Abstract
Background
Despite advances in prevention and treatment, postoperative atrial fibrillation (POAF) is the most common type of complication undergoing cardiac surgery. This study aimed to identify the relationship between POAF and clinical outcomes after coronary artery bypass graft.
Methods
In this cross-sectional study, we retrospectively reviewed the medical records of 324 patients who had undergone coronary artery bypass grafting in an intensive care unit between 2010 and 2019 at a tertiary hospital in Korea. Propensity score matching was used to estimate a 1:1 match (without: with POAF) using seven covariates to overcome selection bias. Kaplan–Meier survival analysis and Cox proportional hazards modeling were performed to determine the effect on intensive care unit readmission and length of hospital stay.
Results
After controlling for covariates, 1:1 matching was performed for 91 patients in each group. The occurrence of postoperative atrial fibrillation was found to increase the probability of readmission to the intensive care unit, with a 23% reduced probability of readmission for every 10% increase in left ventricular ejection fraction. Multivariate analysis indicated that postoperative atrial fibrillation, chronic obstructive pulmonary disease as a comorbidity, and preoperative hemoglobin were factors affecting the length of hospitalization after surgery. The Kaplan–Meier survival analysis results indicated that the without POAF group had a higher survival rate than the with POAF group.
Conclusions
Healthcare professionals should recognize negative factors such as postoperative atrial fibrillation and abnormal hematologic parameters that impact major clinical outcomes in patients and may require closer monitoring before and after coronary artery bypass grafting.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04247-6.
Keywords: Atrial fibrillation, Coronary artery bypass grafting, Clinical outcome, Propensity score matching
Background
Coronary artery bypass graft (CABG) surgery remains the gold standard treatment for multivascular and left main coronary artery diseases [1]. Despite successful revascularization, patients who have undergone CABG remain at risk of recurrent adverse ischemic events and other cardiovascular outcomes, such as coronary revascularization, stroke, and cardiac death [2, 3]. Atrial fibrillation (AF) is the most common perioperative arrhythmia, with an estimated incidence of 11–40% and 3% in patients who have undergone CABG and major noncardiac surgeries, respectively [4].
AF after heart surgery usually presents within the first 48 h [5] and is related to increased sympathetic tone and inflammation. This drives the emergence and maintenance of AF after cardiac surgery [6]. Postoperative atrial fibrillation (POAF) is associated with an increased incidence of short-term complications after CABG [7]. Rapid and irregular ventricular rates in POAF can lead to myocardial ischemia due to insufficient coronary flow to compensate for myocardial oxygen demand [8]. Less frequently, AF manifests as brady arrhythmias, which may decrease cardiac output in patients with fixed stroke volume. Finally, loss of atrial contraction increases pulmonary artery pressure, primarily in patients with hypertension and diastolic dysfunction [9]. Cumulatively, all these effects on cardiopulmonary hemodynamics can lead to hypotension, heart failure, and myocardial infarction [10].
Moreover, POAF not only makes patients anxious but also causes complications, such as additional drug treatment, increased cost, and prolonged length of hospital stay (LOS) [11].
In contrast, POAF prophylaxis can lead to prevent hypotension, bradycardia, and heart blockade. In addition, identifying patients prone to POAF can have a positive impact on clinical outcomes, such as intensive care unit (ICU) readmission rates, LOS, and mortality, and enable personalized preventive treatment [2]. This is a significant challenge for both patients and healthcare professionals because AF is associated with numerous detrimental sequelae, including worsening of patients’ hemodynamic status, increased risk of congestive heart failure (CHF), embolism, stroke, and prolonged ICU stays [12].
Despite efforts to elucidate the optimal management of POAF, the incidence of POAF following cardiac surgery has remained relatively consistent in the past several decades, suggesting that greater efforts must be made to understand the required interventions. In addition, the rates of recurrent AF as well as optimal follow-up and management of adverse effects remain unclear. Most studies have been conducted on the occurrence of AF after CABG have confirmed the mortality rate, readmission rate [7], the effectiveness of various antiarrhythmic therapies [13], and treatment methods for patients who underwent CABG [14, 15]. However, most studies conducted using cross-sectional data with convenience sampling were unable to determine the relationship between POAF and adverse effects, such as readmission to the ICU and prolonged LOS; hence, the results cannot be generalized to the relevance of POAF. For this reason, the occurrence and clinical outcomes of POAF after CABG remain uncertain.
In this study, we compared a group of patients with POAF with a group without POAF following CABG using a propensity score matching (PSM) approach, which can reduce selection bias and capitalize on the randomization effect [16] of this method to determine the correlation between the occurrence of POAF and clinical outcomes after CABG.
The study objectives were as follows: (1) identification of general and clinical characteristics of patients depending on whether POAF occurs or not; (2) differences in control variables before and after 1:1 matching; and (3) identification of the relationship between AF and clinical outcomes (ICU readmission, LOS, survival rate) after CABG.
Methods
Design
A retrospective cohort investigation was performed to identify the association between POAF and clinical outcomes, such as ICU readmission, LOS, and survival rate.
Participants and recruitment
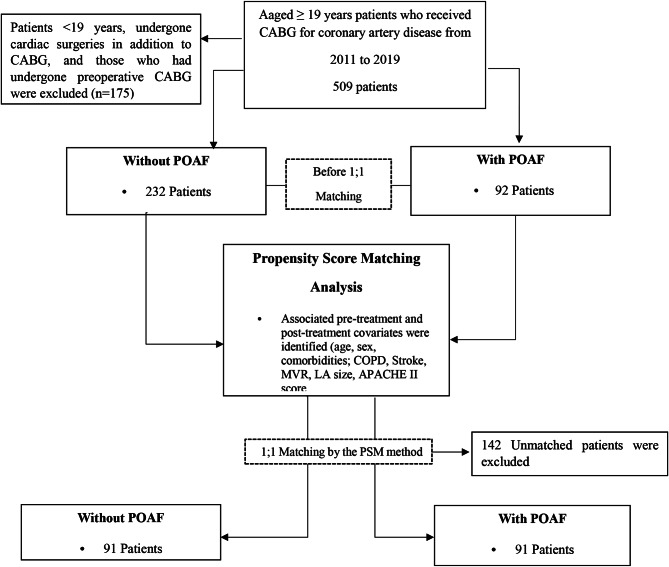
The study participants were adults aged ≥ 19 years who had undergone CABG for coronary artery disease, including those with AF on electrocardiography, those who had undergone reoperation CABG (Redo-CABG) or emergency CABG, and those who had died during hospitalization. Patients < 19 years old, those who had undergone cardiac surgeries in addition to CABG, and those who had undergone preoperative CABG were excluded. Additionally, patients with incomplete electronic medical record data regarding general characteristics, clinical characteristics, and clinical results related to CABG were excluded from the study. The flow chart of this study is shown in the Fig. 1.
Fig. 1.
Flow chart of the present study. PSM: Propensity score matching
Sample size and power
A total of 324 patients met the selection criteria. We estimated the sample size needed for logistic regression using G*Power version 3.1.9.4. Based on achieving 80% power (1 − β) with an alpha of 0.05, a small to medium effect size (odds ratio 1.5) [17], and a two-tailed test, the minimum sample size required for analysis was 308. Accordingly, a sample size of 324 was considered sufficient.
Measures
Clinical and disease-related characteristics
The general and disease-related characteristics of the patients included in the stage were the following: age; sex; smoking and drinking history; medical insurance status; body mass index; comorbidities (hypertension, diabetes, chronic kidney injury, chronic obstructive pulmonary disease [COPD]; mitral valve regurgitation [MVR]); history of acute kidney injury, stroke, and myocardial infarction; left atrium (LA) size; left ventricular ejection fraction (LVEF); physiological parameters such as preoperative hemoglobin, CK-MB, and troponin I; cardiopulmonary bypass use; intraoperative intra-aortic balloon pumping (IABP); APACHE II score at ICU admission; and duration of mechanical ventilation.
POAF was defined as any episode of AF (occurrence of irregular heart rhythm without detectable P-waves) lasting more than 30 s that was recorded on cardiac telemetry or an episode of AF requiring treatment, including antiarrhythmic drugs, such as amiodarone or electrical cardioversion, during hospitalization. This definition is consistent with those of most previous studies [12].
Clinical outcomes
Primary research or clinical outcomes, such as LOS, readmission to the ICU, and survival rate, were used as dependent variables. The period for readmission to the ICU was within 90 days of discharge after CABG.
Data collection
The research data included medical records (admission and discharge data, postoperative clinical records, nursing records from the ICU and general ward, and blood records) of patients who had undergone CABG at a tertiary general hospital in South Korea. Two researchers with experience in ICUs, including conducting examinations, collected data by searching medical records and cross-examined these data to increase reliability.
Statistical analysis and PSM
PSM was used to control and balance existing confounding factors when analyzing the relationship between the occurrence of POAF after CABG and clinical outcomes. Because the data in the current study were population-based, a comparison of clinical outcomes between groups with and without POAF might have been biased. The propensity score was applied to a logistic regression model in which the dependent variables included the LOS and readmission occurrence. As a covariate that adjusts the treatment effect for baseline differences were those with significant differences between the groups in disease-related characteristics, including age, sex, smoking status, comorbidities (COPD, stroke, MVR), LA size, and the APACHE II score upon ICU admission. Based on the propensity score, groups with and without POAF were matched at a 1:1 ratio. The number of matches was optimifized by matching patients with the fewest matches. To compare the results between groups, the “nearest neighbor matching” standard with a caliper value of 0.02 was used, and the balance of the matched data was evaluated by comparing standardized mean differences. A standardized difference between − 0.10 and 0.10 indicated an appropriate balance.
We investigated baseline characteristics as the number of frequencies for categorical variables and as mean values with standard deviations for continuous variables. We compared the mean or frequency of each variable between the POAF and non-POAF groups using an independent two-sample t-test for continuous variables and a chi-squared test for categorical variables. The measurement variables of this study were confirmed to be normally distributed, satisfying the criteria of normal distribution: skewness <|2|, kurtosis <|4|.
PSM was performed using R software (version 3.6.3), and a Kaplan–Meier analysis was performed by log-rank testing to compare survival rates between the POAF and non-POAF groups. A Cox multivariate regression analysis was performed to identify independent variables associated with ICU readmission in the population. Multivariate models were built by forward stepwise (likelihood ratio) selection, with candidate variables included if they met the entry criterion of p < 0.05 in the univariate analysis. All analyses were performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA). Missing information was replaced by multiple imputation.
Ethical considerations
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital Biomedical Research Institute (approval no.: 20200114/10-2020-8/021). Because of the retrospective nature of the study, the requirement for informed consent was waived. The data were analyzed anonymously and kept confidential.
This study was conducted in compliance with ethical principles based on the “Declaration of Helsinki 2013 (Ethical Principles for Medical Research on Human Subjects)” and ICH (International Council for Harmonisation)-GCP (Good Clinical Practice) guidelines. It was conducted after IRB approval for secondary analysis.
Results
General characteristics and distribution of propensity scores before and after matching
Table 1 shows the baseline characteristics of the patients. Of the 324 patients who had undergone CABG, 92 and 232 were included in the POAF and non-POAF groups, respectively, and the statistical differences in patient characteristics between the two groups were compared. The average age of the group that developed POAF after CABG was 69.17 (± 8.07), and that of the group without POAF was 63.78 (± 10.22). The highest age of occurrence of postoperative POAF was in the 70s; 42.39% and 29.74%, in the POAF and non-POAF groups, respectively. More than 50% of patients with POAF (71.55%) were men. By 1:1 matching of patients with the same (or very similar) propensity scores in both groups, 182 people were selected (91 per group) for continued evaluation. Before matching, baseline characteristics, including age, COPD, stroke, MVR, LA size, and APACHE II score at ICU admission, showed statistically significant differences between the groups (p < 0.05).
Table 1.
Baseline characteristics of patients who underwent coronary artery bypass grafting in the original cohort (N = 324)
| Characteristics | Without POAF (n = 232) | With POAF (n = 92) | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (years), M ± SD | 63.78 (10.22) | 69.17 (8.07) | < 0.001 |
| < 60 years | 77 (33.19) | 13 (14.13) | < 0.001 |
| 60–69 years | 78 (33.62) | 30 (32.61) | |
| 70–79 years | 69 (29.74) | 39 (42.39) | |
| ≥ 80 years | 8 (3.45) | 10 (10.87) | |
| Sex, men | 166 (71.55) | 66 (71.74) | 0.973 |
| Smoking | 0.066 | ||
| Non-smoker | 107 (46.12) | 44 (47.83) | |
| Ex-smoker | 56 (24.14) | 31 (33.70) | |
| Smoker | 69 (29.74) | 17 (18.48) | |
| Alcohol drinking, yes | 98 (42.24) | 43 (46.74) | 0.462 |
| Health insurance, medical aids | 187 (80.60) | 65 (70.65) | 0.052 |
| Body mass index (kg/m2) | 24.64 (3.12) | 24.50 (3.52) | 0.725 |
| Comorbidities | |||
| Hypertension, yes | 159 (68.53) | 76 (82.61) | 0.010 |
| Diabetes, yes | 111 (47.84) | 53 (57.61) | 0.113 |
| Chronic kidney injury, yes | 49 (21.12) | 24 (26.09) | 0.335 |
| COPD, yes | 7 (3.02) | 9 (9.78) | 0.011 |
| Mitral valve regurgitation, yes | 22 (9.48) | 17 (18.48) | 0.025 |
| History of acute kidney injury, yes | 18 (7.76) | 6 (6.52) | 0.701 |
| History of stroke, yes | 31 (13.36) | 25 (27.17) | 0.003 |
| History of myocardial infarction, yes | 33 (14.22) | 22 (23.91) | 0.036 |
| Left atrium size (mm) | 39.26 (5.06) | 42.20 (6.01) | < 0.001 |
| LVEF < 30% | 57.19 (12.87) | 54.58 (15.41) | 0.122 |
| Pre-operative hemoglobin (mg/dL) | 12.81 (1.86) | 12.56 (1.75) | 0.276 |
| Pre-operative CK-MB (ng/mL) | 16.12 (65.38) | 8.89 (26.97) | 0.314 |
| Pre-operative troponin I ≥ 0.5 ng/mL, (%) | 3.23 (13.74) | 2.00 (8.03) | 0.428 |
| Cardiopulmonary bypass use | 52 (22.41) | 24 (26.09) | 0.482 |
| Intra-operative IABP | 9 (3.88) | 11 (11.96) | 0.006 |
| APACHE II score at ICU admission | 23.06 (4.20) | 24.71 (4.30) | 0.002 |
| Duration of mechanical ventilation (min) | 20.57 (15.99) | 19.79 (14.96) | 0.687 |
APACHE II, Acute Physiology and Chronic Health Evaluation; CK-MB, creatine kinase MB fraction; COPD, chronic obstructive pulmonary disease; LVEF, Left ventricular ejection fraction; IABP, intra-aortic balloon pump; ICU, intensive care unit; POAF, postoperative atrial fibrillation; M, mean; SD, standard deviation
PSM estimation and balance
To verify the quality of matching, standardized mean difference comparisons and multivariate imbalance tests were performed. Standardized mean differences are shown in Additional File 1. After matching, the overall difference between the two groups was <0.1, which is lower than the standard value of 0.1 [18], confirming that the two groups were balanced and matched. An absolute standardized difference of > 0.1 was considered an acceptable balance. Statistical significance tests (p-values) were also applied. If a variable did not reach an acceptable level of balance (i.e., standardized difference < 0.1 or p-value < 0.05), that variable was included in the outcome model to be adjusted. As shown in Table 2, the multivariate imbalance statistic (L1) before and after matching was 0.94 and 0.59 (close to zero), respectively, confirming that the matching was balanced. The aforementioned verification steps confirmed that each group was matched in a balanced manner and that homogeneity was secured.
Table 2.
Comparison of differences in variables before and after propensity score matching
| Characteristics | Before matching | One-to-one matching | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without POAF (n = 232) |
With POAF (n = 92) |
p-value | SMD | Without POAF (n = 91) |
With POAF (n = 91) |
p-value | SMD | ||||||||||||
| Age (years) | 63.78 (10.22) | 69.17 (8.07) | < 0.001 | 0.044 | 68.69 (8.53) | 69.30 (8.02) | 0.623 | 0.158 | |||||||||||
| Sex, men (%) | 166 (71.55) | 66 (71.74) | 0.973 | 0.014 | 64 (70.33) | 65 (71.43) | 0.870 | 0.027 | |||||||||||
| Comorbidities | |||||||||||||||||||
| COPD, n(%) | 7 (3.02) | 9 (9.78) | 0.011 | 0.175 | 6 (6.59) | 9 (9.89) | 0.419 | 0.055 | |||||||||||
| Stroke, n(%) | 31 (13.36) | 25 (27.17) | 0.003 | 0.056 | 23 (25.27) | 25 (27.47) | 0.737 | 0.009 | |||||||||||
| MVR, n(%) | 22 (9.48) | 17 (18.48) | 0.025 | 0.175 | 12 (13.19) | 17 (18.68) | 0.311 | 0.014 | |||||||||||
| LA size (mm) | 39.26 (5.06) | 42.20 (6.01) | < 0.001 | 0.009 | 41.77 (5.02) | 42.20 (6.01) | 0.601 | 0.021 | |||||||||||
|
APACHEII score at ICU admission |
23.06 (4.20) | 24.71 (4.30) | 0.002 | 0.039 | 24.42 (4.06) | 24.75 (4.30) | 0.596 | 0.154 | |||||||||||
| Balance test | Statistics value | ||||||||||||||||||
|
Multivariate imbalance measure L1 |
0.91 | 0.59 | |||||||||||||||||
APACHEII, Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; LA, left atrium; MVR, mitral valve regurgitation; POAF, postoperative atrial fibrillation; SMD, standardized mean difference
Verification of the homogeneity test
For the matched cohort reconstructed based on the propensity score, homogeneity verification was conducted on age, sex, and comorbidities (i.e., COPD, stroke, MVR, LA size, and APACHE II score at ICU admission). As shown in Table 2, in the original sample before matching, statistically significant differences were found between the groups with and without POAF according to the seven covariates, indicating that the two groups were not homogeneous. However, in the newly constructed matching sample using the propensity score, no statistically significant differences were found between any of the compared covariates, confirming that both groups with and without POAF were homogeneous (Table 2). In addition, to verify matching quality, a balance test was conducted on the final matched data, which consisted of a standardized mean difference comparison and a multivariate imbalance test. After matching, the standardized mean difference between the two groups decreased significantly, and all standardized mean difference values were below the standard value of 0.25, confirming that the groups were balanced and matched (Additional File 1).
Factors influencing ICU readmission
To identify factors affecting ICU readmission, a multivariable Cox proportional hazards regression analysis was performed. The results are shown in Table 3. Looking at risk factors affecting ICU readmission, cases with abnormal LVEF were found to have a lower probability of being readmitted to the ICU compared to cases with normal LVEF (LVEF ≥ 30%) (HR: 0.97; 95% CI 0.95–0.99), the same significant result was shown even after the correction variable was introduced (HR: 0.97; 95% CI 0.95–0.99).
Table 3.
Cox proportional hazards model for intensive care unit readmission in patients with coronary artery bypass graft in the matched cohort (N = 182)
| Characteristics | Crude HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
|---|---|---|---|---|
| POAF | 2.79 (1.23–6.31) | 0.014 | 2.61 (1.13–6.02) | 0.024 |
| Age | ||||
| < 60 years | Reference | Reference | ||
| 60–69 years | 2.03 (0.44–9.38) | 0.366 | 1.23 (0.31–4.84) | 0.768 |
| 70–79 years | 2.78 (0.64–12.07) | 0.174 | 1.46 (0.38–5.66) | 0.581 |
| ≥ 80 years | 1.64 (0.23–11.67) | 0.619 | 0.90 (0.22–7.73) | 0.883 |
| Sex, men | 1.29 (0.55–3.03) | 0.554 | 2.03 (0.55–7.50) | 0.286 |
| Smoking | ||||
| Non-smoker | Reference | Reference | ||
| Ex-smoker | 1.57 (0.72–3.45) | 0.258 | 1.54 (0.36–6.70) | 0.561 |
| Smoker | 0.72 (0.23–2.21) | 0.564 | 0.78 (0.39–1.55) | 0.441 |
| Alcohol drinking, yes | 0.86 (0.41–1.79) | 0.678 | 0.95 (0.32–2.84) | 0.923 |
| Health insurance, medical aids | 0.78 (0.2–1.92) | 0.586 | 0.42 (0.16–1.10) | 0.078 |
| Body mass index | 1.00 (0.90–1.11) | 0.982 | 0.94 (0.81–1.09) | 0.400 |
| Comorbidities | ||||
| Hypertension, yes | 1.52 (0.53–4.37) | 0.436 | 1.03 (0.51–2.04) | 0.945 |
| Diabetes, yes | 0.92 (0.44–1.90) | 0.811 | 0.28 (0.07–1.07) | 0.063 |
| Chronic kidney disease, yes | 1.11 (0.51–2.44) | 0.794 | 1.15 (0.43–3.30) | 0.791 |
| COPD, yes | 1.26 (0.38–4.18) | 0.701 | 0.51 (0.07–3.70) | 0.507 |
| Mitral valve regurgitation, yes | 2.08 (0.92–4.69) | 0.079 | 0.63 (0.18–2.27) | 0.484 |
| History of acute kidney injury, yes | 1.71 (0.52–5.66) | 0.381 | 1.67 (0.38–7.43) | 0.503 |
| History of stroke, yes | 0.70 (0.29–1.73) | 0.442 | 1.01 (0.39–3.12) | 0.866 |
| History of myocardial infarction, yes | 0.80 (0.31–2.11) | 0.656 | 1.05 (0.77–1.44) | 0.759 |
| Left atrium size | 1.01 (0.95–1.08) | 0.765 | 1.02 (0.94–1.11) | 0.668 |
| LVEF < 30% | 0.97 (0.95–0.99) | 0.002 | 0.97 (0.95–0.99) | 0.003 |
| Pre-operative hemoglobin | 1.02 (0.83–1.26) | 0.839 | 1.06 (0.78–1.45) | 0.704 |
| Pre-operative CK-MB | 1.01 (1.00–1.01) | 0.091 | 0.99 (0.96–1.01) | 0.170 |
| Pre-operative troponin I | 1.02 (1.00–1.05) | 0.019 | 1.02 (1.00–1.05) | 0.046 |
| Cardiopulmonary bypass use (%) | 1.04 (0.46–2.34) | 0.934 | 1.55 (0.53–4.50) | 0.419 |
| Intra-operative IABP (%) | 2.09 (0.73–6.01) | 0.172 | 1.66 (0.32–8.59) | 0.545 |
| APACHEII score at ICU admission | 1.08 (0.99–1.18) | 0.078 | 1.03 (0.92–1.15) | 0.626 |
| Duration of mechanical ventilation | 1.01 (0.99–1.03) | 0.196 | 1.02 (0.93–1.11) | 0.706 |
APACHE II, Acute Physiology and Chronic Health Evaluation; CK-MB, Creatine kinase MB fraction; COPD, chronic obstructive pulmonary disease; LVEF, Left ventricular ejection fraction; IABP, Intra-aortic balloon pump; ICU, Intensive care unit; POAF, postoperative atrial fibrillation
The troponin I level was considered normal if < 0.5 ng/mL, and cases with abnormal troponin I were found to have a higher probability of being readmitted to the intensive care unit than normal cervixes (HR: 1.02; 95% CI 1.01–1.05), and the correction variable was even after injection, the same significant result was shown (HR: 1.02; 95% CI 1.01–1.06). (Table 3). Survival analysis using multiple Cox proportional hazards regression analysis with ICU readmission as the dependent variable showed that the POAF group had a significantly higher risk of ICU readmission (log-rank test, p = 0.001). The Kaplan–Meier survival analysis results confirmed that the without POAF group had a higher survival rate than the with POAF group (Fig. 2).
Fig. 2.
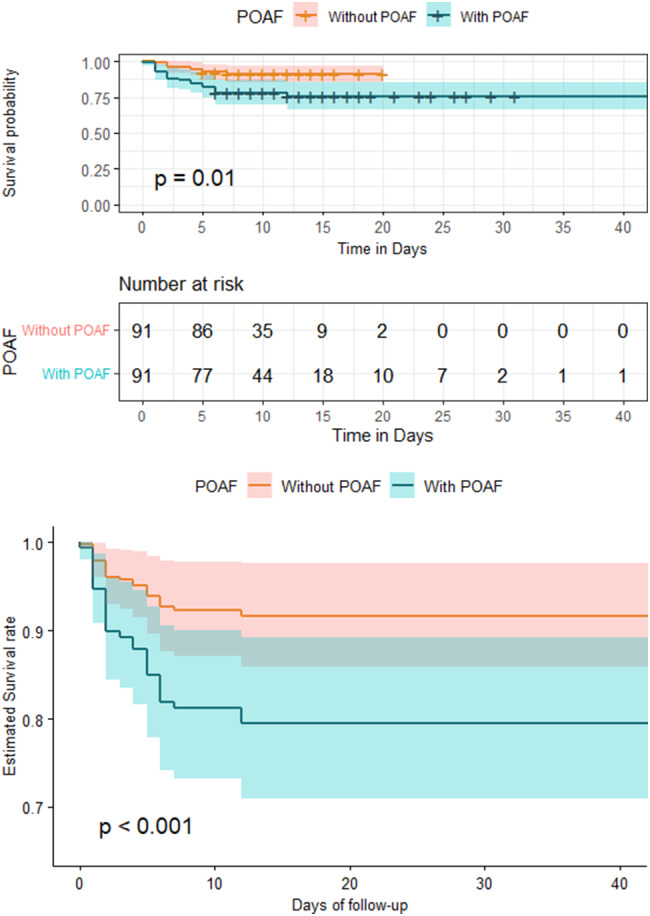
Kaplan–Meier curve (a) and adjusted (b) survival curves with relative 95% CI for the POAF and non-POAF groups. CI: confidence interval, POAF: postoperative atrial fibrillation
Factors affecting LOS
Multivariable linear regression analysis showed that the LOS in the POAF group was significantly longer than that in the non-POAF group (β = 4.36, p = 0.001; Table 4). Univariate analysis identified the following factors influencing the LOS: health insurance, chronic kidney injury, COPD, mitral regurgitation, history of acute kidney injury, LVEF, preoperative hemoglobin, preoperative troponin I, intraoperative IABP, and mechanical implantation. Multivariate analysis was performed after adjusting for the ventilation application period.
Table 4.
Multiple regression model for the length of hospital stay of coronary artery bypass graft patients in the matched cohort (N = 182)
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Standardized beta (95% CI) | p-value | Standardized beta (95% CI) | p-value | ||
| POAF | 4.61 (1.78–7.54) | 0.002 | 4.36 (1.70–7.03) | 0.001 | |
| Age | |||||
| < 60 years | Reference | Reference | |||
| 60–69 years | 1.09 (-3.57–5.75) | 0.646 | 1.06 (0.97–1.15) | 0.207 | |
| 70–79 years | 4.07 (-0.41–8.55) | 0.075 | 4.09 (0.50–33.71) | 0.191 | |
| ≥ 80 years | 2.64 (-3.66–8.95) | 0.409 | 1.57 (0.98–2.52) | 0.059 | |
| Sex, men | 0.77 (-2.49–4.03) | 0.643 | 1.66 (0.56–4.87) | 0.356 | |
| Smoking | |||||
| Non-smoker | Reference | Reference | |||
| Ex-smoker | 2.10 (-1.34–5.54) | 0.229 | 2.25 (0.78–6.47) | 0.130 | |
| Smoker | -1.20 (-5.04–2.65) | 0.541 | 1.10 (0.36–3.39) | 0.861 | |
| Alcohol drinking, yes | -1.89 (-4.85–1.08) | 0.211 | -1.19 (-3.96–1.59) | 0.401 | |
| Health insurance, medical aids | 7.59 (4.29–10.90) | < 0.001 | 4.37 (1.11–7.64) | 0.009 | |
| Body mass index | -0.31 (-0.75–0.12) | 0.160 | -0.29 (0.78–6.46) | 0.131 | |
| Comorbidities | |||||
| Hypertension, yes | 2.05 (-1.70–5.79) | 0.283 | 1.53 (-1.42–4.48) | 0.308 | |
| Diabetes, yes | 0.33 (-2.67-–0.32) | 0.831 | 1.43 (-1.12–3.98) | 0.270 | |
| Chronic kidney injury, yes | 4.02 (0.79–7.25) | 0.015 | -0.35 (-3.65–2.96) | 0.835 | |
| COPD, yes | 5.77 (0.44–11.09) | 0.034 | 6.16 (1.23–11.09) | 0.015 | |
| Mitral valve regurgitation, yes | 4.96 (0.97–8.94) | 0.015 | 2.02 (-1.74–5.78) | 0.290 | |
| History of acute kidney injury, yes | 8.89 (3.07–14.72) | 0.003 | 4.47 (-1.15–10.09) | 0.119 | |
| History of stroke, yes | 1.92 (-1.43–5.27) | 0.261 | 2.59 (-0.69–5.87) | 0.121 | |
| History of myocardial infarction, yes | 3.15 (-0.54–6.84) | 0.094 | 2.22 (0.89–5.56) | 0.090 | |
| Left atrium size | 0.06 (-0.21–0.33) | 0.645 | -0.02 (-0.25–0.21) | 0.870 | |
| Left ventricular ejection fraction | -0.17 (-0.27–0.07) | 0.001 | -0.09 (-0.19–0.01) | 0.079 | |
| Pre-operative hemoglobin | -1.64 (-2.47–0.82) | < 0.001 | -1.28 (-2.120.44) | 0.003 | |
| Pre-operative CK-MB | 0.05 (0.00–0.09) | 0.053 | 0.0.23 (-0.014–0.03) | 0.473 | |
| Pre-operative ≥ 0.5 ng/mL | 0.19 (0.02–0.36) | 0.032 | 0.06 (-0.09–0.22) | 0.421 | |
| Cardiopulmonary bypass use | 3.05 (-0.28–6.38) | 0.073 | 0.46 (0.19–1.12) | 0.083 | |
| Intra-operative IABP | 5.99 (0.50–11.49) | 0.033 | 3.29 (-2.15–8.73) | 0.234 | |
| APACHE II score at ICU admission | 0.20 (-0.1–0.55) | 0.280 | 0.14 (-0.17–0.46) | 0.373 | |
| Duration of mechanical ventilation | 0.09 (0.01–0.18) | 0.026 | 0.06 (-0.01–0.14) | 0.094 | |
APACHE II = Acute Physiology and Chronic Health Evaluation, CK-MB = creatine kinase MB fraction, COPD = chronic obstructive pulmonary disease, IABP = intra-aortic balloon pump, ICU = intensive care unit, POAF = postoperative atrial fibrillation; Health insurance (ref: no health insurance), CI: confidence interval, POAF: postoperative atrial fibrillation
The results of the multivariate analysis indicated that POAF (β = 4.36, p = 0.001), COPD comorbidity (β = 6.16, p = 0.015), and preoperative hemoglobin (β = − 1.28, p = 0.003) were the factors affecting LOS after CABG.
Discussion
In this study, we compared groups using the PSM method, which controls for selection bias. The relationship between the occurrence of POAF after CABG and clinical outcomes (readmission to the ICU and LOS) was confirmed. Additional context to better understand study results is provided below.
First, before matching, the age of patients in the POAF group was significantly higher than that of patients in the non-POAF group (POAF group: 69.17 [8.04] vs. non-POAF group: 63.7 [10.22]). However, this difference was not significant after 1:1 matching. POAF occurred at an older age (POAF group: 70.6 [10.7] vs. non-POAF group: 60.4 [16.4]) [19].
Multivariate analysis controlling for covariates in the Cox proportional hazard model in the matched cohort showed that the occurrence of POAF, LVEF, and preoperative troponin I levels were factors affecting readmission to the ICU. Consistent with the findings of a previous study that retrospectively analyzed the records of 49,264 patients who had undergone cardiac surgery between 2001 and 2012, our study identified POAF as a factor that increases risk-adjusted mortality, and readmission rates.
In agreement with the findings of a previous meta-analysis that confirmed ejection fraction as a predictive factor for POAF, our study implicated LVEF as a variable associated with ICU readmission. Moreover, in patients with LVEF < 30%, the lower the ejection fraction, the higher the incidence of POAF [20]. The occurrence of POAF is a major cause of ICU readmission. In a previous study that preoperatively assessed troponin I level, the patients in the POAF group had higher preoperative troponin I levels, and the cutoff value for preoperative troponin I levels and POAF occurrence was 0.901 ng/mL. Preoperative troponin I levels may serve as an indicator of the need for prompt management to prevent adverse clinical outcomes after CABG [21].
A significant but low positive correlation was observed between admission troponin levels and ICU LOS (r = 0.249, p = 0.045) and discharge (r = 0.312, p = 0.012). No statistically significant correlations were found between troponin levels and other postoperative in-hospital patient outcomes [21, 22]. Additional studies are warranted to validate the relationship between preoperative troponin levels and ICU readmission.
In this study, the occurrence of POAF, COPD comorbidity, and preoperative hemoglobin levels were identified as factors affecting LOS after CABG. POAF is a frequent complication following cardiac surgery, resulting in prolonged LOS and hospital costs [23]. Decreased oxygen supply, hypercapnia, pulmonary hypertension, diastolic dysfunction, oxidative stress, inflammation, changes in atrial size due to changes in respiratory physiology, increased arrhythmia incidence in non-pulmonary venous foci usually located in the right atrium, and respiratory medications may also contribute to the development of AF [24–26]. In a previous multivariate linear regression analysis, COPD with comorbidity was identified as an independent risk factor for the occurrence of POAF [27]. Therefore, when cardiac surgery is performed in patients with a history of COPD, the presence or absence of AF should be monitored more closely. Reducing LOS and medical costs associated with POAF is imperative for patient outcomes.
In this study, preoperative hemoglobin levels were also identified as a factor affecting LOS. According to the multivariate logistic regression analysis of data obtained from a multicenter cohort study, patients with anemia had a higher risk of perioperative blood transfusion, postoperative complications, and in-hospital death [28]. Further, patients with anemia had a poor prognosis, thereby prolonging LOS and increasing ICU admission and in-hospital mortality [28] The results of this study support those of this previous study. Therefore, to improve surgical outcomes and reduce complications, appropriate preoperative hematologic evaluation and maintenance of normal hemoglobin concentrations may be necessary [28].
Strengths and limitations of the work
This study had several limitations. Our findings were based on a retrospective analysis of electronic medical records with inherent variability in accuracy and data availability. Our database lacked granularity and unaccounted for or unmeasured confounders can bias the treatment effect. Other potential factors that may have affected study outcomes include baseline cardiac function, pulmonary artery catheterization, timing of AF onset (particularly in relation to the onset of serious disease), use of anticoagulants, and incidence of other arrhythmias. Furthermore, our study did not include certain outcome data such as stroke incidence. Finally, our data were collected from a single university hospital in Korea; therefore, the results cannot necessarily be generalized.
PSM is still limited by its ability to only control for confounders that are known and measurable in insufficiently balanced groups. Nevertheless, the key finding of this study was a confirmation of the relationship between POAF and clinical negative outcomes after CABG based on PSM, which has the effect of randomization by reducing selection bias. Data from this study provide useful information about the incidence of POAF and the importance of intervention.
Recommendations for further research
We propose a future large-scale, long-term, longitudinal, multicenter study that can determine actual clinical outcomes after CABG.
Conclusions
In this study, using PSM, we analyzed the clinical outcomes of the group that developed POAF and the group that did not develop POAF after CABG surgery. These outcomes included ICU readmission rate, LOS, and survival rate. Specifically, one-to-one pairing (POAF vs. non-POAF) was performed using PSM, and factors affecting the clinical outcomes of the two groups, namely readmission to the ICU and LOS, were identified.
The results of this study showed that the occurrence of POAF, LVEF, and preoperative troponin I levels were independent variables associated with readmission to the ICU after CABG and that factors affecting the hospitalization period of patients after CABG surgery were the occurrence of POAF, presence of medical aid, COPD as a comorbidity, and preoperative troponin hemoglobin levels.
Based on our results, we propose a follow-up study to confirm the relationship between the occurrence of POAF after CABG and clinical outcomes through a large-scale, multicenter, longitudinal study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplementary figure. Covariance=age, sex, COPD, Stroke, MVR, LA size, and APACHE II score at ICU admission
Acknowledgements
We would like to express our gratitude to the patients who underwent CABG surgery who participated in this study.
Abbreviations
- AF
Atrial fibrillation
- CABG
Coronary artery bypass graft
- CHF
Congestive heart failure
- COPD
Chronic obstructive pulmonary disease
- IABP
Intra-aortic balloon pumping
- ICH
International Council for Harmonisation
- ICU
Intensive care unit
- LA
Left atrium
- LOS
Length of stay
- LVEF
Left ventricular ejection fraction
- MVR
Mitral valve regurgitation
- POAF
Postoperative atrial fibrillation
- PSM
Propensity score matching.
- SD
Standard deviation.
Author contributions
Conceptualization, Y.S and J.S ; methodology, Y.S; software, H.C ; validation, Y.S and J.S ; formal analysis, H.C and J.S ; investigation, H.C ; resources, Y.S and H. C; data curation, H.C and J.S ; writing—original draft preparation, Y.S.; writing—review and editing, Y.S and J.S.; visualisation, H.C and J.S.; supervision, Y.S and J.S.; project administration, H.C; funding acquisition, Y.S . All authors reviewed the manuscript . All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation.
of Korea (NRF) grant funded by the Korea government (MSIT) (No.2022R1A2C2092976).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the hospital, with which the primary investigator of this study is affiliated. Because of the retrospective nature of the study, the requirement for informed consent was waived. The data were analyzed anonymously and kept confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Youn-Jung Son, Hong-Jae Choi and JaeLan Shim contributed equally to this study and share the first authorship.
References
- 1.Paquin A, Poirier P, Beaudoin J, Piche ME. Secondary prevention after CABG: do new agents change the paradigm? Curr Opin Cardiol. 2020;35:664–72. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, Che H, Fan Y, Jiang S. Prediction of new onset postoperative atrial fibrillation using a simple Nomogram. J Cardiothorac Surg. 2023;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundaram DM, Vasavada AM, Ravindra C, Rengan V, Sundaram PM. The management of postoperative atrial fibrillation (POAF): a systematic review. Cureus. 2023;15:e42880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AlTurki A, Marafi M, Proietti R, Cardinale D, Blackwell R, Dorian P, Bessissow A, Vieira L, Greiss I, Essebag V, Healey JS. Major adverse cardiovascular events associated with postoperative atrial fibrillation after noncardiac surgery: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2020;13:e007437. [DOI] [PubMed] [Google Scholar]
- 5.Lomivorotov VV, Efremov SM, Pokushalov EA, Karaskov AM. New-onset atrial fibrillation after cardiac surgery: pathophysiology, prophylaxis, and treatment. J Cardiothorac Vasc Anesth. 2016;30:200–16. [DOI] [PubMed] [Google Scholar]
- 6.Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14(2):159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017;52:665–72. [DOI] [PubMed] [Google Scholar]
- 8.Kochiadakis GE, Skalidis EI, Kalebubas MD, Igoumenidis NE, Chrysostomakis SI, Kanoupakis EM, Simantirakis EN, Vardas PE. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J. 2002;23:734–41. [DOI] [PubMed] [Google Scholar]
- 9.Heintz KM, Hollenberg SM. Perioperative cardiac issues: postoperative arrhythmias Surg Clin. N Am. 2005;85:1103–14. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Tiemuerniyazi X, Huang S, Song Y, Xu F, Feng W. Partial cardiac denervation to prevent postoperative atrial fibrillation after coronary artery bypass grafting (pCAD-POAF): study protocol for a randomized controlled trial. Am J Cardiol. 2024;221:120–5. [DOI] [PubMed] [Google Scholar]
- 11.LaPar DJ, Speir AM, Crosby IK, Fonner E Jr, Brown M, Rich JB, Quader M, Kern JA, Kron IL, Ailawadi G. Investigators for the Virginia Cardiac surgery Quality Initiative. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98:527–33. [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom R, Sanjanwala R, Le ML, Yamashita MH, Arora RC. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2012;111:544–54. [DOI] [PubMed] [Google Scholar]
- 13.Taha A, Nielsen SJ, Bergfeldt L, Ahlsson A, Friberg L, Björck S, Franzén S, Jeppsson A. New-onset atrial fibrillation after coronary artery bypass grafting and long‐term outcome: a population‐based nationwide study from the SWEDEHEART registry. J Am Heart Assoc. 2021;10:e017966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai YT, Lai CH, Loh SH, Lin CY, Lin YC, Lee CY, Ke HY, Tsai CS. Assessment of the risk factors and outcomes for postoperative atrial fibrillation patients undergoing isolated coronary artery bypass grafting. Acta Cardiol Sinica. 2015;31:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattartabar B, Ajam A, Pashang M, Jalali A, Sadeghian S, Mortazavi H, Mansourian S, Bagheri J, Karimi AA, Hosseini K. Sex and age difference in risk factor distribution, trend, and long-term outcome of patients undergoing isolated coronary artery bypass graft surgery. BMC Cardiovasc Dis. 2021;21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudino M, Di Franco A, Rahouma M, Tam DY, Iannaccone M, Deb S, D’Ascenzo F, Abouarab AA, Girardi LN, Taggart DP, Fremes SE. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta-analysis. J Am Heart Assoc. 2018;7:e008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen W, Kiger TB, Davies SE, Rasch RL, Simon KM, Ones DS. Samples in applied psychology: over a decade of research in review. J Appl Psychol. 2011;96:1055. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefàno PL, Bugetti M, Del Monaco G, Popescu G, Pieragnoli P, Ricciardi G, Perrotta L, Checchi L, Rondine R, Bevilacqua S, Fumagalli C. Overweight and aging increase the risk of atrial fibrillation after cardiac surgery independently of left atrial size and left ventricular ejection fraction. J Cardiothorac Surg. 2020;15:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariscalco G, Biancari F, Zanobini M, Cottini M, Piffaretti G, Saccocci M, Banach M, Beghi C, Angelini GD. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc. 2014;3:e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leal JC, Petrucci O, Godoy MF, Braile DM. Perioperative serum troponin I levels are associated with higher risk for atrial fibrillation in patients undergoing coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2012;14:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yalçın M, Katircioglu EG, Yazman S, Tayfur KD, Ürkmez M. Troponin I levels before bypass surgery after acute myocardial infarction; when to operate? J Surg Med. 2018;2:228–32. [Google Scholar]
- 23.Ebinger JE, Porten BR, Strauss CE, Garberich RF, Han C, Wahl SK, Sun BC, Abdelhadi RH, Henry TD. Design, challenges, and implications of quality improvement projects using the electronic medical record: case study: a protocol to reduce the burden of postoperative atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2016;9:593–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata Y, Watanabe T, Osaka D, Abe S, Inoue S, Tokairin Y, Igarashi A, Yamauchi K, Kimura T, Kishi H, Aida Y. Impairment of pulmonary function is an independent risk factor for atrial fibrillation: the Takahata study. Int J Med Sci. 2011;8:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konecny T, Park JY, Somers KR, Konecny D, Orban M, Soucek F, Parker KO, Scanlon PD, Asirvatham SJ, Brady PA, Rihal CS. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol. 2014;114:272–7. [DOI] [PubMed] [Google Scholar]
- 26.Simons SO, Elliott A, Sastry M, Hendriks JM, Arzt M, Rienstra M, Kalman JM, Heidbuchel H, Nattel S, Wesseling G, Schotten U. Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. Eur Heart J. 2021;42:532–40. [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi H, Wakejima R, Asakawa A, Baba S, Nakashima Y, Seto K, Kobayashi M, Okubo K. Postoperative atrial fibrillation in lung cancer lobectomy—analysis of risk factors and prognosis. World J Surg. 2020;44:3952–9. [DOI] [PubMed] [Google Scholar]
- 28.Gelebo KG, Neme D, Destaw B, Aweke Z, Kasa SM. The effect of preoperative anemia on perioperative outcomes among patients undergoing emergency surgery: a multicenter prospective cohort study. Heliyon. 2023;9:e17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary figure. Covariance=age, sex, COPD, Stroke, MVR, LA size, and APACHE II score at ICU admission
Data Availability Statement
No datasets were generated or analysed during the current study.