Abstract
Background
Breast cancer (BC) rates have been increasing in young women in the U.S. Alcohol is an established risk factor for breast cancer and has been consistently associated with hormone receptor positive cancers, the type of breast cancer that has been increasing the fastest in young women. Given these trends, we conducted an ecological study to examine whether alcohol consumption, and specifically binge drinking trends, were correlated with female breast cancer diagnosed under 40 years of age using breast cancer data from the Surveillance, Epidemiology, and End Results (SEER) Cancer Registry. We accounted for a latent period before cancer diagnosis by using exposure 10 years before the outcome (lag model); we also conducted a separate cumulative analysis of 10-year aggregate exposure.
Findings
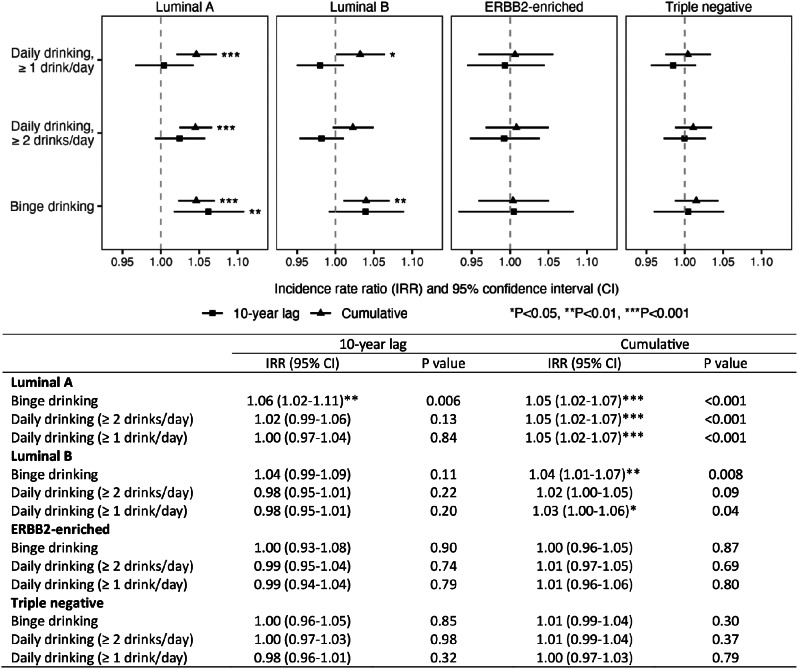
Moderate (Incidence Rate Ratio (IRR) = 1.05, 95% Confidence Interval (CI) = 1.02–1.07) and heavy (IRR = 1.05, 95% CI = 1.02–1.07)(≥ 1 and ≥ 2 drinks/day, respectively) alcohol consumption were each associated with Luminal A breast cancer but not the other molecular subtypes. Binge drinking was associated with an increased rate of early-onset Luminal A BC in both the 10-year lag model (IRR = 1.06, 95% CI = 1.02 to 1.11) and the cumulative model (IRR = 1.05, 95% CI = 1.02–1.07). Binge drinking was also associated with early-onset Luminal B BC in the cumulative model (IRR = 1.04, 95% CI = 1.01–1.07), but not associated with ERBB2-enriched or triple negative early-onset BC in either model.
Conclusion
These trends support the hypothesis that one reason for the increase in early-onset breast cancer is from increased alcohol intake including binge drinking.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01894-7.
Keywords: Breast cancer, Alcohol, Binge drinking, Early onset breast cancer, Luminal A
Background
Alcohol is an established risk factor for breast cancer (BC) and is recognized as such by the International Agency for Research on Cancer (IARC) as well as a risk factor for colorectal cancer, liver cancer, esophageal cancer, oral pharyngeal cancer and head and neck cancer [1, 2]. Epidemiological data supporting the role of alcohol in carcinogenesis have existed for decades (see, e.g., a meta-analysis for BC and alcohol intake in 1988) [3]. Organizations like the American Association of Clinical Oncology and the American Public Health Association have called for provider training [4], and labelling and other awareness campaigns [5], particularly for common cancers like breast and colorectal cancer where awareness of alcohol as a carcinogen is not as high by the general public as for the rarer alcohol-driven cancers (e.g., liver cancer [6]). In addition, to the role of alcohol in breast cancer risk, recently, IARC concluded that there is some, but limited, evidence to support that alcohol cessation and/or reduction decreases BC risk [2].
Early-onset BC has been increasing, including in young women under age 40 where the increase cannot be attributed to population-based BC screening [7, 8]. Just between 2004 and 2017, there was a 7% increase in invasive BC incidence in US women under the age of 40 years. Binge drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as 4 or more drinks on a single occasion for women and five or more for men, has also increased over this time [9]. Approximately two decades ago, men reported binge drinking over three times the rate of women overall and over five times the rate for 55 and older [10], but since then the rates have increased particularly in women such that in some age groups “parity” between the sexes has been achieved [11].
Given these trends, it is important to examine whether the recent increases in early-onset BC may be attributed to the increase in binge drinking. The challenge, however, is that most BC epidemiologic studies ask about regular drinking and drinks per week but have not specifically asked about binge drinking. Among the few studies that evaluated binge drinking [12–18], only three specifically examined associations by important strata relevant to current trends, namely by molecular subype and premenopausal BC [16–18], with two of these finding a significant association [16, 17]. The Sister Study [17] found that this association was specific to HR+ BC; consistent with the large literature on alcohol and breast cancer which supports more consistent increases for HR+ BC. Further, the Spanish study [16] showed that the association between binge drinking and BC was specific to premenopausal women rather than postmenopausal women. As most cohort studies are underpowered and case-control studies may face recall bias in reporting of alcohol behavior, we undertook an ecological study to examine the trends in binge drinking and breast cancer rates by molecular subtype over the last decade. Ecological trend analyses can provide important insights in testing hypotheses particularly for studies of early-onset cancers as most existing cohort studies are underpowered to examine cancer incidence in young adults [19–22].
Findings
Using data from the 17 registries of the Surveillance, Epidemiology, and End Results (SEER) Program, we computed age-adjusted (standardized to the 2000 US standard population) BC incidence rates for molecular subtypes as defined by SEER’s breast subtype variable (Luminal A, Luminal B, ERBB2-enriched (also known as HER2 or neu), and triple negative [23]) for 32 demographic subgroups (i.e., units for ecological analysis) defined by period, age, and race/ethnicity (2 periods [2010–2014 and 2015–2019] × 4 age groups [20–24, 25–29, 30–34, 35–39] × 4 race/ethnicity groups [Hispanic, non-Hispanic White, non-Hispanic Black, and Asian/Pacific Islander]). The study period (Year 2010–2019) was chosen because SEER BC data by subtypes were unavailable before 2010. For exposure variables, we used the Behavioral Risk Factor Surveillance System (BRFSS) data for women during 2000–2019 [24]. We also conducted sensitivity analyses using data after 2006 alone when the same definition for binge drinking was used. The BRFSS had separate questions on daily use and binge drinking. We used negative binomial regression to estimate associations between each exposure and each BC subtype, adjusting for age and race/ethnicity. We accounted for a latent period before cancer diagnosis by using exposure 10 years before the outcome (i.e., 10-year lagged analysis); we also conducted a cumulative analysis, using the sum of exposure over the 10 years before the outcome. In addition, we applied centering methods as described previously to remove the associations of the exposure with the demographic variables [20], and standardized all exposures (mean = 0; SD = 1). For sensitivity and descriptive analyses, we also used BC incidence data from SEER 9 [25], and data for alcohol consumption, body weight, parity, and age of menarche from the National Health and Nutrition Examination Survey (NHANES) [26]. All analyses were conducted using the R statistical language (version 4.2.1).
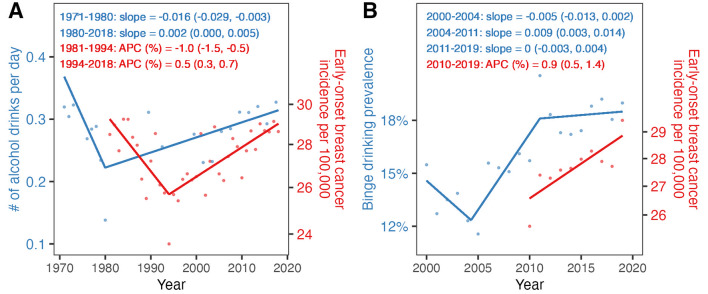
From 2010 to 2019, the incidence increased by 0.9% per year (95% confidence interval [CI] = 0.5–1.4%), and among those cases in the SEER17 registry, 51% were Luminal A, 17% were Luminal B, 7% were ERBB-enriched, 18% were triple negative, and 7% were of an unknown subtype. These increases mapped to overall alcohol consumption (Fig. 1A) and binge drinking (Fig. 1B). The prevalence of binge drinking increased from 2004 to 2011 (slope = 0.008, 95% CI = 0.004 to 0.013), with trends stabilizing from 2011 to 2019 (slope = 0.001, 95% CI = -0.003 to 0.004). The overall prevalence of binge drinking during 2000–2019 was 16.6%. As shown in Fig. 2, daily drinking was associated with Luminal A cancers in the cumulative model (incidence rate ratio [IRR] = 1.05, 95% CI = 1.02–1.07 and IRR = 1.05, 95% CI = 1.02–1.07, for ≥ 2 and ≥ 1 drinks per day, respectively). The associations with daily drinking for the other molecular subtypes were not statistically significant. Binge drinking was associated with an increased rate of early-onset Luminal A BC in both the 10-year lag model (IRR = 1.06, 95% CI = 1.02 to 1.11) and the cumulative model (IRR = 1.05, 95% CI = 1.02–1.07). Binge drinking was also associated with early-onset Luminal B BC in the cumulative model (IRR = 1.04, 95% CI = 1.01–1.07), but not associated with ERBB2-enriched or triple negative early-onset BC in either model. Results were consistent in sensitivity analyses (shorter time period but consistent definition for binge drinking over the entire period; Figure S1). Further, the results remained after adjusting for overweight and obesity (body mass index ≥ 25 kg/m2), as well as after further adjusting for parity and age of menarche (Figures S2-S3, respectively).
Fig. 1.
Trends of alcohol drinking and early-onset breast cancer (BC) incidence rates among 20-39-year-old women. All estimates were age-standardized to the US population in 2000. Panel A shows alcohol consumption estimates (blue dots) from the National Health and Nutrition Examination Survey (NHANES) and BC incidence (red dots) from SEER 9. Panel B shows binge drinking prevalence estimates (blue dots) from BRFSS, and BC incidence (red dots) from SEER 17. Regression models with break points were used to estimate annual percent changes (APCs) and 95% CIs (shown in red) for BC incidence, and slopes and 95% CIs (shown in blue) for alcohol drinking estimates
Fig. 2.
The association of binge and heavy alcohol drinking with BC subtypes among 20-39-year-old women. BC data came from SEER 17 (age: 20–39; period: 2010–2019). Alcohol data came from BRFSS since 2000 (to account for time lags between the exposure and cancer incidence). All exposure variables were centered and standardized
Conclusion
This study provides some of the first data supporting that binge drinking may be associated with early-onset BC incidence rates. Findings were most consistent for Luminal A BC, which accounted for about half of all cases in the analysis. Our findings suggest that the association between binge drinking may be specific to HR+ BC, which is consistent with findings from the Sister Study [17]. This is also consistent with the hypothesis that mechanisms linking alcohol consumption to increased BC risk may be hormonally driven. Animal studies of binge drinking have also demonstrated specific biological effects from binge drinking including DNA damage [27]. Yet, given that there has been limited research to date on the relationship between binge drinking and BC risk, additional studies are needed to further examine whether the association is dependent on molecular subtype.
The increased breast cancer risk in women < 40 years is not due to increased screening as population-based screening is not recommended until after this age. Early-onset BC is increasing across all racial and ethnic subgroups [8], but the highest mortality rates are still seen in Non-Hispanic Black and Hispanic women [8]. Although some have hypothesized that changes in lifestyle factors may play a role in these trends, it is important to note that unlike gastrointestinal cancers, early-onset BC has not been associated with childhood and adolescent obesity [28]. Nor is it due to only one risk factor. For example, we have shown in a systematic review that certain environmental chemicals are associated with early-onset BC [29]. We have also found that changes in parity cannot account for the increase in early-onset cancers using registry data for over 8 decades from the state of Connecticut [19] as well as data from over 185 countries over the last three decades [30]; adjustments for parity in this study did not change any of the findings. Two epidemiological cohort studies [16, 17] support that binge drinking is associated with BC and that this association may be stronger in HR+ BC cancer and premenopausal BC, respectively. Using nationally representative data, we confirm their findings and specifically extend this work to suggest that the increase in binge drinking may be one reason for the increase in early-onset BC diagnosed before age 40 years.
The design of our study has notable strengths compared to traditional ecological designs [21]. By leveraging exposure data up to 10 years before the outcome, the analysis allowed for a latent period before cancer diagnosis. We examined trends in various alcohol consumption habits and found the IRR estimates show a dose-response-like impact (i.e., greater IRRs for ≥ 2 than ≥ 1 drink/day and 10-year cumulative than 10-year lag; Fig. 2). These comprehensive estimates lend further support to the strength of the estimated associations. Moreover, the demographic centering approach helped address potential age-related and other confounding, which are common challenges in ecological studies [20]. We also were able to test two separate hypotheses about effects using lagged and cumulative models and found binge drinking was important under both models whereas daily drinking was limited to the cumulative models. Despite these strengths, we were limited by the lack of molecular subtype data prior to 2010 as well as the lack of individual-level data. Nevertheless, this study is an important first step towards understanding the role of binge drinking in the rise of early-onset BC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the dedicated staff who make SEER, BRFSS and other epidemiological data publicly available.
Abbreviations
- BC
Breast Cancer
- IRR
incidence rate ratio
- IARC
International Agency for Research on Cancer
- HR+
Hormone Receptor Positive
- HR-
Hormone Receptor Negative
- HER2
ERBB2 human epidermal growth factor receptor 2
Author contributions
All authors participated in the data interpretation and in paper writing. MBT had the idea for the study. JC conducted the analysis and statistical test. JC, RK, WY, and MBT designed the study and analytic plan.
Funding
This work was supported by the National Institutes of Health (R01CA257971 for Wan Yang, Mary Beth Terry) and the Breast Cancer Research Foundation.
Data availability
The datasets generated and/or analysed during the current study are publicly available from SEER (Surveillance Research Program NCI. Breast subtype (2010+). Breast Subtype (2010+) - SEER Documentation (cancer.gov) Available at: https://seer.cancer.gov/seerstat/databases/ssf/breast-subtype.html) and from the BRFSS (Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. In Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/brfss/annual_data/annual_data.htm).
Declarations
Ethics approval and consent to participate
This report used only de-identified public data sets which are made availabile by the cited websites.
Consent for publication
N/A as only used publicly available data sets.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer. Agents classified by the IARC monographs, volumes 1–125. March 3. 2020. https://monographs.iarc.who.int/agents-classified-by-the-iarc
- 2.Gapstur SM, Bouvard V, Nethan ST, et al. The IARC Perspective on Alcohol Reduction or Cessation and Cancer Risk. N Engl J Med Dec. 2023;28(26):2486–94. 10.1056/NEJMsr2306723. [DOI] [PubMed] [Google Scholar]
- 3.Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA Aug. 1988;5(5):652–6. [PubMed] [Google Scholar]
- 4.LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and Cancer: A Statement of the American Society of Clinical Oncology. J Clin Oncol Jan. 2018;1(1):83–93. 10.1200/JCO.2017.76.1155. [DOI] [PubMed] [Google Scholar]
- 5.American Institute for Cancer Research; American Public Health Association; Breast Cancer Action. Modernization of the labeling and advertising regulations for wine, distilled spirits, and malt beverages. Fed Regist. 2020;85(64):18704–26. [Google Scholar]
- 6.Strebel J, Terry MB, Alcohol. Binge drinking, and Cancer Risk: accelerating Public Health Messaging through Countermarketing. Am J Public Health May. 2021;111(5):812–4. 10.2105/AJPH.2021.306233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehm RD, Yang W, Tehranifar P, Terry MB. 40 years of change in Age- and stage-specific Cancer Incidence Rates in US women and men. JNCI Cancer Spectr Sep. 2019;3(3):pkz038. 10.1093/jncics/pkz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P. Incidence Trends of Breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US From 2010 to 2016. JAMA Netw Open. Aug 3. 2020;3(8):e2013226. 10.1001/jamanetworkopen.2020.13226 [DOI] [PMC free article] [PubMed]
- 9.Dwyer-Lindgren L, Flaxman AD, Ng M, Hansen GM, Murray CJ, Mokdad AH. Drinking patterns in US counties from 2002 to 2012. Am J Public Health Jun. 2015;105(6):1120–7. 10.2105/AJPH.2014.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. Jan 1. 2003;289(1):70 – 5. 10.1001/jama.289.1.70 [DOI] [PubMed]
- 11.McKetta S, Prins SJ, Bates LM, Platt JM, Keyes KM. US trends in binge drinking by gender, occupation, prestige, and work structure among adults in the midlife, 2006–2018. Ann Epidemiol Oct. 2021;62:22–9. 10.1016/j.annepidem.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA Nov. 2011;2(17):1884–90. 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffreys M, McKenzie F, Firestone R, et al. A multi-ethnic breast cancer case-control study in New Zealand: evidence of differential risk patterns. Cancer Causes Control Jan. 2013;24(1):135–52. 10.1007/s10552-012-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinney AY, Millikan RC, Lin YH, Moorman PG, Newman B. Alcohol consumption and breast cancer among black and white women in North Carolina (United States). Cancer Causes Control Apr. 2000;11(4):345–57. 10.1023/a:1008973709917. [DOI] [PubMed] [Google Scholar]
- 15.Morch LS, Johansen D, Thygesen LC, et al. Alcohol drinking, consumption patterns and breast cancer among Danish nurses: a cohort study. Eur J Public Health Dec. 2007;17(6):624–9. 10.1093/eurpub/ckm036. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Bayona R, Gea A, Gardeazabal I, et al. Binge Drinking and Risk of Breast Cancer: Results from the SUN (‘Seguimiento Universidad de Navarra’) Project. Nutrients Mar. 2020;10(3). 10.3390/nu12030731. [DOI] [PMC free article] [PubMed]
- 17.White AJ, DeRoo LA, Weinberg CR, Sandler DP. Lifetime Alcohol Intake, binge drinking behaviors, and breast Cancer risk. Am J Epidemiol Sep. 2017;1(5):541–9. 10.1093/aje/kwx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirko KA, Lucas DR, Pathak DR, et al. Lifetime alcohol consumption patterns and young-onset breast cancer by subtype among non-hispanic black and white women in the Young women’s Health History Study. Cancer Causes Control Feb. 2024;35(2):377–91. 10.1007/s10552-023-01801-z. [DOI] [PubMed] [Google Scholar]
- 19.Lima SM, Kehm RD, Swett K, Gonsalves L, Terry MB. Trends in parity and breast Cancer incidence in US women younger than 40 years from 1935 to 2015. JAMA Netw open Mar 2. 2020;3(3):e200929. 10.1001/jamanetworkopen.2020.0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Zhang IL, Terry MB, Yang W. Dietary factors and early-onset Colorectal Cancer in the United States-an Ecologic Analysis. Cancer Epidemiol Biomarkers Prev Feb. 2023;6(2):217–25. 10.1158/1055-9965.Epi-22-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni P, Lansdorp-Vogelaar I, Zauber AG, Cao Y. Elucidating the drivers for the rising incidence of early-onset colorectal Cancer: how ecologic studies could help and what is Next. Cancer Epidemiol Biomarkers Prev Feb. 2023;6(2):164–6. 10.1158/1055-9965.Epi-22-1126. [DOI] [PubMed] [Google Scholar]
- 22.Yuan H, Kehm RD, Daaboul JM, et al. Cancer incidence trends in New York State and associations with common population-level exposures 2010–2018: an ecological study. Sci Rep Mar. 2024;26(1):7141. 10.1038/s41598-024-56634-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Data from. Surveillance Research Program NCI. Breast subtype (2010+). Breast Subtype (2010+) - SEER Documentation (cancer.gov) Accessed April 2, 2024. https://seer.cancer.gov/seerstat/databases/ssf/breast-subtype.html
- 24.Data from: Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. In. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/brfss/annual_data/annual_data.htm
- 25.Surveillance E, Surveillance ER, Epidemiology, and End Results (SEER) Program. SEER Stat Database: Incidence - SEER Research Data, 9 Registries, Nov 2020 Sub (1975–2018) - Linked To County Attributes - Time Dependent (1990–2018) Income/Rurality, 1969–2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission. https://seer.cancer.gov/data-software/
- 26.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/default.aspx
- 27.Garaycoechea JI, Crossan GP, Langevin F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nat Jan. 2018;11(7687):171–7. 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terry MB, Colditz GA. Epidemiology and risk factors for breast Cancer: 21st century advances, gaps to address through Interdisciplinary Science. Cold Spring Harb Perspect Med. Sep 2023;1(9). 10.1101/cshperspect.a041317. [DOI] [PMC free article] [PubMed]
- 29.Zeinomar N, Oskar S, Kehm RD, Sahebzeda S, Terry MB. Environmental exposures and breast cancer risk in the context of underlying susceptibility: a systematic review of the epidemiological literature. Environ Res Aug. 2020;187:109346. 10.1016/j.envres.2020.109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine Aug. 2021;38:100985. 10.1016/j.eclinm.2021.100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are publicly available from SEER (Surveillance Research Program NCI. Breast subtype (2010+). Breast Subtype (2010+) - SEER Documentation (cancer.gov) Available at: https://seer.cancer.gov/seerstat/databases/ssf/breast-subtype.html) and from the BRFSS (Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. In Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/brfss/annual_data/annual_data.htm).