Abstract
Background
Walking impairment after stroke is associated with substantial limitations in functional independence, quality of life, and long-term survival. People in the subacute phase after stroke who are unable to walk are most likely to benefit the greatest from use of overground robotic gait training (RGT). This study will provide preliminary evidence regarding the clinical use and efficacy of RGT during the subacute phase of stroke recovery as well as observational findings associated with the safety, tolerability, feasibility, and cost of delivering RGT during inpatient stroke rehabilitation.
Methods
This prospectively registered randomized controlled trial will enroll 54 patients admitted to inpatient rehabilitation within six months of stroke. Admitted patients will be screened at admission to inpatient rehabilitation for eligibility. Consented patients will be randomized based on stroke severity to receive either RGT or usual care for 90 minutes per week of gait training intervention during inpatient rehabilitation length of stay. Patients will complete assessments on walking and health outcomes at admission and discharge from inpatient rehabilitation and at 1- and 3-month follow-up. Intent-to-treat and per protocol analysis will be performed to evaluate safety [rate of adverse events, visual analog scale, and treatment completion rate], walking function [gait speed via 10-Meter Walk Test, Functional Ambulation Category, gait endurance via 6-Minute Walk Test] and health outcomes [Modified Rankin Scale, Stroke Rehabilitation Assessment of Movement, Continuity Assessment Record and Evaluation Tool, 5 Times Sit-to-Stand Test, Berg Balance Scale, and Stroke Impact Scale–16], and cost-analysis.
Discussion
This study will provide foundational evidence regarding the clinical use and efficacy of a RGT program during the subacute phase of stroke recovery with specific findings associated with the safety, tolerability, feasibility, and cost-analysis of delivering RGT during inpatient stroke rehabilitation.
Trial registration
Keywords: Robotic exoskeleton, Brain injury, Neurological rehabilitation, Stroke, Functional recovery
Background
Stroke is estimated to affect 6.6 million Americans, with about 795,000 new cases annually [1, 2]. Gait impairment affects 65% to 80% of people after stroke and is associated with substantial limitations in functional independence, quality of life, and long-term survival [3, 4]. As the number of new cases of stroke is anticipated to climb with an aging population, the growth in related expenses will further increase the already burdensome US healthcare spending [5]. Although financial burden increases with the severity of the stroke, specialized stroke rehabilitation is known to reduce long-term disability and stroke-related costs [6]. Importantly, gait training is a highly prioritized component of specialized rehabilitation for clinicians and patients with stroke alike [6–8].
Overground robotic exoskeletons are a potentially viable technology for delivery of gait training for patients with acute stroke. People with chronic stroke who receive overground robotic gait training (RGT) in combination with physical therapy are more likely to achieve independent walking with greater endurance than people who receive usual care gait training [9]. Further, people in the subacute phase after stroke who are unable to walk are most likely to benefit the greatest from RGT [10]. Specifically, RGT is able to deliver twice the dosage of gait training in comparison to usual care gait training over the average inpatient rehabilitation length of stay [11]. Initial evidence suggests RGT may improve gait function more than usual care gait training approaches [12, 13].
The purposes of this project are to generate novel data regarding the safety, efficacy, and cost-analysis of delivering RGT for people with stroke during inpatient rehabilitation. Importantly, the RGT intervention in this study will be informed by key stakeholders (i.e., researchers, clinicians, people with stroke lived experience) and offers a foundational step to evaluate RGT early after stroke when the neuroplasticity potential is greatest [14]. To meet our study purposes, we established the following aims and hypotheses.
Aim 1
Evaluate the safety, tolerability, and feasibility of delivering RGT that meets the unique needs of people after stroke during inpatient rehabilitation. We will engage our Advisory Board of stakeholders living with stroke to contextualize safety, tolerability, and feasibility considerations of delivery of RGT, and help identify barriers and facilitators to intervention delivery.
Hypothesis 1.1
Delivering RGT therapy during inpatient rehabilitation will be safe, tolerable, and feasible.
Aim 2
Prospectively examine the efficacy of RGT compared to usual care gait training during inpatient rehabilitation in people with stroke.
Hypothesis 2.1
Patients with stroke randomized to receive RGT will demonstrate greater change in walking function at discharge and follow-up at 1 month and 3 months compared to usual care gait training.
Hypothesis 2.2
Patients with stroke randomized to receive RGT will have greater improvement in health outcomes at discharge and follow-up at 1 month and 3 months compared to usual care gait training.
Aim 3
Conduct a cost analysis of delivering RGT during inpatient rehabilitation on functional (walking function) and hospital (fall incidence) metrics compared to usual care gait training.
Hypothesis 3.1
Delivering RGT therapy during inpatient rehabilitation will be cost effective compared to usual care gait training relative to walking function at discharge, 1-month, and 3-month follow-ups.
Hypothesis 3.2
Delivering RGT therapy during inpatient rehabilitation will demonstrate a cost benefit compared to usual care gait training relative to fall incidence at discharge, 1-month, and 3-month follow-ups.
Methods
Design
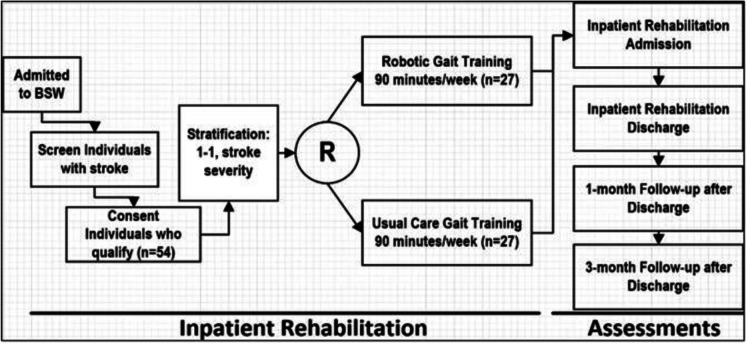
This prospective, randomized controlled trial will enroll patients within our inpatient rehabilitation hospital with stroke into one of two groups: RGT or usual care gait training. Patients will not be blinded to group allocation. Additionally, therapists providing RGT or usual care gait training and completing clinical assessments will not be blinded. However, research staff completing study-specific assessments will be blinded to group allocation. Both groups will complete four assessments at the following timepoints: (1) within seven days of study enrollment, (2) within seven days of discharge from inpatient rehabilitation, (3) one month (± 14 days) after discharge from inpatient rehabilitation, and (4) three months (± 14 days) after discharge from inpatient rehabilitation. Figure 1 details the study design.
Fig. 1.
Study design
To support recruitment, all admitted patients with stroke will be entered into the departmental “Stroke Tracking” database in REDCap for eligibility screening. Demographic information recorded in this database will be obtained from the medical record and include name, birthdate, stroke onset date, admission date, discharge date, and stroke type. The study obtained ethical approval from the local Institutional Research Ethical Committee (023–471) and was prospectively registered at the clinicaltrials.org, Registry: NCT06430632.
Participants
We intend to enroll 54 patients in this study. All patients with stroke will be screened within 24 h of admission to inpatient rehabilitation for the following inclusion criteria:
18–85 years of age;
All types of stroke. In this study, we will include people with stroke regardless of type or location;
Acute/subacute phase. We will include patients with stroke admitted for inpatient rehabilitation within 6 months post-stroke;
Medically stable as deemed by a physician;
Undergoing medical care and rehabilitation at a large urban inpatient rehabilitation hospital;
All genders, races, and ethnicities;
Meet the manufacturer robotic exoskeleton frame limitations;
Continence of or a program for bladder and bowel management;
Capacity and therapeutic goal for walking recovery
Patients will be excluded if they have:
Concurrent neurological diagnoses (e.g., spinal cord injury, degenerative disease, neoplasm)
Profound cognitive impairment;
Pregnancy
Randomization
Randomization will be computer-generated by a blinded researcher. Patients will be assigned to RGT or usual care gait training using 1:1 stratified blocks. Stratification will be based on stroke severity as determined by the modified Rankin Scale: mild (0–3), moderate (4), and severe (5) [15, 16]. Within each stratum, randomization blocks of size 6 will be used to ensure equal distribution between groups. The REDCap Randomization Module will be used to assign participants to their treatment group.
Intervention
Patients in the RGT group will receive 90 min per week of RGT using the EksoNR once patients are deemed clinically appropriate as defined by being able to tolerate standing for 10 min without orthostatic intolerance. The 90 min of RGT will be integrated within the physical therapy plan of care and fall within the Centers for Medicare and Medicaid Services 3-h rule [17].
During the first RGT session, the patient will be fitted for the EksoNR. RGT will be initiated immediately thereafter. While each training session may vary in length due to medical acuity and patient tolerance, RGT will total 90 min/week. RGT intervention progression will be individualized to accommodate stroke severity characteristics and variable rates of functional recovery. The approach to progression of RGT will follow these sequential steps: (1) familiarization with EksoNR stepping and weight shift pattern, (2) standing tasks > walking tasks, (3) walking tasks > standing tasks, (4) program reduced assistance provided by device during walking, (5) program zero assistance provided by device during walking, (6) program resistance provided by device during walking. This progression is based on instruction during manufacturer training, our therapists’ clinical experience and published guidelines [18, 19]. Data for each RGT training session will be recorded including frequency, time standing, time walking, and number of steps. Physical therapists have been trained to use the robotic exoskeleton for mobility and gait training, successfully completing manufacturer Level I (basic) and Level II (advanced) training.
Usual care gait training will adhere to the current stroke-specific clinical practice rehabilitation guidelines. These guidelines recommend body weight support treadmill training (BWSTT) as an option for gait training in addition to conventional overground walking, dependent on resource availability, context, and local expertise [20]. Therapists are trained and proficient in providing usual care gait training interventions to patients with stroke. Patients in the usual care gait training group will receive 90 min per week of usual care gait training.
Outcomes (Table 1)
Table 1.
Outcome measures
| Aim 1 | Safety, tolerability, and feasibility | Metrics include safety (rate of adverse events [e.g., fall incidence, hypotensive episode, skin integrity]), tolerability (visual analog scale of 1 [not tolerable] – 10 [tolerable]), and feasibility (treatment completion rate: proportion of patients completing at least 90% of the inpatient RGT sessions). |
| Aim 2 | Gait speed via 10-Meter Walk Test (10MWT) | Our primary outcome, the 10MWT, assesses gait speed over a short duration. Gait speed (m/s) is correlated with ability to mobilize in the community, capacity to perform activities of daily living, and risk of falls, re-hospitalization, and cognitive decline [21].Score changes >0.16 m/s exceed the MCID [22]. Normal gait speed for adults older than 50 years is >1.27 m/s [23]. |
| Functional Ambulation Category (FAC) | The FAC assesses functional ambulation in patients undergoing rehabilitation and has excellent reliability, good predictive validity, and good responsiveness in patients with stroke [24]. Scores range from 0 (unable to walk) to 5 (independent walking anywhere). After 4 weeks of rehabilitation, FAC scores ≥4 predict community ambulation at 6 months with 100% sensitivity and 78% specificity [25]. | |
| 6-Minute Walk Test (6MWT) | The 6MWT assesses distance walked over 6 minutes as a sub-maximal test of walking capacity. With excellent test-retest reliability (ICC = 0.99) [26] for people with stroke, the established MCID is 34.4 meters [27]. | |
| Gait quality | Gait quality will be measured weekly on a visual analog scale from 1 (“my walking is the worst it has ever been”) to 10 (“my walking is just like before my stroke”). | |
| Modified Rankin Scale (mRS) | The mRS measures the degree of disability or dependence in the daily activities of people who have had a stroke. The mRS is an ordinal scale with 6 categories ranging from 0 (no symptoms) to 5 (complete physical dependence) [28]. | |
| Stroke Rehabilitation Assessment of Movement (STREAM) | The STREAM [28,29] assesses upper and lower limb motor function along with basic mobility in people with stroke and has a very high inter-rater reliability (ICC = 0.96) [30]. MCID values have been established for the upper extremity (2.2 points), lower extremity (1.9 points), and mobility (4.8 points) subscales [31]. | |
| Continuity Assessment Record and Evaluation (CARE) | The Section GG CARE Tool is utilized in post-acute care settings for tracking progress across the continuum of care and is conducted at admission and discharge. The CARE addresses self-care (GG0130, 8 items) and functional mobility (GG0170, 17 items). Scores for each item range from 1 (dependent) to 6 (independent). Total scores for the CARE have strong positive correlations with total scores for the Functional Independence Measure [24]. | |
| 5 Times Sit-to-Stand Test (5TSST) | The 5TSST assesses lower extremity strength and is an indicator of postural control [32]. People with stroke who score >15 seconds are considered at risk for falls [33, 34]. Normal scores for individuals aged 60-80 years range from 11.4 to 12.7 seconds [35]. The 5TSST has demonstrated excellent test-retest reliability (ICC = 0.95) with an established MDC95of 2.3 seconds [36]. | |
| Berg Balance Scale (BBS) | The BBS [37] is a 14-item objective measure that assesses static balance and fall risk in adults. With excellent reliability (ICC = 0.95) [38], the BBS has a large responsiveness for acute stroke (effect size = 0.85) [39] and a minimal detectable change of 6.9 points [40]. Scores <45/56 indicate a risk of falling [41]. | |
| Pain | Pain will be assessed following each RGT and UC session using a pain visual analog scale [19], as per standard of care in our inpatient rehabilitation hospital. | |
| Stroke Impact Scale – 16 (SIS-16) | The SIS-16 assesses 4 dimensions of health-related QOLs specific to people who have had a stroke. Included subscales assess strength, hand function, mobility, and activities of daily living via 5-point Likert scales [42, 43]. | |
| Aim 3 | Cost analysis | Cost-effectiveness will be assessed by comparing RGT to UC relative to walking function as measured by the MCID of the 10MWT (>0.16 m/s). Cost-benefit will be assessed by comparing RGT to UC relative to fall incidence. |
Data monitoring body
The study’s data monitoring body is responsible for designing and maintaining all data collection tools and the study database. Study data are collected and managed using REDCap electronic data capture tools hosted at the local research institution. Following institutional policies and study-specific data management plan, the data monitoring body also conducts quarterly quality assurance checks of all study-related data and is responsible for cleaning the data prior to the final data analysis.
Sample size estimation
We will enroll 54 participants in this study. Sample size calculations were performed based on a test for two means in a repeated measures design with 4 follow-up measures and an autoregression correlation structure. Assuming an autocorrelation of 0.55 and a standard deviation of 0.25,84 we will need 22 participants per group to detect a clinical meaningful difference of 0.16 m/s with 80% power at a 5% significance level. We will plan for an attrition rate of up to 20% and enroll 27 in each group for a total of 54 participants.
Statistical analyses
All analyses will be performed using SAS 9.4 with a 5% significance level. Intent-to-treat and per protocol analysis will be performed. For intent-to-treat analysis participants who completed assessments will be included in the analysis, regardless of the number of RGT sessions completed. The intent-to-treat analysis compares across groups and participants must have completed inpatient admission and outpatient assessments. Per protocol analysis will only include participants who complete the full allotment of RGT sessions assigned through randomization, and who complete inpatient and outpatient assessments. Incomplete assessments will be treated as missing data.
To evaluate safety, tolerance and feasibility will primarily be descriptive in nature by reporting the rate of adverse events, the average pain score based on the visual analog scale, and the treatment completion rate. Additionally, to compare the number of adverse events during inpatient rehabilitation and number of incomplete treatments across groups, simple Poisson regression will be used with the total count of events per person as the outcome and length of inpatient stay included as an offset variable. The average pain rating will be calculated for each person, and comparisons between groups will be conducted using Kruskal–Wallis tests. For participants who are unable to complete the intervention, we will compare demographic and clinical characteristics to assess association with inability to complete RGT during inpatient rehabilitation. This analysis will be performed using t-tests or Mann–Whitney U tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables.
To assess walking function over time, general or generalized mixed models will be conducted for each measure. The choice between a general or generalized model will be based on the distribution of the outcome measure. Each model will include a fixed group and time effect and a group x time interaction to determine if the groups changed differently over time. A random effect will be used to account for repeated measures over time. Health outcomes will be tested using similar mixed effects models. Participant data will be included in the model if at least one follow-up is complete. All models will be adjusted for any demographic or clinical characteristics that were not balanced between groups through randomization.
To determine the cost effectiveness of RGT therapy during inpatient rehabilitation we will calculate the ratio of total therapy time cost during inpatient to improvement in walking function measured by 10MWT from admission to 3-month follow-up (Hypothesis 3.1). This ratio will provide the cost per unit of benefit which will then be compared between groups. Since cost data are often skewed, we will use a log-normal model for analysis and control for patient demographic and clinical characteristics that were not balanced through the randomization process, as well as adjust for the baseline function. To assess potential savings in health care costs due to reduction in number of falls post stroke we will compare the proportion of participants with a fall from admission to 3-month follow-up between treatment groups. Logistic regression will be used for this analysis and the model will adjust for demographic and clinical characteristics that were not balanced through the randomization process.
Discussion
This trial will provide foundational evidence regarding the clinical use and efficacy of RGT during the subacute phase of stroke recovery with specific findings associated with the safety, tolerability, feasibility, and cost-analysis of delivering RGT during inpatient stroke rehabilitation. Currently, there are gaps in the evidence base for RGT related to both clinical benefits and the associated costs, emphasizing the need for systematic research. Best practice in stroke rehabilitation favors a repetitive task-specific approach [44], yet independent community walking and full community reintegration 3-months post-stroke are achieved by less than 65% [45] and 20% [46] of people with stroke, respectively. Moreover, the most effective usual care gait training approach remains unclear [47] and lacks a strong evidence base for people with stroke [48, 49]. Findings from this study will advance the understanding of the potential role of a stakeholder informed RGT program delivered during inpatient rehabilitation for people with subacute stroke. Important foundational data of clinical use (safety, tolerability, feasibility), evidence of effectiveness, and cost of delivering RGT compared to usual care gait training during inpatient rehabilitation will be ascertained. Study findings will be disseminated through peer-reviewed publications, scientific presentations, and consumer-focused fact sheets.
Trial status
Institutional Review Board approvals were obtained on February 02, 2024. This protocol paper reflects the study protocol version 1.2 created in May 2024. The clinical trial was registered at ClinicalTrials.gov (NCT0643063). Recruitment and enrollment was initiated March 1, 2024. At the time of manuscript submission, the expected duration of the study, including enrollment and statistical analysis, will be 3 years. The approximate date of planned recruitment completion is June 2026.
Acknowledgements
None declared.
Abbreviations
- 5TSST
5 Times Sit-to-Stand Test
- 6MWT
6-Minute Walk Test
- 10MWT
10-Meter Walk Test
- BBS
Berg Balance Scale
- BWSTT
Body-weight-supported treadmill training
- CARE
Continuity Assessment Record and Evaluation
- CMS
Centers for Medicare and Medicaid Services
- FAC
Functional Ambulation Category
- IRB
Institutional review board
- LAR
Legally authorized representative
- MCID
Minimal clinically important difference
- mRS
Modified Rankin Scale
- RCT
Randomized controlled trial
- RGT
Robotic gait training
- SIS-16
Stroke Impact Scale – 16
- STREAM
Stroke Rehabilitation Assessment of Movement
Authors’ contributions
F.Z.A. and S.A. wrote the main manuscript text J.G. and R.D. wrote original draft and collect data C.O., T.G., and M.B. review, editing and Statistical Analysis S.D. Wrote original draft and Conceptualization and final edition C.S. Chief Investigator, led the proposal and protocol development, Analysis and Writing – review & editing All authors reviewed the manuscript.
Funding
The contents of this manuscript were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant number 90IFRE0074). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this manuscript do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
Availability of data and materials
De-identified data will be made available in Excel/CSV files and will be divided into subject survey/biometric data, session attendance and tracking data, medication data, and adverse events data. No identifiable information will be included. All elements of date will be calculated (e.g., age, time since injury), and dates will be deleted from the de-identified dataset. Raw data will be stored in a double locked filing cabinet or on password protected data sheets for at least three years after study close out. All data will be sent to ICPSR and made available to interested parties at no cost no later than 24 months after the award’s end date. The dataset will be assigned a Digital Object Identifier (DOI) for future reference and citation.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study obtained ethical approval from the Institutional Research Ethical Committee (023–471) of the Baylor Scott & White Research Institute, Dallas, TX, USA, which means all study procedures remain in accordance with the Declaration of Helsinki concerning Ethical Principles for Medical Research Involving Human Subjects. Informed consent will be obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–743. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinear CM, Lang CE, Zeiler S, et al. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348–60. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BH, Bonafede MM, Watson C. Short-and longer-term health-care resource utilization and costs associated with acute ischemic stroke. Clinicoecon Outcomes Res. 2016;8:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroke Foundation. Clinical guidelines for stroke management—chapter 5 of 8: rehabilitation. 2020. https://files.magicapp.org/guideline/06ee1655-b8ca-4f47-b468-41306dc8efc5/published_guideline_3995-6_0.pdf.
- 6.Grau-Pellicer M, Chamarro-Lusar A, Medina-Casanovas J, Serdà Ferrer BC. Walking speed as a predictor of community mobility and quality of life after stroke. Top Stroke Rehabil. 2019;26(5):349–58. [DOI] [PubMed] [Google Scholar]
- 7.Vahlberg B, Cederholm T, Lindmark B, Zetterberg L, Hellström K. Factors related to performance-based mobility and self-reported physical activity in individuals 1–3 years after stroke: a cross-sectional cohort study. J Stroke Cerebrovasc Dis. 2013;22(8):e426–34. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento LR, Gaviorno LF, de Souza Brunelli M, Goncalves JV, da Areas FZ S. Home-based is a effective as centre-based rehabilitation for improving upper limb motor recovery and activity limitations after stroke: A systematic review with meta-analysis. Clinical Rehabilitation. 2022;36(12):1565–77. [DOI] [PubMed] [Google Scholar]
- 9.Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurological research and practice. 2020Jun 16;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39(5):835–41. [DOI] [PubMed] [Google Scholar]
- 11.Nolan KJ, Karunakaran KK, Chervin K, Monfett MR, Bapineedu RK, Jasey NN, Oh-Park M. Robotic Exoskeleton Gait Training During Acute Stroke Inpatient Rehabilitation. Front Neurorobot. 2020;14:581815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPasquale J, Trammell M, Clark K, et al. Intensity of usual care physical therapy during inpatient rehabilitation for people with neurologic diagnoses. PM&R. 2021;14(1):46–57. [DOI] [PubMed] [Google Scholar]
- 13.Rangaraju S, Haussen D, Nogueira RG, Nahab F, Frankel M. Comparison of 3-month stroke disability and quality of life across modified rankin scale categories. Interv Neurol. 2017;6(1–2):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drum CE, Peterson JJ, Culley C, Krahn G, Heller T, Kimpton T, McCubbin J, Rimmer J, Seekins T, Suzuki R, White GW. Guidelines and criteria for the implementation of community-based health promotion programs for individuals with disabilities. Am J Health Promot. 2009;24(2):93–101. [DOI] [PubMed] [Google Scholar]
- 15.Swank C, Galvan C, DiPasquale J, Callender L, Sikka S, Driver S. Lessons learned from robotic gait training during rehabilitation: Therapeutic and medical severity considerations over 3 years. Technol Disabil. 2020;32(2):103–10. [Google Scholar]
- 16.Louie DR, Mortenson WB, Durocher M, Teasell R, Yao J, Eng JJ. Exoskeleton for post-stroke recovery of ambulation (ExStRA): study protocol for a mixed-methods study investigating the efficacy and acceptance of an exoskeleton-based physical therapy program during stroke inpatient rehabilitation. BMC Neurol. 2020;20(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest, George MDAB, Reppel, Alycia MDC, Kodsi, Mina MSD, Smith, Joshua MDD. Inpatient rehabilitation facilities: The 3-hour rule. Medicine. 2019;98(37):p e17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical‐assisted training for walking after stroke. Cochrane Database Syst Rev. 2020;(10):CD006185. [DOI] [PMC free article] [PubMed]
- 19.Abdollahi M, Rashedi E, Jahangiri S, Kuber PM, Azadeh-Fard N, Dombovy M. Fall risk assessment in Stroke Suvirvors: a machine leaning model usaing detailed motion data from common clinical tests and motor-cognitive duas-tasking. Sensors. 2024;24(3):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell W, Lewis S. Visual analogue measurement of pain. Ulst Med J. 1990;59(2):149. [PMC free article] [PubMed] [Google Scholar]
- 21.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. Aging Phys Ac. 2015;23(2):314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26(1):15–9. [DOI] [PubMed] [Google Scholar]
- 24.Mehrholz J, Wagner K, Rutte K, Meiβner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007;88(10):1314–9. [DOI] [PubMed] [Google Scholar]
- 25.Li C-Y, Mallinson T, Kim H, Graham J, Kuo Y-F, Ottenbacher KJ. Characterizing Standardized Functional Data at Inpatient Rehabilitation Facilities. J Am Med Dir Assoc. 2022;23(11):1845–53.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. Mar2005;37(2):75–82. [DOI] [PubMed] [Google Scholar]
- 27.Tang A, Eng J, Rand D. Relationship between perceived and measured changes in walking after stroke. J Neurol Phys Ther. 2012;36(3):115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40(1):1–8. [DOI] [PubMed] [Google Scholar]
- 29.Daley K, Mayo N, Danys I, Cabot R, Wood-Dauphinee S. The Stroke Rehabilitation Assessment of Movement (STREAM): refining and validating the content. Physiother Can. 1997;49(4):269–78. [Google Scholar]
- 30.Wang C-H, Hsieh C-L, Dai M-H, Chen C-H, Lai Y-F. Inter-rater reliability and validity of the stroke rehabilitation assessment of movement (stream) instrument. J Rehabil Med. 2002;34(1):20–4. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh Y-W, Lin J-H, Wang C-H, Sheu C-F, Hsueh I, Hsieh C-L. Discriminative, predictive and evaluative properties of the simplified stroke rehabilitation assessment of movement instrument in patients with stroke. J Rehabil Med. 2007;39(6):454–60. [DOI] [PubMed] [Google Scholar]
- 32.Newcomer K, Krug H, Mahowald M. Validity and reliability of the timed-stands test for patients with rheumatoid arthritis and other chronic diseases. J Rheumatol. 1993;20(1):21–7. [PubMed] [Google Scholar]
- 33.Cheng P-T, Liaw M-Y, Wong M-K, Tang F-T, Lee M-Y, Lin P-S. The sit-to-stand movement in stroke patients and its correlation with falling. Arch Phys Med Rehabil. 1998;79(9):1043–6. [DOI] [PubMed] [Google Scholar]
- 34.Buatois S, Perret-Guillaume C, Gueguen R, et al. A simple clinical scale to stratify risk of recurrent falls in community-dwelling adults aged 65 years and older. Phys Ther. Apr2010;90(4):550–60. 10.2522/ptj.20090158;10.2522/ptj.20090158. [DOI] [PubMed] [Google Scholar]
- 35.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. PerceptMotSkills. Aug2006;103(1):215–22. [DOI] [PubMed] [Google Scholar]
- 36.Meretta BM, Whitney SL, Marchetti GF, Sparto PJ, Muirhead RJ. The five times sit to stand test: responsiveness to change and concurrent validity in adults undergoing vestibular rehabilitation. J Vestib Res. 2006;16(4, 5):233–43. [PubMed] [Google Scholar]
- 37.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(Suppl 2):S7–11. [PubMed] [Google Scholar]
- 38.Mao H-F, Hsueh I-P, Tang P-F, Sheu C-F, Hsieh C-L. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke. 2002;33(4):1022–7. [DOI] [PubMed] [Google Scholar]
- 39.Chou C-Y, Chien C-W, Hsueh I-P, Sheu C-F, Wang C-H, Hsieh C-L. Developing a short form of the Berg Balance Scale for people with stroke. Phys Ther. 2006;86(2):195–204. [PubMed] [Google Scholar]
- 40.Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Aust J Physiother. 2001;47(1):29–38. [DOI] [PubMed] [Google Scholar]
- 41.Doğğan A, Mengüllüoğlu M, Özgirgin N. Evaluation of the effect of ankle-foot orthosis use on balance and mobility in hemiparetic stroke patients. Disabil Rehabil. 2011;33(15–16):1433–9. [DOI] [PubMed] [Google Scholar]
- 42.Salter KL, Moses MB, Foley NC, Teasell RW. Health-related quality of life after stroke: what are we measuring? Int J Rehabil Res. 2008;31(2):111–7. [DOI] [PubMed] [Google Scholar]
- 43.Duncan PW, Bode RK, Lai SM, Perera S. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84(7):950–63. [DOI] [PubMed] [Google Scholar]
- 44.French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, Sutton CJ, Tishkovskaya S, Watkins CL. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2016;11(11). [DOI] [PMC free article] [PubMed]
- 45.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004Feb;85(2):234–9. [DOI] [PubMed] [Google Scholar]
- 46.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995Jun;26(6):982–9. [DOI] [PubMed] [Google Scholar]
- 47.Belda-Lois JM, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, Iosa M, Molinari M, Tamburella F, Ramos A, Caria A, Solis-Escalante T, Brunner C, Rea M. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil. 2011Dec;13(8):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, Cen SY, Duncan PW, LEAPS Investigative Team. Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS Trial. Neurorehabil Neural Repair. 2013;27(4):370–80. [DOI] [PubMed] [Google Scholar]
- 49.Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352(16):1677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data will be made available in Excel/CSV files and will be divided into subject survey/biometric data, session attendance and tracking data, medication data, and adverse events data. No identifiable information will be included. All elements of date will be calculated (e.g., age, time since injury), and dates will be deleted from the de-identified dataset. Raw data will be stored in a double locked filing cabinet or on password protected data sheets for at least three years after study close out. All data will be sent to ICPSR and made available to interested parties at no cost no later than 24 months after the award’s end date. The dataset will be assigned a Digital Object Identifier (DOI) for future reference and citation.
No datasets were generated or analysed during the current study.