Abstract
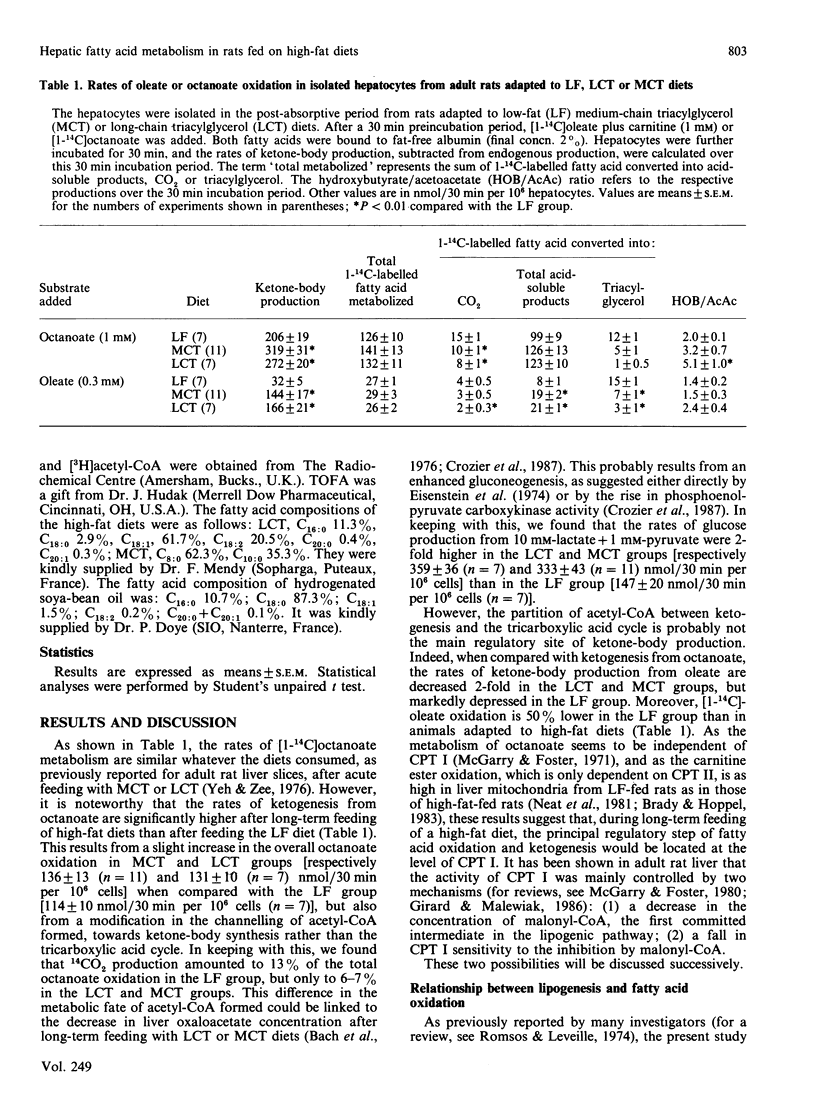
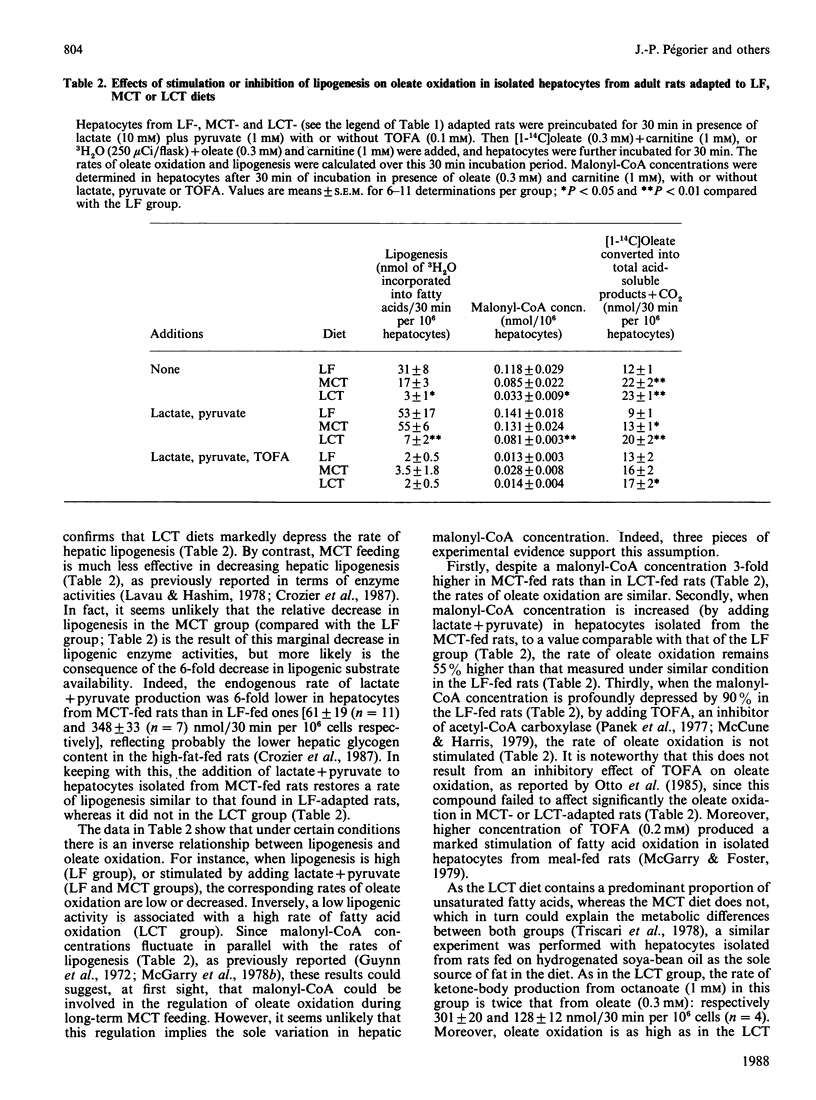
Fatty acid oxidation and synthesis were studied in isolated hepatocytes from adult rats adapted for 44 days on low-fat, high-carbohydrate (LF), diet or high-fat diets, composed of long-chain (LCT) or medium-chain (MCT) triacylglycerols. The rates of [1-14C]octanoate oxidation were almost similar in each group studied, whereas the oxidation of [1-14C]oleate was 50% lower in the LF group than in animals adapted to high-fat diets. The rates of oleate oxidation are inversely correlated with the rates of lipogenesis. However, it seems unlikely that [malonyl-CoA] itself represents the sole mechanism involved in the regulation of oleate oxidation during long-term LCT or MCT feeding, since: (1) despite a 3-fold higher concentration of malonyl-CoA in MCT-fed rats than in LCT-fed ones, the rates of oleate oxidation are similar; (2) when malonyl-CoA concentration is increased after stimulation of lipogenesis (by adding lactate + pyruvate) in MCT-fed rats, to a level comparable with that of the LF group, the rate of oleate oxidation remains 55% higher than that measured under similar conditions in the LF-fed rats; (3) in the LF group, the 90% decrease in malonyl-CoA concentration [by 5-(tetradecyloxy)-2-furoic acid] is not associated with a stimulation of oleate oxidation. By contrast, the sensitivity of carnitine palmitoyltransferase I (CPT I) to malonyl-CoA is markedly decreased in the LCT- and MCT-fed rats, by 90% and 70% respectively. The relevance of this decrease in the sensitivity of CPT I is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach A. C., Babayan V. K. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982 Nov;36(5):950–962. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- Bach A., Phan T., Metais P. Effect of the fatty acid composition of ingested fats on rat liver intermediary metabolism. Horm Metab Res. 1976 Sep;8(5):375–379. doi: 10.1055/s-0028-1093617. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner-membrane properties, beta-oxidation and carnitine palmitoyltransferases A and B. Effects of genetic obesity and starvation. Biochem J. 1986 Jan 15;233(2):427–433. doi: 10.1042/bj2330427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Hoppel C. L. Effect of diet and starvation on hepatic mitochondrial function in the rat. J Nutr. 1983 Nov;113(11):2129–2137. doi: 10.1093/jn/113.11.2129. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Silverstein L. J., Hoppel C. L., Brady P. S. Hepatic mitochondrial inner membrane properties and carnitine palmitoyltransferase A and B. Effect of diabetes and starvation. Biochem J. 1985 Dec 1;232(2):445–450. doi: 10.1042/bj2320445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- Cook G. A., Stephens T. W., Harris R. A. Altered sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA in ketotic diabetic rats. Biochem J. 1984 Apr 1;219(1):337–339. doi: 10.1042/bj2190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier G., Bois-Joyeux B., Chanez M., Girard J., Peret J. Metabolic effects induced by long-term feeding of medium-chain triglycerides in the rat. Metabolism. 1987 Aug;36(8):807–814. doi: 10.1016/0026-0495(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Duee P. H., Pegorier J. P., el Manoubi L., Herbin C., Kohl C., Girard J. Hepatic triglyceride hydrolysis and development of ketogenesis in rabbits. Am J Physiol. 1985 Nov;249(5 Pt 1):E478–E484. doi: 10.1152/ajpendo.1985.249.5.E478. [DOI] [PubMed] [Google Scholar]
- Eisenstein A. B., Strack I., Steiner A. Increased hepatic gluconeogenesis without a rise of glucagon secretion in rats fed a high fat diet. Diabetes. 1974 Nov;23(11):869–875. doi: 10.2337/diab.23.11.869. [DOI] [PubMed] [Google Scholar]
- Escriva F., Ferre P., Robin D., Robin P., Decaux J. F., Girard J. Evidence that the development of hepatic fatty acid oxidation at birth in the rat is concomitant with an increased intramitochondrial CoA concentration. Eur J Biochem. 1986 May 2;156(3):603–607. doi: 10.1111/j.1432-1033.1986.tb09620.x. [DOI] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Harano Y., Kosugi K., Kashiwagi A., Nakano T., Hidaka H., Shigeta Y. Regulatory mechanism of ketogenesis by glucagon and insulin in isolated and cultured hepatocytes. J Biochem. 1982 May;91(5):1739–1748. doi: 10.1093/oxfordjournals.jbchem.a133866. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavau M. M., Hashim S. A. Effect of medium chain triglyceride on lipogenesis and body fat in the rat. J Nutr. 1978 Apr;108(4):613–620. doi: 10.1093/jn/108.4.613. [DOI] [PubMed] [Google Scholar]
- Mannaerts G. P., Debeer L. J., Thomas J., De Schepper P. J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979 Jun 10;254(11):4585–4595. [PubMed] [Google Scholar]
- McCune S. A., Harris R. A. Mechanism responsible for 5-(tetradecyloxy)-2-furoic acid inhibition of hepatic lipogenesis. J Biol Chem. 1979 Oct 25;254(20):10095–10101. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- McMurchie E. J., Abeywardena M. Y., Charnock J. S., Gibson R. A. The effect of dietary lipids on the thermotropic behaviour of rat liver and heart mitochondrial membrane lipids. Biochim Biophys Acta. 1983 Sep 21;734(1):114–124. doi: 10.1016/0005-2736(83)90082-2. [DOI] [PubMed] [Google Scholar]
- McMurchie E. J., Gibson R. A., Abeywardena M. Y., Charnock J. S. Dietary lipid modulation of rat liver mitochondrial succinate: cytochrome c reductase. Biochim Biophys Acta. 1983 Jan 5;727(1):163–169. doi: 10.1016/0005-2736(83)90380-2. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Goodman J., Houk J. M., Anderson S. Studies on the biochemistry of mitochondria and cell morphology in the neonatal swine hepatocyte. J Cell Biol. 1972 May;53(2):335–347. doi: 10.1083/jcb.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neat C. E., Thomassen M. S., Osmundsen H. Effects of high-fat diets on hepatic fatty acid oxidation in the rat. Isolation of rat liver peroxisomes by vertical-rotor centrifugation by using a self-generated, iso-osmotic, Percoll gradient. Biochem J. 1981 Apr 15;196(1):149–159. doi: 10.1042/bj1960149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D. A., Chatzidakis C., Kasziba E., Cook G. A. Reciprocal effects of 5-(tetradecyloxy)-2-furoic acid on fatty acid oxidation. Arch Biochem Biophys. 1985 Oct;242(1):23–31. doi: 10.1016/0003-9861(85)90475-8. [DOI] [PubMed] [Google Scholar]
- Panek E., Cook G. A., Cornell N. W. Inhibition by 5-(tetradecyloxy)-2-furoic acid of fatty acid and cholesterol synthesis in isolated rat hepatocytes. Lipids. 1977 Oct;12(10):814–818. doi: 10.1007/BF02533270. [DOI] [PubMed] [Google Scholar]
- Romsos D. R., Leveille G. A. Effect of diet on activity of enzymes involved in fatty acid and cholesterol synthesis. Adv Lipid Res. 1974;12(0):97–146. [PubMed] [Google Scholar]
- Saggerson E. D., Bird M. I., Carpenter C. A., Winter K. A., Wright J. J. Cycloheximide blocks changes in rat liver carnitine palmitoyltransferase 1 activity in starvation. Biochem J. 1984 Nov 15;224(1):201–206. doi: 10.1042/bj2240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops J. K., Ross P., Arslanian M. J., Aune K. C., Wakil S. J., Oliver R. M. Physicochemical studies of the rat liver and adipose fatty acid synthetases. J Biol Chem. 1979 Aug 10;254(15):7418–7426. [PubMed] [Google Scholar]
- Takase S., Hosoya N. Effect of dietary medium chain triglyceride on lipogenic enzyme activity in rat liver. J Nutr Sci Vitaminol (Tokyo) 1986 Apr;32(2):219–227. doi: 10.3177/jnsv.32.219. [DOI] [PubMed] [Google Scholar]
- Takase S., Morimoto A., Nakanishi M., Muto Y. Long-term effect of medium-chain triglyceride on hepatic enzymes catalyzing lipogenesis and cholesterogenesis in rats. J Nutr Sci Vitaminol (Tokyo) 1977;23(1):43–51. doi: 10.3177/jnsv.23.43. [DOI] [PubMed] [Google Scholar]
- Triscari J., Hamilton J. G., Sullivan A. C. Comparative effects of saturated and unsaturated lipids on hepatic lipogenesis and cholesterogenesis in vivo in the meal-fed rat. J Nutr. 1978 May;108(5):815–825. doi: 10.1093/jn/108.5.815. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Zevenbergen J. L. Effect of dietary fat on total and peroxisomal fatty acid oxidation in rat tissues. Biochim Biophys Acta. 1986 Aug 14;878(1):102–109. doi: 10.1016/0005-2760(86)90348-6. [DOI] [PubMed] [Google Scholar]
- Wiley J. H., Leveille G. A. Metabolic consequences of dietary medium-chain triglycerides in the rat. J Nutr. 1973 Jun;103(6):829–835. doi: 10.1093/jn/103.6.829. [DOI] [PubMed] [Google Scholar]
- Yeh Y. Y., Zee P. Relation of ketosis to metabolic changes induced by acute medium-chain triglyceride feeding in rats. J Nutr. 1976 Jan;106(1):58–67. doi: 10.1093/jn/106.1.58. [DOI] [PubMed] [Google Scholar]


