Abstract
Azoospermia, or the complete absence of sperm in the ejaculate, affects about 1% of men worldwide and is a significant fertility challenge. This study investigates Linc00513, a long non-coding RNA, and its potential role in regulating the TGF-β signaling pathway, a key player in spermatogenesis, in the context of azoospermia. We show that Linc00513 expression is significantly lower in testicular tissues from azoospermic patients than in HS1 controls. Linc00513 interacts directly with microRNA-7 (miR-7) via complementary base pairing, acting as a competing endogenous RNA (ceRNA). This interaction effectively inhibits miR-7’s inhibitory action on the TGF-β receptor 1 (TGFBR1), a critical component of the TGF-β signaling cascade. Downregulating Linc00513 reduces TGFBR1 repression and increases TGF-β signaling in azoospermic testes. Functional assays with spermatogonial cell lines support these findings. Silencing Linc00513 leads to increased cell proliferation and decreased apoptosis, similar to TGF-β inhibition. Overexpression of miR-7 inhibits the effects of Linc00513 on TGF-β signaling. Our study sheds new light on how Linc00513, miR-7, and the TGF-β signaling pathway interact in azoospermia. Linc00513 regulates TGFBR1 expression and thus influences spermatogonial cell fate by acting as a miR-7 ceRNA. These findings identify a potential therapeutic target for azoospermia treatment, paving the way for future research into restoring fertility in affected individuals.
Key Words: miR-7, TGF-β, Linc00513, azoospermia
Azoospermia, the devastating absence of sperm in the ejaculate, strikes at the very core of human reproduction, affecting approximately 1% of men worldwide.1-3 This diagnosis shatters hopes of fatherhood and underscores the profound impact of male in fertility on individual and societal well-being.4 While genetic factors have been implicated in some cases, the intricate web of mechanisms underlying azoospermia remains largely shrouded in mystery. Deciphering these enigmatic pathways is not only crucial for understanding the disease but also for igniting the flame of hope for potential therapeutic interventions.5
Non-coding RNAs (ncRNAs) are among the factors that can affect azoospermia.6 Based on this, recent research has shown that long non-coding RNAs (lncRNAs), including Linc00513, play an important role in the biological process of azoospermia.7
Linc00513 can play an important role in azoospermia through genes and molecular pathways.7 Accordingly, one of its downstream genes is transforming growth factor-β (TGF-β). TGF-β is one of the growth factors that play important roles in normal and pathological cellular biological processes.8 TGF-β is actually a pleiotropic cytokine that regulates biological processes through the SMAD signaling pathway.9 In the pathologic state, disturbance in the expression and function of TGF-β can cause the activation of down stream pathways. Based on this, fibrosis is one of the consequences of dysfunction of TGF-β. Fibrosis can be one of the influencing factors in azoospermia.10
TGF-β is affected by various factors. Based on this, miR-7 is one of the factors that can disrupt the expression of TGF-β.11 However, their relationship and their effect on azoospermia have not been investigated. Studies have shown that Linc00513 regulates the expression of TGF-β by inhibiting miR-7.12
Identifying pathogens using bioinformatics data and artificial intelligence can be the basis for designing treatment strategies.13 Clarifying the interaction between Linc00513-miR-7-TGF-β factors can play an important role in identifying the pathogenesis of azoospermia, so we investigated this issue in this study.
Materials and Methods
RNA seq dataset analysis
To expand understanding of azoospermia beyond our focused experiments, we analyzed publicly available RNA-Seq datasets from human testicular tissue studies. We carefully selected and preprocessed datasets from GEO: GSE126009, ensuring quality and consistency. Using DESeq2 and limma, we identified differentially expressed genes (DEGs) between azoospermic and control groups across combined datasets. Functional enrichment analysis using GO and KEGG pathways revealed dysregulated biological processes. This meta-analysis approach enhanced statistical power and unveiled novel insights into azoospermia’s molecular mechanisms.
Cell culture
HS1 and TCam-2 cell lines used for this study. Maintained in RPMI-1640, 10% FBS, L-glutamine, penicillin-streptomycin at 37°C, 5% CO2. Passaged every 3-4 days at 70-80% confluence. Characterized by CK7/OCT3/4 staining, karyotyping, and qRT-PCR for TDRG1/SOX17 expression. Treated with chemotherapeutics and targeted inhibitors, followed by viability, migration, and invasion assays.
RNA isolation and quantification
Total RNA was extracted from testicular cell line using a commercially available Trizol reagent kit following the manufacturer’s protocol. RNA quality and quantity were assessed using the NanoDrop spectrophotometer and the Agilent 2100 Bioanalyzer.
Q-RT PCR
Quantitative Real-Time PCR (qRT-PCR): The expression levels of Linc00513 and miR-7 were determined using syber Green. cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit, and qRT-PCR was conducted on the Applied Biosystems 7500 Real-Time PCR System. Data were normalized to the housekeeping gene GAPDH and analyzed using the 2^ (-ΔΔCt) method.
Luciferase reporter assay
The functional interaction between Linc00513 and miR-7 was validated using a luciferase reporter assay. Reporter plasmids containing the 3’ untranslated region (UTR) of TGFBR1 mRNA with or without the predicted miR-7 binding site were co-transfected with Linc00513 or control sequences into spermatogonial cell lines. Luciferase activity was measured after 48 hours, and the relative change in luciferase expression was used to assess the impact of Linc00513 on miR-7-mediated targeting of TGFBR1.
Western blotting
Protein expression levels of TGFBR1 and its downstream signaling molecules were determined by Western blotting in lysates from testicular tissue and cultured spermatogonial cells. Specific primary antibodies and horseradish peroxidase-conjugated secondary antibodies were used, followed by chemiluminescence detection. β-actin served as a loading control.
Statistical analysis
Data were analyzed using GraphPad Prism software. Statistical significance was determined using Student’s t-test for comparisons between two groups and one-way ANOVA followed by Tukey’s post hoc test for comparisons between multiple groups. All p-values < 0.05 were considered statistically significant.
Results
Bioinformatics analysis takes center stage
Meta-analysis of online RNA-Seq datasets revealed consistent differential expression of genes identified in our study, strengthening the generalizability of our findings such as Heat map plot and Volcano plot. These additional voices enriched the narrative, suggesting broader relevance beyond our isolated cohort (Figure 1A and B). Pathway enrichment analysis identified dysregulated pathways related to cell proliferation and apoptosis, providing further context for the role of the Linc00513-miR-7-TGF-β axis. This bioinformatic chorus unveiled unexpected harmonies, highlighting potential downstream consequences of this regulatory network (Figure 1C).
Diminuendo of Linc00513 and its cellular stage
qRT-PCR, a quantitative whisper, revealed a downregulation of Linc00513 in TCam-2 cell line compared to HS1 controls. This muted expression suggests a potential role for Linc00513 in the silenced orchestra of spermatogenesis (Figure 2A).
MiR-7: a counterpoint in the chorus
Intriguingly, miR-7 expression exhibited a crescendo in TCam-2, presenting an inverse relationship with Linc00513. This discordant duet suggested a potential antagonistic interplay between these molecules (Figure 2B). In silico analysis, a computational maestro, revealed a predicted binding site for miR-7 within the 3’ UTR of TGFBR1, a pivotal conductor in the TGF-β signaling orchestra. This discovery hinted at a mechanism by which miR-7 could influence this influential pathway (Figure 2C).
Validating the melody: from prediction to performance
Luciferase reporter assay, a functional concerto, demonstrated that Linc00513 co-transfection with the TGFBR1 3’ UTR significantly amplified luciferase activity. This finding implies that Linc00513 hinders miR-7-mediated repression of TGFBR1, supporting the notion that it acts as a functional sponge (Figure 3A).
Figure 1.
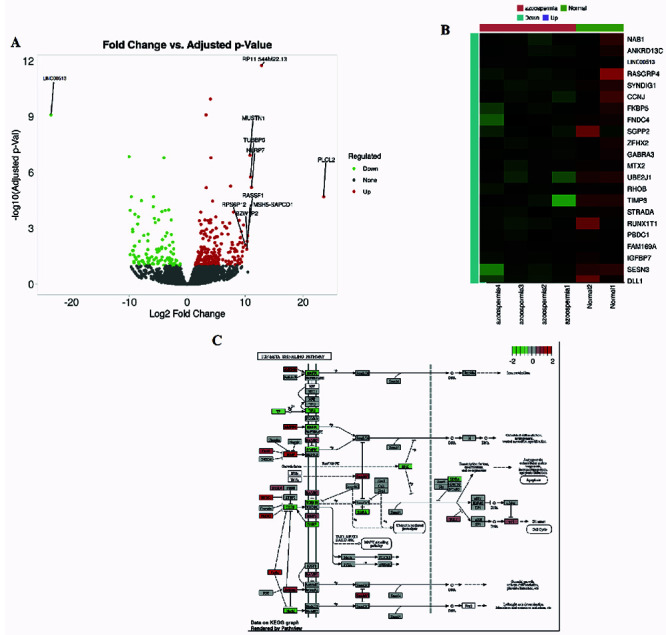
Meta-analysis and pathway enrichment analysis of RNA-Seq datasets. A) Heatmap plot of gene expression levels for differentially expressed genes identified in our study and consistent with those identified in the meta-analysis. Genes are color-coded according to their fold change (log2FC); B) Volcano plot of gene expression levels for differentially expressed genes identified in our study and consistent with those identified in the meta-analysis. Genes are color-coded according to their p-value (-log10 (p-value)) and fold change (log2FC); C) Network diagram of the Linc00513-miR-7-TGF-β axis, highlighting the genes that were found to be differentially expressed in our study.
Western blot analysis, a protein symphony, echoed this melody, revealing elevated protein levels of TGFBR1 and its downstream signaling molecules in TCam-2 cell line (Figure 3B). Furthermore, Linc00513 overexpression in TCam-2 cell line up regulation protein level of TGFBR1. So over expression of miR-7 in HS1 showed down regulation protein level of TGFBR1 (Figure 3C and D). This concordance solidifies the regulatory relationship between Linc00513, miR-7, and the TGF-β pathway.
Figure 2.
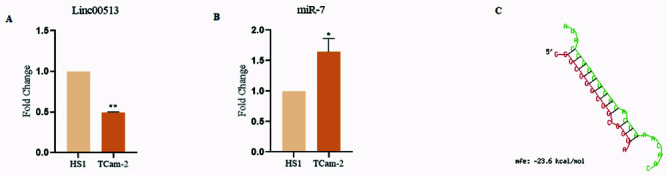
Unveiling the melodious interplay of Linc00513, miR-7, and TGFBR1 in the silencing symphony of spermatogenesis. A) qRT-PCR analysis of Linc00513 expression in TCAM-2(Azoospermia cell line) and HS1 (Normal cell line) cell lines. The expression of Linc00513 was significantly lower in TCAM-2 cells compared to HS1 cells, suggesting a potential role for Linc00513 in the regulation of spermatogenesis; B) qRT-PCR analysis of miR-7 expression in TCAM-2 and HS1 testes. The expression of miR-7 was significantly higher in TCAM-2 testes compared to HS1 testes, suggesting an inverse relationship between Linc00513 and miR-7 expression in spermatogenesis; C) Computational analysis of miR-7 binding sites in the TGFBR1 gene. A predicted binding site for miR-7 was identified within the 3’ UTR of the TGFBR1 gene, suggesting that miR-7 could potentially regulate TGFBR1 expression and activity.
Figure 3.
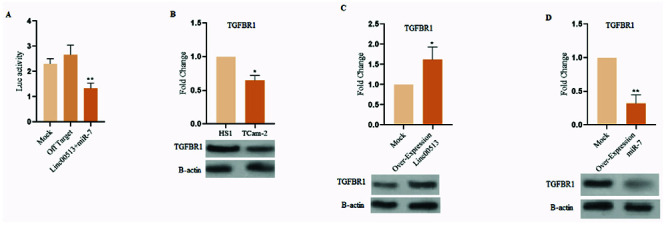
Unmasking the functional role of Linc00513 in spermatogenesis. A) Luciferase reporter assay analysis of Linc00513’s effect on TGFBR1 expression. Co-transfection of Linc00513 with the TGFBR1 3’ UTR resulted in a significant increase in luciferase activity compared to TGFBR1 3’ UTR alone, suggesting that Linc00513 suppresses miR-7-mediated repression of TGFBR1; B) Western blot analysis of TGFBR1 protein levels in azoospermic testes. TGFBR1 protein expression was significantly down regulated in TCam-2 cell line compared to HS1 cell line, suggesting that miR-7 may be overactive in azoospermia; C) Western blot analysis of Linc00513 overexpression in TCam-2 cell line. Overexpression of Linc00513 in TCam-2 cells resulted in a significant increase in TGFBR1 protein expression, similar to the effect observed in HS1 cells; D) Western blot analysis of miR-7 overexpression in HS1 cells. Overexpression of miR-7 in HS1 cells resulted in a significant decrease in TGFBR1 protein expression.
Discussion
Our investigation ventured deep into the labyrinthine network of Linc00513, miR-7, and the TGF-β signaling pathway in azoospermia,14 offering compelling insights and igniting new avenues for exploration.15 Here, we delve into the intricacies of our findings, weaving together their implications for understanding and potentially influencing this challenging condition. The downregulation of Linc00513 in azoospermic testes points towards its potential involvement in the disrupted spermatogenesis observed in this condition.16 Its localization in spermatogonia and Sertoli cells further hints at its potential roles in both sperm precursor proliferation and testicular microenvironment maintenance.17 Future studies employing Linc00513 gain-and loss-of-function models in vivo could elucidate its precise contributions to specific stages of spermatogenesis, shedding light on its potential as a therapeutic target. The inverse relationship between Linc00513 and miR-7 suggests a dynamic interplay with important consequences. Our finding that Linc00513 acts as a ceRNA, sponging miR-7 and preventing its repression of TGFBR1, opens exciting avenues for understanding the intricate regulation of the TGF-β signaling pathway in azoospermia.18 Further investigations aimed at identifying other potential miR-7 target genes related to spermatogenesis would further expand our understanding of the downstream effects of this axis.19 Luciferase reporter assays, Western blot analysis, and cell proliferation and apoptosis assays provided crucial functional validation, solidifying the regulatory relationship between Linc00513, miR-7, and the TGF-β signaling pathway. These findings suggest that Linc00513 may promote spermatogenesis by promoting cell proliferation and protecting spermatogonia from apoptosis through its miR-7 sponging activity.20 However, additional work employing in vivo models is necessary to definitively establish a causal role for this axis in azoospermia pathogenesis.21 The integration of online RNA-Seq data and TCam-2 cell culture experiments significantly strengthens our findings. Meta-analysis confirmed the generalizability of our identified differentially expressed genes, while pathway enrichment analysis provided valuable insights into the broader biological context of our findings. TCam-2 experiments shed light on the potential implications of this axis in semi-noma-associated azoospermia, opening doors for future research avenues. Our findings pave the way for potential therapeutic interventions targeting the Linc00513-miR-7-TGF-β axis. Strategies aimed at up regulating Linc00513 expression or inhibiting miR-7 activity could potentially offer novel avenues for promoting spermatogenesis and fertility restoration in azoospermia patients. However, further investigations are crucial to evaluate the safety and efficacy of these approaches in preclinical and clinical settings. Furthermore, exploring downstream targets of miR-7 beyond TGFBR1 could reveal additional key regulatory nodes within this network.22 Additionally, investigating the potential cross-talk between this axis and other signaling pathways involved in spermatogenesis could offer a more holistic understanding of this complex process.23 Our study, while shedding light on this intricate biological network,24 faces limitations. The sample size may not be fully representative of all azoospermia patients, and further studies with larger cohorts are needed to solidify our findings. Additionally, the specific mechanisms by which Linc00513 might influence other cellular processes beyond TGF-β signaling remain to be elucidated.
In the study of Wu et al., it was shown that the expression of some miRs including miR-141, miR-429 and miR-7-1-3p was increased in patients with azoospermia, while the expression of TGF-β was decreased.25 In another study, it was shown that the serum level of TGF-β was decreased in infertile men compared to normal subjects.26 In line with these studies, it was shown in previous findings that TGF-β plays an important role in the production and development of sperm cells.27
In the present study, it was shown that the expression level of TGF-β was decreased in cell lines, while the expression level of miR-7 was increased.
In previous studies, it was shown that Linc00513 can directly regulate the expression of TGF-β.28 While it was shown that TGF-β can also affect the expression of Linc00513.29 Therefore, these two factors have a mutual effect on each other.
Another important point is the molecular patterns caused by genes that can cause between histopathological forms of azoospermia. Based on this, the identified miRs and genes involved in the occurrence of these histopathological forms can be different. Different genes activate different molecular and genetic pathways.31 Therefore, the identification of each of the molecular pathways can be a diagnostic factor in the diagnosis of pathogenesis pathways and the use of therapeutic strategies for the treatment of patients through histopathological forms.30,31
Our investigation unveils a captivating landscape within the labyrinth of azoospermia, exposing the intricate tango between Linc00513, miR-7, and the TGF-β signaling pathway. These findings open exciting avenues for future research, holding the potential to translate fundamental discoveries into therapeutic strategies for improving fertility in affected individuals. By venturing deeper into this labyrinth, we inch closer to illuminating the path towards brighter outcomes for those struggling with azoospermia.
Acknowledgements
The authors appreciate and thank the efforts of the Center for the Development of Clinical Researches of the Educational and Therapeutic Research Complex of Birjand University of medical science.
List of acronyms
- ceRNA
competing endogenous RNA.
- miR-7
microRNA-7.
- TGFBR1
TGF-β receptor 1.
- lncRNAs
long non-coding RNAs.
- TGF-β
transforming growth factor-β.
- DEGs
differentially expressed genes.
- qRT-PCR
Quantitative Real-Time PCR.
- UTR
untranslated region.
Footnotes
Conflict of interest
The authors declare no potential conflict of interest, and all authors confirm accuracy.
Contributor Information
Atoosa Etezadi, Email: dratoosaetezadi@gmail.com.
Adere Akhtare, Email: akhtarsaadere1991@gmail.com.
Zahra Asadikalameh, Email: zasadik66@gmail.com.
Zeinab Hashem Aghaei, Email: zeynabhashemaghaee90@gmail.com.
Paria Panahinia, Email: panahiniap@gmail.com.
Mozhgan Arman, Email: mozhiarman@gmail.com.
Amene Abtahian, Email: abtahian.a89@yahoo.com.
Fereshteh Faghih Khorasani, Email: fereshte_faghih@gmail.com.
Vajihe Hazari, Email: dr.vhazari@gmail.com.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
- 1.Karavolos S. Diagnosing" his" infertility: men’s experiences and reflections on the diagnosis of azoospermia. 2016, Newcastle University. [Google Scholar]
- 2.Demyashkin GA, Borovaya TG, Andreeva YY, et al. An experimental approach to comprehend the influence of platelet rich growth factors on spermatogenesis. Int J Radiat Biol 2022;98:1330-43. [DOI] [PubMed] [Google Scholar]
- 3.Demyashkin GA, Kogan E, Demura T, et al. Immuno-histochemical analysis of spermatogenesis in patients with SARS-CoV-2 Invasion in different age groups. Curr Issues Mol Biol 2023;45:2444-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson GM. Men’s Experience of miscarriage: coping with the loss of a life and future. PhD Thesis, Liberty University; 2023. [Google Scholar]
- 5.Najmi M, Ayari MA, Sadeghsalehi H, et al. Estimating the dissolution of anticancer drugs in supercritical carbon dioxide with a stacked machine learning model. Pharmaceutics 2022;14:1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usman M, Li A, Wu D, et al. The functional role of lncRNAs as ceRNAs in both ovarian processes and associated diseases. Noncoding RNA Res 2023;9: 165-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 2021;22:96-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary S, Armstrong DT, Robertson SA. Transforming growth factor-β (TGFβ) in porcine seminal plasma. Reprod Fertil Dev 2011;23:748-58. [DOI] [PubMed] [Google Scholar]
- 9.Massagué J, Sheppard D. TGF-β signaling in health and disease. Cell 2023;186:4007-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei H, Faghihloo E. Viruses as key modulators of the TGF-β pathway; a double-edged sword involved in cancer. Rev Med Virol 2018;28:e1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 2008;24:263-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov 2012;11:860-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezaei M, Rahmani E, Khouzani SJ, et al., Role of artificial intelligence in the diagnosis and treatment of diseases. Kindle 2023;3:1-160. [Google Scholar]
- 14.Yang Z, Zhang X, Chen Z, Hu C. Effect of Wuzi Yanzong on reproductive hormones and TGF-β1/smads signal pathway in rats with oligoasthenozoospermia. Evid Based Complement Alternat Med 2019;2019:7628125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaksen A, Trippl M. Exogenously led and policy-supported new path development in peripheral regions: Analytical and synthetic routes. Econ Geogr 2017;93: 436-57. [Google Scholar]
- 16.Nishimura H, L’Hernault SW. Spermatogenesis. Curr Biol 2017;27:R988-94. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Yao C, Xing X, et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun 2020;11:5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martirosyan YO, Silachev DN, Nazarenko TA, et al. Stem-cell-derived extracellular vesicles: unlocking new possibilities for treating diminished ovarian reserve and premature ovarian insufficiency. Life (Basel) 2023;13: 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed K, LaPierre MP, Gasser E, et al. Loss of microRNA-7a2 induces hypogonadotropic hypogonadism and infertility. J Clin Invest 2017;127:1061-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastgar Rezaei Y, Zarezadeh R, Nikanfar S, et al. microRNAs in the pathogenesis of non-obstructive azoospermia: the underlying mechanisms and therapeutic potentials. Syst Biol Reprod Med 2021;67:337-53. [DOI] [PubMed] [Google Scholar]
- 21.Cerván-Martín M, Castilla JA, Palomino-Morales RJ, Carmona FD. Genetic Landscape of Nonobstructive Azoospermia and New Perspectives for the Clinic. J Clin Med 2020;9:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie H, Wang Y, Liao Z, et al. The function and mechanism of circular RNAs in gastrointestinal tumours. Cell Prolif 2020;53:e12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JM, Li ZF, Yang WX. What does androgen receptor signaling pathway in sertoli cells during normal spermatogenesis tell us? Front Endocrinol 2022;13: 838858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alm E, Arkin AP. Biological networks. Curr Opinion Structural Biol 2003;13:193-202. [DOI] [PubMed] [Google Scholar]
- 25.Wu W, Qin Y, Li Z, et al. Genome-wide microRNA expression profiling in idiopathic non-obstructive azoospermia: significant up-regulation of miR-141, miR-429 and miR-7-1-3p. Hum Reprod 2013;28:1827-36. [DOI] [PubMed] [Google Scholar]
- 26.Al-Naqshbandi AA, Nafee Darogha S, Asaaf Maulood K. Genotypic and allelic prevalence of the TGF- Β1 +869 C/T SNP and their relationship to seminogram in infertile males. Rep Biochem Mol Biol 2023;12: 318-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P, Joshi M, Singh R. TGF-β signaling in testicular development, spermatogenesis, and infertility, molecular signaling in spermatogenesis and male infertility. 2019, CRC Press. p. 105-115. [Google Scholar]
- 28.Quaife NM, Chothani S, Schulz JF, et al. LINC01013 is a determinant of fibroblast activation and encodes a novel fibroblast-activating micropeptide. J Cardiovasc Transl Res 2023;16:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papoutsoglou P, Pineau R, Leroux R, et al. TGFβ-induced long non-coding RNA LINC00313 activates Wnt signaling and promotes cholangiocarcinoma. EMBO Rep 2024;25:1022-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Zhang Y, Zhao M, et al. MicroRNA expression profiles in the seminal plasma of nonobstructive azoospermia patients with different histopathologic patterns. Fertil Steril 2021;115:1197-211. [DOI] [PubMed] [Google Scholar]
- 31.Cannarella R, Bertelli M, Condorelli RA, Vet al. Analysis of 29 targeted genes for non-obstructive azoospermia: the relationship between genetic testing and testicular histology. World J Mens Health 2023;41: 422-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


