Abstract
Background
Pregnancy can be a risk factor for the development of more severe COVID-19 with a possible increase in the risk of complications during pregnancy/birth and adverse neonatal outcomes. This study aimed to describe and analyze the clinical and epidemiological aspects of SARS-CoV-2 infection in women in the perinatal period attended in the city of Belém, northern region of Brazil.
Methods
This is a clinical, observational, analytical, and cross-sectional study with a quantitative approach, conducted at the Santa Casa de Misericórdia do Pará Foundation (FSCMPA). It included 230 pregnant women hospitalized at FSCMPA with a positive SARS-CoV-2 RT-PCR molecular test between April 2020 and June 2022. Clinical and epidemiological information (origin, gestational age, prenatal care, comorbidities, birth complications, and chest tomography) were obtained from medical records, and correlation was made between the types of cases (mild, moderate, and severe) and maternal outcome. The chi-square test and G test were used to assess the possibility of association between variables.
Results
Evidence of association was observed between the severity of COVID-19 and the following parameters: gestational age, specific pregnancy comorbidities, baby and maternal death, birth complications, and prematurity. Dyspnea, headache, anosmia, odynophagia, diarrhea, and chest pain were the symptoms most related to disease aggravation. The maternal mortality rate in the study was 8.7%.
Conclusion
Specific pregnancy-related and pre-existing comorbidities associated with SARS-CoV-2 infection directly contribute to the worsening clinical condition, leading to complications such as prematurity, fetal, and maternal death.
Keywords: COVID-19, Pandemic, Maternal death, Women’s health, Brazil
Introduction
Numerous studies have identified that Coronavirus Disease (COVID-19) most critical disease comorbidities and risk factors were advanced age, male gender, obesity, smoking, hypertension, diabetes, hematologic, renal, cardiovascular, respiratory diseases, and pregnancy [1–4]. Evidence supports that pregnancy is a risk factor for severe illness associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), revealing that pregnant women are more likely to be admitted to the Intensive Care Unit (ICU), require mechanical ventilation, and die than non-pregnant women, as well as an increased risk of complications during pregnancy/birth and adverse neonatal outcomes [5, 6].
The clinical manifestations of pregnant women with COVID-19 can vary from asymptomatic cases, mild symptoms such as fever, general discomfort, cough, sore throat, chest pain, chills, myalgia, and diarrhea to the most severe cases, which include heart failure, requiring mechanical ventilation, organ failure, and sepsis [7, 8], especially in women who are infected with the virus in the third trimester of pregnancy.
In theory, COVID-19 is worsened in pregnant women than non-pregnant women due to significant anatomical and physiological changes that happens during pregnancy to nurture and development of fetus, also COVID-19’s severity may be increased if pregnancy was associated with comorbidities, such as diabetes, cardiopathies or chronic lung disease. Between the main changes to occur during pregnancy are in cardiovascular and respiratory systems, in the cardiovascular system there is a peripheral vasodilatation mediated by upregulated nitric oxide synthesis, estradiol and vasodilatory prostaglandins, which results in a 25–30% decreased resistance of the vascular system and to compensate this peripheral vasodilatation, the cardiac output naturally increases by around 40% during pregnancy, also increasing the stroke volume due to the early increase in ventricular wall muscle mass and end-diastolic volume and increases even more during labor and fetus delivery [9–11].
Factors such as social determinants exacerbate the pandemic scenario in the Amazon region, where SARS-CoV-2 infection takes on unequal faces, depicted by the socio-economic, geographical, and environmental conditions of the region. At the onset of the pandemic, the Northern Region experienced the most severe epidemiological situation in Brazil, with the states of Amazonas and Pará presenting the highest numbers of confirmed cases, deaths, lethality, and hospitalizations due to severe acute respiratory syndrome (SARS) caused by COVID-19 [12]. There are limited reports on the clinical impacts and outcomes of COVID-19 during pregnancy with and without pre-existing clinical conditions and comorbidities in emerging regions, such as the Brazilian Amazon, which historically face public health challenges with higher fertility rates, especially among younger women. Therefore, information regarding maternal mortality during the COVID-19 pandemic will help to decrease these fatality rates [13, 14]. This study aims to describe the clinical and epidemiological aspects of SARS-CoV-2 infection in women during the perinatal period in a public maternity hospital in the Brazilian Amazon.
Materials and methods
Study design and area characterization
This is an exploratory, descriptive and cross-sectional study with a quantitative approach, which evaluated pregnant women hospitalized at Santa Casa de Misericórdia do Pará Foundation (FSCMPA), located in the city of Belém, in Pará state, in Amazon region of Brazil. During the COVID-19 pandemic in Pará state, there were around 898.496 confirmed COVID-19 cases and 19.244 deaths due to COVID-19 [15], of which 397 were deaths of pregnant women due to COVID-19 between 2020 and 2022 according Brazilian Obstetric Observatory [16], this high mortality rate among pregnant women are probably due to geographic conditions of Pará state, which causes difficulties for the population, especially pregnant women, to access structured public health services due to lower levels of industrialization, higher rates of poverty and concentration of specialized services in urban areas, such as FSCMPA.
The FSCMPA is the oldest healthcare institution (373 years) and one of the largest maternal and child hospital units (496 beds) in the Brazilian Amazon. It provides medium and high complexity care services, acting on spontaneous demand or referrals from the 144 municipalities of the state of Pará. The FSCMPA has been a reference in women’s and children’s health care in the Brazilian Amazon and was designated as a backup hospital for severe cases of COVID-19 during the critical phase of the pandemic. This study was conducted following the guidelines of the Helsinki Declaration and approved by the Research Ethics Committee of FSCMPA, under approval number 2.174.033.
Sample size and sampling procedure
The sample size determination was based on the estimated prevalence of COVID-19 of pregnant women in Brazil, between 2020 and 2022, which had 22.000 pregnant women COVID-19 infected as population (10.4%) and it was established the sample error (ε) as 5%, and test power was assumed of 95% resulting in a minimum sample size of 225 participants.
In total, 230 pregnant women with COVID-19 hospitalized at FSCMPA participated in the study. The classification of COVID-19 in pregnant women followed the case definition proposed by the Ministry of Health [17]: (i) mild case: cough, sore throat, or runny nose, followed or not by anosmia, ageusia, diarrhea, abdominal pain, fever, chills, myalgia, fatigue, and/or headache; (ii) moderate case: persistent cough and daily persistent fever, adynamia, prostration, hyporexia, diarrhea, and pneumonia without signs or symptoms of severity; (iii) severe case: Severe Acute Respiratory Syndrome (SARS) presenting with dyspnea/respiratory discomfort or persistent chest pressure or oxygen saturation less than 95% in ambient air or bluish discoloration of lips or face.
Based on the COVID-19 classification of Ministry of Health, pregnant women were divided into 3 groups according to the severity of the infection, G1: mild cases; G2: moderate cases and G3: severe cases. The inclusion criteria were all pregnant women of any gestational age attending to FSCMPA; to reside in the Pará state at the time of the study and sign the written consent term. The exclusion criteria were patients with severe morbidities who were not in a condition to participate, pre-existing psychiatric disorders, patients not willing to participate in the study or sign the written consent term.
Obtaining clinical-epidemiological information
Data collection was performed through a search in the electronic medical record system of FSCMPA, where a report with all the data of patients admitted to clinical or intensive care units with symptoms of influenza-like illness from April 2020 to June 2022 was obtained. Pregnant women with a positive result for SARS-CoV-2 using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) were identified in the report. Subsequently, data from these women, such as medical records and examination reports, were accessed and used to present the clinical-epidemiological information in this study. All pregnant women were followed from admission, during the hospitalization period, until discharge (clinical improvement, transfer to another hospital, or death).
Data analysis
All data collected in this study were entered into a spreadsheet in Excel and subsequently converted into a BioEstat file. The clinical and epidemiological variables of pregnant women, types of COVID-19 cases and maternal outcomes were presented using descriptive statistics, using simple frequencies and percentages; in the present study, the variables Age, municipality, ethnicity, gestational age, prenatal care, comorbidities were considered as independent, and the variables maternal clinical outcome, labor complications, lung impairment rate (%) as dependent. The variables were grouped into classes (frequency distribution) or categories (contingency tables). The chi-square test and G test were used to assess the possibility of association between variables. Statistical analysis of the data was conducted using Bioestat 5.3 software, adopting a significance level of 5%.
Results
In total, 295 (100%) pregnant women with positive results for COVID-19 by RT-PCR were initially treated at FSCMPA. However, 40 (61.53%) patients were excluded from this study because they were not admitted to the FSCMPA, 20 (30.76%) due to the absence of data in the electronic medical record system of this public health institution and 5 (7.71%) due to transfer to another hospital during the COVID-19 pandemic, leaving a total of 65/295 (22.03%) excluded. In the final sample, n = 230 (77.97%) were obtained, the average age was 28 years. Most pregnant women came from municipalities Pará state countryside, as Marabá, Parauapebas, Altamira and Santarém and had self-declared mixed ethnicity, we could observe the predominance of COVID-19 severe cases among pregnant women with self-declared mixed ethnicity, from countryside and age between 31 and 39 years old (Table 1).
Table 1.
Epidemiological profile of pregnant and postpartum women with a positive test for SARS Cov-2 and case types from April/2020 to June/2022
| Age range (years) | Total (N = 230) |
% | CI 95% | Light (N = 36) | % | CI 95% | Mild (N = 16) | % | CI 95% | Heavy (N = 178) | % | CI 95% | P-valor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 19 | 23 | 10,0 | 0.061 (6.1%) − 0.139 (13.9%) | 4 | 11,11 | 0.008 (0.8%) − 0.214 (21.4%) | 0 | 0,00 | 0.00% | 19 | 10,67 | 0.061 (6.1%) − 0.152 (15.2%) | 0,2257* |
| Between 20 and 29 | 111 | 48,26 | 0.418 (41.8%) − 0.418 (41.8%) | 19 | 52,78 | 0.365 (36.5%) − 0.691 (69.1%) | 7 | 43,75 | 0.2835 (28.35%) − 0.5915 (59.15%) | 85 | 47,75 | 0.404 (40.4%) − 0.551 (55.1%) | |
| Between 31 and 39 | 85 | 36,96 | 0.307 (30.7%) − 0.432 (43.2%) | 10 | 27,78 | 0.131 (13.1%) − 0.424 (42.4%) | 9 | 56,25 | 0.4090 (40.9%) − 0.7160 (71.6%) | 66 | 37,08 | 0.300 (30.0%) − 0.442 (44.2%) | |
| ³ 40 | 11 | 4,78 | 0.020 (2.0%) − 0.075 (7.5%) | 3 | 8,33 | 0.007 (0.7%) − 0.174 (17.4%) | 0 | 0,00 | 0.00% | 8 | 4,49 | 0.015 (1.5%) − 0.075 (7.5%) | |
| Origin | |||||||||||||
| State’s Capital | 92 | 40,0 | 0.337 (33.7%) − 0.463 (46.3%) | 12 | 33,33 | 0.179 (17.9%) − 0.487 (48.7%) | 7 | 43,75 | 0.2835 (28.35%) − 0.5915 (59.15%) | 73 | 41,01 | 0.338 (33.8%) − 0.482 (48.2%) | 0,6583* |
| Interior | 138 | 60,0 | 0.537 (53.7%) − 0.663 (66.3%) | 24 | 66,67 | 0.513 (51.3%) − 0.821 (82.1%) | 9 | 56,25 | 0.4090 (40.9%) − 0.7160 (71.6%) | 105 | 58,99 | 0.518 (51.8%) − 0.662 (66.2%) | |
| Ethnicity | |||||||||||||
| Brown | 218 | 94,78 | 0.919 (91.9%) − 0.977 (97.7%) | 35 | 97,22 | 0.9621(95.21%) − 0.9823 (92.23%) | 16 | 100,0 | 100% | 167 | 93,82 | 0.903 (90.3%) − 0.974 (97.4%) | 0,4025* |
| White | 6 | 2,61 | 0.005 (0.5%) − 0.047 (4.7%) | 0 | 0,0 | 0.00% | 0 | 0,0 | 0.00% | 6 | 3,37 | 0.007 (0.7%) − 0.060 (6.0%) | |
| Black | 6 | 2,61 | 0.005 (0.5%) − 0.047 (4.7%) | 1 | 2,78 | 0.0177 (1.77%) − 0.0379 (3.79%) | 0 | 0,00 | 0.00% | 5 | 2,81 | 0.0166(1.66%) − 0.0432 (4.32%) |
A higher number of severe cases of COVID-19 was evidenced in the late term of pregnancy (between 41 and 42 weeks), with significant differences observed at the 5% significance level for all studied variables except for the prenatal care attendance. A statistical association between the variables gestational age and severity of COVID-19 was observed (p-value < 0.05). Another significant association was found between the disease and specific pregnancy comorbidities (preeclampsia, eclampsia and hemolysis, elevated liver enzymes and low platelets-HELLP syndrome) and the overlap of other pre-existing conditions (chronic arterial hypertension and diabetes; p-value < 0.05), the most severe cases were associated to fetal and maternal death (p-value < 0.05). Birth complications also showed significant association to maternal and fetal deaths (p-value < 0.05), additionally, when considering birth complications associated with fetal prematurity and intrauterine fetal death, as well as with chest tomography, an association between the percentage of pulmonary involvement and severity in the patients’ evolution was found (p value < 0.05). Thus, greater extents of lung involvement were evidenced in severe cases (Table 2).
Table 2.
Clinical profile of pregnant and postpartum women with a positive test for SARS Cov-2 and case types from April/2020 to June/2022
| Gestational Age | Light (N = 36) | % | CI 95% | Mild (N = 16) | % | CI 95% | Heavy (N = 178) | % | CI 95% | P-valor |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Quarter | 1 | 2,78 | 0.0182 (1.82%) − 0.0374 (3.74%) | 0 | 0,00% | 0.00% | 8 | 4,49% | 0.015 (1.5%) − 0.075 (7.5%) | 0,0386* (b) |
| 2nd Quarter | 3 | 8,33 | 0.0273 (2.73%) − 0.1393 (13.93%) | 4 | 25,00% | 0.0805 (8.05%) − 0.4195 (41.95%) | 47 | 26,40% | 0.199 (19.9%) − 0.329 (32.9%) | |
| 3rd Quarter | 32 | 88,89 | 0.7899 (78.99%) − 0.9879 (98.79%) | 12 | 75,00% | 0.5805 (58.05%) − 0.9195 (91.95%) | 123 | 69,10% | 0.623 (62.3%) − 0.759 (75.9%) | |
| Prenatal | Light (N = 36) | % | Mild (N = 16) | % | Heavy (N = 178) | % | P-valor | |||
| Yes | 30 | 83,33 | 0.7352 (73.52%) − 0.9314 (93.14%) | 15 | 93,75% | 0.8428 (84.28%) − 1 (100%) | 155 | 87,08% | 0.822 (82.2%) − 0.920 (92.0%) | 0,5556 |
| No | 6 | 16,67 | 0.0686 (6.86%) − 0.2648 (26.48%) | 1 | 6,25% | 0 (0.0%) − 0.1572 (15.72%) | 23 | 12,92% | 0.080 (8.0%) − 0.178 (17.8%) | |
| Comorbidities | Light (N = 36) | % | Mild (N = 16) | % | Heavy (N = 178) | % | P-valor | |||
| None | 18 | 50,00 | 0.337 (33.7%) − 0.663 (66.3%) | 13 | 81,25 | 0.5969 (59.69%) − 1.0 (100%) | 86 | 48,31 | 0.410 (41.0%) − 0.557 (55.7%) | 0,0003* (b) |
| Pregnancy-specific | 16 | 44,44 | 0.2893 (28.93%) − 0.5995 (59.95%) | 1 | 6,25 | 0 (0.0%) − 0.1572 (15.72%) | 41 | 23,03 | 0.168 (16.8%) − 0.292 (29.2%) | |
| Of pregnancy superimposed on other pre-existing | 2 | 5,56 | 0 (0%) − 0.1446 (14.46%) | 1 | 6,25 | 0 (0.0%) − 0.1572 (15.72%) | 15 | 8,43 | 0.043 (4.3%) − 0.125 (12.5%) | |
| Pre-existing | 0 | 0,00 | 0.00% | 1 | 6,25 | 0 (0.0%) − 0.1572 (15.72%) | 36 | 20,22 | 0.143 (14.3%) − 0.261 (26.1%) | |
| Mother’s outcome | Light (N = 36) | % | Mild (N = 16) | % | Heavy (N = 178) | % | P-valor | |||
| Discharge | 36 | 100,00 | 100% | 16 | 100,00 | 1 (100%) | 158 | 88,76 | 0.841 (84.1%) − 0.934 (93.4%) | 0,0045* (b) |
| Death | 0 | 0,00 | 0.00% | 0 | 0,00 | 0 (0.0%) | 20 | 11,24 | 0.066 (6.6%) − 0.159 (15.9%) | |
| Birth complications (1) | Light (N = 30) | % | Mild (N = 10) | % | Heavy (N = 122) | % | P-valor | |||
| None | 18 | 60,00 | 0.337 (33.7%) − 0.663 (66.3%) | 4 | 40,00 | 0.0963 (9.63%) − 0.7037 (70.37%) | 63 | 51,64 | 0.428 (42.8%) − 0.605 (60.5%) | 0,0466* (b) |
| Intrauterine Fetal Death | 3 | 10,00 | 0.0273 (2.73%) − 0.1393 (13.93%) | 2 | 20,00 | 0.0482 (4.82%) − 0.4482 (44.82%) | 2 | 1,64 | 0.011 (1.1%) − 0.088 (8.8%) | |
| Prematurity | 9 | 30,00 | 0.1158 (11.58%) − 0.3842 (38.42%) | 4 | 40,00 | 0.0963 (9.63%) − 0.7037 (70.37%) | 57 | 46,72 | 0.379 (37.9%) − 0.556 (55.6%) | |
| Range (1st Chest CT - %) (2) | Light (N = 19) | % | Mild (N = 14) | % | Heavy (N = 155) | % | P-valor | |||
| < 20% | 10 | 52,63 | 0.3727 (37.27%) − 0.6799 (67.99%) | 3 | 21,43 | 0.0020 (0.2%) − 0.4706 (47.06%) | 20 | 12,90 | 0.076 (7.6%) − 0.182 (18.2%) | 0,0013* (b) |
| Between 20% and 40% | 6 | 31,58 | 0.1851 (18.51%) − 0.4465 (44.65%) | 4 | 28,57 | 0.0169 (1.69%) − 0.5545 (55.45%) | 49 | 31,61 | 0.243 (24.3%) − 0.389 (38.9%) | |
| >=40% | 3 | 15,79 | 0.0058 (0.58%) − 0.3100 (31%) | 7 | 50,00 | 0.238 (23.8%) − 0.762 (76.2%) | 86 | 55,48 | 0.477 (47.7%) − 0.633 (63.3%) |
Overall, we can observe an association between several clinical manifestations of pregnant and postpartum women and the severity of disease, considering the classification adopted for COVID-19 cases (Table 3).
Table 3.
Clinical manifestations of pregnant and postpartum women with a positive test for SARS Cov-2 and case types from April/2020 to June/2022
| Symptoms | Light (N = 36) | % | CI 95% | Mild (N = 16) | % | CI 95% | Heavy (N = 178) | % | CI 95% | P-valor |
|---|---|---|---|---|---|---|---|---|---|---|
| Cough – Yes | 26 | 72,22 | 0.514 (51.4%) − 0.930 (93%) | 14 | 87,50 | 0.769 (76.9%) − 0.981 (98.1%) | 157 | 88,20 | 0.835 (83.5%) − 0.929 (92.9%) | 0,0678 (b) |
| Cough – No | 10 | 27,78 | 0.131 (13.1%) − 0.424 (42.4%) | 2 | 12,50 | 0.0787 (7.87%) − 0.1712 (17.12%) | 21 | 11,80 | 0.071 (7.1%) − 0.165 (16.5%) | |
| Fever – Yes | 19 | 52,78 | 0.298 (29.8%) − 0.760 (76%) | 12 | 75,00 | 0.5805 (58.05%) − 0.9195 (91.95%) | 112 | 62,92 | 0.558 (55.8%) − 0.700 (70.0%) | 0,2797 (b) |
| Fever – No | 17 | 47,22 | 0.283 (28.3%) − 0.739 (73.9%) | 4 | 25,00 | 0.0805 (8.05%) − 0.4195 (41.95%) | 66 | 37,08 | 0.300 (30.0%) − 0.442 (44.2%) | |
| Dyspnea – Yes | 0 | 0,00 | 0 (0.0%) | 0 | 0,00 | 0 (0.0%) | 167 | 93,82 | 0.903 (90.3%) − 0.974 (97.4%) | 0,0001* (b) |
| Dyspnea – No | 36 | 100,00 | 1 (100%) | 16 | 100,00 | 1 (100%) | 11 | 6,18 | 0.026 (2.6%) − 0.097 (9.7%) | |
| Myalgia – Yes | 9 | 25,00 | 0.109 (10.9%) − 0.391 (39.1%) | 5 | 31,25 | 0.1436 (14.36%) − 0.4814 (48.14%) | 38 | 21,35 | 0.153 (15.3%) − 0.274 (27.4%) | 0,6342 (b) |
| Myalgia – No | 27 | 75,00 | 0.609 (60.9) − 0.891 (89.1%) | 11 | 68,75 | 0.4950 (49.5%) − 0.8799 (87.9%) | 140 | 78,65 | 0.726 (72.6%) − 0.847 (84.7%) | |
| Headache – Yes | 4 | 11,11 | 0.011 (1.1%) − 0.211 (211%) | 7 | 43,75 | 0.2835 (28.35%) − 0.5915 (59.15%) | 39 | 21,91 | 0.158 (15.8%) − 0.280 (28.0%) | 0,0363* (b) |
| Headache – No | 32 | 88,89 | 0.812 (81.2%) − 0.966 (96.6%) | 9 | 56,25 | 0.4090 (40.9%) − 0.7160 (71.6%) | 139 | 78,09 | 0.720 (72.0%) − 0.842 (84.2%) | |
| Anosmia – Yes | 4 | 11,11 | 0.011 (1.1%) − 0.211 (211%) | 6 | 37,50 | 0.1364 (13.64%) − 0.6136 (61.36%) | 53 | 29,78 | 0.231 (23.1%) − 0.365 (36.5%) | 0,0302*(b) |
| Anosmia – No | 32 | 88,89 | 0.812 (81.2%) − 0.966 (96.6%) | 10 | 62,50 | 0.3864 (38.64%) − 0.8636 (86.36%) | 125 | 70,22 | 0.635 (63.5%) − 0.769 (76.9%) | |
| Ageusia – Yes | 0 | 0,00 | 0 (0.0%) | 2 | 12,50 | 0.0787 (7.87%) − 0.1712 (17.12%) | 10 | 5,62 | 0.022 (2.2%) − 0.090 (9.0%) | 0,0754 (b) |
| Ageusia – No | 36 | 100,00 | 1 (100%) | 14 | 87,50 | 0.769 (76.9%) − 0.981 (98.1%) | 168 | 94,38 | 0.910 (91.0%) − 0.978 (97.8%) | |
| Runny nose – Yes | 9 | 25,00 | 0.109 (10.9%) − 0.391 (39.1%) | 1 | 6,25 | 0.05824 (5.82%) − 0.1832 (18.32%) | 25 | 14,04 | 0.089 (8.9%) − 0.191 (19.1%) | 0,1537 (b) |
| Runny nose – No | 27 | 75,00 | 0.609 (60.9) − 0.891 (89.1%) | 15 | 93,75 | 0.8428 )84.28%) − 1.0 (100%) | 153 | 85,96 | 0.809 (80.9%) − 0.911 (91.1%) | |
| Odynophagia – Yes | 8 | 22,22 | 0.078 (7.8%) − 0.366 (36.6%) | 0 | 0,00 | 0 (0.0%) | 20 | 11,24 | 0.066 (6.6%) − 0.159 (15.9%) | 0,0283* (b) |
| Odynophagia - No | 28 | 77,78 | 0.645 (64.5%) − 0.911 (91.1%) | 16 | 100,00 | 1 (100%) | 158 | 88,76 | 0.841 (84.1%) − 0.934 (93.4%) | |
| Diarrhea - Yes | 0 | 0,00 | 0 (0.0%) | 4 | 25,00 | 0.0805 (8.05%) − 0.4195 (41.95%) | 18 | 10,11 | 0.057 (5.7%) − 0.145 (14.5%) | 0,0053* (b) |
| Diarrhea - No | 36 | 100,00 | 1 (100%) | 12 | 75,00 | 0.5805 (58.05%) − 0.9195 (91.95%) | 160 | 89,89 | 0.855 (85.5%) − 0.943 (94.3%) | |
| Asthenia – Yes | 4 | 11,11 | 0.011 (1.1%) − 0.211 (211%) | 1 | 6,25 | 0.05824 (5.82%) − 0.1832 (18.32%) | 26 | 14,61 | 0.094 (9.4%) − 0.198 (19.8%) | 0,5373 (b) |
| Asthenia - No | 32 | 88,89 | 0.812 (81.2%) − 0.966 (96.6%) | 15 | 93,75 | 0.8428 )84.28%) − 1.0 (100%) | 152 | 85,39 | 0.802 (80.2%) − 0.906 (90.6%) | |
| Chest pain – Yes | 1 | 2,78 | 0.026 (2.6%) − 0.082 (8.2%) | 2 | 12,50 | 0.0787 (7.87%) − 0.1712 (17.12%) | 51 | 28,65 | 0.220 (22.0%) − 0.353 (35.3%) | 0,0003* (b) |
| Chest pain - No | 35 | 97,22 | 0.917(91.7%) − 1.0(100%) | 14 | 87,50 | 0.769 (76.9%) − 0.981 (98.1%) | 127 | 71,35 | 0.647 (64.7%) − 0.780 (78.0%) | |
| Convulsion – Yes | 0 | 0,00 | 0 (0.0%) | 0 | 0,00 | 0 (0.0%) | 8 | 4,49 | 0.015 (1.5%) − 0.075 (7.5%) | 0,1234 (b) |
| Convulsion - No | 36 | 100,00 | 1 (100%) | 16 | 100,00 | 1 (100%) | 170 | 95,51 | 0.925 (92.5%) − 0.985 (98.5%) | |
| Vomiting – Yes | 1 | 2,78 | 0.026 (2.6%) − 0.082 (8.2%) | 0 | 0,00 | 0 (0.0%) | 3 | 1,69 | 0 (0.0%) − 0.0868 (8.68%) | 0,6848 (b) |
| Vomiting - No | 35 | 97,22 | 0.917(91.7%) − 1.0(100%) | 16 | 100,00 | 1 (100%) | 175 | 98,31 | 0.940 (94.0%) − 0.993 (99.3%) | |
| Backache – Yes | 0 | 0,00 | 0 (0.0%) | 0 | 0,00 | 0 (0.0%) | 3 | 1,69 | 0 (0.0%) − 0.0868 (8.68%) | 0,4609 (b) |
| Backache - No | 36 | 100,00 | 1 (100%) | 16 | 100,00 | 1 (100%) | 175 | 98,31 | 0.940 (94.0%) − 0.993 (99.3%) |
When evaluating gestational trimester, prenatal care, comorbidities, birth complications, and chest tomography of pregnant women in relation to their outcome, the influence of the disease on specific pregnancy-related comorbidities and the overlap of other pre-existing conditions was detected, as well as greater lung involvement (Table 4).
Table 4.
Clinical profile of pregnant and postpartum women with a positive test for SARS Cov-2 and the mother’s outcome from April/2020 to June/2022
| Gestational Age | Discharge (N = 210) | % | CI 95% | Death (N = 20) | % | CI 95% | P-valor |
|---|---|---|---|---|---|---|---|
| 1st Quarter | 9 | 4,29 | 0.015 (1.5%) − 0.070 (7.0%) | 0 | 0,00 | 0 | 0,4236 (b) |
| 2nd Quarter | 51 | 24,29 | 0.185 (18.5%) − 0.301 (30.1%) | 3 | 15,00 | 0.0904 (9.04%) − 0.2495 (24.95%) | |
| 3rd Quarter | 150 | 71,43 | 0.653 (65.3%) − 0.775 (77.5%) | 17 | 85,00 | 0.5336 (53.36%) − 0.9664 (96,64%) | |
| Prenatal | Discharge (N = 210) | % | CI 95% | Death (N = 20) | % | P-valor | |
| Yes | 183 | 87,14 | 0.826 (82.6%) − 0.917 (91.7%) | 17 | 85,00 | 0.5336 (53.36%) − 0.9664 (96,64%) | 0,7959 (b) |
| No | 27 | 12,86 | 0.083 (8.3%) − 0.174 (17.4%) | 3 | 15,00 | 0.0904 (9.04%) − 0.2495 (24.95%) | |
| Comorbidities | Discharge (N = 210) | % | CI 95% | Death (N = 20) | % | P-valor | |
| None | 112 | 53,33 | 0.466 (46.6%) − 0.601 (60.1%) | 5 | 25,00 | 0.0215 (2.15%) − 0.2785 (27.85%) | 0,0411* (b) |
| Pregnancy-specific | 51 | 24,29 | 0.185 (18.5%) − 0.301 (30.1%) | 7 | 35,00 | 0.2352 (23.52%) − 0.5648 (56.48%) | |
| Of pregnancy superimposed on other pre-existing | 17 | 8,10 | 0.044 (4.4%) − 0.118 (11.8%) | 1 | 5,00 | 0.0012 (0.12%) − 0.0988 (9.88%) | |
| Pre-existing | 30 | 14,29 | 0.096 (9.6%) − 0.190 (19.0%) | 7 | 35,00 | 0.2352 (23.52%) − 0.5648 (56.48%) | |
| Birth complications | Discharge (N = 210) | % | CI 95% | Death (N = 20) | % | P-valor | |
| None | 78 | 54,93 | 0.306 (30.6%) − 0.437 (43.7%) | 7 | 35,00 | 0.2352 (23.52%) − 0.5648 (56.48%) | 0,0626 (b) |
| Intrauterine Fetal Death | 7 | 4,93 | 0.009 (0.9%) − 0.058 (5.8%) | 0 | 0,00 | 0 | |
| Prematurity | 57 | 40,14 | 0.211 (21.1%) − 0.332 (33.2%) | 13 | 65,00 | 0.3925 (39.25%) − 0.8575 (85.75%) | |
| Not applicable | 68 | 32,38 | 0.261 (26.1%) − 0.387 (38.7%) | 0 | 0 | 0 | |
| Range (1st Chest CT - %) (2) | Discharge (N = 170) | % | CI 95% | Death (N = 18) | % | P-valor | |
| < 20% | 33 | 19,41 | 0.135 (13.5%) − 0.254 (25.4%) | 0 | 0,00 | 0 | 0,0006* (b) |
| Between 20% and 40% | 57 | 33,53 | 0.264 (26.4%) − 0.406 (40.6%) | 2 | 11,11 | 0.0215 (2.15%) − 0.1644 (16.44%) | |
| >=40% | 80 | 47,06 | 0.396 (39.6%) − 0.546 (54.6%) | 16 | 88,89 | 0.4751 (47.51) − 0.9579 (95.79%) |
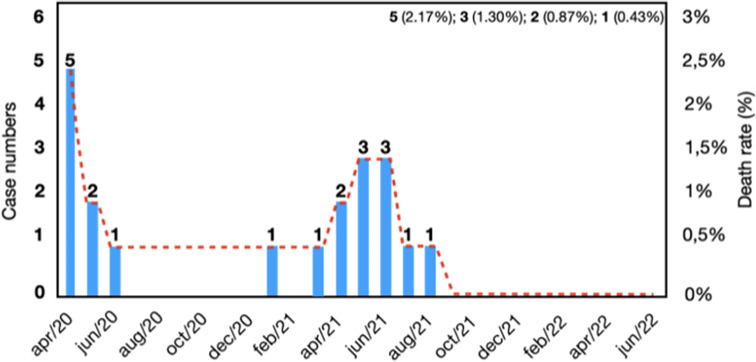
Considering the case fatality rate of 8.7% (n = 20) from April 2020 to June 2022, an increase in the number of deaths is observed from March 2021 to June 2021, corresponding to the increase in the number of cases, followed by a stabilization from July 2021 onwards. After August 2021 and the end of the study period, no deaths related to SARS-CoV-2 infection were recorded (Fig. 1).
Fig. 1.
Histogram showing the evolution of the number of case fatality rate of pregnant women with COVID-19 treated at Fundação Santa Casa de Misericórdia do Pará, Belém, from April/2020 to June/2022
Discussion
The present study describes the clinical-epidemiological profile and maternal outcome of COVID-19 in pregnant women hospitalized at the main maternal and child hospital in the Brazilian state of Pará, especially during the pre-vaccination period against COVID-19. Regarding age range and ethnicity, most participants were between 20 and 29 years old and self-declared as mixed-race, similar to records made in other Brazilian regions [18, 19]. Furthermore, the highest number of COVID-19 cases attended at FSCMPA was found in women from municipalities in the interior of the State of Pará, considering that the hospital served as a regional reference for high-risk maternal and childcare and for severe cases of COVID-19. It is worth noting that by the end of May 2020, severe cases of COVID-19 were more reported in municipalities in the interior of the states of Pará and Amazonas than in the capitals [20].
In the present study, the highest number of serious cases of COVID-19 were recorded in the late term of pregnancy residents of northern Brazil were similar to records made in other regions of Brazil [21] and in other countries such as the Czech Republic [22], United Kingdom [23] and China [24], suggesting that the attention and care of pregnant women during this period requires maximum attention in relation to COVID-19, as reported by Medeiros et al. [25]. The most frequent comorbidities in this study were systemic arterial hypertension and gestational hypertensive syndromes. High blood pressure and diabetes are clinical conditions that can increase the risk of complications from COVID-19, in addition to obesity, elderly age and chronic lung disease [26].
It is known that pregnancy itself is a physiological event, but the anatomical and physiological changes imposed by this period can exacerbate pre-existing comorbidities and make pregnant women susceptible to various infections, including respiratory ones [26]. SARS-CoV-2 infection during pregnancy can have severe consequences for the fetus, as it relies on maternal oxygenation, and if sufficient oxygen supply does not reach through the placenta, fetal circulation may be compromised. This would explain why pregnant women may progress to preterm labor, have babies with low birth weight, or experience intrauterine growth restriction [27]. However, we observed that the symptoms most related to COVID-19 exacerbation and possible maternal death were dyspnea, headache, anosmia, odynophagia, diarrhea, and chest pain. Interestingly, half of the population in this study required ICU admission, but there is a point to consider [28, 29]. The care profile of the ICU at the maternity hospital of FSCMPA is for women in the perinatal period, which means that patients are admitted daily for management of complications related to this period. Thus, it cannot be ensured that women were admitted to the ICU necessarily due to worsening COVID-19 symptoms, as many of them had comorbidities that, in some situations, would require transfer to the ICU.
The most frequent perinatal complications in this study were preterm birth and fetal death. Prematurity during the most critical period of COVID-19 is mentioned in other studies [30–32]; however, one factor to consider is whether preterm birth was triggered by SARS-CoV-2 infection or by complications in the pregnancy itself. Additionally, it should be noted that due to the high rates of cesarean delivery in the hospital where the study was conducted, it cannot be accurately attributed whether prematurity was due to maternal SARS-CoV-2 infection, like what has been observed in other studies [33–35].
The case fatality rate found in this study was 8.7%, with a higher number of deaths observed between March and June 2021, corresponding to the peak of the pandemic in the Amazon region and Brazil, which was significantly higher than observed in other studies worldwide [7, 8, 11, 36]. However, it is not possible to affirm that case fatality rate was solely due to COVID-19, considering that the majority had specific pregnancy-related comorbidities. The high morbidity and mortality rate from COVID-19 among Brazilian pregnant women may be related to both the pathophysiological conditions inherent in the gestational process and the illness caused by SARS-CoV-2 infection, as well as chronic problems faced by Brazilian obstetric care - such as low-quality prenatal care and difficulty accessing emergency and high-complexity care [35].
From this perspective, Villar et al. [11] observed that deaths were more concentrated in institutions in less developed regions, implying that when ICU services and resources are not fully available, COVID-19 in pregnancy can be lethal. This was corroborated by another study [36], stating that SARS-CoV-2 infection is more frequent in people living in socially and economically disadvantaged environments, which was confirmed by Emeruwa et al. [37]. In this study, a stabilization of cases and deaths was observed from July 2021 onwards, which may be justified by the advent of vaccination in the country. This is because vaccination reduces the risk of developing COVID-19 and the severity of the disease if an advanced infection occurs, as well as reducing the risk of stillbirth. Additionally, all available evidence supports the safety of administering currently available vaccines before, during, and after pregnancy. One limitation of the study is that it was conducted in a single setting, which does not allow for the generalization of the information found here and a comprehensive representation of reality. Furthermore, secondary data sources were used, which may introduce information bias due to incompleteness or underreporting of cases.
Conclusion
The dynamics of the COVID-19 pandemic in pregnant women in the Brazilian Amazon affect young adult women of non-white ethnicity in the third trimester of pregnancy. Specific pregnancy-related and pre-existing comorbidities associated with SARS-CoV-2 infection directly contribute to the worsening clinical condition, leading to complications such as prematurity, fetal, and maternal death. There was a tendency towards case stabilization after the vaccination period, and the mortality rate was considered low compared to the total sample.
Acknowledgements
We acknowledge all subjects enrolled in this study and Executive Secretariat of Public Health of the State of Pará.
Author contributions
Conceptualization: A.P.F.M.F. and L.F.A.M.; Data Curation: A.P.F.M.F., J.T.R.P., L.G.F.M.L., A.S.F.V. and F.J.S.F.R.; Investigation and Methodology: A.P.F.M.F., J.T.R.P., L.G.F.M.L., A.S.F.V., F.J.S.F.R., P.F.M.N. and L.F.M.F; Formal Analysis: R.V.L., A.C.F.M.S., A.B.O.-F. and L.F.A.M.; Writing—Original Draft: A.P.F.M.F., R.R.S.F., A.B.O.-F. and L.F.A.M.; Writing—Review and Editing: R.R.S.F., A.B.O.-F. and L.F.A.M.; Project Administration: L.F.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education—Brazil—Grant code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). LFAM is a CNPq Grantee (#314209/2021-2). Publication of the article was supported by Public Notice PAPQ, PROPESP/FADESP of the Federal University of Pará.
Data availability
All related data have been presented within the manuscript. The dataset supporting the conclusions of this article is available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Committee for Ethics in Research of the Research by the Health Sciences Institute, of the Federal University of Pará, Brazil (protocol number: 2.174.033). All participants were included in the study after providing informed and written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Veiga Gonçalves N, De Melo Neto JS, Kazumi da Trindade Noguchi S, Silva Machado A, Da Silva Martins Junior A, Da Silva Peixoto MC, et al. COVID-19 in socially vulnerable quilombola populations in Salvaterra, Para, Eastern Amazon, Brazil. J Infect Dev Ctries. 2021;15(8):1066–73. 10.3855/jidc.14420. [DOI] [PubMed] [Google Scholar]
- 2.da Silva Torres MK, Lopes FT, de Lima ACR, Cordeiro Lima CN, Dos Santos Brito WR, Gonçalves JSS, et al. Changes in the seroprevalence and risk factors between the first and second waves of COVID-19 in a metropolis in the Brazilian Amazon. Front Cell Infect Microbiol. 2022;16(12):932563. 10.3389/fcimb.2022.932563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva HP, Abreu IN, Lima CNC, de Lima ACR, do, Nascimento Barbosa A, de Oliveira LR et al. Migration in times of pandemic: SARS-CoV-2 infection among the Warao indigenous refugees in Belem, Para, Amazonia, Brazil. BMC Public Health. 2021;21(1):1659. 10.1186/s12889-021-11696-7 [DOI] [PMC free article] [PubMed]
- 4.Sousa F, de Araujo LN, de Oliveira TSO, Gomes MC, Ferreira G, Aben-Athar C, et al. Demographic, clinical, and quality of life profiles of older people with diabetes during the COVID-19 Pandemic: cross-sectional study. JMIR Form Res. 2023;16(7):e49817. 10.2196/49817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahromi AS, Jokar M, Sharifi N, Omidmokhtarloo B, Rahmanian V. Global knowledge, attitude, and practice towards COVID-19 among pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2023;23(1):278. 10.1186/s12884-023-05560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qeadan F, Mensah NA, Tingey B, Stanford JB. The risk of clinical complications and death among pregnant women with COVID-19 in the Cerner COVID-19 cohort: a retrospective analysis. BMC Pregnancy Childbirth. 2021;21(1):305. 10.1186/s12884-021-03772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.França APFM, Pereira DDV, Rodrigues EV, Vieira FN, Machado KS, Nogueira PA, et al. Severe COVID-19 in Cardiopath Young Pregnant Patient without Vertical Transmission. Viruses. 2022;14(4):675. 10.3390/v14040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paixão JTR, Santos CJSE, França APFM, Lima SS, Laurentino RV, Fonseca RRS, et al. Association of D-Dimer, C-Reactive Protein, and ferritin with COVID-19 severity in pregnant women: important findings of a cross-sectional study in Northern Brazil. Int J Environ Res Public Health. 2023;20(14):6415. 10.3390/ijerph20146415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purwono A, Agustin H, Lisnawati Y, Faisal HKP. Respiratory perspective of COVID-19 in pregnancy. J Infect Dev Ctries. 2023;17(1):23–36. 10.3855/jidc.16944. [DOI] [PubMed] [Google Scholar]
- 10.Kumar D, Verma S, Mysorekar IU. COVID-19 and pregnancy: clinical outcomes; mechanisms, and vaccine efficacy. Transl Res. 2023;251(4):84–95. 10.1016/j.trsl.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland C, Hammond C, Richmond MM. COVID-19 and pregnancy: risks and outcomes. Nurs Womens Health. 2023;27(1):31–41. 10.1016/j.nwh.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonçalves CWB, Gomes DLF, Pinto Neto AB, Lima GS, Reis KHJF, Cláudio ES. Incidence of COVID-19 in the States of the northern region of Brazil. Rev Pre Infec E Saúde. 2020;6(2):10489. 10.26694/repis.v6i0.10489. [Google Scholar]
- 13.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leal LF, Malta DC, Souza MFM, Vasconcelos AMN, Teixeira RA, Veloso GA, et al. Maternal mortality in Brazil, 1990 to 2019: a systematic analysis of the global burden of Disease Study 2019. Rev Soc Bras Med Trop. 2022;55(suppl 1):e0279. 10.1590/0037-8682-0279-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health Secretariat of the State of Pará. COVID-19 Monitoring. 2024. https://www.covid-19.pa.gov.br/#/. Accessed 7 March 2024.
- 16.Brazilian Obstetric Observatory. OOB presents unprecedented mortality data for pregnant and postpartum women in Brazil. 2024. https://observatorioobstetricobr.org/publicacoes/oobr-apresenta-dados-de-mortalidade-gestantes-e-puerperas-no-brasil. Accessed 7 March 2024.
- 17.Brazilian Ministry of Health. Health Surveillance Secretariat. Department of Health Analysis and Non-Communicable Diseases. Epidemiological surveillance guide Public health emergency of national importance caused by coronavirus disease 2019 – COVID-19. 2021. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/coronavirus/guia-de-vigilancia-epidemiologica-covid-19_2021.pdf/view. Accessed 10 March 2024.
- 18.Cordeiro GTO, Souza RLM, Gazar TN, Menexes SSV, Menezes TAS, Ferreira ATA. Epidemiological profile of Brazilian pregnant and postpartum women in the context of the COVID-19 pandemic, in 2020. Rev Baiana Saude Publica. 2022;46(3):150–66. 10.22278/2318-2660.2022.v46.n3.a3416. [Google Scholar]
- 19.Souza RT, Cecatti JG, Pacagnella RC, Ribeiro-Do-Valle CC, Luz AG, Lajos GJ, et al. The COVID-19 pandemic in Brazilian pregnant and postpartum women: results from the REBRACO prospective cohort study. Sci Rep. 2022;12(1):11758. 10.1038/s41598-022-15647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muniz ES. The internalization of COVID-19 in the Amazon: reflections on the past and present of public health. Hist Cienc Saude-Manguinhos. 2021;28(3):875–8. 10.1590/S0104-59702021005000007. [DOI] [PubMed] [Google Scholar]
- 21.Peres GP, Ferraz JG, Matos AFM, Zöllner MSA. Epidemiological profile of pregnant women infected by COVID-19. Braz J Infect Dis. 2022;26(9):102587. 10.1016/j.bjid.2022.102587. [Google Scholar]
- 22.Ambrož R, Stašek M, Molnár J, Špička P, Klos D, Hambálek J, et al. Spontaneous liver rupture following SARS-CoV-2 infection in late pregnancy: a case report. World J Clin Cases. 2022;10(15):5042–50. 10.12998/wjcc.v10.i15.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population-based cohort study. BMJ. 2020;8(369):m2107. 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Dong L, Ming L, Wei M, Li J, Hu R, et al. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. BMC Pregnancy Childbirth. 2020;20(1):394. 10.1186/s12884-020-03026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Medeiros KS, Sarmento ACA, Costa APF, Macêdo LTA, da Silva LAS, de Freitas CL, et al. Consequences and implications of the coronavirus disease (COVID-19) on pregnancy and newborns: a comprehensive systematic review and meta-analysis. Int J Gynaecol Obstet. 2022;156(3):394–405. 10.1002/ijgo.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton A. Physiological changes and Cardiovascular investigations in pregnancy. Heart Lung Circ. 2021;30(1):e6–15. [DOI] [PubMed] [Google Scholar]
- 27.Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225(1):77. 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease Severity and Perinatal outcomes of pregnant patients with Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. 2021;137(4):571–80. 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Varea A, Satorres E, Florez S, Domenech J, Desco-Blay J, Monfort-Pitarch S, et al. Comparison of maternal-fetal outcomes among unvaccinated and vaccinated pregnant women with COVID-19. J Pers Med. 2022;12(12):2008. 10.3390/jpm12122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Y, Ma H, Suo M, Han F, Wang F, Ji J, et al. Clinical manifestation, outcomes in pregnant women with COVID-19 and the possibility of vertical transmission: a systematic review of the current data. J Perinat Med. 2020;48(9):912–24. 10.1515/jpm-2020-0431. [DOI] [PubMed] [Google Scholar]
- 31.Gurol-Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5):522. 10.1016/j.ajog.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettirosso E, Giles M, Cole S, Rees M. COVID-19 and pregnancy: a review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60(5):640–59. 10.1111/ajo.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura-Pereira M, Amorim MMR, Pacagnella RC, Takemoto MLS, Penso FCC, Rezende-Filho J, et al. COVID-19 and maternal death in Brazil: an invisible tragedy. Rev Bras Ginecol Obstet. 2020;42(8):445–7. 10.1055/s-0040-1715138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheler CA, Discacciati MG, Vale DB, Lajos GJ, Surita F, Teixeira JC. Mortality in pregnancy and the postpartum period in women with severe acute respiratory distress syndrome related to COVID-19 in Brazil, 2020. Int J Gynaecol Obstet. 2021;155(3):475–82. 10.1002/ijgo.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villar J, Soto Conti CP, Gunier RB, Ariff S, Craik R, Cavoretto PI, et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. 2023;401(10375):447–57. 10.1016/S0140-6736(22)02467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–86. 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emeruwa UN, Gyamfi-Bannerman C, Miller RS. Health Care disparities in the COVID-19 pandemic in the United States: a focus on obstetrics. Clin Obstet Gynecol. 2022;65(1):123–33. 10.1097/GRF.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All related data have been presented within the manuscript. The dataset supporting the conclusions of this article is available from the corresponding author upon request.