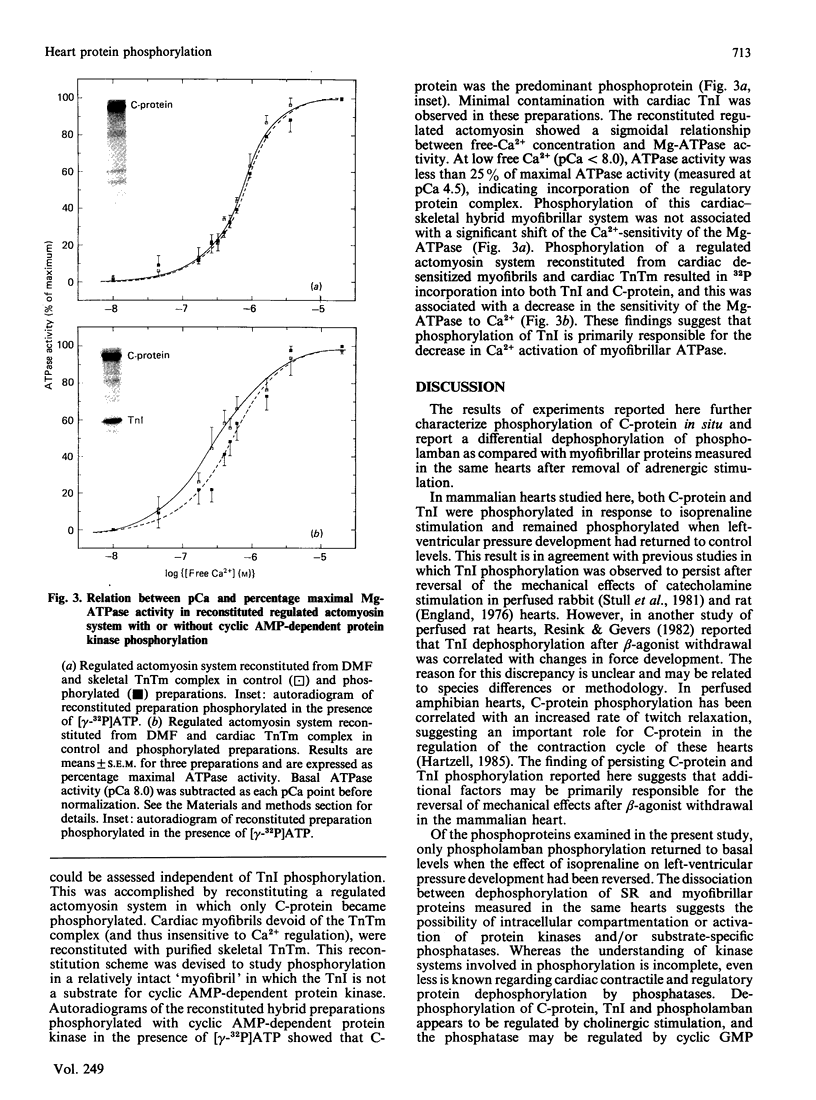
Abstract
Phosphorylation of myofibrillar and sacroplasmic-reticulum (SR) proteins was studied in Langendorff-perfused rabbit hearts subjected to various inotropic interventions. Stimulation of hearts with isoprenaline resulted in the phosphorylation of both troponin I (TnI) and C-protein in myofibrils and phospholamban in SR. Phosphorylation of phospholamban could be reversed by a 15 min perfusion with drug-free buffer, after a 1 minute pulse perfusion with isoprenaline, at which time the mechanical effects of isoprenaline stimulation had also been reversed. However, both TnI and C-protein remained phosphorylated at this time. Moreover, the inhibition of Ca2+ activation of the Mg2+-dependent ATPase (Mg-ATPase) activity associated with myofibrillar phosphorylation persisted in myofibrils prepared from hearts frozen after 15 min of washout of isoprenaline. To assess the contribution of C-protein phosphorylation in the decrease of Ca2+ activation of the myofibrillar Mg-ATPase activity, we reconstituted a regulated actomyosin system in which only C-protein was phosphorylated. In this system, C-protein phosphorylation did not contribute to the decrease in Ca2+ activation of Mg-ATPase activity, indicating that TnI phosphorylation is responsible for the diminished sensitivity of the myofibrils to Ca2+. These observations support the hypothesis that phospholamban phosphorylation plays a more dominant role than TnI or C-protein phosphorylation in the mechanical response of the mammalian heart to beta-adrenergic stimulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailin G. Phosphorylation of a bovine cardiac actin complex. Am J Physiol. 1979 Jan;236(1):C41–C46. doi: 10.1152/ajpcell.1979.236.1.C41. [DOI] [PubMed] [Google Scholar]
- Blanchard E. M., Solaro R. J. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circ Res. 1984 Sep;55(3):382–391. doi: 10.1161/01.res.55.3.382. [DOI] [PubMed] [Google Scholar]
- Carter S. G., Karl D. W. Inorganic phosphate assay with malachite green: an improvement and evaluation. J Biochem Biophys Methods. 1982 Dec;7(1):7–13. doi: 10.1016/0165-022x(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Craig R., Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- England P. J. Correlation between contraction and phosphorylation of the inhibitory subunit of troponin in perfused rat heart. FEBS Lett. 1975 Jan 15;50(1):57–60. doi: 10.1016/0014-5793(75)81040-4. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hartzell H. C. Effects of phosphorylated and unphosphorylated C-protein on cardiac actomyosin ATPase. J Mol Biol. 1985 Nov 5;186(1):185–195. doi: 10.1016/0022-2836(85)90268-2. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Phosphorylation of C-protein in intact amphibian cardiac muscle. Correlation between 32P incorporation and twitch relaxation. J Gen Physiol. 1984 Apr;83(4):563–588. doi: 10.1085/jgp.83.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Titus L. Effects of cholinergic and adrenergic agonists on phosphorylation of a 165,000-dalton myofibrillar protein in intact cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):2111–2120. [PubMed] [Google Scholar]
- Holroyde M. J., Small D. A., Howe E., Solaro R. J. Isolation of cardiac myofibrils and myosin light chains with in vivo levels of light chain phosphorylation. Biochim Biophys Acta. 1979 Nov 1;587(4):628–637. doi: 10.1016/0304-4165(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Jeacocke S. A., England P. J. Phosphorylation of a myofibrillar protein of Mr 150 000 in perfused rat heart, and the tentative indentification of this as C-protein. FEBS Lett. 1980 Dec 15;122(1):129–132. doi: 10.1016/0014-5793(80)80418-2. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Role of the contractile proteins and sarcoplasmic reticulum in the response of the heart to catecholamines: an historical review. Adv Cyclic Nucleotide Res. 1979;11:303–343. [PubMed] [Google Scholar]
- Kranias E. G., Garvey J. L., Srivastava R. D., Solaro R. J. Phosphorylation and functional modifications of sarcoplasmic reticulum and myofibrils in isolated rabbit hearts stimulated with isoprenaline. Biochem J. 1985 Feb 15;226(1):113–121. doi: 10.1042/bj2260113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias E. G., Mandel F., Wang T., Schwartz A. Mechanism of the stimulation of calcium ion dependent adenosine triphosphatase of cardiac sarcoplasmic reticulum by adenosine 3',5'-monophosphate dependent protein kinase. Biochemistry. 1980 Nov 11;19(23):5434–5439. doi: 10.1021/bi00564a044. [DOI] [PubMed] [Google Scholar]
- Kranias E. G. Regulation of calcium transport by protein phosphatase activity associated with cardiac sarcoplasmic reticulum. J Biol Chem. 1985 Sep 15;260(20):11006–11010. [PubMed] [Google Scholar]
- Kranias E. G., Solaro R. J. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982 Jul 8;298(5870):182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman W. Thick-filament-linked calcium regulation in vertebrate striated muscle. Nature. 1978 Jul 6;274(5666):80–81. doi: 10.1038/274080a0. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Watanabe A. M. Muscarinic cholinergic inhibition of beta-adrenergic stimulation of phospholamban phosphorylation and Ca2+ transport in guinea pig ventricles. J Biol Chem. 1985 Oct 25;260(24):13122–13129. [PubMed] [Google Scholar]
- Metzger H., Lindner E. The positive inotropic-acting forskolin, a potent adenylate cyclase activator. Arzneimittelforschung. 1981;31(8):1248–1250. [PubMed] [Google Scholar]
- Mope L., McClellan G. B., Winegrad S. Calcium sensitivity of the contractile system and phosphorylation of troponin in hyperpermeable cardiac cells. J Gen Physiol. 1980 Mar;75(3):271–282. doi: 10.1085/jgp.75.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura J., Wang T., Tsai L. I., Schwartz A. Properties and characterization of a highly purified sarcoplasmic reticulum Ca2+-ATPase from dog cardiac and rabbit skeletal muscle. J Biol Chem. 1983 Apr 25;258(8):5079–5083. [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Gevers W. Dephosphorylation of myofibrillar proteins in actomyosin preparations and in isolated perfused rat hearts after beta-agonist withdrawal. J Mol Cell Cardiol. 1982 Jun;14(6):329–337. doi: 10.1016/0022-2828(82)90248-6. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Holroyde M. J., Kranias E. G., Potter J. D., Solaro R. J. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982 Jan 10;257(1):260–263. [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Pang D. C., Briggs F. N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971 Aug 6;245(1):259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. Polypeptide chains of intermediate molecular weight in myosin preparations. FEBS Lett. 1971 Jun 2;15(1):40–44. doi: 10.1016/0014-5793(71)80075-3. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Buss J. E. Phosphorylation of cardiac troponin by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Feb 10;252(3):851–857. [PubMed] [Google Scholar]
- Tada M., Inui M. Regulation of calcium transport by the ATPase-phospholamban system. J Mol Cell Cardiol. 1983 Sep;15(9):565–575. doi: 10.1016/0022-2828(83)90267-5. [DOI] [PubMed] [Google Scholar]
- Tada M., Yamada M., Ohmori F., Kuzuya T., Inui M., Abe H. Transient state kinetic studies of Ca2+-dependent ATPase and calcium transport by cardiac sarcoplasmic reticulum. Effect of cyclic AMP-dependent protein kinase-catalyzed phosphorylation of phospholamban. J Biol Chem. 1980 Mar 10;255(5):1985–1992. [PubMed] [Google Scholar]