Abstract
Rationale and objective
To investigate the MR characteristics of phlegmonous stage and abscess stage primary spinal epidural abscess.
Materials and methods
This study retrospectively analyzed the clinical and imaging characteristics of 27 cases of pathologically confirmed primary spinal epidural abscess. Predisposing conditions of all patients were collected. All patients underwent conventional magnetic resonance imaging, while fifteen patients also underwent post-contrast magnetic resonance imaging.
Results
The initial symptoms included back pain in 25 patients, fever in 18, motor deficit in five, and sensory changes in 13. Underlying diseases included distant site of infection in seven, injection therapy in five, neoplasm in five, chronic inflammatory disease in five, diabetes mellitus in four, alcoholism in three, metabolic disorder in three, hepatopathy in three, and obesity in two. Abscess location was ventral epidural space in 15 patients (55.6%) and dorsal epidural space in 12 (44.4%). On T1-weighted image, the abscess was hypointense to the spinal cord in 23 patients (85%) and isointense in four (15%). All abscesses were hyperintense to the spinal cord on T2-weighted image. Among the 15 patients who underwent contrast-enhanced imaging, ring enhancement was present in 13 and homogeneous enhancement in two. Adjacent vertebrae body edema was present in four patients. The abscess was purely intraspinal in 25 patients (92.6%). Paraspinal extension was present in two (7.4%).
Conclusion
Primary spinal epidural abscess patients have one or more predisposing conditions. Phlegmonous stage primary spinal epidural abscess appears isointense on T1WI and hyperintense on T2WI and enhancement is homogeneous. Abscess stage primary spinal epidural abscess hyperintense on T2WI and hypointense on T1WI and ring enhancement. Presence of vertebral body edema is an important sign to help diagnose primary spinal epidural abscess.
Keywords: Spinal epidural abscess, Primary, Magnetic resonance imaging, Diagnosis
Introduction
Spinal epidural abscess (SEA) represents an accumulation of purulent fluid or infected granulation tissue within the spinal epidural space [1]. It is a rare and challenging entity which presents with nonspecific signs and symptoms. Most patients with an SEA have one or more predisposing conditions [2, 3]. Primary SEA (PSEA) is caused by hematogenous spread of pathogens from a distant focus of infection into the spinal epidural space [4]. In general, patients with PSEA have different underlying diseases and a wider range of pathogens than those with secondary SEA. Here, we summarize the imaging manifestations of 27 cases of PSEA.
Materials and methods
Patients
We retrospectively reviewed the clinical and imaging data of 27 patients with surgically and pathologically confirmed PSEA who were treated in our hospital between September 2015 and November 2023. All patients had undergone magnetic resonance imaging (MRI) performed before surgery. Hospital ethics committee approval was obtained. The requirement for informed consent was waived owing to its retrospective design.
Etiologic agents
Causative agents of spinal epidural abscess are listed in Table 1.The most common pathogen causing SEA was Staphylococcus aureus in 16 cases. This S aureus is followed by Streptococcus in 4 cases. Less common causative pathogens include Enterococcus, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis,and Salmonella enteridis.
Table 1.
Etiologic agents in the 27 PSEA patients
| cause | Number of patients |
|---|---|
|
Staphylococcus aureus Streptococcus Enterococcus Escherichia coli Pseudomonas aeruginosa Proteus mirabilis Salmonella enteridis |
16 4 2 2 1 1 1 |
MRI technique
Sagittal T1-weighted imaging (T1WI), sagittal and transverse T2WI, and sagittal short tau inversion recovery (STIR) imaging were performed. Fifteen patients also underwent additional contrast-enhanced axial, sagittal, and coronal T1WI using intravenous gadolinium diethylenetriamine pentaacetate (0.2 mmol/kg). Slice thickness and slice gap was 3 mm and 0.8 mm, respectively.
Image analysis
Region of PSEA, number of lesions, affected level, location of PSEA relative to the thecal sac, lesion signal intensity, presence of edema in the adjacent vertebrae body, and presence of paraspinal extension were independently analyzed by two radiologists. Any discrepancies were resolved by discussion and consensus.
Results
Patient characteristics
Fifteen were men and 12 were women. Mean patient age was 47.18 ± 6.35 years (range, 35–58). The most common symptom was back pain (25 patients, 92.6%), followed by fever (18 patients, 66.7%). Five patients presented with a motor deficit (18.5%) and 13 with sensory change (48.1%).
Underlying disease
Underlying diseases are shown in Table 2. Twenty-one patients (78%) had at least one comorbidity. Twenty (74%) had a disease associated with altered immune function, including malignancy, diabetes mellitus, alcoholism, metabolic disorders, hepatopathy, and obesity. Chronic inflammatory diseases or another septic condition was present in 12 patients (44%). Iatrogenic infection arising from an injection at a distant site was present in five (19%). The source of infection was unclear in six patients.
Table 2.
Underlying disease in 27 patients with primary spinal epidural abscess
| Comorbidity | Frequency |
|---|---|
|
septic disease in case history injections therapy neoplasma chronic inflammatory disease diabetes mellitus alcoholism metabolic disorders hepatopathy obesity endocarditis |
7 5 5 4 4 3 3 3 2 1 |
Imaging features
PSEA imaging characteristics are shown in Table 3. PSEA location was thoracic in nine patients (33.3%), thoracolumbar in five (18.5%), lumbar in 12 (44.4%), and lumbosacral in one (3.7%). The abscess spanned two vertebral levels in six patients (22.2%), three levels in 10 (37%), and four levels in nine (33.3%). The abscess was ventral to the thecal sac in 15 patients (55.6%)(Figs. 1 and 2) and dorsal in 12 (44.4%)(Figs. 3 and 4).
Table 3.
Magnetic resonance imaging characteristics in 27 patients with primary spinal epidural abscess
| Characteristics | Frequency (%) |
|---|---|
| Region of spine | |
|
Thoracic Thoracolumbar Lumbar Lumbosacral |
9(33.3) 5(18.5) 12(44.4) 1(3.7) |
| No. of affected levels | |
|
2 3 4 |
6(22.2) 12(44.4) 9(33.3) |
| Location of abscess relative to thecal sac | |
|
Ventral Dorsal |
15(55.6) 12(44.4) |
|
Signal intensity T1WI |
|
|
hypo-intense iso-intense T2WI |
23(85) 4(15) |
|
hyperintense Contrast enhanced* |
27(100) |
|
ring-enhanced homogeneous |
13(87) 2(13) |
|
Vertebrae body edema Confined intraspinal Paraspinal extension |
4(14.8) 25(92.6) 2(7.4) |
T1WI, T1-weighted imaging; T2WI, T2-weighted imaging
*Fifteen patients underwent contrast-enhanced imaging
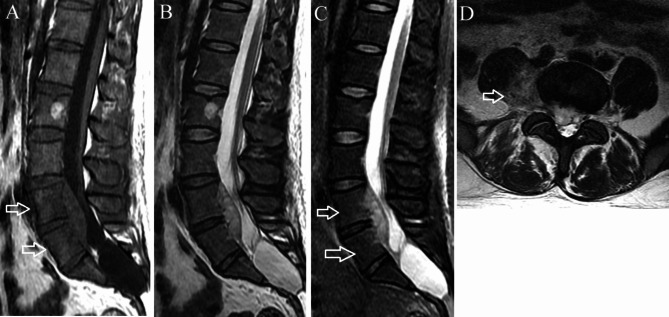
Fig. 1.
Phlegmonous stage primary L4-5 spinal epidural abscess in a 37-year-old man. (A) Sagittal T1-weighted imaging shows a fusiform homogeneously isointense lesion ventral to the thecal sac at L4-5. Edema is present in the L4 and L5 vertebral bodies (arrows). (B) On sagittal T2-weighted imaging, the lesion appears homogeneously hyperintense. (C) On sagittal short tau inversion recovery imaging, the vertebral body edema appears mildly hyperintense (arrows). (D) Axial T2-weighted imaging demonstrates the lesion extending through the neural foramina on the right into the paraspinal region (arrow)
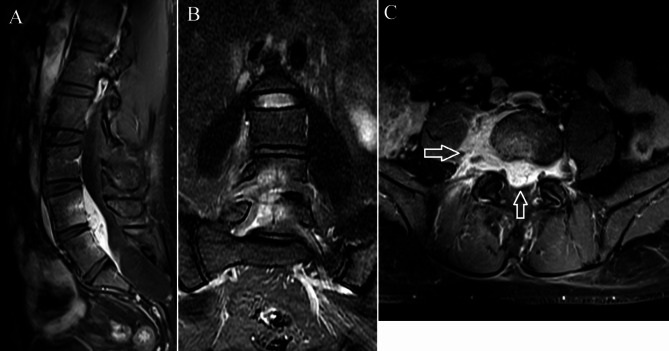
Fig. 2.
Contrast-enhanced T1-weighted imaging of the same patient as in Fig. 1. (A) The abscess and the L4 and L5 vertebral bodies exhibit enhancement on the sagittal imaging. (B) Coronal imaging also demonstrates L4-5 vertebral body enhancement. (C) Both the epidural and paraspinal components of the abscess show considerable homogeneous enhancement on the axial imaging (arrow)
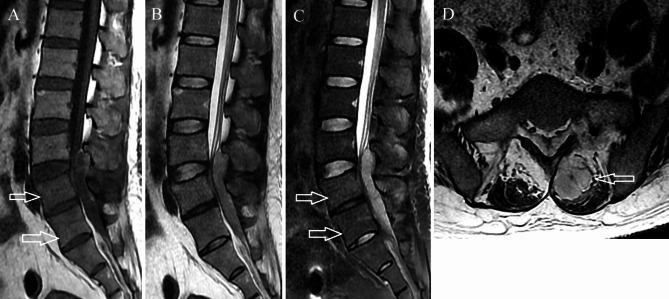
Fig. 3.
Abscess stage primary L3-5 spinal epidural abscess in a 41-year-old man. (A) On axial T1-weighted imaging, the abscess is hypointense and dorsal to thecal sac. Mild vertebral body edema is seen in the L4 and L5 vertebral bodies (arrows). (B) The abscess appears hyperintense on sagittal T2-weighted imaging. (C) Sagittal short tau inversion recovery imaging shows mild hyperintensity within the L4 and L5 vertebral bodies (arrows). (D) Axial T2-weighted imaging shows an abscess in the posterior paraspinal muscles (arrow)
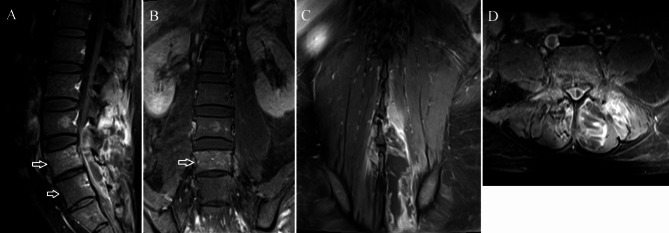
Fig. 4.
Contrast-enhanced imaging of the same patient as in Fig. 3. The vertebral body edema mildly enhances (arrows) on the sagittal (A) and coronal (B) images. The posterior paraspinal muscle abscess exhibits ring enhancement on the coronal (C) and axial (D) images
On T1WI, the PSEA was isointense to the spinal cord in 4 patients (15%)(Fig. 1A) and hypointense in twenty-three (85%)(Fig. 3A). All PSEAs were hyperintense to the spinal cord on T2WI. Among the 15 patients who underwent contrast-enhanced imaging, ring enhancement was present in 13 and homogeneous enhancement in two.
Vertebral body edema was present in four patients. Two vertebral body edemas in three patients and one vertebral body edema in one patient. Vertebral body edema appeared as mildly hypointense on T1WI and mildly hyperintense on STIR imaging(Figs. 1C and 3C). The involved vertebral body exhibited mild enhancement on contrast-enhanced imaging(Figs. 2A and B and 4A and B). Among these, the abscess was located ventral to the thecal sac in three and dorsal in one.
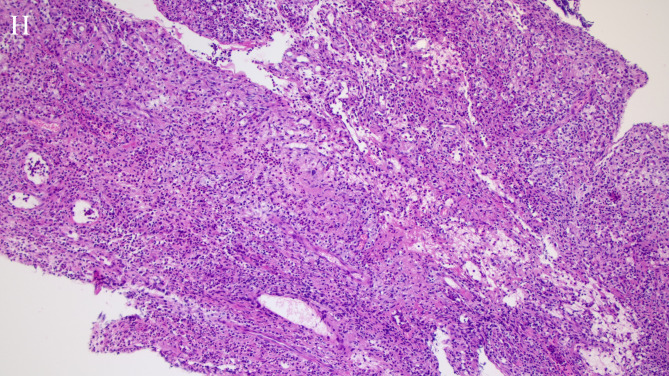
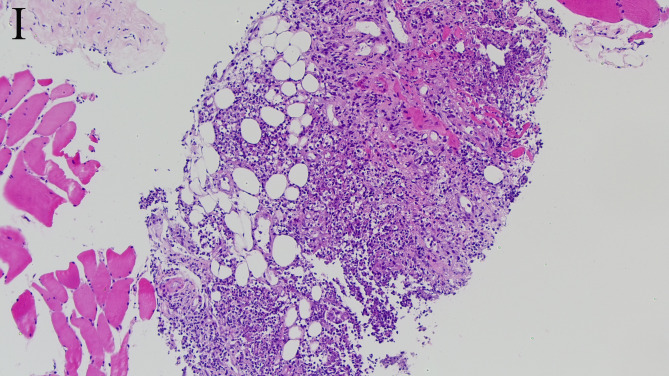
The abscess was purely intraspinal in 25 patients (92.6%). Paraspinal extension was present in two (7.4%). One of the abscesses with paraspinal extension was located ventral to the thecal sac, extended along both intervertebral foramina, and compressed the right psoas major(Figs. 1D and 2C). This abscess appeared homogeneously and moderately hyperintense on T2WI and strongly enhanced on contrast-enhanced images. Histological features consistent with phlegmonous stage SEA(Fig. 5).The other with paraspinal extension was located dorsal to the thecal sac and involved the paraspinal muscles bilaterally(Figs. 3D and 4D). The paraspinal muscles appeared hypointense on T1WI and hyperintense on T2WI. On contrast-enhanced imaging, this abscess exhibited ring enhancement(Fig. 4C). Histological features consistent with abscess stage SEA(Fig. 6).
Fig. 5.
Hematoxylin-eosin staining of a histopathologic specimen shows chronic granulomatous inflammation and fibrinoid exudation. A large number of neutrophils, plasma cells, lymphocyte infiltrates, and small vessel proliferation are also demonstrated (×100)
Fig. 6.
Hematoxylin-eosin staining of a histopathologic specimen demonstrates pyogenic inflammation and multiple areas of neutrophilic infiltration (×100)
Discussion
Infections of the spine present with a variety of nonspecific signs and symptoms, which makes them challenging to diagnose. Such infections include osteomyelitis and epidural, subdural, and intradural abscesses [5]. SEA represents the accumulation of purulent material in the space between the dura matter and the osseoligamentous confines of the vertebral canal and is relatively uncommon. The annual incidence of SEA is 2 to 3 cases per 10,000 hospital admissions [5]. SEA is associated with a relatively high rate of morbidity and mortality. Although first mentioned by Morgagni in 1761, the condition was not clearly defined as a clinical entity until 1820 by Bergamaschi [6, 7].
Most SEA patients have one or more predisposing conditions such as an underlying disease (diabetes mellitus, alcoholism, human immunodeficiency virus infection) or a local or systemic source of infection (skin or soft-tissue infection, osteomyelitis, urinary tract infection); invasive procedures such as acupuncture and various types of diagnostic or therapeutic injection may also be a source of infection associated with SEA [3]. Patients with PSEA have different underlying diseases and a wider range of pathogens than those with secondary SEA [4].
The study of cerebrospinal fluid (CSF) is helpful in contributing to the etiology of the infectious disease [8].In three quarters of patients whose CSF is evaluated, CSF analysis shows a high level of protein and pleocytosis (with either a polymorphonuclear or a mononuclear predominance) [3].Chemical analysis almost uniformly shows a protein level of > 0.45 g/l and a lactate value > 2µmol/l [9].The concentration of protein was not related to the severity of the spinal block as shown by myelography. Glucose was low (< 50 mg/dl) in 54% patients. Cultures of CSF were positive in 25% patients [10]. The findings are suggestive of parameningeal inflammation but are not specific for epidural infection [3]. However, CSF cultures are positive in less than 25% of patients whose CSF is microbiologically assessed [3]. Needle puncture of the dura carries the risk of seeding the intrathecal compartment with resultant meningitis and the procedures should not be done until after spinal epidural abscess has been ruled out [11].
SEA may be difficult to diagnose because the early signs and symptoms are nonspecific and shared by other types of pathology. As a result, the diagnosis may be delayed. The most common symptoms are fever, motor deficit, and back pain. However, this classic clinical triad is present only in a minority of patients [4]. The triad may indicate a progression to irreversible symptoms [12]. SEA-related motor deficit is believed to be caused by a combination of mechanical compression of the spinal cord and/or nerve roots as well as ischemia from vascular compromise [13, 14].
SEA may occur after spinal trauma, spinal injection, spinal surgery, or after the direct introduction of pathogens into the epidural space. This type of SEA is called secondary SEA. In contrast, PSEA results from hematogenous spread of pathogens from a distant focus of infection into the spinal epidural space [4]. Many cases of PSEA represent local extension of vertebral body osteomyelitis. Cases in which microorganisms gain access to the epidural space and proliferate without vertebral body involvement are rare and particularly challenging to diagnose.
MRI is the imaging method of choice to diagnose SEA because it delineates both the longitudinal and paraspinal extension of the abscess [15] and can demonstrate spinal cord compression [14]. Because SEA is more likely to develop in areas with a larger epidural space that contain infection-prone fat, it is more commonly located posterior to the thecal sac and in the thoracolumbar region. One study reported that 82% of SEAs are posterior to the thecal sac and 18% are anterior [13].
Two main SEA patterns can be observed on MRI: phlegmonous stage and abscess stage. The phlegmonous stage is seen as a homogeneously enhancing area which represents granulomatous tissue, microabscess, and pus collection. This stage appears isointense on T1WI and hyperintense on T2WI and enhancement is homogeneous. The abscess stage is surrounded by inflammatory tissue which shows a heterogenous degree of peripheral enhancement. In this stage, the collection appears hyperintense on T2WI and hypointense on T1WI and ring enhancement is common. Diffusion-weighted imaging frequently demonstrates restricted diffusion within the abscess [16]. The imaging findings in our patients in this series are consistent with those reported by Numaguchi et al. [16]. However, diffusion-weighted sequences were not performed in our study.
The spinal epidural space is a potential space containing a rich venous plexus. The venous drainage of the spinal column and epidural space communicates with the systemic circulation via Batson’s plexus, a bidirectional, valveless venous network [14]. The venous plexus allowed the SEA result in vertebral body edema. Four patients in our study exhibited vertebral body edema, including ones with an SEA located both dorsal and ventral to the thecal sac. Vertebral body edema appeared as mildly hyperintense signal on STIR and enhanced slightly. The presence of such edema may differentiate SEA from neurogenic tumors. One patient in our series was in the phlegmonous stage of infection—the lesion was isointense on T1WI and extended along the intervertebral foramen. We initially misdiagnosed it as a neurogenic tumor. When re-reviewing the images after surgery, we noted that the vertebral body edema should have indicated that it was SEA. In another patient, the SEA was dorsal to the thecal sac and edema in two vertebral bodies was present.
In conclusion, SEA is challenging to diagnose owing to its rarity and insidious presentation. Its ability to cause a rapid precipitous neurologic decline emphasizes the importance of accurate diagnosis and early intervention. Risk factor screening is far more sensitive than the classic triad because the triad is usually absent in most patients. Misdiagnosis of PSEA mainly occurs in the phlegmonous stage because of atypical imaging findings. However, those in the abscess stage usually demonstrate typical imaging findings, such as peripheral or ring enhancement. Presence of vertebral body edema is an important sign to help diagnose PSEA, especially for those in the phlegmonous stage.
Acknowledgements
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Abbreviations
- SEA
Spinal epidural abscess
- PSEA
Primary spinal epidural abscess
- MRI
Magnetic resonance imaging
- T1WI
T1-weighted imaging
- T2WI
T2-weighted imaging
- STIR
Short time of inversion recovery
Author contributions
Author contributions: Jiang Gang and Gao Chuanping completed the experimental design and manuscript review. Sun wrote the main manuscript. Yang and Cui have completed data analysis and image drawing; Zhang completed the editing work of the manuscript, Gao Chuanping provided experimental guidance, and the manuscript was reviewed. All authors have read and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
The data used for the analysis are available from the corresponding authors upon request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Review Board of the Affiliated hospital of Qingdao University, and all patient requirements for informed consent were waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Connor DE Jr, Chittiboina P, Caldito G, et al. Comparison of operative and nonoperative management of spinal epidural abscess: a retrospective review of clinical and laboratory predictors of neurological outcome. J Neurosurg Spine. 2013;19:119–27. [DOI] [PubMed] [Google Scholar]
- 2.Pereira CE, Lynch JC. Spinal epidural abscess: an analysis of 24 cases. Surg Neurol. 2005;63:S26–9. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355:2012–20. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerer SME, Conen A, Müller AA, et al. Spinal epidural abscess: aetiology, predisponent factors and clinical outcomes in a 4-year prospective study. Eur Spine J. 2011;20:2228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson KG. Spinal epidural abscess. Crit Care Nurs Clin North Am. 2013;25:389–97. [DOI] [PubMed] [Google Scholar]
- 6.Feldenzer JA, McKeever PE, Schaberg DR, et al. Experimental spinal epidural abscess: a patho-physiological model in the rabbit. Neurosurgery. 1987;20:859–67. [DOI] [PubMed] [Google Scholar]
- 7.Browder J, Meyers R. Infection of the spinal epidural space. Am J Surg. 1937;37:4. – 26. [Google Scholar]
- 8.Miyoshi IC, de Toledo AHN, Pereira FV, et al. Infectious myelitis. Semin Ultrasound CT MR. 2023;44:424–35. [DOI] [PubMed] [Google Scholar]
- 9.Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008;101:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche RO, Hamill RJ, Greenberg SB, et al. Bacterial spinal epidural abscess: review of 43 cases and literature survey. Med (Baltim). 1992;71:369–85. [PubMed] [Google Scholar]
- 11.Tompkins M, Panuncialman I, Lucas P, et al. Spinal Epidural Abscess J Emerg Med. 2010;39:384–90. [DOI] [PubMed] [Google Scholar]
- 12.Davis D, Salazar A, Chan T, et al. Progressive evaluation of clinical decision guideline to diagnose spinal epidural abscess in patients who present to the emergency department with spine pain. J Neurosurg Spine. 2011;14:765–70. [DOI] [PubMed] [Google Scholar]
- 13.Shah AA, Ogink PT, Harris MB, et al. Development of Predictive algorithms for Pre-treatment Motor Deficit and 90-Day mortality in spinal epidural abscess. J Bone Joint Surg Am. 2018;100:1030–38. [DOI] [PubMed] [Google Scholar]
- 14.Schwab JH, Shah AA. Spinal epidural abscess: diagnosis, management, and outcomes. J Am Acad Orthop Surg. 2020;28:e929–38. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson JF, Sekhon LH. Spinal epidural abscess: appearance on magnetic resonance imaging as a guide to surgical management. Neurosurg Focus. 2004;17:E12. [DOI] [PubMed] [Google Scholar]
- 16.Numaguchi Y, Rigamonti D, Rothman MI et al. Spinal epidural abscess: evaluation with gadolinium-enhanced MR imaging. RadioGraphics.1993,13:545–59. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the analysis are available from the corresponding authors upon request.