Summary
Introduction
Respiratory Syncytial Virus (RSV) predominantly affects young children, with a peak incidence in temperate regions of the northern hemisphere from October to May. Children under 24 months of age are particularly vulnerable because of the immaturity of their lungs and immune systems, often leading to severe respiratory infections. The World Health Organization (WHO) recognizes RSV as a global health priority. Recently, Nirsevimab, a long-acting monoclonal antibody (mAb), was authorised to prevent RSV disease in infants.
Methods
Our narrative review brings together the effectiveness data of Nirsevimab available in the literature, highlighting the strengths and weaknesses of the published studies and the prevention opportunities represented by the new preparation.
Results
All reviewed studies provide evidence for the effectiveness of immunisation with Nirsevimab in real-world settings, beyond the controlled conditions of clinical trials, and highlight its safety and feasibility. Nirsevimab significantly reduces RSV hospitalisations and Intensive Care Unit (ICU) admissions. High coverage and high efficacy of immunisation have been reported, although supply issues and variability in studies present challenges.
Conclusions
Continued research and surveillance are critical to understanding the long-term effectiveness of Nirsevimab. Overall, available data provide valuable insights into the efficacy, safety, and impact of immunisation with Nirsevimab in preventing severe RSV infections in infants, highlighting its potential to reduce the burden of RSV-related hospitalisations and improve paediatric health outcomes.
Keywords: Respiratory syncytial virus (RSV), Nirsevimab, Effectiveness, Infants, Prophylaxis
Introduction
Respiratory Syncytial Virus (RSV) is a single-stranded RNA virus belonging to the Paramyxoviridae family, with two main antigenic subtypes (A and B). RSV is a seasonal virus with epidemiology that strongly depends on the climatic zone concerned; in temperate regions of the Northern Hemisphere, it generally circulates from October to early May, with a typical peak between December and February [1].
RSV appears to be the most common etiological agent identified in respiratory infections among young children, with greater severity in infants up to 24 months, probably due to incomplete lung and immune system development. RSV disease is often not self-limiting and may have long-term consequences, as suggested by the fact that approximately 30-40% of children with previous bronchiolitis-related hospitalisations are likely to experience recurrent episodes of bronchospasm and asthma as long-term complications [2, 3].
All newborns are at risk of developing Lower Respiratory Tract Infection with RSV (RSV-LRTI), mainly due to seasonality and being under one year of age. Other risk factors include prematurity, congenital heart malformations/diseases (CHD), chronic lung disease (CLD), bronchopulmonary dysplasia (BPD), and other severe conditions that compromise the immune and neuromuscular systems [2, 4]. However, research conducted by the Centers for Disease Control and Prevention (CDC) in the United States, confirmed by recent studies conducted in Italy, revealed that almost 90% of infants hospitalised for RSV and up to 90% of infants with a lower respiratory tract infection seen on an outpatient basis were born at term and in apparently normal health [5, 6].
Globally, RSV causes a significant burden of respiratory disease annually, with 33 million cases of Lower Respiratory Tract Infections (LRTIs) requiring outpatient medical care and 3.6 million hospital admissions, causing more than 100,000 deaths, including more than 26,000 in hospital settings [7].
In Europe, RSV causes, on average, about 250,000 hospitalisations in children under five years of age, with most cases occurring in children under one year of age. Epidemiological studies conducted in Italy have shown an increased incidence of RSV infections and an increased need for Paediatric Intensive Care (PICU), especially in infants ≤ 3 months [8, 9]. According to the Italian RespiVirNet surveillance network, during the 2022-2023 influenza season, RSV caused 49.1% and 22.3% of influenza-like illnesses in children under 2 years of age and between 2 and 4 years of age [10].
A recent study conducted in the winter season 2022-2023, described a baseline of the RSV disease burden in primary care in Italy prior to the introduction of upcoming immunization strategies. Specifilly, fifty-five paediatricians from five Italian regions collected nasal swabs from 650 children under 5 years of age with acute respiratory infections (ARI). The results showed 37.8% of ARI cases were RSV-positive, with subtype B comprising 65.4%. RSV-positive children were younger and had symptoms lasting 11.47 ± 6.27 days. RSV-A cases required more paediatric visits than RSV-B. The impact included 53% of children missing school, 46% of parents losing workdays, and 25% of families facing extra costs [11].
In the 2023-2024 season, the total number of RSV-positive samples corresponded to 4,341 on a total of 15,684 ARI positive samples, with a clear prevalence in the 0-2 age group [12].
Given the substantial clinical burden in terms of morbidity and mortality, RSV disease is recognised by the World Health Organisation (WHO) as a global public health priority [13]. In 2016, the WHO Strategic Advisory Group on Immunisation recommended that efforts be made to identify and address gaps in evidence needed for regulation, pre-qualification, and policy guidance for RSV prevention interventions, including maternal and paediatric immunisation with RSV vaccines and passive childhood immunisation with long-acting RSV monoclonal antibodies (mAb) [14].
Palivizumab, the first mAb approved in 1999, requires monthly injections at a dose of 15 mg/kg body weight to provide protection during the typical 5-month RSV season. This drug is only recommended for high-risk infants in a limited age group, leaving most children vulnerable to RSV infection [6, 15].
Between 2022 and 2023, two novel products received authorisation to prevent RSV disease in neonates: Nirsevimab and a bivalent recombinant vaccine.
Nirsevimab, a long-acting mAb, was licensed to prevent lower respiratory tract disease caused by RSV in infants and children during their first RSV season [16, 17]. The recombinant bivalent RSVpreF vaccine has been approved for administration during pregnancy to prevent lower respiratory tract disease in children from birth to 6 months of age [18, 19].
European Centre for Disease prevention and Control (ECDC), WHO and the Italian Guidelines on the Management of RSV Bronchiolitis identified Nirsevimab as a promising preventive strategy that could soon be included in routine immunisation programs to protect all infants and children during their first RSV season [2].
This narrative review describes the available effectiveness data of Nirsevimab, highlighting the strengths and weaknesses of the published studies and the prevention opportunities represented by the new preparation.
Methods
This narrative review is structured into three main sections: the characteristics of the Nirsevimab (MEDI8897) and available efficacy studies, real-world effectiveness data and trials in Progress.
In the first section, we reported the main technological characteristics and some clinical practice aspects (dosage, administration, and usage recommendations). Moreover, we synthetically described the available efficacy studies, identified through the same search performed for effectiveness studies and described below.
The second section represents the core of our work and summarises all the available evidence on the real-world effectiveness of Nirsevimab.
The references for this article were identified through PubMed, GoogleScholar and Clinicaltrials.gov with the search terms: “effectiveness”, “efficacy”, “real-world”, “Nirsevimab”, “Beyfortus”, “Respiratory Syncytial Virus” and “RSV”, over the period from 2000 until the end of June 2024.
In the third section, we discussed ongoing clinical trials evaluating the efficacy and effectiveness of Nirsevimab: the search was performed on Clinicaltrials.gov with the methodology described above.
Results and discussion
NIRSEVIMAB (MEDI8897)
Nirsevimab, marketed under the brand name Beyfortus® and manufactured by AstraZeneca, is distributed by Sanofi Pasteur, Inc [16, 20, 21].
It is indicated for infants and children up to 12 months of age and for children at increased risk of RSV up to 24 months. Safety and efficacy in children over 24 months have not been established.
Nirsevimab is not indicated for pregnant or lactating individuals or those with reproductive potential. Clinical studies have shown that neither race nor increased susceptibility to severe RSV significantly affects the pharmacokinetics of Nirsevimab [16, 20].
Structure
Nirsevimab is a human G1 kappa-type monoclonal antibody (IgG1κ) produced in animal cells using recombinant DNA technology and designed to have sustained action [22].
It provides passive immunity against RSV infections by binding to a highly conserved epitope within the Ø site of the pre-fusion conformation (PreF) of the RSV fusion protein (F) inhibiting viral fusion and thus preventing virus entry into the host cell. The enhanced neutralising activity of Nirsevimab is due to the addition of the triple amino acid substitution M252Y/S254T/T256E (YTE) to the crystallisable region of the fragment (Fc). This modification extends the serum half-life in vivo approximately 3-fold (average 71 days), allowing a single intramuscular injection of Nirsevimab to provide protection for an entire RSV season [23, 24] (Fig. 1).
Fig. 1.
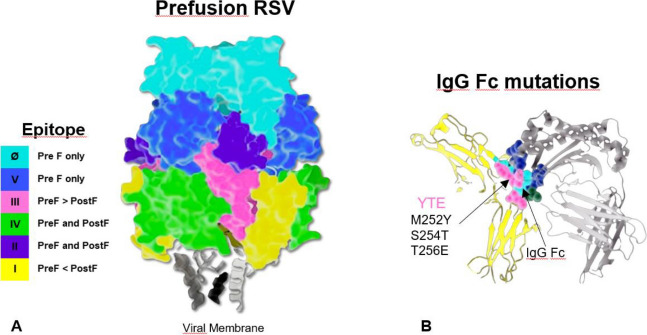
Mechanism of action of Nirsevimab. A) Ø site of the PreF of the RSV fusion protein (F). B) The addition of the triple amino acid substitution M252Y/S254T/T256E (YTE) to the crystallisable region of the fragment (Fc).
Dosage, administration, and usage recommendations
Nirsevimab is available in sterile prefilled syringes of 50 mg/0.5 ml or 100 mg/ml, without preservatives, for intramuscular injection. The dosage is weight-based: for infants and children weighing < 5 kg, the recommended dose is 50 mg; for children weighing > 5 kg, the recommended dose is 100 mg [20].
The Advisory Committee on Immunisation Practices (ACIP) and the American Academy of Pediatrics (AAP) recommend 1 dose of Nirsevimab for all children <8 months of age during or entering their first RSV season [25, 26]. The same recommendations are also suggested by the European Medicines Agency (EMA) and the Italian Medicines Agency (AIFA) [16, 17].
Children born shortly before or during the RSV season are recommended to receive their initial dose of Nirsevimab within the first week of life, either before hospital discharge or during their first outpatient visit. For those eligible by age who have not yet received a dose, administration is advised at any point during the season [25, 27].
The AAP also recommends administering Nirsevimab to children between 8 and 19 months of age during their second RSV season. However, providers may decide to administer the drug up to 24 months of age for those children considered to be at higher risk. Children who received less than five doses of Palivizumab during the season should switch to one dose of Nirsevimab, and those entering the second RSV season after receiving Palivizumab in the first season should receive Nirsevimab, otherwise continue with Palivizumab. A child receiving Nirsevimab should not concurrently receive Palivizumab in the same season [26].
For children at increased risk of severe RSV disease entering their second RSV season (up to 24 months of age) or children undergoing cardiac surgery with cardiopulmonary bypass, an additional dose of 200 mg (two 100 mg IM injections administered simultaneously) is recommended [20]. The AAP defines children at consistently increased risk as severely immunocompromised children, those with cystic fibrosis, children with severe or chronic premature lung disease (for which they required medical support in the 6 months preceding the RSV season) or those at increased risk of developing severe RSV disease because they live in communities in remote areas [26-28] (Tab. I).
Tab. I.
Dosage, administration, and usage recommendations.
| Patient Type | Schedule | Notes |
|---|---|---|
| Infants < 8 months at first RSV season* and < 5 kg | 50 mg | For infants born just before or during the RSV season (within the first week of life), it is advisable to administer the dose before discharge from the hospital or during their initial outpatient visit |
| Children < 8 months at first RSV season and ≥ 5 kg | 100 mg | |
| Children between 8 and 19 months to second RSV season | 1 dose** | Recommended for the second RSV season but may be given up to 24 months of age for children considered at high risk |
| (50 or 100 mg) | ||
| Children who have received fewer than five doses of Palivizumab during the season | 1 dose** | It is recommended to switch to Nirsevimab if not enough doses of Palivizumab have been received |
| (50 or 100 mg) | ||
| Children entering the second season of RSV after receiving Palivizumab in the first season | 1 dose** | It is recommended to switch to Nirsevimab in the second season; otherwise, continue with Palivizumab |
| (50 or 100 mg) | ||
| Children who have received Palivizumab (less than five doses) and can receive Nirsevimab | 1 dose** | Giving one dose of Nirsevimab instead of additional doses of Palivizumab is recommended |
| (50 or 100 mg) | ||
| Children £ 24 months at high risk of severe RSV disease in their second RSV season or undergoing cardiac surgery/cardiopulmonary bypass | 2 doses** | Two doses were administered simultaneously. In children undergoing cardiac surgery/cardiopulmonary bypass who have received a first dose within 90 days, the additional dose should be administered as soon as the child is stable after surgery [16] |
| (50 or 100 mg each) | ||
| * In temperate regions of the Northern Hemisphere, the RSV season typically spans from October to early May, experiencing its peak between December and February. ** 1 dose 50 mg/0.5 ml for children < 5 kg; 1 dose 100 mg/ml for children ≥ 5 kg. | ||
Potential adverse effects
Nirsevimab may cause common adverse effects such as pain, swelling, hardness, or redness at the injection site and rashes in the first 7-14 days after administration. Rare adverse effects include hypersensitivity reactions, such as anaphylaxis, and thrombocytopenia. Severe allergic reactions observed with other mAb products may manifest with symptoms such as malaise, headache, arthralgias, muscle weakness, severe skin rashes, urticaria, swelling of the face, difficulty swallowing or breathing and blue discolouration of the lips or nails [16, 22, 29].
Contraindications and co-administration with other drugs
Nirsevimab is contraindicated in children who have experienced severe hypersensitivity reactions to the active substance or its excipients. Caution should be exercised in children with bleeding disorders, thrombocytopenia, coagulation disorders, or on anticoagulant therapy [16, 20].
Since Nirsevimab is a mAb, that generates an RSV-specific passive immunisation, it is not expected to interfere with the active immune response generated by concomitantly administered vaccines. Clinical studies show that the safety and reactogenicity profile of concomitantly administered vaccines is similar to that of vaccines administered individually, although experience with co-administration is limited. Therefore, Nirsevimab can be administered concomitantly with childhood vaccines [30].
EFFICACY STUDIES
Several clinical trials have studied the efficacy of Nirsevimab: NCT02878330 (2016-2018) [22]; NCT03979313 - MELODY (2019-2023) [29, 31]; NCT03959488 - MEDLEY (2019-2023) [32] and NCT04484935 - MUSIC (2023) [33].
The study NCT02878330 (randomised, double-blind, placebo-controlled phase 2b study), conducted in the USA, confirmed a 78.4% (95% CI: 51.9-90.3) reduction in the incidence of RSV-associated LRTIs and hospitalisations and a 70.1% (95% CI: 52.3-81.2) reduction in physician-assisted LRTIs in healthy preterm infants of Gestational Age (GA) between 29 and 35 weeks at their first RSV season [22].
The phase 3 of the MELODY (NCT03979313) study examined the efficacy of Nirsevimab in infants born at a gestational age of at least 35 weeks who were not eligible for RSV prophylaxis with Palivizumab. In the first cohort, the efficacy against physician-assisted RSV-associated LRTI was 74.5% (95% CI: 49.6-87.1) [29]. In the secondary exploratory cohort, the efficacy against hospitalisation for RSV-associated LRTI was 76.8% (95% CI: 49.4-89.4) and the efficacy against very severe RSV-associated LRTI attended by a physician was 78.6% (95% CI: 48.8-91.0) [31].
A comparison of safety, tolerability and pharmacokinetics of Nirsevimab and Palivizumab, was evaluated in preterm infants in their first RSV season, as well as in infants with CLD and CHD in their first and second RSV season, in the phases 2/3, randomised, double-blind study, NCT03959488-MEDLEY. The incidence of adverse events was similar between treatment groups and cohorts [32].
In the Simoes analysis, the efficacy of Nirsevimab was evaluated using a weight-based dosing regimen in infants born between 29 weeks gestational age and term. A total of 2,350 infants (1,564 in the Nirsevimab group and 786 in the placebo group) were included in the phase 2b and MELODY studies. Nirsevimab demonstrated significant efficacy compared to placebo in the primary endpoint of LRTI associated with physician-assisted RSV, with a relative risk reduction of 79.5%. In infants with risk conditions such as chronic pulmonary disease, CHD, or extreme preterm delivery (MEDLEY), serum exposures to Nirsevimab were like those of the general study population, with above-target exposures in more than 80% of cases [34].
The MUSIC study (NCT04484935) is a 12-month Phase 2 study in which a single dose of Nirsevimab was administered. This uncontrolled, open-label study was conducted to examine safety, pharmacokinetics, and the occurrence of anti-drug antibodies (ADA) in immunocompromised children aged ≤ 24 months. It was observed that children who were ADA-positive at day 361 tended to have lower Nirsevimab levels than those who were ADA-negative, especially between day 151 and day 361, suggesting an influence of ADA on pharmacokinetics.
Nirsevimab serum concentrations at day 151 were like those shown to be effective in preventing RSV LRTI in healthy patients enrolled in the Phase 3 MELODY study. In addition, some children with underlying conditions involving protein loss showed a rapid decline in serum concentrations of Nirsevimab [33].
REAL-WORLD EFFECTIVENESS DATA
The EMA approved the use of Nirsevimab shortly before the 2022-2023 RSV season (one year before the US FDA authorisation). Due to the short period between the EMA authorisation and the start of the RSV season, public health policies on universal immunisation of newborns have not been finalised. This delay has postponed the implementation in the relevant European countries until the 2023-2024 season [16].
However, during the 2022-2023 RSV season, a phase 3b efficacy study under real-world conditions on Nirsevimab - HARMONIE (NCT05437510) [35] - was initiated involving several European countries: the UK, France, and Germany. This pragmatic study evaluated Nirsevimab in the prevention of hospitalisation due to RSV-associated LRTI in neonates. Infants with gestational age ≥ 29 weeks were randomised openly in a 1:1 ratio to receive a single dose of Nirsevimab (< 5 kg 50 mg; ≥ 5 kg 100 mg) or no intervention (standard of care) before or during the RSV season. In the primary analysis of 8,058 healthy infants, an efficacy of 83% (95% CI: 67.8-92.0) was observed against hospitalisations for RSV-related LRTIs and 76% (95% CI: 32.8-92.9) against very severe LRTIs confirming consistency with the efficacy endpoints of randomised clinical trials. Furthermore, 58% (95% CI: 39.7-71.2) efficacy prevented real-world hospitalisation for all causes of LRTIs [35].
For the 2023-2024 RSV season, the global implementation of Nirsevimab was very successful in several countries. In Europe, France and Spain were the first countries to adopt a seasonal program. For logistical convenience, the UK opted for an annual immunisation programme with mAb or maternal vaccination. In the US, the CDC recommend the seasonal use of Nirsevimab or maternal vaccination against RSV, with no clear preference for one method over the other [36].
Early results on the effectiveness of Nirsevimab come from Luxembourg [37], Spain [38-43], the US [44], France [45], and Italy [46] (Tab. II).
Tab. II.
Real-world effectiveness data with a single dose of Nirsevimab.
| Country | Study Details | Population | Main Results | Strengths |
|---|---|---|---|---|
| Luxembourg | Ernst et al. 2024 [36] Paediatric RSV-H in children ≤ 5 years |
Infants born between Oct. 1, 2023, and Mar. 31, 2024: N = 1524 4 hospitals, the entire country |
nIC coverage with Nirsevimab from Oct to mid-Dec.: 1277/1524 (84%) Reduction in RSV-H: children ≤ 5 years -38%, infants < 6 months -69% Reduction in the proportion of hospitalised children < 5 years in PICUs: 2022: 36/389 (9.3%), 2023: 15/241 (6.2%) Increase in the average age of children hospitalised in 2023: 7.8 months in 2022, 14.4 months in 2023 |
First real-world data on the effectiveness of Nirsevimab in protecting infants from severe RSV disease |
| Spain | Lopez-Lacort et al. 2024 [38] Paediatric RSV-LRTI-H in infants < 9 months |
Infants during their first RSV season (born on or after Apr. 1, 2023) N = 15676 9 hospitals, 3 regions (Valencia, Murcia, Valladolid) |
nIC with Nirsevimab ranges from 78.7% to 98.6% Effectiveness in preventing RSV-LRTI-H in infants < 9 months: 70.2% (95% CI: 38.3-88.5) Valencia: 69.3% (95% CI: 36.4-86.2), Murcia: 86.9% (95% CI: 77.1-92.9), Valladolid: 97.0% (95% CI: 87.7-99.6) Effectiveness in preventing LRTI-negative RSV admissions: 32.4% (95% CI: -27.5-63.4). Reduction in RSV-H in infants: 70.2% (95% CI: 38.3-88.5) |
Uniform methodology to detect hospitalised RSV cases in three Spanish regions Simultaneous use of screening approach and test-negative design Active surveillance of respiratory infections in all nine hospitals |
| Ezpeleta et al. 2024 [39] Paediatric RSV-H in children born in Navarra |
Infants born between Oct. 2023 and Jan. 2024 N = 1771 All hospitals, 1 region (Navarra) |
nIC with Nirsevimab from Oct 2023 to Jan.2024: 1083/1771 (92%) Effectiveness in preventing RSV-H in infants: 88.7% (95% CI: 69.6-95.8) Reduction in the risk of RSV-H: Immunised 8/1083 (0.7%), Non-immunised 8/94 (8.5%) Reduction in the risk of RSV-ICU admission: Immunised 3/1083 (0.3%), Non-immunised 2/94 (2.1%) Vaccination of infants born from Sep. 2023 to Jan. 2024 prevents one hospitalisation per 15.3 immunized infants and avoids 77.5% (95.4/121.5) of the hospitalisations |
Effectiveness and impact of the prospective immunisation strategy against RSV-H Provides a benchmark to compare the effectiveness of different immunisation strategies against RSV |
|
| Martinón-Torres et al. 2023 [37] Mallah et al. 2024 [40] Ares-Gómez et al. 2024 [41] Paediatric RSV-LRTI-H in children Very severe RSV-LRTI ICU admissions LRTI admission for all causes |
Infants born between Sep. 25, 2023, and Mar. 31, 2024 N = 10259 14 hospitals, 1 region (Galicia) |
nIC with Nirsevimab ranges from 81.4% to 97.5% Effectiveness in preventing RSV-LRTI-H in infants: 82.0% (95% CI 65.6-90.2%) Reduction in the risk of RSV-LRTI-H: Immunised 30/9408 (0.3%), Non-immunised 16/851 (1.9%) Effectiveness in preventing RSV-LRTI-H in ICU: 86.9% (95% CI 69.1-94.2%) Reduction in the risk of RSV-LRTI ICU admission: Immunised 15/9408 (0.16%), Non-immunised 10/851 (1.18%) Average RSV-LRTI-H prevented per 1000 infants: 407 (Effectiveness: 89.84%) |
Rapid and effective implementation of Nirsevimab in the Galician neonatal population A robust information and education campaign and a flexible booking system facilitated high adherence Demonstration of Nirsevimab’s effectiveness in preventing RSV-LRTI-H and hospitalisations for all causes, consistent with previous clinical and real-world studies [28, 34, 38] |
|
| Coma et al. 2024 [42] Effectiveness against: RSV infection Primary care attended bronchiolitis Viral Pneumonia Hospital Emergency Visit Hospital admission ICU admission |
Infants born between Apr. and Sep. 2023 N = 26525 All hospitals, 1 region (Catalonia) |
nIC with Nirsevimab: 23127/26525 (87.2%) Reduction in the risk of RSV-related hospital and ICU admissions: Hospital admission: 87.6% (95% CI: 82.1-91.4%) ICU admission: 90.1% (95% CI: 76.3-95.9%) Reduction in the risk of: RSV Infection: 68.9% (95% CI: 51.7-80%) Primary care attended bronchiolitis: 48.1% (95% CI: 42.4-53.3%) Viral Pneumonia: 60.7% (95% CI: 24.2-79.7%) Hospital Emergency Visit: 55.4% (95% CI: 48.4-61.5%) |
Use of an integrated database allowing comprehensive analysis of the impact of the Catalan immunisation program on the reduction of RSV-related outcomes The analysis included a wide range of outcomes, offering a comprehensive evaluation of the efficacy of Nirsevimab across different levels of healthcare |
|
| United States | Moline et. Al 2024 [43] RSV-H among infants in their first RSV season |
Infants born after Oct. 1, 2023, or aged less than 8 months on Oct. 1, 2023, N = 699 7 paediatric academic medical centres (Missouri, Ohio, New York, Washington, Texas, Pennsylvania, Tennessee) |
nIC with Nirsevimab: High- Risk conditions 46% (18/39), No risk conditions 6% (41/660) Reduction in RSV-H: 90% (95% CI: 75-96%) Time elapsed between Nirsevimab administration and onset of ARI symptoms: 7-127 days; Median= 45 days (IQR = 19-76 days) |
Inclusion of infants according to a standardised definition of ARI, ensuring uniformity of inclusion criteria Systematic testing for the presence of RSV reduces the risk of misdiagnosis or missed diagnosis |
| France | Paireau et al. 2024 [44] RSV bronchiolitis hospitalised in ICU from Sep. 15, 2023, to Jan. 31, 2024 (TND) |
Infants (0-8 months) admitted to ICU in the period Sep. 15, 2023, to Jan. 31, 2024 N = 288 20 PICUs, metropolitan areas of the entire country |
nIC with Nirsevimab: 58/288 (20.1%) Effectiveness in preventing severe RSV bronchiolitis in neonates admitted to the PICU: 75.9% (95%CI 48.5-88.7%) |
Use TND to quickly estimate effectiveness based on surveillance data, reducing confounding bias and providing meaningful results |
| Italy | Consolati et. Al 2024 [45] RSV-H among infants in their first RSV season 2023-2024 |
Infants born between May 1, 2023, and Feb. 15, 2024, N = 537 1 hospital, 1 region (Valle d’Aosta) |
nIC with Nirsevimab: in RSV season 77/89 (86%), out of RSV season 292/448 (65%) Reduction in hospitalisation cases for RSV bronchiolitis: -54% (47 cases in 2022-2023 season vs 18 cases in 2023-2024) Hospitalisations for bronchiolitis during the RSV season 2023-2024 after Nirsevimab introduction: Treated with Nirsevimab 0/369 (0%), No Prophylaxis 14/168 (8.3%) |
Demonstration of the effectiveness and safety of universal prophylaxis with Nirsevimab in infants, with a marked reduction in the risk of RSV-H |
| RSV-H: RSV-related hospitalisation; RSV-LRTI-H: RSV-LRTI paediatric hospitalisation; nIC: neonatal immunisation coverage. | ||||
Luxembourg
In July 2023, the Luxembourg Infectious Diseases Advisory Group recommended prophylaxis with one dose of Nirsevimab in all infants born between October 1, 2023, and March 30, 2024 (weeks 39/2023-13/2024); infants born between 1 January and 30 September 2023 for catch-up immunisation; and children under 2 years of age with risk factors for severe respiratory infections. A national immunisation campaign followed this recommendation in October 2023. Vaccination coverage was calculated weekly by dividing the doses of Nirsevimab administered in hospital maternity and neonatology wards by the number of births during the corresponding period. Between October and mid-December 2023, national coverage was estimated at 84% (1277 doses per 1524 births), varying from 66% to 94% between maternity wards, with no reports of adverse events associated with immunisation. However, in other contexts, coverage could not be monitored due to the absence of a vaccination register.
Results: by comparing weeks 39-52/2022 with the same weeks of the 2023/2024 RSV season, a reduction of 38% in cases of hospitalisation for RSV infection was observed in children under 5 years of age (389 cases in 2022 and 241 cases in 2023) and 69% in infants under 6 months of age compared to the previous season (232 (59.6%) in 2022 and 72 (29.9%) in 2023). In particular, during the study periods, the average age of children hospitalised in 2023 was significantly higher than in 2022 (14.4 months in 2023 compared to 7.8 months in 2022). Of the children admitted in 2023, 213 (88.4%) had not been immunised with Nirsevimab, and of these 47 (65.3%) were aged ≤ 6 months. The average length of hospital stay was significantly reduced in 2023 compared to 2022, especially in infants aged ≤ 6 months, as well as in the total number of RSV-related hospitalisation days. In 2023, most infants who required oxygen supplementation or high-flow nasal therapy had not been immunised. The proportion of hospitalised children under 5 years of age admitted to PICUs decreased from 9.3% (36/389) in 2022 to 6.2% (15/241) in 2023.
Strengths: this study provides the first solid evidence that immunisation with Nirsevimab protects infants against severe RSV disease in the context of relatively high immune coverage, as previous clinical studies have shown similar efficacy of Nirsevimab against LRTIs caused by RSV only in healthy patients.
Limitations: the comparison was only made between two seasons, hence it would be important to examine a broader period. Furthermore, the intensity of the 2022 epidemic could be influenced by the reduced circulation of RSV due to immunity depletion [37].
Spain
At the end of September 2023, Spain introduced universal prophylaxis against RSV in its national immunisation program for all infants born on or after 1 April 2023 [38]. Consequently, most of the effectiveness results stem from this country.
The initial results originate from a hospital-based multicentre active surveillance conducted in nine hospitals across three autonomous regions in Spain (five hospitals in Valencia, three in Murcia, and one in Valladolid) [38]. The study population included all infants born on or after 1 April 2023 eligible for immunisation with Nirsevimab during their first RSV season, totalling 15,676 infants (6.4% of the Spanish child population eligible for immunisation). The surveillance period was from 1 October 2023 to 31 December 2023 or 10 January 2024, depending on the hospital.
Results: a total of 166 LRTI admissions were included, of which 95 were positive for RSV and 73 were between 0 and 3 months old. Among all 95 RSV cases, 56 (59%) had received immunisation. Population coverage with Nirsevimab was high, varying from 78.7% to 98.6% depending on the hospital. The effectiveness of immunoprophylaxis with Nirsevimab was assessed by region, resulting in 69.3% (95% CI: 36.4-86.2), 86.9% (95% CI: 77.1-92.9), and 97.0% (95% CI: 87.7-99.6) in Valencia, Murcia, and Valladolid, respectively. By using a test-negative design (TND), the immunisation odds of infants testing RSV-positive by PCR (N = 77) were compared to those of infants who tested negative (N = 71). The overall effectiveness of Nirsevimab in preventing RSV-LRTI hospitalisations was 70.2% (95% CI: 38.3-88.5). In a sensitivity analysis, the effectiveness in preventing LRTI-negative RSV admissions was 19.6% (95% CI: -180.8-82.3) in Valencia, 27.5% (95% CI: -47.3-66.2) in Murcia and, considering both regions, 32.4% (95% CI: -27.5-63.4). Data from Valladolid was not included due to the lack of RSV-negative cases.
Strengths: the study integrated data from three Spanish regions using a uniform methodology to detect hospitalised RSV cases. A screening approach and a TND were applied simultaneously, including cases with a negative outcome. Nirsevimab coverage in the eligible cohorts in the participating regions was high in the first three months, averaging around 90%, and effectiveness estimates in immunisation candidate infants (under 9 months of age) were above 70%. In addition to the clinical trial data, a significant reduction in RSV-related hospitalisations in infants under real-world conditions, was demonstrated. Active surveillance of respiratory infections in all nine hospitals provided reliable estimates of Nirsevimab’s effectiveness.
Limitations: variations in RSV circulation, hospital admission policies, and case definitions between hospitals and regions influenced the reported effectiveness estimates. Due to the short period since the implementation of the immunisation program and the limited number of cases, it was not possible to assess regional effectiveness using the TND [39].
A second Spanish study aimed to examine the effectiveness of Nirsevimab was conducted in the region of Navarra [40]. Also, in Navarra, immunoprophylaxis with Nirsevimab was publicly funded and it was offered prospectively to all infants born in maternity wards between October 2023 and January 2024 and to those born abroad but residing in Navarra during the period under review. The follow-up of infants ended after two consecutive weeks without hospitalisation for RSV or after eight weeks without further hospitalisation (28 January 2024). The study design included an analysis of epidemiological and immunisation data, a prospective cohort to assess the effectiveness of Nirsevimab, and an integration of the results to compare the immunisation’s impact with other strategies. The proportion of hospitalisations for confirmed RSV infection in infants was calculated according to immunisation status with Nirsevimab.
Results: of the 1,177 infants examined, 1,083 (92.0%) received Nirsevimab and 21 went to the emergency room for RSV infection (11 immunised and 10 not). Only 16 babies were hospitalised (8 immunised and 8 not) and of these, 3 immunised and 2 non-immunised babies were transferred to intensive care. The estimated effectiveness of Nirsevimab was 88.7% (95% CI: 69.6-95.8), with a significantly lower risk of hospitalisation for RSV in immunised (8/1083) infants than in non-immunised (8/94) infants (0.7% and 8.5%, respectively). Vaccination of infants born between September 2023 and January 2024 prevents one hospitalisation per 15.3 vaccinated infants and avoids 77.5% (95.4/121.5) hospitalisations. In a prospective analysis combining the immunisation of babies born from September to January and the recovery of those born from April to August, the estimate was 81.8% (94.4/115.3). These results support immunisation with Nirsevimab at birth, to prevent severe RSV infections and alleviate paediatric hospital resource overload. The absence of reported adverse effects confirms the safety of this immunoprophylaxis.
Strengths: the study evaluates the efficacy of Nirsevimab and the impact of the use strategy against RSV-related hospitalisations, providing an important evaluation of the effectiveness of this immunoprophylaxis. The efficiency of the prospective immunisation strategy is evident, as only few doses of Nirsevimab were sufficient to prevent hospitalisation. Although the study’s statistical power was limited by the low number of parents who refused vaccination, it allowed accurate estimates to be obtained. These results provide a good benchmark for comparing the effectiveness of passive immunisation of children and vaccination of pregnant women with new RSV vaccines.
Limitations: the absence of complete information on comorbidities and prematurity for all infants may influence the study to evaluate the efficacy of Nirsevimab and the impact of the use strategy against RSV-related hospitalisations, providing an important evaluation of the effectiveness of this immunoprophylaxis. Overlaps with other high-risk groups were not considered, limiting the understanding of efficacy in these populations. The potential impact of immunoprophylaxis was estimated considering immunisation coverage and the observed epidemiological pattern, which may vary in future years. There is the possibility of variability in the distribution of childhood hospitalisations for RSV according to the month of birth, but extending immunoprophylaxis to children born in different months could cover a significant proportion of infants at risk. Although the study was conducted in a specific region and season, the results could be valid for other similar scenarios. The analysis of emergency room patients may be biased due to intermittent routine testing for RSV, although the results are consistent with other findings of hospital admissions. Finally, while the effectiveness of immunoprophylaxis is high, the cost of Nirsevimab may require economic evaluation to determine the optimal use strategy in different national settings.
The NIRSE-GAL (NCT06180993) study is an impressive prospective longitudinal initiative in the Galicia region, Spain, with a follow-up period of three years [38, 41, 42]. Galicia offers a public health system with universal access, facilitating the involvement and monitoring of study participants. In 2022, 14,495 births occurred in Galicia, with a paediatric vaccination coverage rate of 90%. The Galician public health system (SERGAS), provides care through 14 public hospital complexes and a fully digital, centralised system.
For the 2023-2024 season, RSV cases were actively monitored from 25 September 2023 to 31 March 2024. The campaign end date was decided based on the expected conclusion of the RSV season, using data from the previous 12 seasons (excluding those during the 2020/2021 and 2021/2022 COVID-19 pandemic). Testing for RSV is routinely performed in hospital and emergency room settings, and a primary health surveillance program was developed for NIRSE-GAL that retrieves data on all eligible study participants from several registries in the SERGAS information system. Thanks to this continuous surveillance system, it will be possible to change the start and end dates of the campaigns in the coming years. RSV cases will be identified through hospital registers and laboratory tests, with a thorough evaluation by public health specialists. All the necessary electronic documents for NIRSE-GAL are updated weekly through the Galician Regional Surveillance Information System [47].
The NIRSE-GAL study involves approximately 14,000 children per RSV season, divided into three categories seasonal group (infants born during the RSV season), catch-up group (children aged less than six months at the start of the RSV season), and high-risk group (children aged between 6 and 24 months at the start of the RSV season who have CHD, BPD, severe immunosuppression, congenital metabolic disorders, neuromuscular diseases, severe pulmonary diseases, genetic syndromes causing significant respiratory problems, trisomy 21, cystic fibrosis, palliative care patients). If the immunisation recommendation changes in future seasons, the stratification strategy will be adjusted accordingly to the updated recommendation.
Nirsevimab is administered through the SERGAS network of public hospitals and primary health centres, with an educational campaign for health workers and the general public. Infants will receive a dose of Nirsevimab depending on weight and risk conditions. Depending on the children’s specific situation, the follow-up period varies from the immunisation date until the event of interest, death, or end of the observation period.
The primary objective of the NIRSE-GAL study is to evaluate the effectiveness of Nirsevimab on RSV-related LRTI admissions during the 2023-2024, 2024-2025, and 2025-2026 RSV seasons in the different patient groups. Secondary objectives, again subdivided according to enrolment group, includes: (I) to assess the effectiveness of Nirsevimab against 4 key secondary endpoints (a) very severe RSV-related LRTI (Padmissions), (b) very severe LRTI related to RSV is defined by the label of severity, (c) LRTI admission for all causes, (d) hospitalisation for any cause); (II) to assess the impact of Nirsevimab on primary care for any reason, primary care for respiratory disease, acute otitis media diagnosis, pneumonia diagnosis, acute respiratory diagnosis and antibiotic use in children; (III) to evaluate the impact of Nirsevimab on RSV-related visits in emergency departments and all-cause visits in emergency departments; (IV) longitudinal assessment of Nirsevimab’s effect on wheezing and asthma; (V) safety evaluation of Nirsevimab and reporting of adverse events; (VI) assessment of absorption.
There are also composite endpoints including various respiratory conditions and infections along with exploratory objectives. These include evaluation of the RSV-related LRTI hospitalisation rate in infants and subsequently in children, exploring the impact of different primary and secondary endpoints, Nirsevimab’s impact evaluation on bacterial respiratory infections, study of the reinfection/readmission rate and duration of hospitalisations for RSV and evaluation of trends in the duration of hospitalisations for RSV. Therefore, the results of this study will be significant for the conduct of appropriate cost-effectiveness studies [38, 41].
Results: preliminary results of the NIRSE-GAL study were published 3 weeks after the beginning of the immunisation campaign (31 October 2023). Immunisation adherence in infants born since the start of the campaign (seasonal group) reached 92.6% (1,104/1,192). In the recovery and high-risk infant groups, coverage was 81.4% (5,820/7,150) and 97.5% (317/325), respectively. No serious adverse effects were reported in this first analysis.
A second part of the results of the first season of the study, were published in May 2024 [42]. These were based on data collected done by 15 December 2023. 10,259 infants were eligible for Nirsevimab administration: 6,919 (67.4%) in the recovery group and 3,340 (32.6%) in the seasonal group; of these, 9,408 (91.7%) received Nirsevimab: 6,220 (89.9%) in the recovery group and 3,188 (95.4%) in the seasonal group. Immunisation was also offered to 360 high-risk infants achieving a coverage rate of 97.0% (348/360). 83.8% of infants in the recovery group, 96.4% in the high-risk group, and 92.6% in the seasonal group were vaccinated before the start of the RSV season (by 20 October 2023). Most of the missed vaccinations were due to missed appointments. Only 2.0% of households refused vaccination, with no significant differences between the seasonal and catch-up groups. The distribution by sex and age was even, with an average gestational age at birth of 4 months. 656 (6.6%) of the infants were premature, but 93.9% (616/656) still received Nirsevimab.
During the reporting period, 46 RSV-LRTI hospitalisations (30 in immunised and 16 in non-immunised children) and 25 ICU admissions (15 immunised and 10 non-immunised children) were observed. The effectiveness of Nirsevimab against RSV-related LRTI hospitalisations was 82.0% (95% CI: 65.6-90.2) and 86.9 (95% CI: 69.1-94.2). In addition, protection was found for both all-cause and LRTI hospitalisations. Sensitivity analysis confirmed these results. The 2023-24 RSV season showed significantly lower hospitalisation rates in infants treated with Nirsevimab compared to previous seasons. The immunisation campaign prevented an average of 407 RSV-related LRTI hospitalisations per 1,000 infants, with an effectiveness of 89.84% (IQR 87.58-90.30). Only five adverse events were reported, none of these related to Nirsevimab. The high-risk group was not included in the efficacy analysis because of the sample size (including only 12 in the non-Nirsevimab group) and the low number of events in this group: 22 all-cause hospitalisations, nine LRTI hospitalisations for all causes and three RSV-related LRTI hospitalisations.
Strengths: rapid and effective implementation of Nirsevimab occurred in the Galician neonatal population, with more than 90% of eligible infants immunised within 3 months of the campaign’s start. The effectiveness of Nirsevimab in preventing RSV-related LRTI admissions and hospitalisations for all causes is comparable to the results of previous clinical and real-world studies [29, 31, 35, 39]. Finally, the use of historical RSV season data, which incorporates the variability of viral circulation over time, was essential to provide adequate context for the results.
Limitations: the report is based on data from the first 3 months of the immunisation campaign; therefore, the results may be unrepresentative of the entire 2023-24 RSV season. It was impossible to assess specific endpoints (e.g. ICU admissions or mechanical ventilation) to estimate the effectiveness of Nirsevimab in these conditions. Limitations related to sample size and missing data for some variables at birth, could influence the interpretation of the results (prematurity, birth weight, and gestational age at birth). Finally, while the data support the effectiveness of Nirsevimab, the analysis has not yet considered the cost-effectiveness, which will be evaluated in the future.
A final Spanish study is conducted in Catalonia by Coma et al. from 1 October 2023 to 31 January 2024 [43]. The study analysed the effectiveness of Nirsevimab against RSV infection and its potential severity using data collected from different Catalan healthcare databases from October 1, 2023, to January 31, 2024. In this retrospective cohort study, 26,525 infants born between April and September 2023 resident in Catalonia and registered in the health databases were included. Several outcomes related to RSV infection and severity were examined including primary care-assisted bronchiolitis, RSV infection, viral pneumonia, hospital emergency room visits, and RSV-related hospital or ICU admissions. Other infectious diseases were also examined for unmeasured confounders. Covariates included sociodemographic data such as age, gender, residence, nationality, rurality, and socioeconomic status. The study included both infants immunised with Nirsevimab and non-immunised infants, following them until the event of interest, death, or the end of the study.
Results: 23,127 infants (87.2%) included in the study, were immunised with Nirsevimab. Vaccine coverage was rapid, with 76.3% immunised infants within the first month of the immunisation campaign. The control and Nirsevimab groups were adjusted by gender, rurality, and socio-economic status, but differed in age and nationality. In the control group, higher incidence rates for all outcomes were observed, especially severe cases, such as hospital and ICU admissions.
Nirsevimab demonstrated significant effectiveness in reducing the risk of hospitalisation and ICU admission, with an adjusted hazard ratio (HR) of 0.124 (0.086-0.179; immunised 52/control 76) and 0.099 (0.041-0.237; immunised 8/control 17), respectively. Higher incidence rates were also observed in the control group for all other outcomes: RSV infection HR 0.311 (0.2-0.483; immunised 71/control 31), primary care attended bronchiolitis HR 0. 519 (0.467-0.576; immunised 1560/control 617), viral pneumonia HR 0.393 (0.203-0.758; immunised 42/control 14), emergency room visits HR 0.446 (0.385-0.516; immunised 604/control 354).
The estimated effectiveness of Nirsevimab was 90.1% (95% CI: 76.3-95.9) versus ICU admission and 87.6% (95% CI: 82.1-91.4) versus hospital admission. Impetigo diagnoses were used as negative controls and were similar in the two groups. A sensitivity analysis confirmed the Nirsevimab’s effectiveness in preventing hospitalisation for bronchiolitis caused by other pathogens.
Two deaths were reported in the study population, both in infants who were not immunised with Nirsevimab and unrelated to RSV infection. Therefore, these deaths were not considered significant study outcomes.
Strengths: the study utilises a linked database that integrates primary care and hospital data (including intensive care), allowing a comprehensive analysis of the impact of the Catalan immunisation program on the reduction of RSV-related outcomes. It offers a comprehensive view of Nirsevimab’s impact across a wide range of outcomes of different severity and across all levels of care. The robustness of the results is confirmed by the comparability of the cohorts, which includes unobserved covariates. The conclusions are strengthened by the absence of an association between immunisation with Nirsevimab and impetigo, used as a negative control outcome.
Limitations: the analysis may be subject to confounding due to the observational nature of the data. A comprehensive analysis of less specific outcomes, such as bronchiolitis diagnosed in primary care or viral pneumonia, was not included due to the lack of complete testing for all patients in these settings. The analysis is based on the RSV epidemic period 2023-2024, potentially limiting the extensibility of the results to other periods.
United States
In the United States, the New Vaccine Surveillance Network (NVSN) evaluated the efficacy of Nirsevimab against RSV-associated hospitalisation among infants in their first RSV season during the period 1 October 2023-29 February 2024 at the seven US academic paediatric medical centres. Each site included in the analysis, had to have enrolled at least five infants who were treated with Nirsevimab at least 7 days before symptom onset [44].
The analysis involved infants who were born after 1 October 2023 or were less than 8 months of age on 1 October 2023, and who were admitted with an ARI and received the drug Nirsevimab. Infants who received Palivizumab maternal RSV vaccination or had inconclusive RSV test results were excluded.
The efficacy of Nirsevimab in avoiding RSV-related hospitalisation was evaluated in a TND case-control study. Cases included infants with positive RSV test results, while controls had test negative results. Effectiveness was calculated using multivariable logistic regression models, controlling age at recruitment, month of illness, enrolment site, and medical conditions at high risk for severe RSV disease. Prematurity status and insurance type were examined as potential confounding factors, but did not influence the estimates and therefore were not included in the final model.
Results: among the 1,036 eligible infants, 699 infants from four sites met the inclusion criteria: 146 preterm births (< 37 weeks), 551 term births 2 with unknown gestational age, and 39 with at least one risk factor. There were 407 (58%) patients with a positive RSV test result (cases) and 292 (42%) patients with a negative result (controls). Nirsevimab administration was more common among infants with high-risk medical conditions (18/39; 46%; p < 0.001) than among healthy infants 41/660 (6%). No significant differences were found in the frequency of Nirsevimab administration according to prematurity status or insurance type. The time elapsed between Nirsevimab administration, and the onset of ARI symptoms ranged from 7 to 127 days with a median of 45 days (IQR = 19-76 days). The effectiveness of Nirsevimab in preventing RSV-associated hospitalisation was 90% (95% CI: 75-96).
Strengths: infants were included according to a standardised definition of ARI, ensuring uniformity of the inclusion criteria. The presence of RSV was systematically tested, reducing the risk of misdiagnosis or missed diagnosis. The presence of Nirsevimab in the immunisation computer systems or medical records of all newborns was verified, ensuring adequate recording of treatments. Estimating the effectiveness of Nirsevimab under real-world conditions was crucial, especially for children at high risk of severe disease (aged between 8 and 19 months) for whom Nirsevimab administration before the start of the second RSV season is recommended.
Limitations: only a small proportion of infants hospitalised with ARI received Nirsevimab, likely due to delayed and intermittent supply availability, as well as selection criteria favouring infants with baseline conditions. This prevented stratified estimates based on time since Nirsevimab administration. Some infants may have contracted RSV before receiving Nirsevimab, which could impact the estimated effectiveness of the drug. Since the dosage of Nirsevimab has not been established, it was impossible to assess dose-related effectiveness. Finally, effectiveness was only evaluated in preventing RSV-associated hospitalisation, without considering other outcomes such as outpatient and emergency room visits, which could be equally significant.
France
In France, in response to the increased intensity of recent RSV outbreaks, bronchiolitis surveillance has been reinforced for the 2023-2024 season through a multicentre network of volunteer PICUs coordinated by Santé Publique France [45].
Using surveillance data from this network, a case-control study based on the TND was conducted to evaluate the effectiveness of Nirsevimab in PICU hospitalisation for RSV bronchiolitis in metropolitan France between 15 September 2023 and 31 January 2024. Infants aged 0 to 8 months (including premature and comorbid) who had received Nirsevimab were included (319). Infants who tested positive for RSV were considered cases, while negative infants were considered controls. Exclusion criteria included lack of etiological research, unknown prior treatment against RSV other than Nirsevimab, unknown comorbidities/ prematurity, or missing data. In the main analysis, 31 infants who had received Nirsevimab <8 days before PICU admission or those with an unknown date of receipt were excluded. A second sensitivity analysis included 312 infants (aged > 1 month) in whom Nirsevimab administration had occurred more than 8 days before PICU admission. A final sensitivity analysis included all infants (319) who had received Nirsevimab, regardless of the time elapsed between administration and PICU admission. Two periods were defined based on RSV detection rates: low circulation from 15 September to 29 October 2023 and high circulation from 30 October 2023 to 07 January 2024. The effectiveness of Nirsevimab was estimated with a logistic regression model, adjusted for age group, gender, comorbidity, prematurity, and period.
Results: among the 542 reported cases of severe bronchiolitis, 288 infants admitted to 20 PICUs were included in the main analysis, of whom the majority (91%) were aged 0-3 months and 55% were male. RSV was identified in 83% of the infants, while rhinovirus was the most common pathogen in the controls. The cases were younger and had fewer males than the controls. During the study, 20% of the infants had received Nirsevimab at least 8 days before PICU admission, with an average delay of 35 days.
In the main analysis, the adjusted effectiveness of Nirsevimab against RSV bronchiolitis cases admitted to the PICU was estimated at 75.9% (95% CI: 48.5-88.7). In sensitivity analyses, the effectiveness of Nirsevimab was estimated at 80.6% (95% CI: 61.6-90.3%) for SA1 and 80.4% (95% CI: 61.7-89.9%) for SA2.
Strengths: the study found the high effectiveness of Nirsevimab in preventing severe RSV bronchiolitis in infants admitted to the PICU, with estimates ranging from 75.9% (95% CI: 48.5-88.7) to 80.6% (95% CI: 61.6-90.3) depending on the assumptions (≤ 8 days vs ≥ 8 days) in line with results from other clinical and surveillance studies. The TND allowed rapid estimation of effectiveness based on surveillance data, reducing confounding bias and providing meaningful results.
Limitations: The small sample size, especially for controls, limited the ability to perform subgroup analyses or to match cases and controls, thereby reducing the precision of the estimates. Although sensitivity analyses were conducted, there could be a bias if administration dates and prior treatment were reported less frequently when the pathogen was not RSV, leading to an underestimation of effectiveness. Furthermore, while the TND reduced confounding bias, bias may still exist if the use of healthcare differs between infants treated or not treated with Nirsevimab depending on the severity of the disease.
Italy
This is prospective observational cohort study examines the incidence of hospitalisation for RSV bronchiolitis or pneumonia in two groups of children, one receiving Nirsevimab prophylaxis and the other not [46]. The objectives include assessing Nirsevimab’ safety and optimising its distribution. In Italy Nirsevimab became available from 20 December 2023, however, all children born in Valle d’Aosta between 1 May 2023 and 15 February 2024(556) were included in the study, excluding those with risk factors already treated with Palivizumab and non-residents [20]. The subjects were categorised into in-season RSV births (95, for those born between 19/12/2023 and 15/02/2024) and out-of-season RSV births (461, for those born between 01/05/2023 and 18/12/2023). Eligibility for prophylaxis was determined via local information systems, and distribution was ensured by the Service of Hygiene and Public Health. Telephone interviews were conducted 7 and 14 days after Nirsevimab administration to monitor side effects, while data on hospitalisations for RSV bronchiolitis were obtained from local information systems. This approach allowed a comprehensive evaluation of the efficacy and safety of Nirsevimab, providing crucial information on its impact on hospitalisations and RSV-related adverse events.
Results: 69% of the 537 candidates for Nirsevimab’s prophylaxis (89 born in the RSV season and 448 born out of RSV season) adhered with a coverage rate of 86% (77/89) in in-season births and 65% (292/448) in out-of-season births. During the RSV season 2023-2024, 29 RSV bronchiolitis hospitalisations occurred (18 recorded for children born after May 1, 2023), compared to 61 (47 recorded for children born after May 1, 2022) in the previous season. Up to 20 December 2023, no infants treated with Nirsevimab were hospitalised for bronchiolitis, compared to 8.3% (14/168) of those not treated. Side effects of Nirsevimab were mild and short-lived, with no cases of serious complications. These results suggest effective prevention of RSV infections and good tolerability of Nirsevimab in the study population.
Strengths: the study demonstrates the effectiveness and safety of universal prophylaxis with Nirsevimab in infants, positioning Valle d’Aosta as pioneering Italian region in extending universal prophylaxis to infants without risk factors for RSV complications. Infants who received Nirsevimab showed a significant reduction in the risk of RSV-related hospitalization compared to those who did not, consistent with findings from other countries. No infants immunised with Nirsevimab required hospitalisation for RSV bronchiolitis, indicating robust effectiveness in preventing RSV infections in this vulnerable population. The universal prevention approach emerges as a compelling strategy, ensuring broad coverage and equitable access, and significantly improving health protection of infants and children. Evidence is the significant drop in hospitalisations for RSV infection in children not in their first epidemic season, suggesting that the program has had a real impact regardless of the reduction in epidemic frequency. The cost-effectiveness of universal prophylaxis with Nirsevimab is underlined by the direct savings resulting directly from the hospitalisation reduction and the discontinuation of selective prophylaxis with Palivizumab, as well as by the indirect savings resulting from decreased social care costs incurred by parents for RSV-infected children.
Limitations: the study was conducted in a small Italian region, so it may not fully reflect the complexities of larger and more diverse populations. Despite the promising results, further economic evaluations are needed to assess the cost-effectiveness of Nirsevimab compared to standard therapy. While the results are encouraging, it is important to continue surveillance to monitor any emergent patterns of resistance or changes in RSV epidemiology following the widespread adoption of Nirsevimab prophylaxis.
TRIALS IN PROGRESS
There are ongoing clinical trials evaluating efficacy and effectiveness: JUBILUS (NCT06042049) – Japan [48] CHIMES (NCT05110261) – China [49]; ENVIE (NCT06030505) – France [50]; EPINIR-BRON (NCT06185647) – France [51] and BEAR (NCT06325332) – US [52].
The JUBILUS (NCT06042049) clinical trial is a phase 3, single-arm, open-label study aimed at evaluating the safety, pharmacokinetics, and immune response to the administration of two doses of Nirsevimab to infants with immunodeficiency, CLD, CHD, Down syndrome or premature birth. The goal is to recruit 33 patients by April 2025, excluding those with a seizure disorder, on respiratory support, scheduled for cardiac surgery within six months [48].
The CHIMES study (NCT05110261) is a phase 3, double-blind, placebo-controlled trial evaluating the efficacy and safety of a single dose of Nirsevimab in healthy preterm and term infants in the first year of life. It is expected to enrol 800 children, with an estimated completion date of November 2025. Subjects meeting the criteria for treatment with Palivizumab are excluded from the study [49].
ENVIE (NCT06030505) is an observational case-control study to assess the effectiveness of Nirsevimab in children hospitalised with RSV bronchiolitis. The study plans to enrol 963 children < 12 months treated for RSV bronchiolitis (cases) and hospitalised for other causes (controls). Subjects who received Palivizumab or maternal RSV vaccination were excluded from the study. Enrolment started on October 17, 2023, with an estimated completion date of July 15, 2026. Secondary outcomes included the proportion of children hospitalised for RSV bronchiolitis requiring invasive or non-invasive ventilation, the duration of hospitalisation, the time between immunisation with Nirsevimab and hospitalisation for RSV bronchiolitis, the monthly frequency of hospitalisation for RSV bronchiolitis, and the proportion of children hospitalised for RSV bronchiolitis with concomitant viral detection or other than RSV on nasopharyngeal swab [50].
The retrospective observational study EPINIR-BRON (NCT06185647) started on October 14, 2023, and ended on February 29, 2024. The aim is to evaluate the Nirsevimab’s ffectiveness in a real-life setting in the use of emergency rooms to treat bronchiolitis and to reduce hospitalisations in France. For the study, all infants aged < 12 months were enrolled, and two separate analyses were performed: a comparative analysis between infants who received Nirsevimab and those who did not receive it before their ER visit for bronchiolitis, and a comparison between infants diagnosed with bronchiolitis who received Nirsevimab and those who did not, to assess the drug’s effectiveness on ER use and hospitalisation. The results have not yet been made public [51].
Lastly, the BEAR trial (NCT06325332) conducted in the United States (start 01/4/2024 and end 01/04/2025), involves the inclusion of 33000 infants born ≥ 37 weeks’ gestation and aged 0-12 months facing their first season of RSV, with no significant medical history. The study is a retrospective observational cohort investigation designed to evaluate the effectiveness of Nirsevimab in preventing RSV-related medical visits and healthcare utilisation in infants facing their first season of RSV in the United States. Current primary outcome measures include the incidence of PCR-confirmed first RSV episodes with an ICD-identified diagnosis of LRTD, along with the number of physician visits associated with these episodes. Current secondary outcome measures assess the number of respiratory and LRTD-related physician visits, along with the effect of Nirsevimab on RSV confirmation by PCR, otitis media diagnoses, and antibiotic prescribing [52].
Conclusions
Studies examining the effectiveness of Nirsevimab in preventing hospitalisations due to RSV infections in infants provide a detailed overview of the benefits of this immunoprophylaxis.
Consistently high immunisation coverage rates with Nirsevimab have been reported, indicating successful implementation of immunisation programs with efficient and effective distribution among target populations in different countries.
Effectiveness estimates slightly varied from region to region, but demonstrated significant protection against RSV hospitalisations. Data on a meaningful reduction in hospitalisations for RSV infection among immunised infants were reported in all studies. In particular, the Spanish – Galician and Catalan – studies reported significant effectiveness of Nirsevimab in reducing the risk of hospitalisation and admission to PCIU for RSV- LRTIs, with variations from 86.9 % (95% CI: 69.1-94.2) to 90.1% (95% CI: 76.3-95.9) against PICU admissions and from 82.0% (95% CI: 65.6-90.2) to 87.6% (95% CI: 82.1-91.4) against hospital admissions [42, 43].
All the studies reviewed provide evidence of the effectiveness of immunisation with Nirsevimab in real-world settings, beyond the controlled conditions of clinical trials, and highlight its safety and feasibility. Once again, the Galician study -NIRSE-GAL- and the Catalan study stood out as they integrated primary care data with hospital data allowing a comprehensive analysis of the impact of the immunisation program on the reduction of RSV-related outcomes. Specifically, the NIRSE-GAL study established and utilised a primary care surveillance program that retrieves data on all participants eligible for Nirsevimab from several registries in the SERGAS information system [38, 41-43].
Although the studies provide valuable insights into the efficacy of Nirsevimab, there are limitations to consider.
Delayed and intermittent availability of Nirsevimab supply, selection criteria favouring infants with underlying conditions, and small sample sizes limited the ability to conduct subgroup analyses or match cases and controls in some studies. Furthermore, despite efforts to standardise inclusion criteria and verify the presence of Nirsevimab in immunisation registries, hospital admission policies, and case definitions differed between countries. Potential confounding due to the observational nature of the data and the limited duration of the analysis should also be considered, resulting in a lack of long-term data that could limit the understanding of Nirsevimab’s long-term effectiveness. Another consideration is the variation in RSV circulation across seasons. To achieve effective immunisation, it will be essential to accurately determine the beginning and end of the RSV season, which could be complicated, as evidenced by the disruptions caused by the COVID-19 pandemic [53]. As it is already the case in Galicia, ongoing surveillance will be necessary to comprehensively assess the circulation of RSV and monitor any emerging patterns of resistance or changes in RSV epidemiology following widespread vaccine uptake.
Our work has not included and examined cost-effectiveness studies: a thorough economic evaluation will be necessary to ensure the best use of Nirsevimab in different settings. Additional data that could complement and confirm the already proven effectiveness of the product are represented by long-term effects and epidemiological trends of RSV.
To comprehensively evaluate the effectiveness of Nirsevimab, it is essential to emphasise the importance of continuous research and surveillance in various settings. The results of ongoing clinical trials will be crucial, as they will examine the effectiveness of Nirsevimab in several different medical situations and conditions. Specifically, the JUBILUS trial in Japan will provide data on the safety, pharmacokinetics, and immune response of two doses of Nirsevimab administered to high-risk newborns. Similarly, the CHIMES trial in China will provide valuable information on effectiveness in preterm infants [48, 49].
Most of the results summarised in this work were instrumental in enabling international health authorities to promote the recommendation of Nirsevimab use in all infants and children in their first season of RSV. The first countries to recommend its use were the USA [21, 25, 26], France [54], Luxembourg [37], Spain [55], Chile [56] and Australia [57]. For the 2024/2025 season, seven other countries followed suit, recommending the universal implementation of Nirsevimab: Netherlands [58], England [59], Germany [60], Austria [61], Canada [62], Belgium [63] and Italy [64].
In particular, in Italy, the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI) and the Italian Society of Infectious and Tropical Diseases (SIMIT) suggest considering the availability of Nirsevimab as an important universal preventive tool against RSV diseases. They emphasise the need to consider Nirsevimab immunisation as a vaccine programme in regulatory and organisational documents, intended to cover the entire cohort of infants, recognising Nirsevimab as prevention and not treatment [65].
Overall, available data provide valuable insights into the efficacy, safety, and impact of immunisation with Nirsevimab in preventing severe RSV infections in infants, highlighting its potential to reduce the burden of RSV-related hospitalisations and improve paediatric health outcomes.
Fundings
This study did not receive any specific funding.
Conflict of interest statement
AO, V.B, MC, SG, EM, EP, CR, DP and GI: provided consultation and/or received speaker fees and/or received grants to conduct experimental and/or observational studies from CSL Seqirus, GSK, Moderna, Novavax, Pfizer, Sanofi, Viatris, MSD and AstraZeneca. MS and FB: declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author’s contributions
AO, MS, GI: conceptualization; AO, MS, DP: methodology; AO, MS: formal analysis; AO, MS: investigation; AO, DP, GI: resources; AO, MS: data analysis; AO, MS: writing-original draft preparation; VB, FB, MC, SG, EM, EP, CR: writing-review and editing; AO, DP, GI: visualization; DP, GI: supervision. All authors have read and agreed to the current version of the manuscript.
History
Received on July 1, 2024. Accepted on July 15, 2024.
Figures and tables
References
- [1].Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M, Manzoni P, Mazzone T, Sandri F, Checcucci Lisi G, Parisi S, Piacentini G, Mosca F. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr 2021;47:198. https://doi.org/10.1186/s13052-021-01148-8 10.1186/s13052-021-01148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dovizio M, Veronesi C, Bartolini F, Cavaliere A, Grego S, Pagliaro R, Procacci C, Ubertazzo L, Bertizzolo L, Muzii B, Parisi S, Perrone V, Baraldi E, Bozzola E, Mosca F, Esposti LD. Clinical and economic burden of respiratory syncytial virus in children aged 0-5 years in Italy. Ital J Pediatr 2024;50:57. https://doi.org/10.1186/s13052-024-01628-7 10.1186/s13052-024-01628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baraldi E, Lanari M, Manzoni P, Rossi GA, Vandini S, Rimini A, Romagnoli C, Colonna P, Biondi A, Biban P, Chiamenti G, Bernardini R, Picca M, Cappa M, Magazzù G, Catassi C, Urbino AF, Memo L, Donzelli G, Minetti C, Paravati F, Di Mauro G, Festini F, Esposito S, Corsello G. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital J Pediatr 2014;40:65. https://doi.org/10.1186/1824-7288-40-65 10.1186/1824-7288-40-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sommer C, Resch B, Simões EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J 2011;5:144-54. https://doi.org/10.2174/1874285801105010144 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barbieri E, Cavagnis S, Scamarcia A, Cantarutti L, Bertizzolo L, Bangert M, Parisi S, Cantarutti A, Baraldi E, Giaquinto C, Baldo V. Assessing the burden of bronchiolitis and lower respiratory tract infections in children ≤ 24 months of age in Italy, 2012-2019. Front Pediatr 2023;11:1143735. https://doi.org/10.3389/fped.2023.1143735 10.3389/fped.2023.1143735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rha B, Curns AT, Lively JY, Campbell AP, Englund JA, Boom JA, Azimi PH, Weinberg GA, Staat MA, Selvarangan R, Halasa NB, McNeal MM, Klein EJ, Harrison CJ, Williams JV, Szilagyi PG, Singer MN, Sahni LC, Figueroa-Downing D, McDaniel D, Prill MM, Whitaker BL, Stewart LS, Schuster JE, Pahud BA, Weddle G, Avadhanula V, Munoz FM, Piedra PA, Payne DC, Langley G, Gerber SI. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015-2016. Pediatrics 2020;146:e20193611. https://doi.org/10.1542/peds.2019-3611 10.1542/peds.2019-3611. [DOI] [PubMed] [Google Scholar]
- [7].Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simões EAF, Campbell H, Pariente AB, Bardach D, Bassat Q, Casalegno JS, Chakhunashvili G, Crawford N, Danilenko D, Do LAH, Echavarria M, Gentile A, Gordon A, Heikkinen T, Huang QS, Jullien S, Krishnan A, Lopez EL, Markić J, Mira-Iglesias A, Moore HC, Moyes J, Mwananyanda L, Nokes DJ, Noordeen F, Obodai E, Palani N, Romero C, Salimi V, Satav A, Seo E, Shchomak Z, Singleton R, Stolyarov K, Stoszek SK, von Gottberg A, Wurzel D, Yoshida LM, Yung CF, Zar HJ, Respiratory Virus Global Epidemiology Network. Nair H, RESCEU investigators . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:2047-64. https://doi.org/10.1016/S0140-6736(22)00478-0 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuhdari P, Brosio F, Malaventura C, Stefanati A, Orsi A, Icardi G, Gabutti G. Human respiratory syncytial virus and hospitalization in young children in Italy. Ital J Pediatr 2018;44:50. https://doi.org/10.1186/s13052-018-0492-y 10.1186/s13052-018-0492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barbati F, Moriondo M, Pisano L, Calistri E, Lodi L, Ricci S, Giovannini M, Canessa C, Indolfi G, Azzari C. Epidemiology of Respiratory Syncytial Virus-Related Hospitalization Over a 5-Year Period in Italy: Evaluation of Seasonality and Age Distribution Before Vaccine Introduction. Vaccines (Basel) 2020;8:15. https://doi.org/10.3390/vaccines8010015 10.3390/vaccines8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fortunato F, Campanozzi A, Maffei G, Arena F, Carri VD, Rollo T, Lopalco PL, Martinelli D. Respiratory syncytial virus-associated hospitalizations among children: an Italian retrospective observational study. Ital J Pediatr 2024;50:45. https://doi.org/10.1186/s13052-024-01617-w 10.1186/s13052-024-01617-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scarpaci M, Bracaloni S, Esposito E, De Angelis L, Baglivo F, Casini B, Panatto D, Ogliastro M, Loconsole D, Chironna M, Pariani E, Pellegrinelli L, Pandolfi E, Croci I, Rizzo C, RSVComNet Italia . RSV Disease Burden in Primary Care in Italy: A Multi-Region Pediatric Study, Winter Season 2022-2023. Influenza Other Respir Viruses 2024;18:e13282. https://doi.org/10.1111/irv.13282 10.1111/irv.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Istituto Superiore di Sanità. Rapporto RespiVirNet Virologico 2024-17. Available at: https://respivirnet.iss.it/pagine/rapportoInflunet.aspx (Accessed on: 14/05/2024).
- [13].Sparrow E, Adetifa I, Chaiyakunapruk N, Cherian T, Fell DB, Graham BS, Innis B, Kaslow DC, Karron RA, Nair H, Neuzil KM, Saha S, Smith PG, Srikantiah P, Were F, Zar HJ, Feikin D. WHO preferred product characteristics for monoclonal antibodies for passive immunization against respiratory syncytial virus (RSV) disease in infants - Key considerations for global use. Vaccine 2022;40:3506-10. https://doi.org/10.1016/j.vaccine.2022.02.040 10.1016/j.vaccine.2022.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, April 2016 - Conclusions and recommendations. Wkly Epidemiol Rec 2016;91:265-84.27236868 [Google Scholar]
- [15].Esposito S, Abu Raya B, Baraldi E, Flanagan K, Martinon Torres F, Tsolia M, Zielen S. RSV Prevention in All Infants: Which Is the Most Preferable Strategy? Front Immunol. 2022;13:880368. https://doi.org/10.3389/fimmu.2022.880368 10.3389/fimmu.2022.880368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].EMA. European Medicines Agency: Beyfortus. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus (Accessed on: May 14, 2024).
- [17].AIFA. Agenzia Italiana del Farmaco: Beyfortus. Available at: https://www.aifa.gov.it/documents/20142/1805944/DETERMINA_9-2023_BEYFORTUS.pdf (Accessed on: 14/05/2024).
- [18].EMA. European Medicines Agency: Abrysvo. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo (Accessed on: 14/05/2024).
- [19].AIFA. Agenzia Italiana del Farmaco: Abrysvo. Available at: https://www.aifa.gov.it/documents/20142/2128501/DETERMINA_127-2023_ABRYSVO.pdf (Accessed on: 14/05/2024).
- [20].AstraZeneca Sanofi Pasteur Beyfortus (nirsevimab - alip) [Prescribing information]. 2023. Available at: https://products.sanofi.us/beyfortus/beyfortus.pdf (Accessed on: 14/05/2024).
- [21].US Sanofi-Aventis. Available at: https://www.news.sanofi.us/2023-08-03-U-S-CDC-Advisory-Committee-unanimously-recommends-routine-use-of-Beyfortus-TM-nirsevimab-alip-to-protect-infants-against-RSV-disease [Online] 03-08-2023 (Accessed on: 14/05/2024).
- [22].Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, Villafana T, DeVincenzo JP, Nirsevimab Study Group . Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020;383:415-25. https://doi.org/10.1056/NEJMoa1913556 10.1056/NEJMoa1913556. [DOI] [PubMed] [Google Scholar]
- [23].Zhu Q, McLellan JS, Kallewaard NL, Ulbrandt ND, Palaszynski S, Zhang J, Moldt B, Khan A, Svabek C, McAuliffe JM, Wrapp D, Patel NK, Cook KE, Richter BWM, Ryan PC, Yuan AQ, Suzich JA. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017;9:eaaj1928. https://doi.org/10.1126/scitranslmed.aaj1928 10.1126/scitranslmed.aaj1928. [DOI] [PubMed] [Google Scholar]
- [24].Brady T, Cayatte C, Roe TL, Speer SD, Ji H, Machiesky L, Zhang T, Wilkins D, Tuffy KM, Kelly EJ. Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection. Front Immunol 2023;14:1283120. https://doi.org/10.3389/fimmu.2023.1283120 10.3389/fimmu.2023.1283120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].ACIP. Evidence to Recommendations for Use of Nirsevimab in Infants born during the RSV season or entering their first RSV season. Available at: https://www.cdc.gov/vaccines/acip/recs/grade/Nirsevimab-season1-rsv-infants-children-etr.html (Accessed on: 14/05/2024).
- [26].AAP. Recommendations for the Prevention of RSV Disease in Infants and Children. Available at: https://publications.aap.org/redbook/resources/25379/AAP-Recommendations-for-the-Prevention-of-RSV?autologincheck=redirected (Accessed on: 14/05/2024).
- [27].Jones JM, Fleming-Dutra KE, Prill MM, Roper LE, Brooks Sánchez PJ, Kotton CN, Mahon BE, Meyer S, Long SS, McMorrow ML. Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2023;72:920-5. https://doi.org/10.15585/mmwr.mm7234a4 10.15585/mmwr.mm7234a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balbi H. Nirsevimab: A Review. Pediatr Allergy Immunol Pulmonol 2024;37:3-6. https://doi.org/10.1089/ped.2024.0025 10.1089/ped.2024.0025. [DOI] [PubMed] [Google Scholar]
- [29].Hammitt LL, Dagan R, Yuan Y, Cots MB, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Brooks D, Grenham A, Wählby Hamrén U, Mankad VS, Ren P, Takas T, Abram ME, Leach A, Griffin MP, Villafana T, MELODY Study Group . Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N Engl J Med 2022;386:837-46. https://doi.org/10.1056/NEJMoa2110275 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- [30].Esposito S, Abu-Raya B, Bonanni P, Cahn-Sellem F, Flanagan KL, Martinon Torres F, Mejias A, Nadel S, Safadi MAP, Simon A. Coadministration of Anti-Viral Monoclonal Antibodies With Routine Pediatric Vaccines and Implications for Nirsevimab Use: A White Paper. Front Immunol 2021;12:708939. https://doi.org/10.3389/fimmu.2021.708939 10.3389/fimmu.2021.708939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muller WJ, Madhi SA, Seoane Nuñez B, Baca Cots M, Bosheva M, Dagan R, Hammitt LL, Llapur CJ, Novoa JM, Saez Llorens X, Grenham A, Kelly EJ, Mankad VS, Shroff M, Takas T, Leach A, Villafana T, MELODY Study Group . Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. N Engl J Med 2023;388:1533-34. https://doi.org/10.1056/NEJMc2214773 10.1056/NEJMc2214773. [DOI] [PubMed] [Google Scholar]
- [32].Domachowske J, Madhi SA, Simões EAF, Atanasova V, Cabañas F, Furuno K, Garcia-Garcia ML, Grantina I, Nguyen KA, Brooks D, Chang Y, Leach A, Takas T, Yuan Y, Griffin MP, Mankad VS, Villafana T, MEDLEY Study Group . Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N Engl J Med 2022;386:892-94. https://doi.org/10.1056/NEJMc2112186 10.1056/NEJMc2112186. [DOI] [PubMed] [Google Scholar]
- [33].Domachowske J, Wählby Hamren U, Basavaraju B, Koen A, Leach A, Mankad VS, Mori M, Ndibmun C, Soler-Palacin P, Pannaraj PS, Takas T, Villafanaet T. Safety, Tolerability, and Pharmacokinetics of Nirsevimab for the Prevention of RSV Disease in Immunocompromised Children Aged ≤ 24 Months: Music, an Open Label, Phase 2 Trial. Blood 2023;142(Suppl 1):1173. https://doi.org/10.1182/blood-2023-189096 10.1182/blood-2023-189096.37768692 [DOI] [Google Scholar]
- [34].Simões EAF, Madhi SA, Muller WJ, Atanasova V, Bosheva M, Cabañas F, Baca Cots M, Domachowske JB, Garcia-Garcia ML, Grantina I, Nguyen KA, Zar HJ, Berglind A, Cummings C, Griffin MP, Takas T, Yuan Y, Wählby Hamrén U, Leach A, Villafana T. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc Health 2023;7:180-9. https://doi.org/10.1016/S2352-4642(22)00321-2 10.1016/S2352-4642(22)00321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, Kaiser F, Cohen R, Pinquier D, Felter CT, Vassilouthis NC, Jin J, Bangert M, Mari K, Nteene R, Wague S, Roberts M, Tissières P, Royal S, Faust SN, HARMONIE Study Group . Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N Engl J Med 2023;389:2425-35. https://doi.org/10.1056/NEJMoa2309189 10.1056/NEJMoa2309189. [DOI] [PubMed] [Google Scholar]
- [36].Hak SF, Venekamp RP, Wildenbeest JG, Bont LJ. Outpatient respiratory syncytial virus infections and novel preventive interventions. Curr Opin Pediatr 2024;36:171-81. https://doi.org/10.1097/MOP.0000000000001323 10.1097/MOP.0000000000001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ernst C, Bejko D, Gaasch L, Hannelas E, Kahn I, Pierron C, Del Lero N, Schalbar C, Do Carmo E, Kohnen M, Andlauer E, Hublart P, Masi S, de la Fuente Garcia I, Vergison A, Mossong J. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Euro Surveill 2024;29:2400033. https://doi.org/10.2807/1560-7917.ES.2024.29.4.2400033 10.2807/1560-7917.ES.2024.29.4.2400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martinón-Torres F, Mirás-Carballal S, Durán-Parrondo C. Early lessons from the implementation of universal respiratory syncytial virus prophylaxis in infants with long-acting monoclonal antibodies, Galicia, Spain, September and October 2023. Euro Surveill 2023;28:2300606. https://doi.org/10.2807/1560-7917.ES.2023.28.49.2300606 10.2807/1560-7917.ES.2023.28.49.2300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].López-Lacort M, Muñoz-Quiles C, Mira-Iglesias A, López-Labrador FX, Mengual-Chuliá B, Fernández-García C, Carballido-Fernández M, Pineda-Caplliure A, Mollar-Maseres J, Shalabi Benavent M, Sanz-Herrero F, Zornoza-Moreno M, Pérez-Martín JJ, Alfayate-Miguelez S, Pérez Crespo R, Bastida Sánchez E, Menasalvas-Ruiz AI, Téllez-González MC, Esquiva Soto S, Del Toro Saravia C, Sanz-Muñoz I, Eiros JM, Matías Del Pozo V, Toquero-Asensi M, Pastor-Villalba E, Lluch-Rodrigo JA, Díez-Domingo J, Orrico-Sánchez A. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Euro Surveill 2024;29:2400046. https://doi.org/10.2807/1560-7917.ES.2024.29.6.2400046 10.2807/1560-7917.ES.2024.29.6.2400046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ezpeleta G, Navascués A, Viguria N, Herranz-Aguirre M, Juan Belloc SE, Gimeno Ballester J, Muruzábal JC, García-Cenoz M, Trobajo-Sanmartín C, Echeverria A, Martínez-Baz I, Vera-Punzano N, Casado I, López-Mendoza H, Ezpeleta C, Castilla J. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines (Basel) 2024;12:383. https://doi.org/10.3390/vaccines12040383 10.3390/vaccines12040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mallah N, Ares-Gómez S, Pardo-Seco J, Malvar-Pintos A, Santiago-Pérez MI, Pérez-Martínez O, Otero-Barrós MT, Suárez-Gaiche N, Kramer R, Jin J, Platero-Alonso L, Alvárez-Gil RM, Ces-Ozores OM, Nartallo-Penas V, Mirás-Carballal S, Piñeiro-Sotelo M, González-Pérez JM, Rodríguez-Tenreiro C, Rivero-Calle I, Salas A, Durán-Parrondo C, Martinón-Torres F. Assessment of effectiveness and impact of universal prophylaxis with nirsevimab for prevention of hospitalizations due to respiratory syncytial virus in infants. The NIRSE-GAL study protocol. Hum Vaccin Immunother 2024;20:2348135. https://doi.org/10.1080/21645515.2024.2348135 10.1080/21645515.2024.2348135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ares-Gómez S, Mallah N, Santiago-Pérez MI, Pardo-Seco J, Pérez-Martínez O, Otero-Barrós MT, Suárez-Gaiche N, Kramer R, Jin J, Platero-Alonso L, Alvárez-Gil RM, Ces-Ozores OM, Nartallo-Penas V, Mirás-Carballal S, Piñeiro-Sotelo M, Malvar-Pintos A, González-Pérez JM, Rodríguez-Tenreiro-Sánchez C, Rivero-Calle I, Salas A, Durán-Parrondo C, Martinón-Torres F, NIRSE-GAL study group . Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study. Lancet Infect Dis 2024;30:S1473-3099(24)00215-9. https://doi.org/10.1016/S1473-3099(24)00215-9 10.1016/S1473-3099(24)00215-9. Erratum in: Lancet Infect Dis 2024;23:S1473-3099(24)00355-4. https://doi.org/10.1016/S1473-3099(24)00355-4. [DOI] [PubMed] [Google Scholar]
- [43].Coma E, Martinez-Marcos M, Hermosilla E, Mendioroz J, Reñé A, Fina F, Perramon-Malavez A, Prats C, Cereza G, Ciruela P, Pineda V, Antón A, Ricós-Furió G, Soriano-Arandes A, Cabezas C. Effectiveness of nirsevimab immunoprophylaxis against respiratory syncytial virus-related outcomes in hospital and primary care settings: a retrospective cohort study in infants in Catalonia (Spain). Arch Dis Child 2024;10:archdischild-2024-327153. https://doi.org/10.1136/archdischild-2024-327153 10.1136/archdischild-2024-327153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moline HL, Tannis A, Toepfer AP, Williams JV, Boom JA, Englund JA, Halasa NB, Staat MA, Weinberg GA, Selvarangan R, Michaels MG, Sahni LC, Klein EJ, Stewart LS, Schlaudecker EP, Szilagyi PG, Schuster JE, Goldstein L, Musa S, Piedra PA, Zerr DM, Betters KA, Rohlfs C, Albertin C, Banerjee D, McKeever ER, Kalman C, Clopper BR, New Vaccine Surveillance Network Product Effectiveness Collaborators. McMorrow ML, Dawood FS. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus-Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season - New Vaccine Surveillance Network, October 2023-February 2024. MMWR Morb Mortal Wkly Rep 2024;73:209-14. https://doi.org/10.15585/mmwr.mm7309a4 10.15585/mmwr.mm7309a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Paireau J, Durand C, Raimbault S, Cazaubon J, Mortamet G, Viriot D, Milesi C, Daudens-Vaysse E, Ploin D, Tessier S, Vanel N, Chappert JL, Levieux K, Ollivier R, Daoudi J, Coignard B, Leteurtre S, Parent-du-Châtelet I, Vaux S. Nirsevimab Effectiveness Against Cases of Respiratory Syncytial Virus Bronchiolitis Hospitalised in Paediatric Intensive Care Units in France, September 2023-January 2024. Influenza Other Respir Viruses 2024;18:e13311. https://doi.org/10.1111/irv.13311 10.1111/irv.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Consolati A, Farinelli M, Serravalle P, Rollandin C, Apprato L, Esposito S, Bongiorno S. Safety and Efficacy of Nirsevimab in a Universal Prevention Program of Respiratory Syncytial Virus Bronchiolitis in Newborns and Infants in the First Year of Life in the Valle d’Aosta Region, Italy, in the 2023-2024 Epidemic Season. Vaccines (Basel) 2024;12:549. https://doi.org/10.3390/vaccines12050549 10.3390/vaccines12050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].NIRSE-GAL Available at: https://www.nirsegal.es/en (Accessed on: 20/05/2024).
- [48].NCT06042049 Clinical Trial. Available at: https://clinicaltrials.gov/study/NCT06042049?cond=NCT06042049.&rank=1 (Accessed on: 22/05/2024).
- [49].NCT05110261 Clinical Trial. Available at: https://clinicaltrials.gov/study/NCT05110261?cond=NCT05110261&rank=1 (Accessed on: 22/05/2024).
- [50].NCT06030505 Clinical Trial. Available at: https://clinicaltrials.gov/study/NCT06030505?cond=NCT06030505&rank=1 (Accessed on: 22/05/2024).
- [51].NCT06185647 Clinical Trial. Available at: https://clinicaltrials.gov/study/NCT06185647?cond=NCT06185647&rank=1 (Accessed on: 22/05/2024).
- [52].NCT06325332 Clinical Trial. Available at: https://clinicaltrials.gov/study/NCT06325332?cond=NCT06325332&rank=1 (Accessed on: 22/05/2024).
- [53].Gebretekle GB, Yeung MW, Ximenes R, Cernat A, Simmons AE, et al. Cost-effectiveness of RSVpreF vaccine and nirsevimab for the prevention of respiratory syncytial virus disease in Canadian infants. MedRxiv 2024.03.21.24304675. https://doi.org/10.1101/2024.03.21.24304675 10.1101/2024.03.21.24304675. [DOI] [PubMed] [Google Scholar]
- [54].Position de la SP2A sur les stratégies de prévention de la Bronchiolite. Available at: https://afpa.org/recommandation/position-de-la-sp2a-sur-les-strategies-de-prevention-de-la-bronchiolite (Accessed on: 28/06/2024).
- [55].Recomendaciones de utilización de nirsevimab para la temporada 2024-2025 en España. Available at: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab.pdf (Accessed on: 28/06/2024).
- [56].Chile adquiere medicamento Nirsevimab para enfrentar virus respiratorio sincicial (VRS). Available at: https://www.gob.cl/noticias/chile-adquiere-medicamento-nirsevimab-para-enfrentar-virus-respiratorio-sincicial-vrs (Accessed on: 28/06/2024).
- [57].Western Australian children first to access protection from RSV. Available at: https://www.wa.gov.au/government/media-statements/Cook-Labor-Government/Western-Australian-children-first-to-access-protection-from-RSV-20240305 (Accessed on: 28/06/2024).
- [58].Immunisation against RSV in the first year of life. Available at: https://www.healthcouncil.nl/binaries/healthcouncil/documenten/advisory-reports/2024/02/14/immunisation-against-rsv-in-the-first-year-of-life/Immunisation-against-RSV-in-the-first-year-of-life-Summary.pdf (Accessed on: 28/06/2024).
- [59].Joint Committee on Vaccination and Immunisation. Available at: https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation (Accessed on: 28/06/2024).
- [60].RSV-Infektionen (Respiratorische Synzytial-Viren). Available at: https://www.rki.de/SharedDocs/FAQ/RSV/FAQ_Liste_RSV.html#FAQId16747596 (Accessed on: 28/06/2024).
- [61].Respiratorisches Synzytial-Virus. Available at: https://www.sozialministerium.at/dam/jcr:eb64732e-1747-400a-beeb6d069f781182/Impfplan_%C3%96sterreich_2023_2024_Version1.0.pdf (Accessed on: 28/06/2024).
- [62].Respiratory syncytial virus (RSV): Canadian Immunization Guide. Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/respiratory-syncytial-virus.html (Accessed on: 28/06/2024).
- [63].Preventive strategies against RSV disease in children. Available at: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/20231222_shc-9760_advice_rsv_children_vweb.pdf. Accessed on: 28/06/2024.
- [64].Misure di prevenzione e immunizzazione contro il virus respiratorio sinciziale (VRS). Available at: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2024&codLeg=99716&parte=1%20&serie=null (Accessed on: 28/06/2024).
- [65].Prevenzione delle infezioni da Virus Respiratorio Sinciziale nella popolazione italiana Available at: https://www.igienistionline.it/docs/2024/03rsv.pdf?_sm_nck=1 (Accessed on: 28/06/2024).


