Abstract
Congenital diaphragmatic hernia (CDH) is a rare heterogenous disorder with varying degrees of severity. Infant survival rates in high-income countries are approaching 80% in isolated CDH; however, over 50% will have long-term morbidities. Advanced antenatal imaging, including ultrasound and magnetic resonance imaging, has made it possible to prognosticate severity of CDH and to stratify risk when counseling expectant parents. Risk stratification can also better prepare healthcare teams to enable optimal neonatal management, and provide options for fetal intervention or, where legally permitted, pregnancy termination. Factors that may affect the immediate and long-term prognosis for CDH include prenatal diagnosis, gestational age at detection and delivery, side of the defect, presence of additional structural or genetic abnormalities, defect size, estimation of fetal lung volume, the extent of visceral herniation, and the delivery center’s experience in caring for neonates with CDH. Optimizing the outcome for families and infants begins with an early prenatal diagnosis followed by referral to a diverse and inclusive multidisciplinary center with CDH expertise. Prediction of disease severity is supported by accurate fetal imaging and comprehensive genetic testing, and allows the care team to provide realistic outcome expectations during the counseling of expectant parents of all racial and ethnic backgrounds.
Keywords: fetal medicine, congenital abnormalities
Introduction
Congenital diaphragmatic hernia (CDH) is a rare disorder with an estimated prevalence of 2.3–2.6 per 10 000 births.1 2 In countries with established tertiary treatment centers, 70%–80% of children born with isolated CDH survive. CDH is not a single clinical entity, but a heterogenous disorder with highly variable outcomes ranging from perinatal death to mild, if any, respiratory issues. Most (85%) diaphragmatic defects are left-sided, 13% are right-sided, and 2% are bilateral.2 Reported mortality rates for CDH vary, with survival rates of ≥90% when only liveborn infants are considered compared with 30%–40% when prenatally diagnosed cases are included.3,6 The latter figure accounts for the ‘hidden mortality’ associated with CDH, which includes fetal and early neonatal deaths as well as pregnancies that are terminated.7
Rates of neonatal death are higher in those affected by multiple congenital anomalies and syndromes than those with isolated CDH (45% vs. 29%).7,9 With contemporary antenatal ultrasound (US) screening, 60%–70% of CDH cases are now detected antenatally, enabling early referral to experienced multidisciplinary centers which counsel families, optimize peripartum and neonatal care, and in recent years, provide an opportunity for fetal intervention in the most severe cases.10
Advanced antenatal imaging has made it possible to prognosticate severity of CDH and to stratify risk when counseling expectant parents. Risk stratification can also better prepare healthcare teams to enable optimal neonatal management, and provide options for fetal intervention or, where legally permitted, pregnancy termination.10 11 Factors that may affect the immediate and long-term prognosis for CDH include prenatal diagnosis4; gestational age (GA) at detection12; laterality13; presence of additional structural or genetic abnormalities14 15; defect size,16 which is indirectly assessed by US and magnetic resonance imaging (MRI) estimation of fetal lung volumes17 and the extent of visceral herniation18; GA at delivery19 20; and the delivery center’s experience in caring for neonates affected by CDH.21
This review aims to highlight the components of prenatal diagnosis that may play a role in risk stratification in pregnancies complicated by CDH.
Prenatal detection
In high-income countries, the incorporation of US into routine obstetric care has significantly increased the rates of prenatal detection of CDH from 15% to over 60%,22 23 which is likely a contributing factor to enhanced postnatal survival.24
GA at diagnosis is a likely determinant of CDH mortality. Previous studies have reported prenatal diagnosis prior to 25 weeks as being indicative of a poor prognosis.25 26 Other reports have not found GA at diagnosis to be a significant predictor of perinatal outcome,27 28 however those studies were small and treated GA as a categorical, rather than continuous variable. In an analysis of 377 cases of prenatally diagnosed isolated left and right CDH, mortality rates at 28 days and at 6 months of age differed significantly based on the trimester in which the diagnosis was made. At 28 days of life, mortality rates for CDH detected in the first, second, and third trimesters were 61%, 39%, and 10% (p<0.001), respectively and this difference was maintained at 6 months of age. Earlier GA at detection also correlated with higher rates of respiratory morbidity and a greater need for patch repair.29
The impact of CDH laterality on outcome remains controversial. Increasing evidence suggests that right-sided CDH (RCDH) is not simply a variant of left-sided CDH (LCDH) with a similar response to treatment.13 30 Several reports have suggested a worse prognosis for RCDH,31 32 while others have found no difference or even better outcomes in RCDH compared with LCDH.33 Higher perinatal morbidity may be partly related to later GA at diagnosis and to a larger defect. In the absence of intrathoracic herniation of the stomach, and due to the sonographic similarity of the fetal liver and lung, RCDH goes undetected more often than LCDH.4 34 RCDH usually have larger defects than LCDH, with nearly all cases having liver herniation. When corrected for defect size and liver herniation, RCDH does not appear to have a higher mortality than LCDH.34 35
Malformations associated with congenital diaphragmatic hernia
Additional structural and/or genetic anomalies may be seen in 25%–60% of CDH cases,14 36 which can substantially influence perinatal outcome.5 37 The most frequently associated anomalies involve the cardiovascular system, followed by urogenital, limb, orofacial, and gastrointestinal malformations.15 38 Accumulation of fluid in different body cavities may also be seen in CDH (e.g., pleural or pericardial effusions, skin edema), but these do not appear to affect survival.39 Intrathoracic herniation of the stomach, seen more often in LCDH, may result in kinking of the distal esophagus, which can compromise fetal swallowing, resulting in polyhydramnios. Although a risk factor for prematurity, earlier studies have not consistently found polyhydramnios to be associated with perinatal outcome in LCDH.40,43
All fetuses with prenatally diagnosed CDH should undergo a detailed anatomical survey and echocardiogram, with referral to a tertiary care fetal medicine center that has expertise in CDH.3 44 Differences in predicting CDH severity and detecting additional anomalies occur frequently between referring centers and fetal surgery centers.6 Of note, ‘isolated’ CDH can only truly be ascertained after birth, as additional anomalies may be detected in up to 4% of cases postnatally.11
Genetics
A detectable genetic etiology will be found in 30%–57% of CDH cases with multiple anomalies (i.e., complex/syndromic cases). Routine chromosomal analysis in CDH can detect a chromosomal abnormality in up to 10% of cases, most commonly trisomy 18 and isochromosome 12p (Pallister-Killian syndrome) in addition to trisomies 13 and 21. Commonly associated syndromes also include Cornelia de Lange syndrome, Donnai-Barrow syndrome, Fryn’s syndrome, Denys-Drash syndrome, craniofrontonasal syndrome, Beckwith-Wiedemann syndrome, CHARGE syndrome, Simpson-Golabi-Behmel syndrome, and Wolf- Hirschhorn syndrome.45 46 Chromosomal microarray (CMA) with targeted next-generation sequencing can detect pathological and likely pathological variants, which occur in 9%–13% of suspected ‘isolated’ CDH cases.47 The expanded use of single gene testing, gene panels, exome sequencing, and family trio whole exome sequencing (WES) has been shown to identify a genetic etiology in over 30% of non-isolated, prenatally detected CDH cases.48 WES can be concurrent or sequential with trio (parental) testing preferred over fetal-only testing, due to the high rate of de novo sequence variants in both complex and isolated CDH.47 Postnatally, whole genomic sequencing (WGS) has been used as second-line or third-line testing when prior testing has been negative. Recent reports have found that WGS provides incrementally more accurate and likely more cost-effective genetic information in fetuses with structural malformations, including CDH, and may replace CMA and karyotyping as first-line for prenatal diagnosis.49 50 Until then, comprehensive genetic evaluation should include CMA, with consideration of WES where available, as part of prenatal risk assessment.51 52
Once structural and genetic abnormalities have been excluded, the neonatal prognosis in ‘isolated’ CDH is based primarily on estimation of the severity of pulmonary hypoplasia (PH), pulmonary hypertension (pHTN), and cardiac dysfunction, the triad of factors that determine the severity of CDH.17 31 In the sections that follow, we will discuss each factor and the prenatal imaging modalities and metrics that are currently used to quantify them.
Prediction of pulmonary hypoplasia
Ultrasound
Metkus et al. first described the sonographic prediction of PH by obtaining the area of the lung contralateral to the diaphragmatic defect and the fetal head circumference, to determine the lung-to-head ratio (LHR), with severe hypoplasia defined as an LHR of <1.26 Subsequently, in a study of normal lung growth in 650 fetuses, Peralta et al., demonstrated a fourfold increase in lung size compared with head circumference with advancing GA.53 To account for this exponential growth in normal fetal lung volume, the observed-to-expected LHR (o/e LHR) was subsequently introduced by Jani et al., which is independent of GA and expresses the observed LHR as a percentage of the expected mean LHR for a given GA.32
Systematic reviews and meta-analyses have found that a threshold o/e LHR of <25% is associated with a postnatal mortality OR of 11.98 (95% CI 4.64 to 30.89) and mortality >70%.54 55 In practice, severe hypoplasia in LCDH is defined as an o/e LHR of ≤25%; moderate hypoplasia as an o/e LHR of 25%–34.9% or 35%–44.9% with liver herniation, with postnatal survival rates of approximately 40%–60%, and mild hypoplasia as an o/e LHR of >45% or an o/e LHR of 35%–45% in the absence of liver herniation, with postnatal survival approaching 65%–90%.32 56 Severe hypoplasia in RCDH is predicted by an o/e LHR <50%.31 A reduced o/e LHR is also associated with significant neonatal morbidity, including greater use of extracorporeal lifet support (ECLS), need for prosthetic patch repair, prolonged duration of assisted ventilation, need for supplemental oxygen at 28 days, and incidence of feeding problems.57 58
Although o/e LHR has become the most commonly used screening tool for outcome prediction, it should be noted that variability in lung area measurement methods, experience, and the specific formula used influence the accuracy of o/e LHR for prognosticating outcomes in CDH.59 60 The preferred method for measuring lung area involves tracing of the lung perimeters (figure 1), as this is more reproducible than other methods, including both the longest and anterior-posterior diameter methods, which can overestimate lung area by 45% and 35%, respectively.61 Moreover, the calculator used for the Tracheal Occlusion for To Acclerate Lung grow (TOTAL) trial should be used to determine o/e LHR, to ensure that consistent reference standards for determination of expected lung areas are used. As there is a significant learning curve for reliably measuring o/e LHR,62 it is recommended that this assessment be performed in experienced centers, where its predictive value may be better compared with smaller, less experienced centers.59
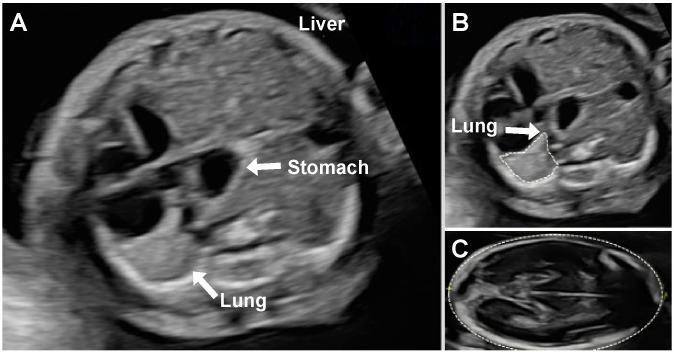
Figure 1. (A) Prenatal ultrasound image of left congenital diaphragmatic hernia with intrathoracic stomach and liver herniation on axial view. (B) Lung area obtained by trace method for determination of the lung-to-head ratio. (C) Head circumference, which is measured in the standard biparietal view. These measurements are typically acquired at 24–32 weeks’ gestation.32 61 62.
Additional methods of assessing lung volume have been proposed, including lung-thorax (L/T) ratio,62,64 three-dimensional (3D) US,65 quantitative lung index (QLI),66 and mediastinal shift angle (MSA).67 The L/T ratio is independent of GA and has a linear correlation with the o/e LHR, such that an o/e LHR threshold of <25% is comparable to an L/T ratio cut-off of <0.08.63 64 A systematic review and meta-analysis of the five studies of L/T ratio found a predictive OR of mortality of 10.28 (95% CI 3.38 to 31.31).55 63 64 The ratio has been used primarily in Japan and has yet to be validated in other countries as a predictor of perinatal outcome in prenatally diagnosed CDH. The use of 3D US to predict total fetal lung volume has proven to be inadequate in obtaining ipsilateral lung measurements in >40% of cases.17 65 Further studies are needed to evaluate the clinical accuracy of L/T ratio, QLI, and MSA as prognostic indicators in CDH.66 67 We recommend that o/e LHR be used in counselling as it is the most widely studied and validated US predictor of postnatal CDH outcomes.
Liver position: up or down
In addition to reduced o/e LHR, intrathoracic liver herniation (ILH) is a well-established predictor of perinatal mortality in LCDH.28 54 68 In a systematic review and meta-analysis of 20 studies including >700 fetuses with CDH, survival was significantly lower in fetuses when liver herniation was documented by either US or MRI (45.4% vs. 73.9% p<0.005).68 US assessment of the degree of ILH is feasible, however reproducibility of accurate measurements is limited due to the similar echogenicity of lung and liver.28 ILH by US has been primarily reported as a binary variable, ‘up’ (intrathoracic) or ‘down’ (intra-abdominal). Intrathoracic stomach herniation (ISH) indirectly approximates ILH,69 70 which, as a screening tool, can serve as a surrogate for quantifying ILH in LCDH, with progressive ISH displacement being associated with more ILH.71 72 There is a linear association between the degree of ISH and mortality, ECLS use, need for prolonged mechanical ventilation, and respiratory support.6873,76 ILH in RCDH has not been shown to be predictive of outcome, as liver herniation is present in essentially all such cases.
Quantitative liver herniation
When compared with dichotomous reporting of liver herniation as ‘up’ or ‘down’ in LCDH, quantifying the percentage of liver herniation (%LH) (figure 2) or liver/thoracic volume ratio (LiTR) by MRI has proven superior in predicting survival and need for ECLS.73 When MRI and US parameters were compared, the best combination of measurements for prediction of mortality were observed-to-expected total fetal lung volume (o/e TFLV), discussed below, with %LH.74 Furthermore, MRI parameters (including o/e TFLV and %LH) were found to have greater sensitivity and specificity for the prediction of mortality when compared with US-derived parameters (including LHR and o/e LHR).77
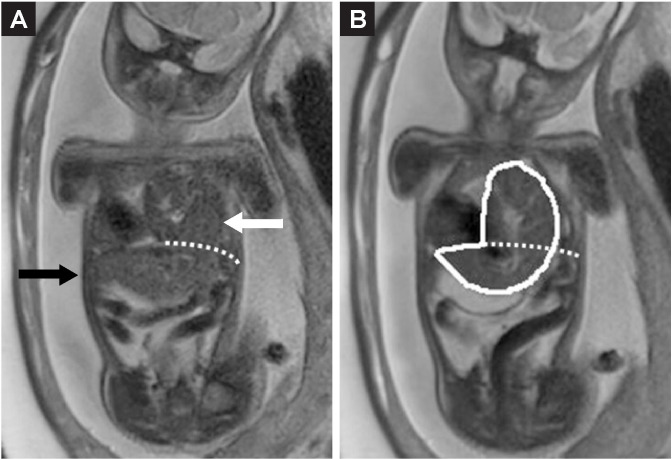
Figure 2. Percentage of liver herniation is defined as the volume of liver herniating into the thoracic cavity (white arrow) divided by the total liver volume.74.
A %LH >21% is associated with higher mortality and a greater need for ECLS, with an accuracy of 87% and 79%, respectively. LiTR >14% is associated with greater mortality and ECLS use, with an accuracy of 85% and 72%, respectively.73 Chronic lung disease is strongly predicted by %LH (OR=10.96, 95% CI 2.5 to 48.9, p=0.002) and, when combined with o/e TFLV, %LH predicts mortality or need for ECLS better than all other MRI and US parameters.7477,79
Magnetic resonance imaging
Over the past decade, MRI has become complementary to US for antenatal prognostication of CDH in most referral centers. MRI allows for more accurate imaging of most fetal structures than US because of its superior tissue contrast, wider field of view, and image quality that is independent of maternal body habitus, fetal position, or abnormalities of amniotic fluid. MRI provides a more reliable measurement of lung area, especially on the ipsilateral side, for assessing the TFLV, that is, the sum of both lung volumes (figure 3). The methodologies, techniques, and formulae used to assess fetal lung volume on MRI vary.80 MRI can assess fetal lung volume with TFLV, o/e TFLV, or per cent predicted lung volume (PPLV).81,83 Systematic reviews and meta-analyses have found that o/e TFLV performs well in predicting perinatal mortality, with area under the curve (AUC) of 0.8.54 An o/e TFLV threshold of <25% for predicting perinatal mortality has an OR of 11.14 (95% CI 5.19 to 23.89).54 82 The optimal window for MRI in the prenatal risk assessment of CDH is generally prior to 30 weeks of gestation, with the ideal timing falling between 25 and 28 weeks’ gestation, especially if there is the option for fetal intervention.83,85 The value of serial MRI remains unclear. Some studies have suggested that MRI should be repeated >30 weeks due to the finding of progressive reduction in lung volumes with advancing GA.86 87 Others have found no difference in the predictive value of MRI when comparing early (<28 weeks) and late (>32 weeks) gestational ages.88
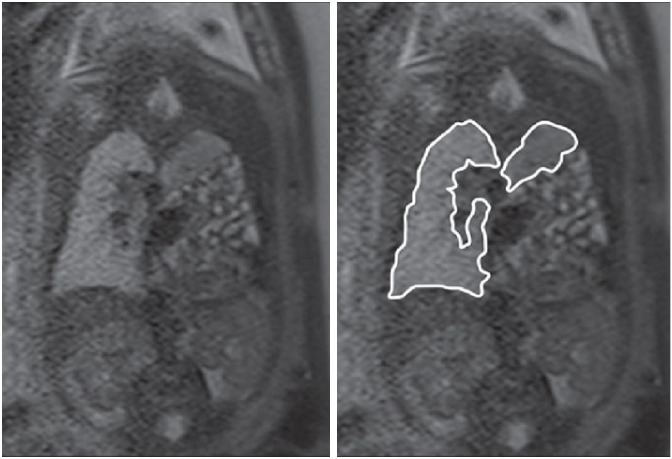
Figure 3. Total fetal lung volume is defined as the sum of both lung volumes measured by MRI (as traced in this image).82,85.
PPLV is the sum of the right and left lung volumes divided by the predicted lung volume, multiplied by 100. A PPLV <15% is associated with a greater use of ECLS, longer hospital length of stay, and a 60% mortality rate.80 82 86 PPLV decreases throughout pregnancy in fetuses with CDH.82 A meta-analysis found that PPLV effectively predicted mortality (overall effect 3.95, p<0.001), but was less discriminatory than o/e TFLV. While not all studies have agreement, a growing body of evidence indicates that o/e TFLV is a better discriminator of survival than PPLV and predictor of respiratory morbidity up to 2 years of age.31 81 83 89 90 Ruano et al. reported a good association between o/e TFLV and PPLV in predicting mortality with no statistical difference between the techniques; however, o/e TFLV combined with %LH was found to be the most accurate in predicting mortality and need for ECLS.74
Right-sided versus left-sided CDH
Compared with LCDH, the threshold for the prediction of severe PH using o/e LHR (on US) is higher but inconsistent between reports, which may reflect the comparatively small numbers of cases. The initial reports from the Eurofetus antenatal CDH registry found that survival with an o/e LHR ≤45% was 17% (3/18), compared with 69% (18/26) in cases with an o/e LHR >45%.91 A recent European report involving 86 expectantly managed RCDH cases further affirmed that the o/e LHR threshold for predicting mortality is higher in RCDH versus LCDH. With an AUC of 0.77 (95% CI 0.64 to 0.89), the best cut-off for mortality prediction was an o/e LHR >50% (sensitivity 78%, specificity 72%).31 In a single US center study of 24 cases of prenatally detected RCDH with an overall survival of 60%, o/e LHR, o/e TFLV, and liver herniation were not predictive of outcome.90 Similarly, a recent report from the CDH study group (CDHSG) did not identify an o/e LHR threshold that was predictive of survival in RCDH, but did find that ECLS use was more common in neonates with a fetal o/e LHR <45% (60%) vs. fetal o/e LHR ≥45% (29%; p=0.0027).33 ILH has not been found to be a predictive parameter prenatally in RCDH, since liver herniation is present in essentially all cases.
The reported thresholds for prediction of mortality of the various prenatal US and MRI parameters are listed in table 1.
Table 1. Prenatal imaging parameter thresholds for prediction of mortality in expectantly managed cases of CDH.
| Prenatal marker | Cut-off | Mortality (%) |
| o/e LHR (on US) | ||
| LCDH54 55 120 | <25% | 50–70 |
| RCDH31 | <50% | 80 |
| L/T ratio on US63 | <0.08% | 53 |
| o/e TLFV (on MRI)55 | <25%<35% | 75–10030–75 |
| PPLV (on MRI)80 82 86 | <15% | 60–87 |
| Liver herniation on MRI73 74 | >21% | 20–52.4 |
| Combined (on MRI) | ||
| o/e TFLV with liver herniation121 | <35% with liver ‘up’ | 75 |
| o/e TFLV %LH78 | <32% and >21% | 52 |
| Stomach herniation (on US)122 | Grade 3/Retrocardiac | 61 |
| GA at delivery19 20 | <32 weeks | 52–68 |
GAgestational ageLCDHleft-sided congenital diaphragmatic herniaLHliver herniationL/Tlung-thoraxL/T ratiolung to thoraxo/e LHRobserved-to-expected lung-to-head ratioPPLVper cent predicted lung volumeRCDHright-sided CDHTFLVtotal fetal lung volumeUSultrasound
Prediction of pulmonary hypertension
Given the importance of pHTN in determining postnatal outcome, there has been interest in determining whether its severity could be predicted prenatally using a variety of fetal pulmonary artery vascular Doppler measurements.4292,96
Pulmonary vascular index of the contralateral lung determined by 3D power Doppler has been reported to be significantly lower in fetal CDH cases that end with perinatal death or severe pHTN, however, these findings have not been reproducible. 92 Measurement of the proximal main, right and left pulmonary artery diameters were found to be predictive of perinatal death but not postnatal pHTN.93 Spectral Doppler intrapulmonary artery (IPA) indices, specifically pulsatility index (PI) and peak early diastolic reversed flow have been shown to be predictive of survival and neonatal morbidity in fetuses with severe CDH that undergo fetal tracheal occlusion.94 95 A multicenter retrospective report found that IPA Doppler in combination with o/e LHR was predictive of neonatal outcome in mild and moderate LCDH. Increased IPA PI was associated with an increased risk of mortality, pHTN, and the need for oxygen supplementation at discharge, with an OR of 3.96 (95% CI 1.62 to 9.70); 2.20 (95% CI 1.01 to 4.59); and 1.90 (95% CI 1.10 to 3.40), respectively. When combined with o/e LHR, the AUC was 0.917 for the prediction of pHTN.96 A recent meta-analysis by Russo et al. found that Doppler and US assessment of pulmonary vascular indices for the prediction of pHTN has yet to be proven beyond small single-center series, with variability in technique and gestational timing of measurement as well as inconsistent findings. Further work is needed to derive and validate fetal vascular Doppler measurements as potential antenatal predictors of pHTN.57
Cardiac parameters
Fetuses with CDH are at risk of developmental cardiac chamber abnormalities, particularly left ventricular hypoplasia, with resultant right and left ventricular dysfunction after birth. There is growing evidence that cardiac dysfunction is a major contributor to the pathophysiology of CDH. It is unclear whether the morphological cardiac changes are part of the primary embryological disorder or whether they are secondary to redistributed cardiac flow in utero and/or cardiac compression from visceral herniation. Postnatal ventricular disproportion and ventricular dysfunction have been associated with increased mortality and an increased need for ECLS,97 98 however, there is not a consistent relationship between fetal cardiac dimensions and outcome. Some studies have found an association between pHTN severity with fetal echocardiographic evidence of right ventricular enlargement and left ventricular hypoplasia, which are both predictors of overall prognosis and outcome,99,101 while others have not replicated this correlation.102 103 Further research is needed to determine which fetal cardiac parameters may be beneficial in predicting neonatal outcome in prenatally diagnosed CDH.
Risk stratification
Models based on known risk factors for CDH have been developed by various groups, including the CDHSG, the Canadian Neonatal Network, and the Japanese CDH study group for risk stratification of severity to predict neonatal mortality and morbidity.104,106 Combining prenatal and postnatal markers have been shown to be predictive of long-term outcomes,107 however, all of these systems depend on early neonatal assessment. To enable feasible antenatal counseling, to prepare healthcare teams for delivery and neonatal care, and to evaluate candidacy for fetal intervention, strategies for the accurate prenatal assessment of CDH severity are needed.108
Combining prognostic parameters may improve prenatal risk stratification. A review of 81 cases of isolated CDH that had undergone MRI and US reported that the combination of o/e TFLV and %LH best predicted neonatal mortality and the need for ECLS.74 Subsequently, that center proposed a classification of severity based on a combination of these imaging parameters at different thresholds. Mild disease was predicted by an o/e TFLV >32% and %LH <21%. Moderate disease was predicted by either TFLV >32% with %LH >21% or TFLV <32% with %LH <21%. Severe CDH was predicted by TFLV <32% and %LH >21%.74 109
Other investigators have reported better prediction of neonatal survival and long-term morbidity by combining multiple prenatal imaging parameters. A proposed risk stratification system based on a retrospective multicenter analysis of an isolated CDH cohort, combined US markers of L/T ratio (threshold <0.08) and ILH (threshold >1/3) to predict neonatal mortality at 90 days of age: OR=9.34 (95% CI 1.92 to 70.2, p=0.011) and 8.28 (95% CI 2.33 to 33.3, p=0.002), respectively. Stratifying cases into ‘low’ (neither threshold is met), ‘moderate’ (one threshold is met), and ‘high risk’ (both thresholds are met) yielded mortality rates of 0%, 20%, and 65%, respectively, with ECLS use in 2.1%, 14.3%, and 40% of neonates, respectively.41
An alternative prenatal risk stratification model has been proposed that uses calculated ORs from five US parameters that have been reported to be associated with poor outcomes.110 The prenatal risk factors with weighted scores include: o/e LHR <25% (+1), intrathoracic herniation of either the liver (where it occupies one-third of the thoracic space) (+1) and/or stomach (+1), RCDH (+2), and presence of other severe malformations (cardiac, central nervous system, or ventral wall defects) (+3). Exclusion criteria include: chromosome abnormalities, bilateral CDH, and cases treated with fetal endoluminal tracheal occlusion (FETO). Adverse neonatal outcomes were defined as death within 90 days or hospitalization >180 days. The latter served as a surrogate to integrate the morbidities associated with prolonged hospitalization. Adverse outcome was scaled as ‘low’, (0–2 points), ‘intermediate’ (3–5 points), or ‘high’ (6–8 points).110 All cases were treated aggressively (ie, without intentional palliation) following delivery. The model’s prognostic performance was better than that of any single predictor; the C-statistic (ability of the model to rank patients from high to low risk) for derivation and validation datasets was 0.83 and 0.80, respectively. Observed rates of adverse outcome in predicted low, intermediate, and high-risk groups were 12%, 56%, and 100%, respectively, in the derivation dataset and 17%, 46%, and 100%, respectively, in the validation dataset (p<0.001).110 As with each of the proposed predictive models, further prospective studies are needed to validate them.
While there is general agreement on the imaging parameters and their respective thresholds for predicting severity of CDH, an important determinant of predictive accuracy is the effectiveness of postnatal care and the potential contribution of non-standardization to observed outcome differences between centers.111 This was highlighted recently in a response to publication of a 15% (6/40) survival rate in the expectantly managed ‘severe’ arm of the TOTAL trial.10 In comparison, using the same o/e LHR threshold for severity, the North American Fetal Therapy Network (NAFTNet) FETO Consortium cohort study reported a survival rate of 58% (25/43) in expectantly managed severe cases.112 Acknowledging that the NAFTNet cohort was non-randomized, there are other potential explanations for the disparity in outcomes between the two ‘severe’ cohorts. In the NAFTNet study, prenatal care, delivery, and neonatal management for all patients (FETO and expectantly managed cases) were provided in 10 FETO centers with CDH expertise.112 In contrast, the care for patients in the severe arm of the TOTAL trial was distributed across 10 FETO and 26 delivery/neonatal centers.10 Additionally, there were postnatal differences in care between NAFTNet and TOTAL trial centers in terms of ECLS use (52% vs. 29%) and rates of non-repair (47% vs. 63%).10 112 Systematic review and meta-analysis have shown that institutional integration of prenatal and postnatal care results in better survival rates in prenatally diagnosed severe CDH.113 Furthermore, aggressive surgical management, with higher rates of surgical repair, has been shown to improve risk-adjusted mortality rates.114
Therefore, interpretation of the prediction of CDH severity by prenatal diagnosis should also take into consideration peri-operative and surgical management protocols and their degree of standardization.
Antenatal counseling
Following the initial diagnosis of CDH, families begin a long emotional journey of dealing with an unknown and the subsequent trajectory is characterized by clinical parameters that may not be available at the first evaluation. Thus, avoidance of speculation on severity and outcome should be avoided with prompt referral to a CDH specialist center to minimize stress and the uncertainty burdening the expectant parents. The treatment center should have expertise in prenatal imaging modalities, including obstetric US, fetal echocardiography, and fetal MRI. In both isolated and non-isolated CDH, patients should be offered comprehensive genetic evaluation. Sufficient and thorough information is crucial to guide families in making informed decisions. Families should be provided with an understanding of the fetal condition, neonatal risks, and immediate and long-term outcomes. This should include center-specific outcomes, as each center has its own unique practices and experiences. The integration of a racially and ethnically diverse prenatal and postnatal team in both counseling and care results in a multidisciplinary care model that has been proven to improve survival outcomes and the overall satisfaction of these families with the experience of care.113 115 CDH centers of excellence include a unique infrastructure with collaboration between fetal medicine, maternal medicine, pediatric surgery, neonatology, pediatric subspecialists, and nursing.116 The need for care coordinators, social workers, and access to psychosocial support should also be part of the standard of care in CDH and other complex prenatal diagnosis.117 While most neonates do not benefit from ECLS, prenatally detected severe CDH cases show a significant survival benefit with ELCS use. Additionally, center experience and high case volume play a major role in improving outcomes.118 119 If ECLS is not available at the planned birthing site of a high-risk CDH fetus, then transfer to a delivery center that can offer ECLS must be initiated. Risks and benefits of FETO should be included in the counseling and management options when discussing tailored treatment plans with families. If antenatal intervention is not provided at the treatment center, referral to a level III fetal center should be a consideration in cases which meet criteria.116 Critical to antenatal counseling is the availability of center-specific outcomes. These require multidisciplinary and long-term surveillance of patients with CDH and transparency in reporting. Multidisciplinary clinics should include pediatric surgery, pulmonary medicine, gastroenterology, nutrition, and developmental specialists to address the long-term sequelae of CDH beyond the delivery unit.113 Parents may choose to pursue expectant management, fetal therapy, pregnancy termination, active neonatal care, or palliation. Respecting parental preferences through unconditional acceptance of their choices and needs is the duty of the treatment center, regardless of the management approach selected.113 116
Conclusion
Prenatal risk stratification goes hand-in-hand with postnatal management of CDH. Despite improvements in prenatal detection and prognostication of CDH disease severity via fetal imaging and genetic testing, uncertainty remains regarding the accuracy of mortality predictions and the severity of morbidity in survivors. Optimizing the prenatal care of a fetal CDH pregnancy begins with an early diagnosis, followed by referral to a CDH center experienced in both fetal imaging and advanced genetic diagnosis to allow families to be counselled accurately and comprehensively. Although US alone supports accurate prenatal diagnosis, the current standard of prenatal CDH care includes fetal MRI assessments of lung volume and liver herniation as a means of refining the accuracy of prenatal prediction.
Although existing clinical practice guidelines for CDH management include recommendations for standardized prenatal diagnosis, there is a need for international consensus on imaging modalities and thresholds for risk stratification. Additionally, there should be a commitment to validate these thresholds through outcome measurement in high-volume CDH centers of excellence that offer standardized care.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Provenance and peer review: Part of a Topic Collection; not commissioned; externally peer reviewed.
Contributors: All authors contributed to writing original draft, conceptualization, supervision, writing review and editing.
Contributor Information
Nimrah Abbasi, Email: nimrah.abbasi@sinaihealth.ca.
Sami Backley, Email: sami.backley@uth.tmc.edu.
Greg Ryan, Email: Greg.Ryan@sinaihealth.ca.
Anthony Johnson, Email: anthony.johnson@uth.tmc.edu.
References
- 1.Paoletti M, Raffler G, Gaffi MS, et al. Prevalence and risk factors for congenital diaphragmatic hernia: A global view. J Pediatr Surg. 2020;55:2297–307. doi: 10.1016/j.jpedsurg.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Politis MD, Bermejo-Sánchez E, Canfield MA, et al. Prevalence and mortality in children with congenital diaphragmatic hernia: a multicountry study. Ann Epidemiol. 2021;56:61–9. doi: 10.1016/j.annepidem.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HS, Dickinson JE, Tan JK, et al. Congenital diaphragmatic hernia: Impact of contemporary management strategies on perinatal outcomes. Prenat Diagn. 2018;38:1004–12. doi: 10.1002/pd.5376. [DOI] [PubMed] [Google Scholar]
- 4.Burgos CM, Frenckner B, Luco M, et al. Prenatally versus postnatally diagnosed congenital diaphragmatic hernia - Side, stage, and outcome. J Pediatr Surg. 2019;54:651–5. doi: 10.1016/j.jpedsurg.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Gallot D, Boda C, Ughetto S, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol. 2007;29:276–83. doi: 10.1002/uog.3863. [DOI] [PubMed] [Google Scholar]
- 6.Downard CD, Jaksic T, Garza JJ, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–32. doi: 10.1016/jpsu.2003.50194. [DOI] [PubMed] [Google Scholar]
- 7.Harrison MR, Bjordal RI, Langmark F, et al. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg. 1978;13:227–30. doi: 10.1016/s0022-3468(78)80391-1. [DOI] [PubMed] [Google Scholar]
- 8.Harrison MR, Adzick NS, Estes JM, et al. A prospective study of the outcome for fetuses with diaphragmatic hernia. JAMA. 1994;271:382–4. [PubMed] [Google Scholar]
- 9.Garne E, Haeusler M, Barisic I, et al. Congenital diaphragmatic hernia: evaluation of prenatal diagnosis in 20 European regions. Ultrasound Obstet Gynecol. 2002;19:329–33. doi: 10.1046/j.1469-0705.2002.00635.x. [DOI] [PubMed] [Google Scholar]
- 10.Deprest JA, Nicolaides KH, Benachi A, et al. Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia. N Engl J Med. 2021;385:107–18. doi: 10.1056/NEJMoa2027030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deprest JA, Benachi A, Gratacos E, et al. Randomized Trial of Fetal Surgery for Moderate Left Diaphragmatic Hernia. N Engl J Med. 2021;385:119–29. doi: 10.1056/NEJMoa2026983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doné E, Gratacos E, Nicolaides KH, et al. Predictors of neonatal morbidity in fetuses with severe isolated congenital diaphragmatic hernia undergoing fetoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2013;42:77–83. doi: 10.1002/uog.12445. [DOI] [PubMed] [Google Scholar]
- 13.Schaible T, Kohl T, Reinshagen K, et al. Right- versus left-sided congenital diaphragmatic hernia: postnatal outcome at a specialized tertiary care center. Pediatr Crit Care Med. 2012;13:66–71. doi: 10.1097/PCC.0b013e3182192aa9. [DOI] [PubMed] [Google Scholar]
- 14.Menon SC, Tani LY, Weng HY, et al. Clinical characteristics and outcomes of patients with cardiac defects and congenital diaphragmatic hernia. J Pediatr. 2013;162:114–9. doi: 10.1016/j.jpeds.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Zaiss I, Kehl S, Link K, et al. Associated malformations in congenital diaphragmatic hernia. Am J Perinatol. 2011;28:211–8. doi: 10.1055/s-0030-1268235. [DOI] [PubMed] [Google Scholar]
- 16.The Congenital Diaphragmatic Hernia Study Group Defect Size Determines Survival in Infants With Congenital Diaphragmatic Hernia. Pediatrics. 2007;120:e651–7. doi: 10.1542/peds.2006-3040. [DOI] [PubMed] [Google Scholar]
- 17.Avena-Zampieri CL, Hutter J, Rutherford M, et al. Assessment of the fetal lungs in utero. Am J Obstet Gynecol MFM . 2022;4:100693. doi: 10.1016/j.ajogmf.2022.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odibo AO, Najaf T, Vachharajani A, et al. Predictors of the need for extracorporeal membrane oxygenation and survival in congenital diaphragmatic hernia: a center’s 10‐year experience. Prenat Diagn. 2010;30:518–21. doi: 10.1002/pd.2508. [DOI] [PubMed] [Google Scholar]
- 19.Tsao K, Allison ND, Harting MT, et al. Congenital diaphragmatic hernia in the preterm infant. Surgery. 2010;148:404–10. doi: 10.1016/j.surg.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn-Oudshoorn EJJ, Russo FM, Deprest JA, et al. Survival in very preterm infants with congenital diaphragmatic hernia and association with prenatal imaging markers: A retrospective cohort study. BJOG. 2023;130:1403–11. doi: 10.1111/1471-0528.17497. [DOI] [PubMed] [Google Scholar]
- 21.Cordier AG, Russo FM, Deprest J, et al. Prenatal diagnosis, imaging, and prognosis in Congenital Diaphragmatic Hernia. Semin Perinatol. 2020;44:51163. doi: 10.1053/j.semperi.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 22.LaRusso K, Baird R, Keijzer R, et al. Standardizing congenital diaphragmatic hernia care in Canada: Implementing national clinical practice guidelines. J Pediatr Surg. 2020;55:835–43. doi: 10.1016/j.jpedsurg.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Doné E, Gucciardo L, Van Mieghem T, et al. Prenatal diagnosis, prediction of outcome and in utero therapy of isolated congenital diaphragmatic hernia. Prenat Diagn. 2008;28:581–91. doi: 10.1002/pd.2033. [DOI] [PubMed] [Google Scholar]
- 24.Gupta VS, Harting MT, Lally PA, et al. Mortality in Congenital Diaphragmatic Hernia: A Multicenter Registry Study of Over 5000 Patients Over 25 Years. Ann Surg. 2023;277:520–7. doi: 10.1097/SLA.0000000000005113. [DOI] [PubMed] [Google Scholar]
- 25.Adzick NS, Vacanti JP, Lillehei CW, et al. Fetal diaphragmatic hernia: ultrasound diagnosis and clinical outcome in 38 cases. J Pediatr Surg. 1989;24:654–7. doi: 10.1016/s0022-3468(89)80713-4. [DOI] [PubMed] [Google Scholar]
- 26.Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996;31:148–51. doi: 10.1016/s0022-3468(96)90338-3. [DOI] [PubMed] [Google Scholar]
- 27.Datin-Dorriere V, Rouzies S, Taupin P, et al. Prenatal prognosis in isolated congenital diaphragmatic hernia. Am J Obstet Gynecol. 2008;198:80. doi: 10.1016/j.ajog.2007.06.069. [DOI] [PubMed] [Google Scholar]
- 28.Werneck Britto IS, Olutoye OO, Cass DL, et al. Quantification of liver herniation in fetuses with isolated congenital diaphragmatic hernia using two-dimensional ultrasonography. Ultrasound Obstet Gynecol. 2015;46:150–4. doi: 10.1002/uog.14718. [DOI] [PubMed] [Google Scholar]
- 29.Bouchghoul H, Senat MV, Storme L, et al. Congenital diaphragmatic hernia: does gestational age at diagnosis matter when evaluating morbidity and mortality? Am J Obstet Gynecol. 2015;213:535. doi: 10.1016/j.ajog.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Berdan EA, Saltzman DA. Right- versus left-sided congenital diaphragmatic hernia--can we trust the data? Pediatr Crit Care Med. 2012;13:103–4. doi: 10.1097/PCC.0b013e31823886ee. [DOI] [PubMed] [Google Scholar]
- 31.Russo FM, Cordier AG, Basurto D, et al. Fetal endoscopic tracheal occlusion reverses the natural history of right-sided congenital diaphragmatic hernia: European multicenter experience. Ultrasound Obstet Gynecol. 2021;57:378–85. doi: 10.1002/uog.23115. [DOI] [PubMed] [Google Scholar]
- 32.Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol . 2007;30:67–71. doi: 10.1002/uog.4052. [DOI] [PubMed] [Google Scholar]
- 33.Danzer E, Chock VY, Chung S, et al. Image-based prenatal predictors of postnatal survival, extracorporeal life support, and defect size in right congenital diaphragmatic hernia. J Perinatol. 2022;42:1202–9. doi: 10.1038/s41372-022-01470-x. [DOI] [PubMed] [Google Scholar]
- 34.Abramov A, Fan W, Hernan R, et al. Comparative outcomes of right versus left congenital diaphragmatic hernia: A multicenter analysis. J Pediatr Surg. 2020;55:33–8. doi: 10.1016/j.jpedsurg.2019.09.046. [DOI] [PubMed] [Google Scholar]
- 35.Partridge EA, Peranteau WH, Herkert L, et al. Right- versus left-sided congenital diaphragmatic hernia: a comparative outcomes analysis. J Pediatr Surg. 2016;51:900–2. doi: 10.1016/j.jpedsurg.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 36.Fauza DO, Wilson JM. Congenital diaphragmatic hernia and associated anomalies: their incidence, identification, and impact on prognosis. J Pediatr Surg. 1994;29:1113–7. doi: 10.1016/0022-3468(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 37.Shanmugam H, Brunelli L, Botto LD, et al. Epidemiology and Prognosis of Congenital Diaphragmatic Hernia: A Population-Based Cohort Study in Utah. Birth Defects Res. 2017;109:1451–9. doi: 10.1002/bdr2.1106. [DOI] [PubMed] [Google Scholar]
- 38.Stoll C, Alembik Y, Dott B, et al. ASSOCIATED NON DIAPHRAGMATIC ANOMALIES AMONG CASES WITH CONGENITAL DIAPHRAGMATIC HERNIA. Genet Couns. 2015;26:281–98. [PubMed] [Google Scholar]
- 39.Van Mieghem T, Cruz-Martinez R, Allegaert K, et al. Outcome of fetuses with congenital diaphragmatic hernia and associated intrafetal fluid effusions managed in the era of fetal surgery. Ultrasound Obstet Gynecol. 2012;39:50–5. doi: 10.1002/uog.10097. [DOI] [PubMed] [Google Scholar]
- 40.Shojai R, Gire C, Chaumoître K, et al. Right diaphragmatic hernia and hydrops: is it always fatal? Ultrasound Obstet Gynecol. 2004;24:803–4. doi: 10.1002/uog.1757. [DOI] [PubMed] [Google Scholar]
- 41.Usui N, Kitano Y, Okuyama H, et al. Prenatal risk stratification for isolated congenital diaphragmatic hernia: results of a Japanese multicenter study. J Pediatr Surg. 2011;46:1873–80. doi: 10.1016/j.jpedsurg.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Ruano R, Takashi E, da Silva MM, et al. Prediction and probability of neonatal outcome in isolated congenital diaphragmatic hernia using multiple ultrasound parameters. Ultrasound Obstet Gynecol . 2012;39:42–9. doi: 10.1002/uog.10095. [DOI] [PubMed] [Google Scholar]
- 43.Berger V, Sparks T, Gosnell K, et al. Does Polyhydramnios Predict Adverse Perinatal Outcome in Congenital Diaphragmatic Hernia? [10P] Obstet Gynecol. 2017;129:S166. doi: 10.1097/01.AOG.0000514083.36462.f3. [DOI] [Google Scholar]
- 44.Skari H, Bjornland K, Haugen G, et al. Congenital diaphragmatic hernia: A meta-analysis of mortality factors. J Pediatr Surg. 2000;35:1187–97. doi: 10.1053/jpsu.2000.8725. [DOI] [PubMed] [Google Scholar]
- 45.Longoni M, Pober BR, High GM, et al. In: GeneReviews®. Adam MP, Feldman J, Mirzaa GM, editors. University of Washington, Seattle; 2006 Feb 1 [Updated 2020 Nov 5]. Congenital diaphragmatic hernia overview. [PubMed] [Google Scholar]
- 46.Adam MP, Feldman J, Mirzaa GM, editors. GeneReviews®. Seattle (WA): University of washington; 1993. https://Www.ncbi.nlm.nih.gov/books/NBK1359/ Available. [Google Scholar]
- 47.Zhu Q, High FA, Zhang C, et al. Systematic analysis of copy number variation associated with congenital diaphragmatic hernia. Proc Natl Acad Sci U S A. 2018;115:5247–52. doi: 10.1073/pnas.1714885115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannata G, Caporilli C, Grassi F, et al. Management of Congenital Diaphragmatic Hernia (CDH): Role of Molecular Genetics. Int J Mol Sci. 2021;22:6353. doi: 10.3390/ijms22126353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wapner RJ, Giordano JL, de Voest J, et al. 1 Multicenter, prospective cohort of genome sequencing in 750 fetal structural anomalies. Am J Obstet Gynecol. 2024;230:S2. doi: 10.1016/j.ajog.2023.11.015. [DOI] [Google Scholar]
- 50.Jobanputra V, Giordano JL, Pervola J, et al. 86 Genome sequencing as a first-tier prenatal diagnostic test: Is it time to change? Am J Obstet Gynecol. 2024;230:S64. doi: 10.1016/j.ajog.2023.11.110. [DOI] [Google Scholar]
- 51.Yu L, Hernan RR, Wynn J, et al. The influence of genetics in congenital diaphragmatic hernia. Semin Perinatol. 2020;44:151169. doi: 10.1053/j.semperi.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiao L, Wynn J, Yu L, et al. Likely damaging de novo variants in congenital diaphragmatic hernia patients are associated with worse clinical outcomes. Genet Med. 2020;22:2020–8. doi: 10.1038/s41436-020-0908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peralta CFA, Cavoretto P, Csapo B, et al. Assessment of lung area in normal fetuses at 12-32 weeks. Ultrasound Obstet Gynecol. 2005;26:718–24. doi: 10.1002/uog.2651. [DOI] [PubMed] [Google Scholar]
- 54.Oluyomi-Obi T, Kuret V, Puligandla P, et al. Antenatal predictors of outcome in prenatally diagnosed congenital diaphragmatic hernia (CDH) J Pediatr Surg. 2017;52:881–8. doi: 10.1016/j.jpedsurg.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Masahata K, Yamoto M, Umeda S, et al. Prenatal predictors of mortality in fetuses with congenital diaphragmatic hernia: a systematic review and meta-analysis. Pediatr Surg Int. 2022;38:1745–57. doi: 10.1007/s00383-022-05232-w. [DOI] [PubMed] [Google Scholar]
- 56.Deprest J, Brady P, Nicolaides K, et al. Prenatal management of the fetus with isolated congenital diaphragmatic hernia in the era of the TOTAL trial. Semin Fetal Neonatal Med. 2014;19:338–48. doi: 10.1016/j.siny.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Russo FM, Eastwood MP, Keijzer R, et al. Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49:704–13. doi: 10.1002/uog.16000. [DOI] [PubMed] [Google Scholar]
- 58.Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33:64–9. doi: 10.1002/uog.6141. [DOI] [PubMed] [Google Scholar]
- 59.Senat MV, Bouchghoul H, Stirnemann J, et al. Prognosis of isolated congenital diaphragmatic hernia using lung-area-to-head-circumference ratio: variability across centers in a national perinatal network. Ultrasound Obstet Gynecol. 2018;51:208–13. doi: 10.1002/uog.17463. [DOI] [PubMed] [Google Scholar]
- 60.Abbasi N, Cortes MS, Ruano R, et al. Variability in antenatal prognostication of fetal diaphragmatic hernia across the North American Fetal Therapy Network (NAFTNet) Prenat Diagn. 2020;40:342–50. doi: 10.1002/pd.5560. [DOI] [PubMed] [Google Scholar]
- 61.Abbasi N, Ryan G, Johnson A, et al. Reproducibility of fetal lung-to-head ratio in left diaphragmatic hernia across the North American Fetal Therapy Network (NAFTNet) Prenat Diagn. 2019;39:188–94. doi: 10.1002/pd.5413. [DOI] [PubMed] [Google Scholar]
- 62.Cruz‐Martinez R, Figueras F, Moreno‐Alvarez O, et al. Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol . 2010;36:32–6. doi: 10.1002/uog.7577. [DOI] [PubMed] [Google Scholar]
- 63.Usui N, Okuyama H, Sawai T, et al. Relationship between L/T ratio and LHR in the prenatal assessment of pulmonary hypoplasia in congenital diaphragmatic hernia. Pediatr Surg Int. 2007;23:971–6. doi: 10.1007/s00383-007-1980-0. [DOI] [PubMed] [Google Scholar]
- 64.Usui N, Okuyama H, Kanamori Y, et al. The lung to thorax transverse area ratio has a linear correlation with the observed to expected lung area to head circumference ratio in fetuses with congenital diaphragmatic hernias. J Pediatr Surg. 2014;49:1191–6. doi: 10.1016/j.jpedsurg.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Jani JC, Cannie M, Peralta CF, et al. Lung volumes in fetuses with congenital diaphragmatic hernia: comparison of 3D US and MR imaging assessments. Radiology. 2007;244:575–82. doi: 10.1148/radiol.2442061158. [DOI] [PubMed] [Google Scholar]
- 66.Quintero RA, Quintero LF, Chmait R, et al. The quantitative lung index (QLI): a gestational age–independent sonographic predictor of fetal lung growth. Am J Obstet Gynecol. 2011;205:544. doi: 10.1016/j.ajog.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 67.Romiti A, Viggiano M, Conforti A, et al. Ultrasonographic assessment of mediastinal shift angle (MSA) in isolated left congenital diaphragmatic hernia for the prediction of postnatal survival. J Matern Fetal Neonatal Med. 2020;33:1330–5. doi: 10.1080/14767058.2018.1517329. [DOI] [PubMed] [Google Scholar]
- 68.Mullassery D, Ba’ath ME, Jesudason EC, et al. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol . 2010;35:609–14. doi: 10.1002/uog.7586. [DOI] [PubMed] [Google Scholar]
- 69.Cerbelle V, Le Duc K, Lejeune S, et al. Fetal Lung Volume Appears to Predict Respiratory Morbidity in Congenital Diaphragmatic Hernia. J Clin Med. 2023;12:1508. doi: 10.3390/jcm12041508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cordier AG, Cannie MM, Guilbaud L, et al. Stomach position versus liver-to-thoracic volume ratio in left-sided congenital diaphragmatic hernia. J Matern Fetal Neonatal Med. 2015;28:190–5. doi: 10.3109/14767058.2014.906576. [DOI] [PubMed] [Google Scholar]
- 71.Tsuda H, Kotani T, Miura M, et al. Observed-to-expected MRI fetal lung volume can predict long-term lung morbidity in infants with congenital diaphragmatic hernia. J Matern Fetal Neonatal Med. 2017;30:1509–13. doi: 10.1080/14767058.2017.1299126. [DOI] [PubMed] [Google Scholar]
- 72.Cordier AG, Jani JC, Cannie MM, et al. Stomach position in prediction of survival in left-sided congenital diaphragmatic hernia with or without fetoscopic endoluminal tracheal occlusion. Ultrasound Obstet Gynecol. 2015;46:155–61. doi: 10.1002/uog.14759. [DOI] [PubMed] [Google Scholar]
- 73.Lazar DA, Ruano R, Cass DL, et al. Defining “liver-up”: does the volume of liver herniation predict outcome for fetuses with isolated left-sided congenital diaphragmatic hernia? J Pediatr Surg. 2012;47:1058–62. doi: 10.1016/j.jpedsurg.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Ruano R, Lazar DA, Cass DL, et al. Fetal lung volume and quantification of liver herniation by magnetic resonance imaging in isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol . 2014;43:662–9. doi: 10.1002/uog.13223. [DOI] [PubMed] [Google Scholar]
- 75.Weller K, Peters NCJ, van Rosmalen J, et al. Prenatal stomach position and volume in relation to postnatal outcomes in left-sided congenital diaphragmatic hernia. Prenat Diagn. 2022;42:338–47. doi: 10.1002/pd.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou MC, Wang J, Song S, et al. The grading of stomach position for postnatal outcomes in isolated left‐sided congenital diaphragmatic hernia: A systematic review and meta‐analysis. Prenat Diagn. 2023;43:1008–17. doi: 10.1002/pd.6383. [DOI] [PubMed] [Google Scholar]
- 77.Bebbington M, Victoria T, Danzer E, et al. Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2014;43:670–4. doi: 10.1002/uog.13271. [DOI] [PubMed] [Google Scholar]
- 78.Alfaraj MA, Shah PS, Bohn D, et al. Congenital diaphragmatic hernia: lung-to-head ratio and lung volume for prediction of outcome. Am J Obstet Gynecol. 2011;205:43. doi: 10.1016/j.ajog.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 79.Zamora IJ, Olutoye OO, Cass DL, et al. Prenatal MRI fetal lung volumes and percent liver herniation predict pulmonary morbidity in congenital diaphragmatic hernia (CDH) J Pediatr Surg. 2014;49:688–93. doi: 10.1016/j.jpedsurg.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 80.Deshmukh S, Rubesova E, Barth R. MR Assessment of Normal Fetal Lung Volumes: A Literature Review. Am J Roentgenol. 2010;194:W212–7. doi: 10.2214/AJR.09.2469. [DOI] [PubMed] [Google Scholar]
- 81.Victoria T, Bebbington MW, Danzer E, et al. Use of magnetic resonance imaging in prenatal prognosis of the fetus with isolated left congenital diaphragmatic hernia. Prenat Diagn. 2012;32:715–23. doi: 10.1002/pd.3890. [DOI] [PubMed] [Google Scholar]
- 82.Barnewolt CE, Kunisaki SM, Fauza DO, et al. Percent predicted lung volumes as measured on fetal magnetic resonance imaging: a useful biometric parameter for risk stratification in congenital diaphragmatic hernia. J Pediatr Surg. 2007;42:193–7. doi: 10.1016/j.jpedsurg.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Cannie M, Jani J, Meersschaert J, et al. Prenatal prediction of survival in isolated diaphragmatic hernia using observed to expected total fetal lung volume determined by magnetic resonance imaging based on either gestational age or fetal body volume. Ultrasound Obstet Gynecol. 2008;32:633–9. doi: 10.1002/uog.6139. [DOI] [PubMed] [Google Scholar]
- 84.Kastenholz KE, Weis M, Hagelstein C, et al. Correlation of Observed-to-Expected MRI Fetal Lung Volume and Ultrasound Lung-to-Head Ratio at Different Gestational Times in Fetuses With Congenital Diaphragmatic Hernia. AJR Am J Roentgenol. 2016;206:856–66. doi: 10.2214/AJR.15.15018. [DOI] [PubMed] [Google Scholar]
- 85.Nawapun K, Eastwood MP, Diaz-Cobos D, et al. In vivo evidence by magnetic resonance volumetry of a gestational age dependent response to tracheal occlusion for congenital diaphragmatic hernia. Prenat Diagn. 2015;35:1048–56. doi: 10.1002/pd.4642. [DOI] [PubMed] [Google Scholar]
- 86.Shieh HF, Barnewolt CE, Wilson JM, et al. Percent predicted lung volume changes on fetal magnetic resonance imaging throughout gestation in congenital diaphragmatic hernia. J Pediatr Surg. 2017;52:933–7. doi: 10.1016/j.jpedsurg.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 87.Coleman A, Phithakwatchara N, Shaaban A, et al. Fetal lung growth represented by longitudinal changes in MRI-derived fetal lung volume parameters predicts survival in isolated left-sided congenital diaphragmatic hernia. Prenat Diagn. 2015;35:160–6. doi: 10.1002/pd.4510. [DOI] [PubMed] [Google Scholar]
- 88.Walleyo A, Debus A, Kehl S, et al. Periodic MRI lung volume assessment in fetuses with congenital diaphragmatic hernia: prediction of survival, need for ECMO, and development of chronic lung disease. AJR Am J Roentgenol. 2013;201:419–26. doi: 10.2214/AJR.12.8655. [DOI] [PubMed] [Google Scholar]
- 89.Kilian AK, Büsing KA, Schuetz EM, et al. Fetal MR lung volumetry in congenital diaphragmatic hernia (CDH): prediction of clinical outcome and the need for extracorporeal membrane oxygenation (ECMO) Klin Padiatr. 2009;221:295–301. doi: 10.1055/s-0029-1192022. [DOI] [PubMed] [Google Scholar]
- 90.Victoria T, Danzer E, Oliver ER, et al. Right Congenital Diaphragmatic Hernias: Is There a Correlation between Prenatal Lung Volume and Postnatal Survival, as in Isolated Left Diaphragmatic Hernias? Fetal Diagn Ther. 2018;43:12–8. doi: 10.1159/000464246. [DOI] [PubMed] [Google Scholar]
- 91.DeKoninck P, Gomez O, Sandaite I, et al. Right‐sided congenital diaphragmatic hernia in a decade of fetal surgery. BJOG. 2015;122:940–6. doi: 10.1111/1471-0528.13065. [DOI] [PubMed] [Google Scholar]
- 92.Ruano R, Aubry MC, Barthe B, et al. Quantitative analysis of fetal pulmonary vasculature by 3-dimensional power Doppler ultrasonography in isolated congenital diaphragmatic hernia. Am J Obstet Gynecol. 2006;195:1720–8. doi: 10.1016/j.ajog.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 93.Ruano R, Aubry MC, Barthe B, et al. Predicting perinatal outcome in isolated congenital diaphragmatic hernia using fetal pulmonary artery diameters. J Pediatr Surg. 2008;43:606–11. doi: 10.1016/j.jpedsurg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Cruz‐Martinez R, Castañon M, Moreno‐Alvarez O, et al. Usefulness of lung‐to‐head ratio and intrapulmonary arterial Doppler in predicting neonatal morbidity in fetuses with congenital diaphragmatic hernia treated with fetoscopic tracheal occlusion. Ultrasound Obstet Gynecol . 2013;41:59–65. doi: 10.1002/uog.11212. [DOI] [PubMed] [Google Scholar]
- 95.Cruz‐Martinez R, Moreno‐Alvarez O, Hernandez‐Andrade E, et al. Contribution of intrapulmonary artery Doppler to improve prediction of survival in fetuses with congenital diaphragmatic hernia treated with fetal endoscopic tracheal occlusion. Ultrasound in Obstet & Gyne. 2010;35:572–7. doi: 10.1002/uog.7593. [DOI] [PubMed] [Google Scholar]
- 96.Basurto D, Fuenzalida J, Martinez‐Portilla RJ, et al. Intrapulmonary artery Doppler to predict mortality and morbidity in fetuses with mild or moderate left‐sided congenital diaphragmatic hernia. Ultrasound in Obstet & Gyne. 2021;58:590–6. doi: 10.1002/uog.23701. [DOI] [PubMed] [Google Scholar]
- 97.Pugnaloni F, Bo B, Hale L, et al. Early Postnatal Ventricular Disproportion Predicts Outcome in Congenital Diaphragmatic Hernia. Am J Respir Crit Care Med. 2023;208:325–8. doi: 10.1164/rccm.202212-2306LE. [DOI] [PubMed] [Google Scholar]
- 98.Fraga MV, Hedrick HL, Rintoul NE, et al. Congenital Diaphragmatic Hernia Patients with Left Heart Hypoplasia and Left Ventricular Dysfunction Have Highest Odds of Mortality. J Pediatr. 2024;271:114061. doi: 10.1016/j.jpeds.2024.114061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sokol J, Bohn D, Lacro RV, et al. Fetal pulmonary artery diameters and their association with lung hypoplasia and postnatal outcome in congenital diaphragmatic hernia. Am J Obstet Gynecol. 2002;186:1085–90. doi: 10.1067/mob.2002.122413. [DOI] [PubMed] [Google Scholar]
- 100.Massolo AC, Romiti A, Viggiano M, et al. Fetal cardiac dimensions in congenital diaphragmatic hernia: relationship with gestational age and postnatal outcomes. J Perinatol. 2021;41:1651–9. doi: 10.1038/s41372-021-00986-y. [DOI] [PubMed] [Google Scholar]
- 101.Yamoto M, Tanaka Y, Fukumoto K, et al. Cardiac fetal ultrasonographic parameters for predicting outcomes of isolated left-sided congenital diaphragmatic hernia. J Pediatr Surg. 2015;50:2019–24. doi: 10.1016/j.jpedsurg.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 102.Vogel M, McElhinney DB, Marcus E, et al. Significance and outcome of left heart hypoplasia in fetal congenital diaphragmatic hernia. Ultrasound in Obstet & Gyne. 2010;35:310–7. doi: 10.1002/uog.7497. [DOI] [PubMed] [Google Scholar]
- 103.Moon-Grady AJ, Byrne FA, Lusk LA, et al. Expected small left heart size in the presence of congenital diaphragmatic hernia: Fetal values and Z-scores for infants confirmed to have no heart disease postnatally. Front Pediatr. 2022;10:1083370. doi: 10.3389/fped.2022.1083370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terui K, Nagata K, Kanamori Y, et al. Risk stratification for congenital diaphragmatic hernia by factors within 24 h after birth. J Perinatol. 2017;37:805–8. doi: 10.1038/jp.2017.11. [DOI] [PubMed] [Google Scholar]
- 105.Brindle ME, Cook EF, Tibboel D, et al. A clinical prediction rule for the severity of congenital diaphragmatic hernias in newborns. Pediatrics. 2014;134:e413–9. doi: 10.1542/peds.2013-3367. [DOI] [PubMed] [Google Scholar]
- 106.Skarsgard ED, MacNab YC, Qiu Z, et al. SNAP-II predicts mortality among infants with congenital diaphragmatic hernia. J Perinatol. 2005;25:315–9. doi: 10.1038/sj.jp.7211257. [DOI] [PubMed] [Google Scholar]
- 107.Akinkuotu AC, Cruz SM, Abbas PI, et al. Risk-stratification of severity for infants with CDH: Prenatal versus postnatal predictors of outcome. J Pediatr Surg. 2016;51:44–8. doi: 10.1016/j.jpedsurg.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 108.Deprest JA, Flake AW, Gratacos E, et al. The making of fetal surgery. Prenat Diagn. 2010;30:653–67. doi: 10.1002/pd.2571. [DOI] [PubMed] [Google Scholar]
- 109.Mehollin-Ray AR. Prenatal lung volumes in congenital diaphragmatic hernia and their effect on postnatal outcomes. Pediatr Radiol. 2022;52:637–42. doi: 10.1007/s00247-021-05153-1. [DOI] [PubMed] [Google Scholar]
- 110.Terui K, Nagata K, Hayakawa M, et al. Novel Risk Score for Fetuses with Congenital Diaphragmatic Hernia Based on Ultrasound Findings. Eur J Pediatr Surg. 2020;30:51–8. doi: 10.1055/s-0039-1698768. [DOI] [PubMed] [Google Scholar]
- 111.Stolar CJH, Flake AW, Losty PD, et al. Fetal Surgery for Severe Left Diaphragmatic Hernia. N Engl J Med. 2021;385:2111–2. doi: 10.1056/NEJMc2115673. [DOI] [PubMed] [Google Scholar]
- 112.Bergh E, Baschat AA, Cortes MS, et al. Fetoscopic Endoluminal Tracheal Occlusion for Severe, Left-Sided Congenital Diaphragmatic Hernia: The North American Fetal Therapy Network Fetoscopic Endoluminal Tracheal Occlusion Consortium Experience. Obstet Gynecol. 2024;143:440–8. doi: 10.1097/AOG.0000000000005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sferra SR, Miller JL, Cortes M S, et al. Postnatal care setting and survival after fetoscopic tracheal occlusion for severe congenital diaphragmatic hernia: A systematic review and meta-analysis. J Pediatr Surg. 2022;57:819–25. doi: 10.1016/j.jpedsurg.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 114.Harting MT, Hollinger L, Tsao K, et al. Aggressive Surgical Management of Congenital Diaphragmatic Hernia: Worth the Effort?: A Multicenter, Prospective, Cohort Study. Ann Surg. 2018;267:977–82. doi: 10.1097/SLA.0000000000002144. [DOI] [PubMed] [Google Scholar]
- 115.Sferra SR, Salvi PS, Penikis AB, et al. Racial and Ethnic Disparities in Outcomes Among Newborns with Congenital Diaphragmatic Hernia. JAMA Netw Open . 2023;6:e2310800. doi: 10.1001/jamanetworkopen.2023.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baschat AA, Blackwell SB, Chatterjee D, et al. Care Levels for Fetal Therapy Centers. Obstet Gynecol. 2022;139:1027–42. doi: 10.1097/AOG.0000000000004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crombag N, Ceulemans V, Debeer A, et al. Prenatal diagnosis of congenital diaphragmatic hernia: Parental counselling and support needs. Prenat Diagn. 2022;42:387–97. doi: 10.1002/pd.6118. [DOI] [PubMed] [Google Scholar]
- 118.Jancelewicz T, Langham MR, Jr, Brindle ME, et al. Survival Benefit Associated With the Use of Extracorporeal Life Support for Neonates With Congenital Diaphragmatic Hernia. Ann Surg. 2022;275:e256–63. doi: 10.1097/SLA.0000000000003928. [DOI] [PubMed] [Google Scholar]
- 119.Gien J, Kinsella JP, Behrendt NJ, et al. Improved survival for infants with severe congenital diaphragmatic hernia. J Perinatol. 2022;42:1189–94. doi: 10.1038/s41372-022-01397-3. [DOI] [PubMed] [Google Scholar]
- 120.Basurto D, Russo FM, Van der Veeken L, et al. Prenatal diagnosis and management of congenital diaphragmatic hernia. Best Pract Res Clin Obstet Gynaecol. 2019;58:93–106. doi: 10.1016/j.bpobgyn.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 121.Jani J, Cannie M, Sonigo P, et al. Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;32:793–9. doi: 10.1002/uog.6234. [DOI] [PubMed] [Google Scholar]
- 122.Basta AM, Lusk LA, Keller RL, et al. Fetal Stomach Position Predicts Neonatal Outcomes in Isolated Left-Sided Congenital Diaphragmatic Hernia. Fetal Diagn Ther. 2016;39:248–55. doi: 10.1159/000440649. [DOI] [PMC free article] [PubMed] [Google Scholar]