Abstract
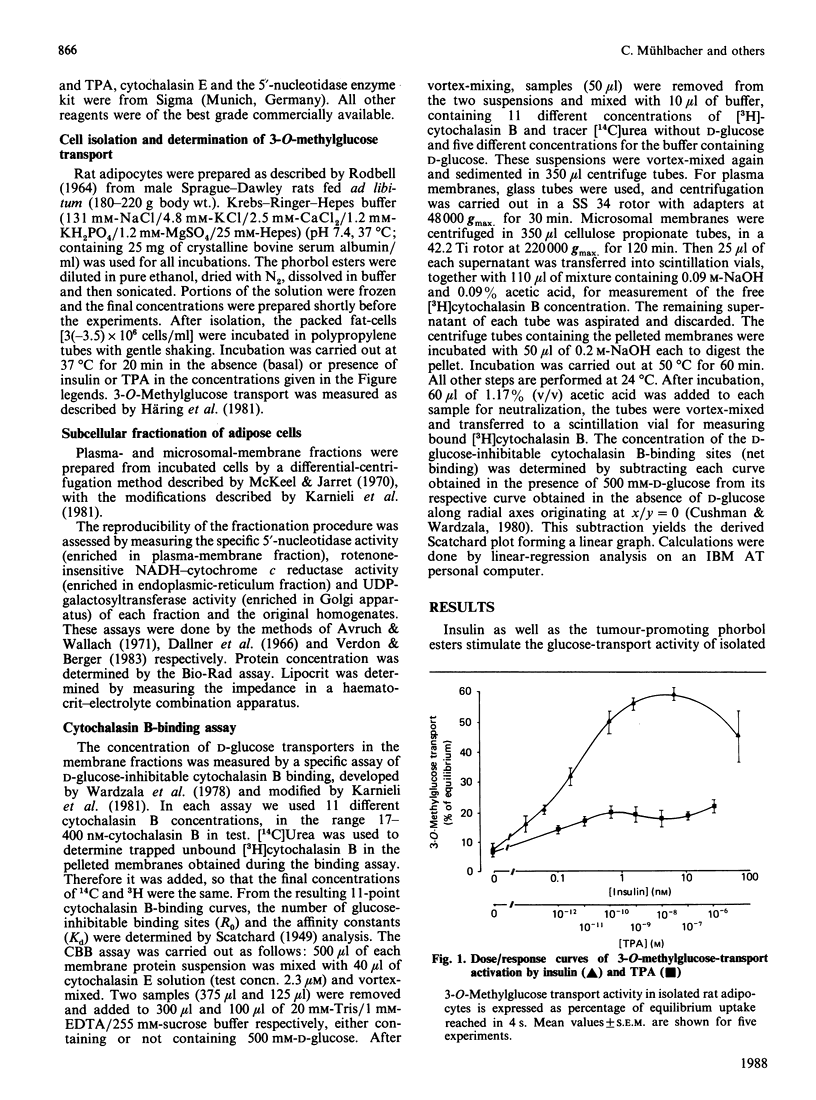
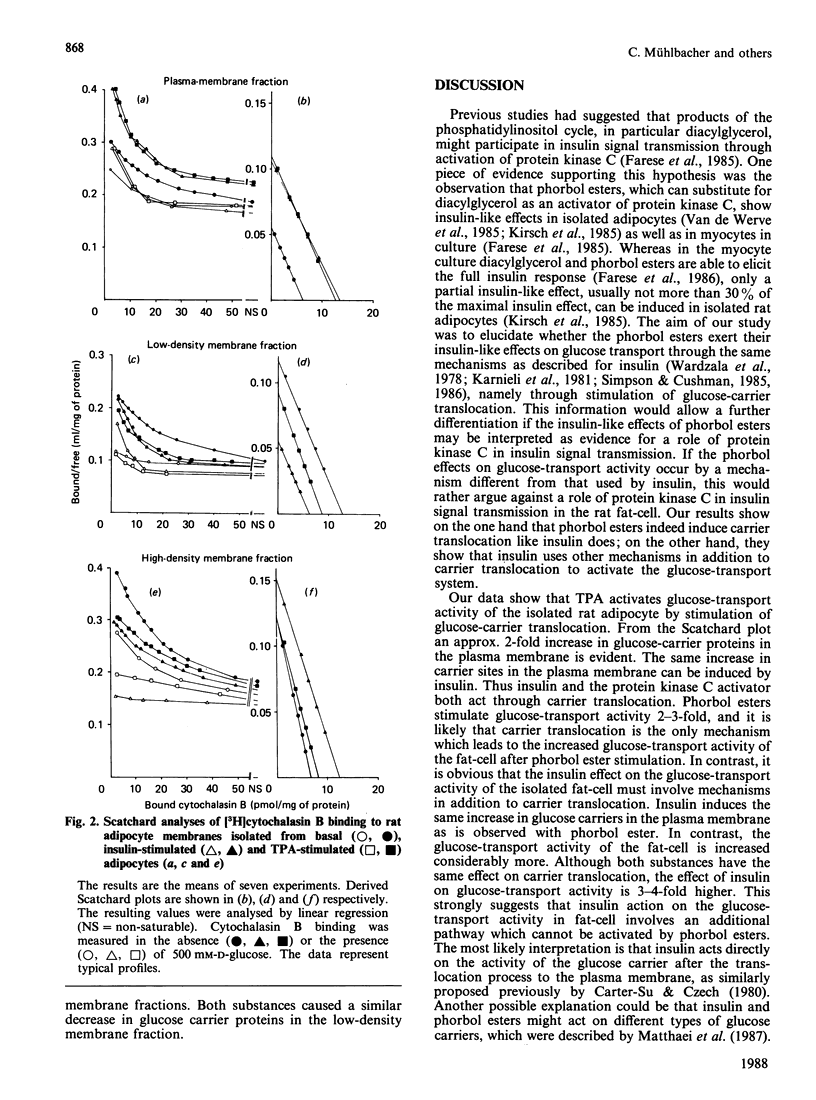
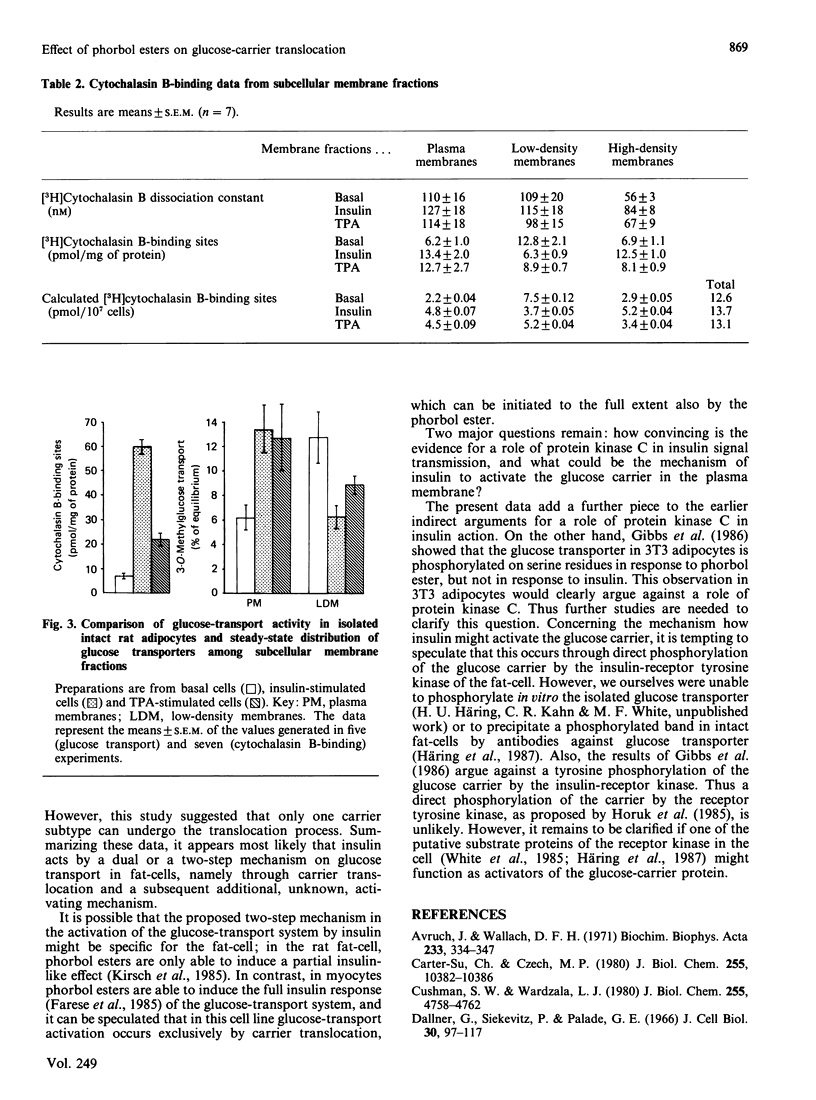
Tumour-promoting phorbol esters have insulin-like effects on glucose transport and lipogenesis in adipocytes and myocytes. It is believed that insulin activates the glucose-transport system through translocation of glucose transporters from subcellular membranes to the plasma membrane. The aim of the present study was to investigate if phorbol esters act through the same mechanism as insulin on glucose-transport activity of rat adipocytes. We compared the effects of the tumour-promoting phorbol ester tetradecanoylphorbol acetate (TPA) and of insulin on 3-O-methylglucose transport and on the distribution of D-glucose-inhibitable cytochalasin-B binding sites in isolated rat adipocytes. Insulin (100 mu units/ml) stimulated 3-O-methylglucose uptake 9-fold, whereas TPA (1 nM) stimulated the uptake only 3-fold (mean values of five experiments, given as percentage of equilibrium reached after 4 s: basal 7 +/- 1.3%, insulin 60 +/- 3.1%, TPA 22 +/- 2.3%). In contrast, both agents stimulated glucose-transporter translocation to the same extent [cytochalasin B-binding sites (pmol/mg of protein; n = 7): plasma membranes, basal 6.2 +/- 1.0, insulin 13.4 +/- 2.0, TPA 12.7 +/- 2.7; low-density membranes, basal 12.8 +/- 2.1, insulin 6.3 +/- 0.9, TPA 8.9 +/- 0.7; high-density membranes, 6.9 +/- 1.1; insulin 12.5 +/- 1.0, TPA 8.1 +/- 0.9]. We conclude from these data: (1) TPA stimulates glucose transport in fat-cells by stimulation of glucose-carrier translocation; (2) insulin and TPA stimulate the carrier translocation to the same extent, whereas the stimulation of glucose uptake is 3-fold higher with insulin, suggesting that the stimulatory effect of insulin on glucose-transport activity involves other mechanisms in addition to carrier translocation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Czech M. P. Reconstitution of D-glucose transport activity from cytoplasmic membranes. Evidence against recruitment of cytoplasmic membrane transporters into the plasma membrane as the sole action of insulin. J Biol Chem. 1980 Nov 10;255(21):10382–10386. [PubMed] [Google Scholar]
- Champion M. C., Keen H., Pickup J. C., Tamborlane W. V., Dupre J. Conference on insulin pump therapy in diabetes. Multicenter study of effect on microvascular disease. Origin and design of the Kroc Collaborative Study. Diabetes. 1985 Aug;34 (Suppl 3):5–12. doi: 10.2337/diab.34.3.s5. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Rosic N., Standaert M., Babischkin J., Cooper D. R., Davis J. S., Pollet R. J. Further evidence implicating diacylglycerol generation and protein kinase C activation in agonist-induced increases in glucose uptake. Insulin-like effects of phenylephrine in BC3H-1 myocytes. Diabetes. 1986 Sep;35(9):951–957. doi: 10.2337/diab.35.9.951. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Barnes D. E., Davis J. S., Pollet R. J. Phorbol ester provokes insulin-like effects on glucose transport, amino acid uptake, and pyruvate dehydrogenase activity in BC3H-1 cultured myocytes. Endocrinology. 1985 Jun;116(6):2650–2655. doi: 10.1210/endo-116-6-2650. [DOI] [PubMed] [Google Scholar]
- Gibbs E. M., Allard W. J., Lienhard G. E. The glucose transporter in 3T3-L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J Biol Chem. 1986 Dec 15;261(35):16597–16603. [PubMed] [Google Scholar]
- Häring H. U., Biermann E., Kemmler W. Coupling of insulin binding and insulin action on glucose transport in fat cells. Am J Physiol. 1981 May;240(5):E556–E565. doi: 10.1152/ajpendo.1981.240.5.E556. [DOI] [PubMed] [Google Scholar]
- Häring H. U., White M. F., Machicao F., Ermel B., Schleicher E., Obermaier B. Insulin rapidly stimulates phosphorylation of a 46-kDa membrane protein on tyrosine residues as well as phosphorylation of several soluble proteins in intact fat cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):113–117. doi: 10.1073/pnas.84.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch D., Obermaier B., Häring H. U. Phorbolesters enhance basal D-glucose transport but inhibit insulin stimulation of D-glucose transport and insulin binding in isolated rat adipocytes. Biochem Biophys Res Commun. 1985 Apr 30;128(2):824–832. doi: 10.1016/0006-291x(85)90121-4. [DOI] [PubMed] [Google Scholar]
- Koepfer-Hobelsberger B., Wieland O. H. Insulin activates phospholipase C in fat cells: similarity with the activation of pyruvate dehydrogenase. Mol Cell Endocrinol. 1984 Jun;36(1-2):123–129. doi: 10.1016/0303-7207(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Blevins T. L., Ezaki O. Evidence that translocation of the glucose transport activity is the major mechanism of insulin action on glucose transport in fat cells. J Biol Chem. 1982 Sep 25;257(18):10942–10947. [PubMed] [Google Scholar]
- Machicao F., Häring H., White M. F., Carrascosa J. M., Obermaier B., Wieland O. H. An Mr 180,000 protein is an endogenous substrate for the insulin-receptor-associated tyrosine kinase in human placenta. Biochem J. 1987 May 1;243(3):797–801. doi: 10.1042/bj2430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei S., Garvey W. T., Horuk R., Hueckstaedt T. P., Olefsky J. M. Human adipocyte glucose transport system. Biochemical and functional heterogeneity of hexose carriers. J Clin Invest. 1987 Mar;79(3):703–709. doi: 10.1172/JCI112874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Proietto J., Jeanrenaud B. Tumour-promoting phorbol esters increase basal and inhibit insulin-stimulated lipogenesis in rat adipocytes without decreasing insulin binding. Biochem J. 1985 Jan 15;225(2):523–527. doi: 10.1042/bj2250523. [DOI] [PMC free article] [PubMed] [Google Scholar]


