45) | (-95.39, +125.35) | 0.2212 | 0.8652 | 0.6476 | 0.3932 | 0.0961 | |
| FMV | 317 | 333.1 | 347.4 | 324.8 | |||||
| (-70.69, +90.98) | (-93. 8, +130.58) | (-91.77, +124.72) | (-93.3, +130.89) | 0.7687 | 0.7447 | 0.3364 | 0.2208 | 0.9721 | |
| NT | 283.2 | 324.6 | 337.8 | 322 | |||||
| (-82.49, +116.4) | (-96.65, +137.63) | (-74.51, +95.6) | (-71.42, +91.77) | 0.2347 | 0.9984 | 0.4873 | 0.0659 | 0.1562 | |
Data are summarized by geometrical mean and standard deviation calculated from the standard deviation factor. The p-value was calculated in for data the logarithmic domain. The conditions were analyzed with Repeated measure one-way ANOVA and the post-hoc Tukey’s multiple comparison test. Statistical difference was set for a p-value<0.05. ns, p≥0.05; *p<0.05.
Figure 3.
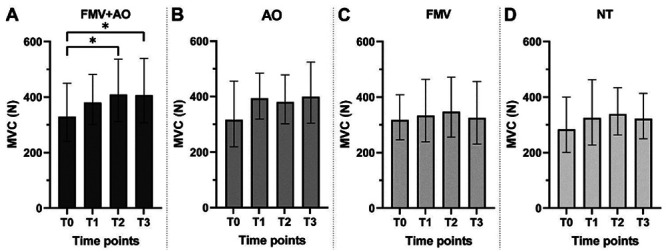
MVC barplots for each condition. Data presented as geometric mean±SDf. To compare difference between timepoints within a condition we used the Repeated Measures one-way ANOVA test with Geisser-Greenhouse correction, with Tukey multiple comparison test with individual variances computed for each comparison on data converted to the logarithmic domain (see Materials and Methods). *p<0.05.
The effects of each treatment – FMV+AO, AO, and FMV – were then analyzed to assess their relative effect on both MVC and FC. We normalized the values of the MVC timepoints to each group’s baseline (as described in the Materials and Methods section) and assessed the differences in FCs between the baseline and each evaluation timepoint (Figure 5A-B). After one week of treatment, the impact of AO on the MVC is significantly more pronounced compared to the FMV treatment. By the end of the protocol (T2), the combination of FMV and AO led to a significantly greater enhancement in the MVC compared to FMV alone. This trend continues even one week after treatment cessation (T3), where both the FMV+AO and AO conditions still showed statistically significant differences compared to the FMV (Figure 5A). There is no statistically significant difference observed in terms of modifying the FC (Figure 5B).
Figure 4.
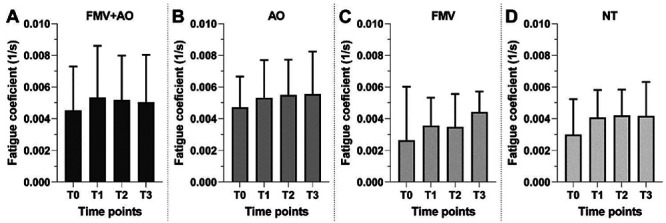
FC barplots for each condition. Data presented as mean±SD. To compare differences between timepoints within a condition we used the Friedman test with Dunn’s multiple comparison test.
Figure 5.
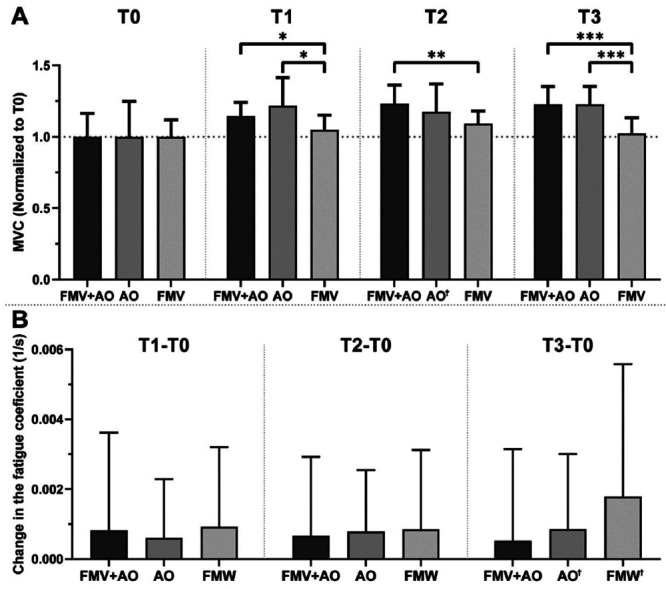
Comparison of treatment effect on MVC (5A) and FC (5B). Data presented as mean±SD. To compare differences in effectiveness treatments we used Repeated Measures two-way ANOVA test with Geisser-Greenhouse correction, with Tukey multiple comparison test with individual variances computed for each comparison. Ϯ datasets failing Shapiro-Wilk normality test. *p<0.05; **p<0.01; ***p<0.001.
Discussion
Our study demonstrated the synergistic effect of combining the AO with FMV to strengthen the quadriceps. This innovative treatment yields superior results in terms of muscle strengthening without the need for active training. The results confirmed that, in healthy subjects, the combination of FMV as a Bottom-Up approach and AO as a Top-Down approach significantly enhances the MVC. The hypothesis that the integration of these two treatments could influence plasticity through a bimodal approach is supported by the already known mechanism of the two techniques, further corroborated by our experiment and results.
The bottom-up effect of focal muscle vibration
Thanks to the safety, tolerability, and versatility of FMV, its use in clinical settings increased over time. According to the Literature, FMV applied to muscles activates muscle-tendon receptors and reflexes, capable of inducing peripheral and central changes thanks to Long-Term Potentiation (LTP).16 The proprioceptive stimulus, caused by muscle vibration at low amplitudes, produces Ia fiber afferent input and reaches both the primary somatosensory and motor cortex.17 Despite this, a clear consensus about the most effective frequency to be used for muscular strengthening has not been established, with multiple studies showing results for frequencies ranging from 80 to 300 Hz.9,16 The chosen vibration frequency of 100 Hz for this study is the most commonly used in the field of rehabilitation.17 Traditionally, this frequency has been described as appropriate for eliciting the phenomenon of “spindle driving”, driving the same stimulation frequency to Ia fibers afferent discharge.18,19 Interestingly, 100 Hz is also employed in other stimulation techniques to induce plastic reorganization of central nervous networks, both in vitro20 and in vivo.21 This suggests that this kind of stimulation might lead to synaptic events such as LTP and result in an immediate and sustained change in synaptic responsiveness, followed by sustained reorganization of the synaptic pathway. However, further studies on the actual mechanism underlying the central effect of FMV are needed.
The described mechanism suggests that the MVC improvement of the vibrated limb in our study, both in the intervention and in the control group, might be rather due to a modification and a greater efficiency in the neural control of the muscle, than to a peripheral muscular adaptation. This also seems to be supported by the analyses of the fatigue, which did not show any significant effect in any of the treatments. Furthermore, the short duration of the protocol and the underlying neural mechanism are not likely to determine a change in the muscle structure, cross-sectional area, or fiber proportion. Therefore, the overall sustained force in time is not as affected as the MCV which relies more on the motoneuron firing efficiency than on the fiber muscle resistance.
Notably, an increase in the MVC is evident not only in the limb muscle subjected to vibration but also in the left quadriceps muscle, which was un-vibrated in all groups. This might be due to the so-called cross-education effect, already described in a unilateral vibrational or electrical neuromuscular stimulation.22 Cross-education seems to rely on adaptations in the Central Nervous System induced by the peripheral stimulation, even if there is no concordance on the actual mechanism.23 Our findings further expand this concept, showing a slight, improvement in the no-treated limb, more evident in the AO condition.
This result confirms the efficacy of muscle stimulation as an adjunct therapy, especially in people with fatigue syndromes who spend less time exercising, thus exacerbating motor difficulties.24 Although exercising is the best countermeasure to muscle deterioration, safe and toll-free rehabilitation training might benefit from alternative strategies aimed at facilitating initial strength gains. Previous studies have shown that other bottom-up approaches, such as Functional Electrical Stimulation (FES) and Neuro-Muscular Electrical Stimulation (NMES), represent effective techniques for immobilized patients or those with limited exercise capacity.24,25
FMV is a relatively new option that, together with FES, NMES, and brief training sessions, completes the arsenal available to clinicians for ensuring muscle and joint maintenance and providing initial reinforcement.
The top-down effect of action observation
With its effect of stimulating cortical pathways associated with voluntary movement and activating mirror neurons, AO showed great results in skill learning, training, and recovery.26 Former studies showed that AO can lead not only to the acquisition of skill12 and improvement in motor performance after periods of inactivity and disuse13 but also to muscular strengthening,27 especially when associated with motor imagery.28 In our study, the Intervention Group watched and imagined movements for quadriceps strengthening. Even if the presented protocol did not follow the canonical features for the motor imagery practice,29 the results showed the efficacy of the AO paradigm in strengthening the extension of the limb, especially when combined with FMV. As said, the improvement of the non-vibrated limb in both experimental groups might be then explained by the “cross-education” in the NT condition, but in the AO condition both the “cross-education” mechanism and the action observation mechanism should be acknowledged. Thus, the AO condition also highlights the synergic effect of the two approaches on the non-vibrated limb.
It is worth noting that, to enhance the effect of AO, the replication of observed actions should be performed right afterward. This contributes to the formation of motor memory and motor learning.12,30 Additionally, it is known that tendon vibration can induce a kinaesthetic illusion modifying the cortical plasticity evoked by AO.31 Given the placement of the transducers, we may assume that in our study, the movement has been substituted by the vibratory stimulus provided during the video watching, thereby enhancing the effect of the AO itself on the vibrated limb. This is supported also by the superior result observed in the FMV+AO condition.
The effect of the combination
Utilizing combined approaches in both physiological and clinical settings to enhance performance and outcomes might be a powerful strategy for synergistically amplifying the effects of individual approaches. However, the choice of the combination must rely on the knowledge and the integration of the underlying mechanism rather than the sole availability of the different techniques. In fact, the second approach might lead to an increase in the heterogeneity of findings, preventing the possibility of reproducibility of results and making it difficult to draw specific guidelines and indications. For their versatility, both FMV and AO can be combined with several other strategies. Few studies proposed a safe combination of AO with Non-Invasive Brain Stimulation (NIBS) techniques32,33 and of FMV with robotic-assisted treatment.34 However, the AO-NIBS combination studies found either interference of the NIBS on the positive effects of AO alone33 or did not find a clear effect for the lack of homogeneity in the sample.32
Regarding the association of FMV and robotic-assisted treatment, the two approaches had two different aims: modulating spasticity for FMV and improving motor control for robotic training. The use of FMV facilitates the rehabilitation and consequently the effect of the robotic rehabilitation, though, the integration is not explained by a synergistic combination at a neurophysiological level.34
In our work, for the first time, the two treatments address the same aim, driving two different mechanisms of plasticity at the same time (Bottom-Up and Top-Down), to reach an integrated result. Furthermore, the maintenance of the effect on the MCV in the FMV+AO condition at follow-ups suggests that the integrated treatment might induce a long-lasting plasticity.
The study carried out in healthy subjects, sheds light on possible extension of the application of the combined FMV+AO treatment in the clinical rehabilitation field. With the proposed explanation it is not difficult to think about the use of a similar combined treatment in patients bedded due to chronic disease, to initiate a rehabilitative treatment when the execution of a standard therapy might be an excessive effort. This treatment would be easy to perform at the bedside, even in acute patients. Other studies already highlighted the effectiveness of FMV a complementary non-pharmacological therapy to promote motor recovery in acute stroke patients, thanks to its ability to modulate the excitatory/inhibitory state of the primary motor cortex.35,36 This effect can be enhanced by the association of a Top-Down approach - like AO in our study - administered at the same time as the peripheral stimulation.
The two, integrated here, treatments convey an already known mechanism called “learning without training”,37,38 according to which repetition of sensory stimulation (tactile, visual, or auditory) might induce LTP and Long-term Depression (LTD) mechanisms, which are the key factors in the rehabilitation of neurological disease. Furthermore, the known modulatory effect on motoneuron firing, driven especially by FMV,39 justifies the potential application of the proposed treatment in the recovery of motor impairments in neurological disease,40 especially in stroke, where the modification in the firing rate induced by a rehabilitative treatment is positively correlated with recovery.41
Likely, a lack of a statistical difference in the treatment with FMV and AO alone in our study can be attributed to the small number of participants. Since these are both already proven-efficacy treatments, increasing the group size might reinforce already known findings. On the other hand, discovering a significant effect of the combination of the two treatments already at this sample-size further underlines the significance of our finding, which can gain a higher resonance with a bigger sample.
Limitations
The main limitation of the present study is the number of participants, which may have partly contributed to the low statistical significance observed. Additionally, our study did not show any significant change in muscle fatigue, likely due to the relatively short duration of the treatment. Further studies with a larger sample size can be aimed at confirming and better characterizing the observed changes. Furthermore, an extension of the duration of the study on the proposed treatment can ensure functional and long-lasting outcomes.
Our study presents cues about the potential central plastic changes; however, our findings are based on behavioral data and may not fully capture the physiological dynamic of neural adaptations. Therefore, future research employing specific methodologies, such as neuroimaging techniques, electroencephalography, and NIBS, is needed to provide a deeper understanding of central modifications and their implications.
Conclusions
Our study shows the synergistic benefits arising from the integration of Top-Down and Bottom-Up approaches, combining FMV and AO. This combination demonstrated a substantial enhancement in muscular strength among healthy subjects compared to the isolated application of each strategy. The observed effect likely stems from the potentiation of plasticity phenomena induced by the simultaneous implementation of both approaches. Our proposed treatment not only achieves the challenging goal of minimizing training duration in healthy subjects but also holds promise for reducing recovery time in rehabilitation settings. This innovative approach enhances outcomes through non-invasive complementary strategies, contributing to an advanced and more effective model of rehabilitation care.
Acknowledgments
The authors acknowledge all the healthy volunteers involved in the study. Special thanks to Mario Oneglia for carefully following the participants during training and evaluation, and to Chiara Notarstefano for her insights during the design of the experiment.
List of acronyms
- FMV
Focal Muscle Vibration.
- AO
Action Observation.
- AOT
Action Observation Therapy.
- NT
No-Treatment.
- MVC
Maximum Voluntary Contraction.
- FC
Fatigue Coefficient.
- CV
Coefficient of Variation.
- BMI
Body Mass Index.
- LTP
Long Term Potentiation.
- NIBS
Non-Invasive Brain Stimulation.
- NTT
Number To be Treated.
- FES
Functional Electrical Stimulation.
- NMES
Neuro-Muscular Electrical Stimulation.
Footnotes
Conflict of interest
The authors declare no potential conflict of interest, and all authors confirm accuracy.
Contributor Information
Valentina Azzollini, Email: valentina.dr.azzollini@gmail.com.
Noemi Fragapane, Email: n.fragapane@studenti.unipi.it.
Zbigniew Baster, Email: baster.zbigniew@gmail.com.
Simone Carozzo, Email: simone.carozzo@gmail.com.
Stefania Dalise, Email: stefania.dalise@ao-pisa.toscana.it.
Carmelo Chisari, Email: carmelo.chisari@unipi.it.
References
- 1.Wang DXM, Yao J, Zirek Y, et al. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle 2020;11:3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suchomel TJ, Nimphius S, Bellon CR, Stone MH. The importance of muscular strength: training considerations. Sports Med 2018;48:765-85. [DOI] [PubMed] [Google Scholar]
- 3.Horlings CGC, van Engelen BGM, Allum JHJ, Bloem BR. A weak balance: the contribution of muscle weakness to postural instability and falls. Nat Clin Pract Neurol 2008;4:504–515. [DOI] [PubMed] [Google Scholar]
- 4.Del Vecchio A, Casolo A, Negro F, et al. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol 2019;597:1873–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis SK, Mujika I, Gentles JA, Stone MH, Bazyler CD. Tapering and peaking maximal strength for power-lifting performance: a review. Sports (Basel) 2020;8: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storey A, Smith HK. Unique aspects of competitive weightlifting. Sports Med 2012;42:769–90. [DOI] [PubMed] [Google Scholar]
- 7.Morone G, Spitoni GF, De Bartolo D, et al. Rehabilitative devices for a top-down approach. Expert Rev Med Devices 2019;16:187-95. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez N, Winstein CJ. Lost in translation: simple steps in experimental design of neurorehabilitation-based research interventions to promote motor recovery post-stroke. Front Hum Neurosci 2021;15:644335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattorini L, Rodio A, Pettorossi VE, Filippi GM. Is the focal muscle vibration an effective motor conditioning intervention? a systematic review. J Funct Morphol Kinesiol 2021;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahir S, Baig MO, Rathore FA, Aslam H. The emerging role of focal muscle vibration in rehabilitation of neurological disorders. J Pak Med Assoc 2022;72:2126–8. [DOI] [PubMed] [Google Scholar]
- 11.Buccino G. Action observation treatment: a novel tool in neurorehabilitation. Philos Trans R Soc Lond B Biol Sci 2014;369:20130185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakadjian A, Panchuk D, Pearce AJ. I look, therefore i see. Using action observation in improving strength and conditioning techniques. Strength Conditioning J 2013; 35:33–8. [Google Scholar]
- 13.Sarasso E, Gemma M, Agosta F, et al. Action observation training to improve motor function recovery: a systematic review. Arch Physiother 2015;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales JU, Scheuermann BW. Absence of gender differences in the fatigability of the forearm muscles during intermittent isometric handgrip exercise. J Sports Sci Med 2007;6:98–105. [PMC free article] [PubMed] [Google Scholar]
- 15.Potvin JR, Fuglevand AJ. A motor unit-based model of muscle fatigue. PLoS Comput Biol 2017;13:e1005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feltroni L, Monteleone S, Petrucci L, et al. Potentiation of muscle strength by focal vibratory stimulation on quadriceps femoris. G Ital Med Lav Ergon 2018;40: 90-96. [PubMed] [Google Scholar]
- 17.Filippi GM, Rodio A, Fattorini L, et al. Plastic changes induced by muscle focal vibration: A possible mechanism for long-term motor improvements. Front Neurosci 2023;17:1112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roll J P, Vedel J P. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 1982;47:177–90. [DOI] [PubMed] [Google Scholar]
- 19.Bianconi R, Van Der Meulen JP. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol 1963;26:177–90. [DOI] [PubMed] [Google Scholar]
- 20.Abraham WC, Williams JM. Properties and Mechanisms of LTP Maintenance. Neuroscientist 2003;9: 463–74. [DOI] [PubMed] [Google Scholar]
- 21.Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation of thalamic input to the motor cortex induced by coactivation of thalamocortical and corticocortical afferents. J Neurophysiol 1991;65:1435–41. [DOI] [PubMed] [Google Scholar]
- 22.Minetto MA, Botter A, Gamerro G, et al. Contralateral effect of short-duration unilateral neuromuscular electrical stimulation and focal vibration in healthy subjects. Eur J Phys Rehabil Med 2018;54:911-20. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Zhang SS, Crowley-McHattan ZJ. A scoping review of the contralateral effects of unilateral peripheral stimulation on neuromuscular function. PLoS One 2022;17:e0263662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravara B, Giuriati W, Maccarone MC, et al. Optimized progression of Full-Body In-Bed Gym workout: an educational case report. Eur J Transl Myol 2023;33:11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carraro U, Marcante A, Ravara B, et al. Skeletal muscle weakness in older adults home-restricted due to COVID-19 pandemic: a role for full-body in-bed gym and functional electrical stimulation. Aging Clin Exp Res 2021;33:2053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.di Pellegrino G, Fadiga L, Fogassi L, et al. Understanding motor events: a neurophysiological study. Exp Brain Res 1992;91:176–80. [DOI] [PubMed] [Google Scholar]
- 27.Salama IM, Turner S, Edwards MG. Rapid communication: Automatic priming of grip force following action observation. Qtly J Experiment Psychol 2011;64: 833–38. [DOI] [PubMed] [Google Scholar]
- 28.Scott M, Taylor S, Chesterton P, et al. Motor imagery during action observation increases eccentric hamstring force: an acute non-physical intervention. Disabil Rehabilit 2018;40:1443–51. [DOI] [PubMed] [Google Scholar]
- 29.Bovend’Eerdt TJH, Dawes H, Sackley C, Wade DT. Practical research-based guidance for motor imagery practice in neurorehabilitation. Disabil Rehabilit 2012;34:2192–200. [DOI] [PubMed] [Google Scholar]
- 30.Celnik P, Webster B, Glasser D, Cohen L. Effects of action observation on physical training after stroke. Stroke 2008;39:1814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisio A, Biggio M, Avanzino L, et al. Kinaesthetic illusion shapes the cortical plasticity evoked by action observation. J Physiol 2019;597:3233–45. [DOI] [PubMed] [Google Scholar]
- 32.Noh JS, Lim JH, Choi TW, et al. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: A randomized controlled trial. Restor Neurol Neurosci 2019;37:219–30. [DOI] [PubMed] [Google Scholar]
- 33.Schwell G, Kozol Z, Tarshansky D, et al. The effect of action observation combined with high-definition transcranial direct current stimulation on motor performance in healthy adults: A randomized controlled trial. Front Hum Neurosci 2023;17:1126510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrò RS, Naro A, Russo M, et al. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS One 2017;12:e0185936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marconi B, Filippi GM, Koch G, et al. Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci 2008;275:51-9. [DOI] [PubMed] [Google Scholar]
- 36.Toscano M, Celletti C, Viganò A, et al. Short-term effects of focal muscle vibration on motor recovery after acute stroke: a pilot randomized sham-controlled study. Front Neurol 2019;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunetti O, Filippi GM, Lorenzini M, et al. Improvement of posture stability by vibratory stimulation following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2006;14:1180-7. [DOI] [PubMed] [Google Scholar]
- 38.Beste C, Dinse HR. Learning without training. Current Biol 2013;23:R489–R499. [DOI] [PubMed] [Google Scholar]
- 39.Lapole T, Mesquita RNO, Baudry S, et al. Can local vibration alter the contribution of persistent inward currents to human motoneuron firing? J Physiol 2023;601: 1467–82. [DOI] [PubMed] [Google Scholar]
- 40.Azzollini V, Dalise S, Chisari C. How does stroke affect skeletal muscle? State of the art and rehabilitation perspective. Front Neurol 2021;12:797559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chisari C, Bertolucci F, Monaco V, et al. Robot-assisted gait training improves motor performances and modifies Motor Unit firing in poststroke patients. Eur J Phys Rehabil Med 2015;51:59-69. [PubMed] [Google Scholar]


