Abstract
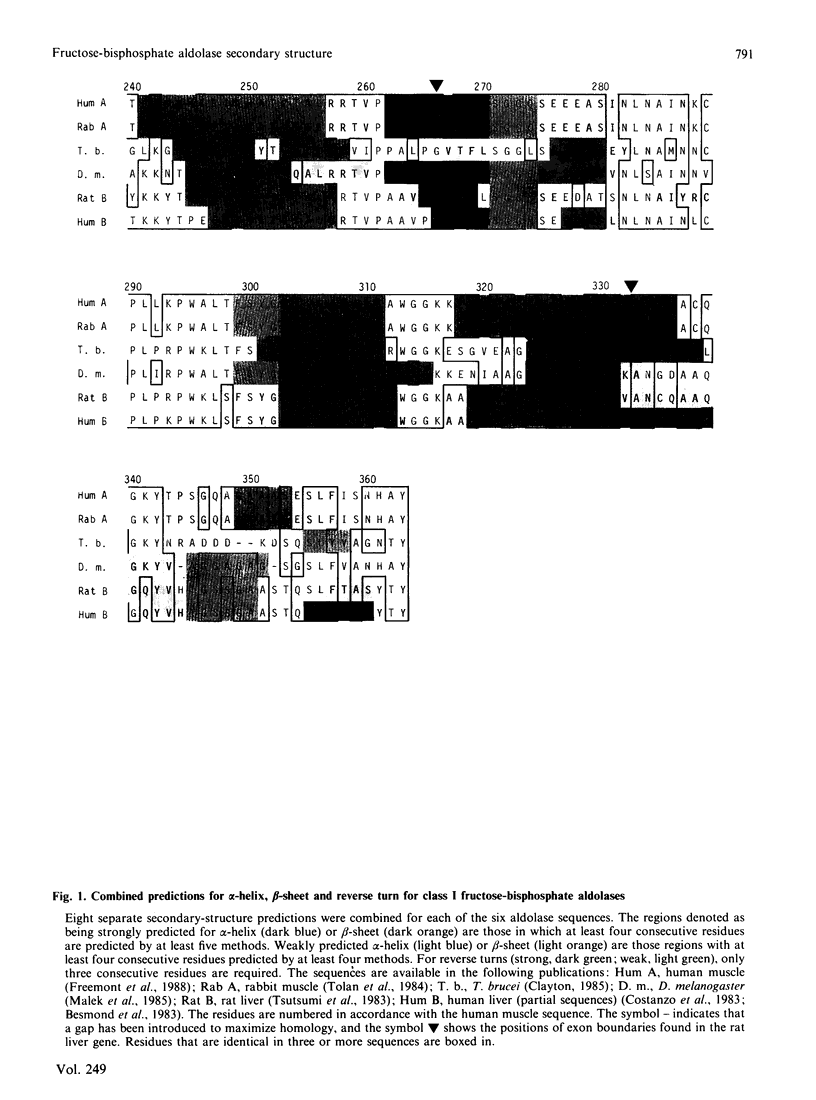
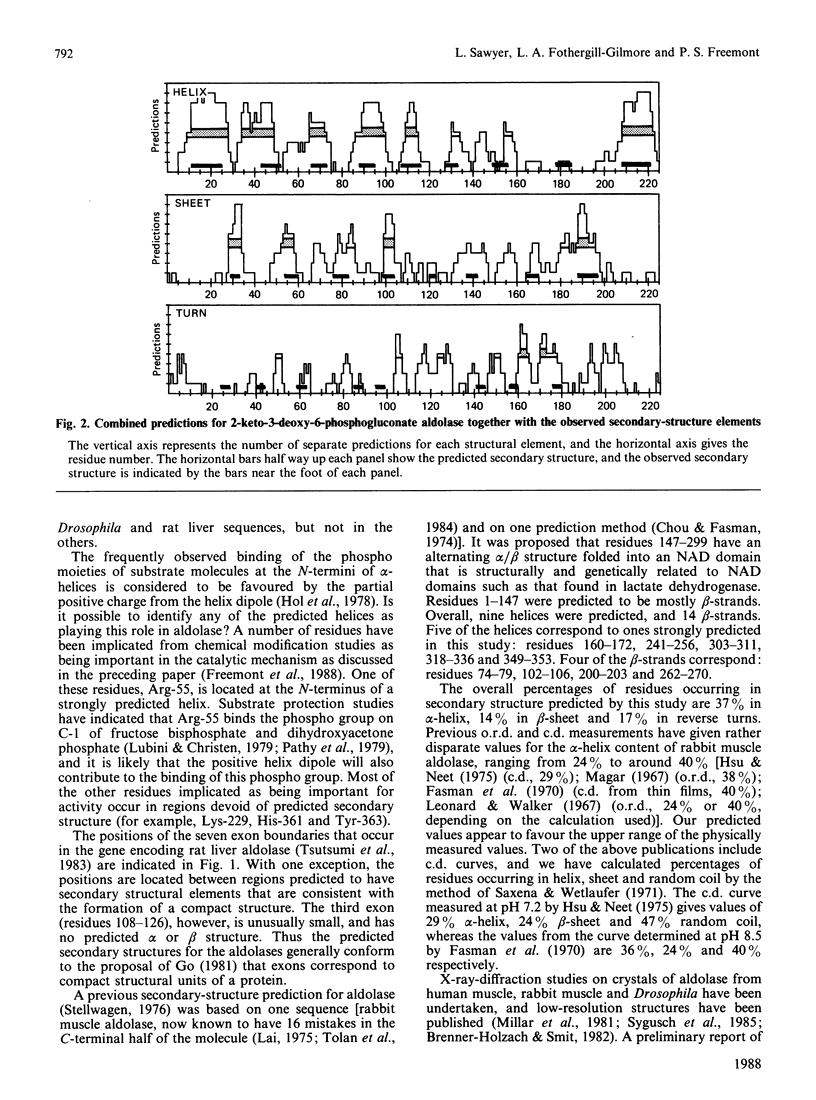
The results of several secondary-structure prediction programs were combined to produce an estimate of the regions of alpha-helix, beta-sheet and reverse turns for fructose-bisphosphate aldolases from human and rat muscle and liver, from Trypanosoma brucei and from Drosophila melanogaster. All the aldolase sequences gave essentially the same pattern of secondary-structure predictions despite having sequences up to 50% different. One exception to this pattern was an additional strongly predicted helix in the rat liver and Drosophila enzymes. Regions of relatively high sequence variation generally were predicted as reverse turns, and probably occur as surface loops. Most of the positions corresponding to exon boundaries are located between regions predicted to have secondary-structural elements consistent with a compact structure. The predominantly alternating alpha/beta structure predicted is consistent with the alpha/beta-barrel structure indicated by preliminary high-resolution X-ray diffraction studies on rabbit muscle aldolase [Sygusch, Beaudry & Allaire (1986) Biophys. J. 49, 287a].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner D. W., Bloomer A. C., Petsko G. A., Phillips D. C., Pogson C. I., Wilson I. A., Corran P. H., Furth A. J., Milman J. D., Offord R. E. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 angstrom resolution using amino acid sequence data. Nature. 1975 Jun 19;255(5510):609–614. doi: 10.1038/255609a0. [DOI] [PubMed] [Google Scholar]
- Besmond C., Dreyfus J. C., Gregori C., Frain M., Zakin M. M., Sala Trepat J., Kahn A. Nucleotide sequence of a cDNA clone for human aldolase B. Biochem Biophys Res Commun. 1983 Dec 16;117(2):601–609. doi: 10.1016/0006-291x(83)91243-3. [DOI] [PubMed] [Google Scholar]
- Brenner-Holzach O., Smit J. D. Crystallization and preliminary crystallographic data for fructose-1,6-bisphosphate aldolase from Drosophila melanogaster. J Biol Chem. 1982 Oct 10;257(19):11747–11749. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Clayton C. E. Structure and regulated expression of genes encoding fructose biphosphate aldolase in Trypanosoma brucei. EMBO J. 1985 Nov;4(11):2997–3003. doi: 10.1002/j.1460-2075.1985.tb04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Castagnoli L., Dente L., Arcari P., Smith M., Costanzo P., Raugei G., Izzo P., Pietropaolo T. C., Bougueleret L. Cloning of several cDNA segments coding for human liver proteins. EMBO J. 1983;2(1):57–61. doi: 10.1002/j.1460-2075.1983.tb01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufton M. J., Hider R. C. Snake toxin secondary structure predictions. Structure activity relationships. J Mol Biol. 1977 Sep 15;115(2):177–193. doi: 10.1016/0022-2836(77)90095-x. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Hoving H., Timasheff S. N. Circular dichroism of polypeptide and protein conformations. Film studies. Biochemistry. 1970 Aug 18;9(17):3316–3324. doi: 10.1021/bi00819a005. [DOI] [PubMed] [Google Scholar]
- Freemont P. S., Dunbar B., Fothergill-Gilmore L. A. The complete amino acid sequence of human skeletal-muscle fructose-bisphosphate aldolase. Biochem J. 1988 Feb 1;249(3):779–788. doi: 10.1042/bj2490779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Go M. Correlation of DNA exonic regions with protein structural units in haemoglobin. Nature. 1981 May 7;291(5810):90–92. doi: 10.1038/291090a0. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Hsu L., Neet K. E. Subunit interactions of rabbit muscle aldolase. Conformational change of aldolase in magnesium chloride and its relationship to the dissociation of subunits. J Mol Biol. 1975 Sep 25;97(3):351–367. doi: 10.1016/s0022-2836(75)80045-3. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. The influence of nearest-neighboring amino acid residues on aspects of secondary structure of proteins. Attempts to locate -helices and -sheets. Biopolymers. 1973 Apr;12(4):751–774. doi: 10.1002/bip.1973.360120406. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. Studies on the structure of rabbit muscle aldolase. Determination of the primary structure of the COOH-terminal BrCN peptide; the complete sequence of the subunit polypeptide chain. Arch Biochem Biophys. 1975 Jan;166(1):358–368. doi: 10.1016/0003-9861(75)90398-7. [DOI] [PubMed] [Google Scholar]
- Lenstra J. A., Hofsteenge J., Beintema J. J. Invariant features of the structure of pancreatic ribonuclease. A test of different predictive models. J Mol Biol. 1977 Jan 15;109(2):185–193. doi: 10.1016/s0022-2836(77)80028-4. [DOI] [PubMed] [Google Scholar]
- Leonard K. R., Walker I. O. Circular dichroism and Cotton effects in the near-ultraviolet spectral region of aldolase. Biochim Biophys Acta. 1967 Feb 21;133(2):366–368. doi: 10.1016/0005-2795(67)90077-3. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Lubini D. G., Christen P. Paracatalytic modification of aldolase: a side reaction of the catalytic cycle resulting in irreversible blocking of two active-site lysyl residues. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2527–2531. doi: 10.1073/pnas.76.6.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magar M. E. Optical rotatory dispersion of aldolase and glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1967 May 25;242(10):2517–2521. [PubMed] [Google Scholar]
- Malek A. A., Suter F. X., Frank G., Brenner-Holzach O. Amino acid sequence of an invertebrate FBP aldolase (from Drosophila melanogaster). Biochem Biophys Res Commun. 1985 Jan 16;126(1):199–205. doi: 10.1016/0006-291x(85)90591-1. [DOI] [PubMed] [Google Scholar]
- Mavridis I. M., Hatada M. H., Tulinsky A., Lebioda L. Structure of 2-keto-3-deoxy-6-phosphogluconate aldolase at 2 . 8 A resolution. J Mol Biol. 1982 Dec 5;162(2):419–444. doi: 10.1016/0022-2836(82)90536-8. [DOI] [PubMed] [Google Scholar]
- Millar J. R., Shaw P. J., Stammers D. K., Watson H. C. The low-resolution structure of human muscle aldolase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):209–214. doi: 10.1098/rstb.1981.0074. [DOI] [PubMed] [Google Scholar]
- Nagano K. Logical analysis of the mechanism of protein folding. I. Predictions of helices, loops and beta-structures from primary structure. J Mol Biol. 1973 Apr 5;75(2):401–420. doi: 10.1016/0022-2836(73)90030-2. [DOI] [PubMed] [Google Scholar]
- Patthy L., Váradi A., Thész J., Kovács K. Identification of the C-1-phosphate-binding arginine residue of rabbit-muscle aldolase. Isolation of 1,2-cyclohexanedione-labeled peptide by chemisorption chromatography. Eur J Biochem. 1979 Sep;99(2):309–313. doi: 10.1111/j.1432-1033.1979.tb13258.x. [DOI] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. A new basis for interpreting the circular dichroic spectra of proteins. Proc Natl Acad Sci U S A. 1971 May;68(5):969–972. doi: 10.1073/pnas.68.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen E. Predicted structure for aldolase. J Mol Biol. 1976 Sep 25;106(3):903–911. doi: 10.1016/0022-2836(76)90273-4. [DOI] [PubMed] [Google Scholar]
- Stuart D. I., Levine M., Muirhead H., Stammers D. K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J Mol Biol. 1979 Oct 15;134(1):109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- Sygusch J., Boulet H., Beaudry D. Structure of rabbit muscle aldolase at low resolution. J Biol Chem. 1985 Dec 5;260(28):15286–15290. [PubMed] [Google Scholar]
- Tolan D. R., Amsden A. B., Putney S. D., Urdea M. S., Penhoet E. E. The complete nucleotide sequence for rabbit muscle aldolase A messenger RNA. J Biol Chem. 1984 Jan 25;259(2):1127–1131. [PubMed] [Google Scholar]
- Tsutsumi K., Mukai T., Hidaka S., Miyahara H., Tsutsumi R., Tanaka T., Hori K., Ishikawa K. Rat aldolase isozyme gene. J Biol Chem. 1983 May 25;258(10):6537–6542. [PubMed] [Google Scholar]




