Abstract
Objectives
To identify the predictive factors of first hospitalization and associated variables to the main causes of hospitalizations in lupus patients from a Latin American cohort.
Methods
The first hospitalization after entry into the cohort during these patients’ follow-up due to either lupus disease activity and/or infection was examined. Clinical and therapeutic variables were those occurring prior to the first hospitalization. Descriptive statistical tests, multivariable logistic, and Cox regression models were performed.
Results
1341 individuals were included in this analysis; 1200 (89.5%) were women. Their median and interquartile range (IQR) age at diagnosis were 27 (20–37) years and their median and IQR follow up time were 27.5 (4.7–62.2) months. A total of 456 (34.0%) patients were hospitalized; 344 (75.4%), 85 (18.6%) and 27 (5.9%) for disease activity, infections, or both, respectively. The predictors of the first hospitalization regardless of its cause were: medium (HR 2.03(1.27–3.24); p = 0.0028) and low (HR 2.42(1.55–3.79); p < 0.0001) socioeconomic status, serosal (HR 1.32(1.07–1.62); p = 0.0074) and renal (HR 1.50(1.23–1.82); p < 0.0001) involvement. Antimalarial (AM) use (HR 0.61(0.50–0.74); p < 0.0001) and achieving remission (HR 0.80(0.65–0.97); p = 0.0300) were negative predictors.
Conclusions
The first hospitalization was associated with worse socioeconomic status and serosal and renal involvement. Conversely, AM use and achieving remission were associated with a lower risk of hospitalizations.
Keywords: Lupus erythematosus systemic, epidemiology, hospitalization
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystemic disease that has diverse clinical manifestations. Although the survival rate of patients with SLE has improved since the 1950s due to advances in diagnosis and therapy, morbidity-mortality remain high compared with that of the general population.1–3
SLE patients experience frequent hospitalizations associated with several factors, including disease activity, infections, adverse drug reactions, and comorbidities. Hospitalizations not only reflect the severity of the disease, but also limit the patient’s social and economic activities, contributing to further reduce the patients’ health-related quality of life (HRQoL).4–7
The positive impact of achieving remission or Low Disease Activity State (LDAS) in lupus patients has been studied previously. The Grupo Latinoamericano de Estudio del Lupus (GLADEL) cohort,8,9 the Lupus in Minority populations, Nature versus nurture (LUMINA) 10 and the Hopkins Lupus cohorts 11 have shown that patients who achieve remission during their disease course accrue less damage.
The relationship between achieving remission or LDAS and hospitalizations has been described by Reátegui-Sokolova et al 4 in the Almenara Lupus Cohort (a Peruvian prevalent lupus cohort), resulting in a diminished hospitalization rate.
The aim of this study is to identify the predictive and associated factors of first hospitalization and the main causes of hospitalizations in lupus patients from a Latin American (LA) cohort.
Methods
GLADEL is an observational, multiethnic, longitudinal inception cohort study started in 1997 and constituted by patients from 34 centers from nine Latin American countries under local institutional review boards’ regulations and the Declaration of Helsinki’s guidelines. 12 The first patients were enrolled in October 1997; to insure their recent disease onset they could only had been included if the diagnosis of SLE had been made after 1 January 1996, either by a rheumatologist or by a qualified internist with experience in SLE. Fulfillment of 4 1982 ACR 13 SLE criteria at the time of enrollment was not mandatory; nevertheless, 95.9% of the patients met ≥4 criteria during the follow-up period. Patients included in this cohort had to have a disease duration ≤24 months.
The first hospitalization after entry into the cohort during these patients’ follow-up due to either SLE disease activity and/or infection was examined; other causes, which constituted less than 1% of them, were not included in this analysis.
Socioeconomic demographic variables included are age at SLE diagnosis, gender, ethnicity [Caucasian, Mestizo, and African Latin American (ALA)], residence (rural vs urban), time to diagnosis (the time elapsing from when a patient met the first ACR criterion to SLE diagnosis), socioeconomic status (SES) (by the Graffar’s method), medical insurance (full vs partial/no coverage), and years of education.
The variables included were: previous hospitalizations for SLE (before entry into the cohort), cumulative ACR and non-ACR clinical manifestations, immunological variables, disease activity [SLE Disease Activity Index (SLEDAI)] 14 and damage accrual [Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (SDI)] at the baseline visit (1 year after entry into the cohort) and at the last visit. 15
Renal involvement was defined as the presence of any of the following: persistent proteinuria greater than 0.5 g/day on two or more occasions or red or cellular casts or histologically (renal biopsy compatible with LN histopathology classes II, III, IV, V according to the WHO). Neurologic involvement was defined as the presence of any of the following: headache, seizures, longitudinal and transverse myelitis, peripheral neuropathy, optic neuritis, cranial nerve involvement, stroke or psychosis.
Therapeutic variables included are glucocorticoid and antimalarial (AM) use. Clinical and therapeutic variables were those occurring at baseline, prior to the first hospitalization.
Based on the definitions of remission in SLE (DORIS) framework, 16 remission on treatment was defined as a SLEDAI (excluding serology) of 0, prednisone ≤5 mg/day and maintenance treatment with immunosuppressants. 8 LDAS was defined as a SLEDAI ≤4 with no SLEDAI scores for renal, central nervous system, serositis, vasculitis, and constitutional components, no increase in any SLEDAI component since the previous visit, and prednisone dose ≤7.5 mg/day. Immunosuppressants at maintenance doses were allowed for LDAS and AM were allowed in both groups.
Statistical analyses
Patient characteristics were compared between the two groups: hospitalizations versus no hospitalizations due to SLE (activity, infections or both). Categorical variables were examined by the Chi-square test and continuous variables by the Kruskal–Wallis test; categorical variables are presented as frequencies and percentages while continuous variables are presented as medians and their interquartile ranges (IQRs).
Univariable and multivariable Cox regression models were used to study time to first hospitalization regardless of its cause. Univariable and multivariable logistic regression analysis were used to study the associated factors of hospitalization due to disease activity and infections but hospitalizations due to both were excluded. Disease activity state, SDI and AM use were evaluated as time-dependent covariates. Potential confounders of hospitalization included sociodemographic factors and the baseline SDI; previous hospitalizations (before entry into the cohort) and AM use and were examined at the same visit as disease activity state. A multivariable model was built by selecting covariates using a backward elimination procedure. A probability value less than 0.05 was considered significant. Statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
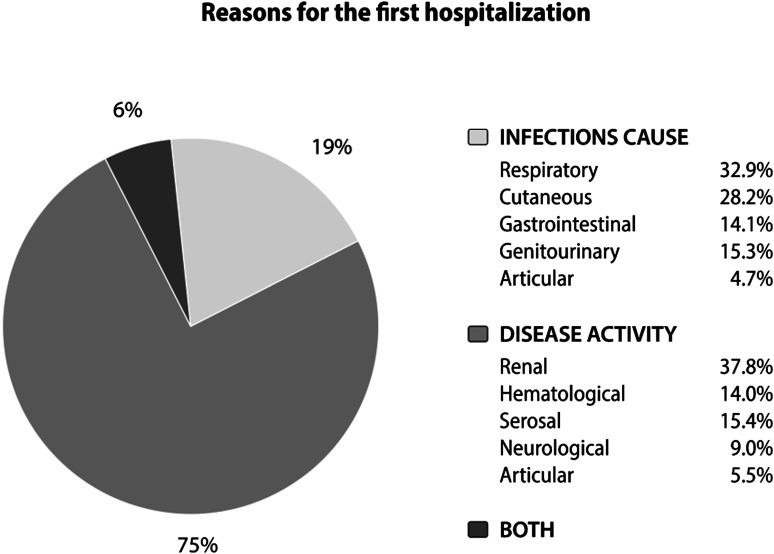
A total of 1480 patients were included in the GLADEL cohort; 1341 individuals with sufficient data on the variables of interest were included in this analysis; of them, 1200 (89.5%) were women. Their median and interquartile range (IQR) age at diagnosis were 27 (20–37) years and their median and IQR follow up time were 27.5 (4.7–62.2) months. A total of 456 (34.0%) patients were hospitalized; 344 (75.4%), 85 (18.6%) and 27 (5.9%) for disease activity, infections, or both, respectively, as depicted in Figure 1. The most common infection sites that led to hospitalizations were respiratory and cutaneous.
Figure 1.
Reasons for the first hospitalization.
Table 1 compares hospitalized patients, regardless of the cause, with those not hospitalized. Compared to the non-hospitalized patients, hospitalized patients were younger (25.0 vs 29.0; p = 0.0002), exhibited a shorter time to diagnosis (5.3 vs 6.0 months; p = 0.0191), had a lower SES (69.5% vs 55.8%; p < 0.0001) and had a higher frequency of previous hospitalizations (before entry into the cohort) due to SLE (44.7% vs 33.7%; p < 0.0001).
Table 1.
Characteristics of the population and comparison between hospitalized versus non-hospitalized patients.
| Feature | Patients hospitalized (456) | Patients non- hospitalized (885) | p value | Total (1341) |
|---|---|---|---|---|
| Age at diagnosis; median (IQR) | 25.0 (20.0 – 34.0) | 29.0 (21.0 – 38.0) | 0.0002 a | 28.0 (20.0 – 37.0) |
| Gender Female, n (%) | 407 (89.3) | 794 (89.7) | 0.7927 b | 1201 (89.6) |
| Time to diagnosis(months); median (IQR) | 5.3 (2.0 – 14.0) | 6.0 (2.7 – 16.4) | 0.0191 a | 5.8 (2.3 – 16.0) |
| Ethnicity, n (%) (c10) | 0.1263 b | |||
| Caucasian | 170 (37.4) | 389 (44.1) | 559 (41.8) | |
| Mestizo | 209 (46.0) | 366 (41.5) | 575 (43.0) | |
| ALA | 57 (12.6) | 100 (11.3) | 157 (11.8) | |
| Residence, urban n (%)(c20) | 411 (90.7) | 797 (90.8) | 0.9781 b | 1208 (90.8) |
| Socioeconomic status, n (%)(c9) | <.0001 b | |||
| High | 21 (4.6) | 112 (12.7) | 133 (10.0) | |
| Medium | 118 (25.9) | 277 (31.4) | 395 (29.6) | |
| Low | 316 (69.5) | 492 (55.8) | 808 (60.5) | |
| Partial medical insurance, n(%)(c26) | 188(41.4) | 335(38.3) | 0.2758 b | 523(39.4) |
| Educational level, in year, n (%)(c132) | 0.6057 b | |||
| 0–7 | 134 (30.8) | 251 (29.8) | 385 (30.1) | |
| 8–12 | 205 (47.1) | 384 (45.6) | 589 (46.1) | |
| More than 12 | 96 (22.1) | 207 (24.6) | 303 (23.7) | |
| Previous hospitalization due to SLE d , n (%) | 204 (44.7) | 298 (33.7) | <.0001 b | 502 (37.4) |
| ACR criteria at baseline, n (%) | ||||
| Malar rash | 239 (52.4) | 491 (55.5) | 0.2852 b | 730 (54.4) |
| Discoid lupus | 237 (52.0) | 293 (33.1) | <.0001 b | 530 (39.5) |
| Photosensitivity | 218 (47.8) | 479 (54.1) | 0.0283 b | 697 (52.0) |
| Oral ulcers | 174 (38.2) | 314 (35.5) | 0.3343 b | 488 (36.4) |
| Synovitis | 357 (78.3) | 696 (78.6) | 0.8809 b | 1053 (78.5) |
| Serositis | 138 (30.3) | 185 (20.9) | 0.0001 b | 323 (24.1) |
| Renal involvement | 237 (52.0) | 293 (33.1) | <.0001 b | 530 (39.5) |
| Neurologic involvement | 50 (11.0) | 73 (8.2) | 0.1026 b | 123 (9.2) |
| Hematological | 322(70.6) | 530(59.9) | 0.0001 b | 852(63.5) |
| Hemolytic anemia | 72(15.8) | 72(8.1) | <0.0001 b | 144 (10.7) |
| Leucopenia | 168(36.8) | 392(34.1) | 0.3231 b | 470(35.0) |
| Lymphopenia | 248(54.4) | 400(45.2) | 0.0014 b | 648(48.3) |
| Thrombocytopenia | 80(17.5) | 140(15.8) | 0.4192 b | 220(16.4) |
| Immunological | 297 (81.1) | 543 (76.7) | 0.0938 b | 840 (78.2) |
| Serological features at baseline, n (%) | ||||
| Anti-dsDNA (c381) | 243 (73.9) | 418 (66.2) | 0.0156 b | 661 (68.9) |
| Anti-Sm (c382) | 97 (59.1) | 160 (46.4) | 0.0071 b | 257 (50.5) |
| Hypocomplementemia (c442) | 209 (70.1) | 343 (57.1) | 0.0002 b | 552 (61.4) |
| Antiphospholipid antibodies, n (%)(c778) | 104 (52.8) | 201 (54.9) | 0.6292 b | 305 (54.2) |
| SLEDAI at cohort intro; median (IQR) | 12.0(8.0 -19.0) | 8.0 (4.0 -12.0) | <0.0001 a | 9.0 (4.0 -15.0) |
| Baseline SDI; median (IQR) | 1.0 (0.0 -2.0) | 0.0 (0.0–1.0) | <0.0001 a | 1.0 (0.0 -1.0) |
| SDI at the last visit; median (IQR) | 2.0 (1.0 -4.0) | 1.0 (0.0–2.0) | <0.0001 a | 1.0 (0.0 -2.0) |
| Prednisone (baseline), n (%) | 0.9433 b | |||
| ≤7.5 mg/d | 10 (2.2) | 19 (2.1) | 29 (2.2) | |
| ˃7.5; ≤15 mg/d | 35 (7.7) | 70 (7.9) | 105 (7.8) | |
| ˃15; ≤60 mg/d | 98 (21.5) | 173 (19.5) | 271 (20.2) | |
| ˃60 mg/d | 56 (12.3) | 108 (12.2) | 164 (12.2) | |
| AM (baseline) use | 118 (25.9) | 315 (35.6) | 0.0003 b | 433 (32.3) |
| Mortality at last visit, n (%) | 48 (10.5) | 27 (3.1) | <0.0001 b | 75 (5.6) |
| Disease state at baseline | 0.3562 b | |||
| Remission | 28 (6.1) | 73 (8.2) | 101 (7.5) | |
| LDAS | 124 (27.2) | 227 (25.6) | 351 (26.2) | |
| Active | 304 (66.7) | 585 (66.1) | 889 (66.3) | |
| Disease state at the last visit | 0.0145 b | |||
| Remission | 217 (47.6) | 451 (51.0) | 668 (49.8) | |
| LDAS | 98 (21.5) | 224 (25.3) | 322 (24.0) | |
| Active | 141 (30.9) | 210 (23.7) | 351 (26.2) |
ALA: African Latin American; LDAS: Low disease activity state; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; SDI: Systemic Lupus International Collaborating Clinics/American College of Rheumatology Disease Index; AM: Antimalarials. Bold type was used for the p value that were statistically significant.
bChi-Square p-value.
aKruskal-Wallis p-value.
cmissing data.
dPrevious at entry to the cohort.
In relation to the clinical variables, the presence of discoid lupus (52.0% vs 33.1%; p < 0.0001), serosal (30.3% vs 20.9; p = 0.0001), renal (52.0% vs 33.1%; p < 0.0001), hematological (70.6% vs 59.9%; p = 0.0001) and serological involvement (70.1% vs 57.1%; p = 0.0002) were more frequent at baseline in the hospitalized versus the non-hospitalized patients. Hospitalized patients also had a higher baseline SLEDAI (12.0 vs 8.0; p < 0.0001), as well as a higher SDI at baseline (1.0 vs 0.0; p < 0.0001) and at the end of follow-up (2.0 vs 1.0; p < 0.0001); they also experienced a higher mortality (10.5% vs 3.1; p < 0.0001) and a lower AM use before the baseline visit (25.9% vs 35.6%; p = 0.0003) compared with non-hospitalized patients. In the same way, these patients had reached remission (47.6% vs 51.0; p = 0.0145) and LDAS (21.5 vs 25.3; p = 0.0145) less frequently at the end of the follow-up period than the non-hospitalized patients.
Table 2 describes the predictors of the first hospitalization regardless of its cause. The variables that maintained statistical significance in the multivariable analysis were: medium (HR 2.03(1.27–3.24); p = 0.0028) and low (HR 2.42(1.55–3.79); p < 0.0001) SES; serosal (HR 1.32(1.07–1.62); p = 0.0074) and renal (HR 1.50(1.23–1.82); p < 0.0001) involvement at baseline. AM use (HR 0.61(0.50–0.74); p < 0.0001), and achieving remission (HR 0.80(0.65-0.97); p = 0.0300) decreased the risk of hospitalization.
Table 2.
Predictive factors of the first hospitalization in SLE patients (n = 1341).
| Variable | Univariable HR (95% CI) | p value | Multivariable HR (95% CI) | p value |
|---|---|---|---|---|
| Gender, Female | 0.94 (0.70–1.27) | 0.6901 | 1.03 (0.76–1.39) | 0.8367 |
| Age at diagnosis | 0.87 (0.81–0.95) | 0.0011 | ||
| Ethnicity | 0.0674 | 0.7207 | ||
| Caucasian | Ref. | |||
| Mestizo | 1.21 (0.99–1.48) | 0.0652 | 0.99 (0.80–1.22) | 0.9664 |
| ALA | 1.28 (0.95–1.73) | 0.1011 | 1.04(0.77–1.42) | 0.7654 |
| Socioeconomic status | <0.0001 | 0.0003 | ||
| High | Ref. | |||
| Medium | 2.12 (1.33–3.37) | 0.0015 | 2.03(1.27–3.24) | 0.0028 |
| Low | 2.81 (1.81–4.37) | <0.0001 | 2.42(1.55–3.79) | <0.0001 |
| Medical insurance, full coverage | 0.92 (0.76–1.11) | 0.3940 | ||
| SLEDAI | 1.05 (1.04–1.06) | <0.0001 | ||
| SDI a, b | 1.33 (1.26–1.41) | <0.0001 | ||
| Previous hospitalization due to SLE c | 1.39 (1.16–1.67) | 0.0005 | ||
| Arthritis d | 0.91 (0.72–1.13) | 0.3808 | ||
| Serositis d | 1.55 (1.27–1.89) | <0.0001 | 1.32(1.07–1.62) | 0.0074 |
| Renal d | 1.90 (1.58–2.29) | <0.0001 | 1.50(1.23–1.82) | <0.0001 |
| Anti-dsDNA d | 1.38 (1.08–1.77) | 0.0096 | ||
| Antimalarial use b | 0.55 (0.46–0.66) | <0.0001 | 0.61(0.50–0.74) | <0.0001 |
| Prednisone e | 0.9974 | |||
| None | Ref. | |||
| <7.5 mg/d | 0.96 (0.51–1.81) | 0.9103 | ||
| 7.5–15 mg/d | 0.95 (0.67–1.36) | 0.8002 | ||
| 15–60 mg/d | 1.02 (0.81–1.29) | 0.8620 | ||
| ≥60 mg/d | 0.98 (0.73–1.31) | 0.8884 | ||
| Disease State b | 0.0046 | 0.0929 | ||
| Remission | 0.73 (0.60–0.88) | 0.0012 | 0.80(0.65–0.97) | 0.0300 |
| LDAS | 0.74 (0.35–1.57) | 0.4327 | 0.85(0.40–1.82) | 0.6846 |
| Active | Ref. |
LDAS: low disease activity state; ALA: Afro–Latin American; SDI: SLICC/ACR damage index; HR: Hazard ratio; 95% CI: 95% confidence interval; Ref: reference group. Bold type was used for the p value that were statistically significant.
aIncrease on per 1 unit.
bTime–dependent covariates.
cPrevious at entry to the cohort.
dAt baseline.
ehighest dose.
Finally, Table 3 shows the factors associated with hospitalizations due to either disease activity or infections. In terms of disease activity, variables independently associated with them in the multivariable analysis were: medium (OR 2.77 (1.40–5.23); p = 0.0030) and low (OR 3.13 (1.64–5.98); p = 0.0006) SES, increase on the SDI per 1 unit (OR1.35 (1.20–1.52); p < 0.0001) and baseline SLEDAI (OR1.05 (1.03-1.07); p < 0.0001) while age at diagnosis (OR 0.98 (0.97–0.99); p = 0.0234) and AM use were protective of their occurrence (OR 0.69 (0.50–0.96); p = 0.0253).
Table 3.
Univariable and multivariable logistic regression analysis of factors associated with hospitalization due to disease activity and infections in patients with SLE a .
| Activity hospitalization (n: 344) | Infections hospitalization (n: 85) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value | Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value |
| Gender, female | 0.89 (0.60–1.32) | 0.5568 | 0.89 (0.57–1.39) | 0.6111 | 1.14 (0.54–2.41) | 0.7347 | 1.26 (0.59–2.70) | 0.5554 |
| Age at diagnosis | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.97–0.99) | 0.0234 | 1.00 (0.98–1.02) | 0.7790 | ||
| Ethnicity | 0.1630 | 0.9190 | 0.1580 | 0.3398 | ||||
| Caucasian | Ref. | Ref. | Ref. | Ref. | ||||
| Mestizo | 1.26 (0.96–1.65) | 0.1000 | 0.91 (0.67–1.24) | 0.5590 | 1.47 (0.90–2.38) | 0.1234 | 1.33(0.81–2.19) | 0.2648 |
| ALA | 1.51 (1.02–2.23) | 0.0403 | 1.03 (0.66–1.61) | 0.8965 | 0.84 (0.36–1.95) | 0.6777 | 0.75(0.32–1.77) | 0.5079 |
| Socioeconomic status | <.0001 | 0.0026 | 0.1552 | 0.4332 | ||||
| High | Ref. | Ref. | Ref. | Ref. | ||||
| Medium | 3.16 (1.67–5.97) | 0.0004 | 2.77 (1.40–5.23) | 0.0030 | 1.06 (0.41–2.70) | 0.9102 | 1.01(0.39–2.62) | 0.9807 |
| Low | 4.23 (2.29–7.81) | <0.0001 | 3.13 (1.64–5.98) | 0.0006 | 1.67 (0.71–3.96) | 0.2417 | 1.40(0.58–3.37) | 0.4520 |
| Medical insurance, full coverage | 0.86 (0.67–1.05) | 0.2387 | 0.97 (0.62–1.53) | 0.9114 | ||||
| SLEDAIb, c | 1.07 (1.05–1.09) | <0.0001 | 1.05 (1.03–1.07) | <0.0001 | 1.02 (0.99–1.04) | 0.2036 | ||
| SDIb, c | 1.52 (1.37–1.70) | <0.0001 | 1.35 (1.20–1.52) | <0.0001 | 1.11 (0.93–1.33) | 0.2469 | ||
| Previous hospitalization due to SLE d | 1.24(0.96–1.60) | 0.0922 | 2.57(1.64–4.02) | <0.0001 | 2.52(1.59–4.02) | <0.0001 | ||
| Arthritis b | 0.83 (0.62–1.11) | 0.2119 | 1.90 (0.99–3.64) | 0.0515 | 2.09(1.08–4.03) | 0.0279 | ||
| Serositis b | 1.62 (1.23–2.14) | 0.0006 | 1.27 (0.78–2.07) | 0.3420 | ||||
| Renal b | 2.31 (1.79–2.96) | <.0001 | 1.38 (0.89–2.15) | 0.1501 | ||||
| Anti–dsDNA b | 1.40 (1.01–1.94) | 0.0412 | 1.38 (0.76–2.52) | 0.2908 | ||||
| Prednisone e | 0.7971 | 0.0769 | ||||||
| None | Ref | Ref. | ||||||
| <7.5 mg/d | 0.76 (0.30–1.90) | 0.5492 | 1.56 (0.36–.86) | 0.5522 | ||||
| 7.5–15 mg/d | 0.86 (0.53–1.38) | 0.5289 | 1.44 (0.62–.32) | 0.3908 | ||||
| 15–60 mg/d | 0.84 (0.61–1.16) | 0.2945 | 2.07 (1.22–.52) | 0.0068 | ||||
| ≥60 mg/d | 0.89 (0.60–1.31) | 0.5469 | 1.84 (0.97–.49) | 0.0621 | ||||
| Antimalarial use b | 0.52 (0.39–0.70) | <0.0001 | 0.69 (0.50–0.96) | 0.0253 | 1.35 (0.86–2.12) | 0.1934 | ||
| Disease state at baseline | 0.7698 | 0.1930 | ||||||
| Remission | 0.96 (0.60–1.55) | 0.8713 | 0.27 (0.07–1.12) | 0.0720 | ||||
| LDAS | 1.10 (0.83–1.46) | 0.5050 | 0.91 (0.55–1.50) | 0.7016 | ||||
| Active | Ref. | Ref. | ||||||
ALA: African Latin American; LDAS: Low disease activity state; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; SDI: Systemic Lupus International Collaborating Clinics/American College of Rheumatology Disease Index. Bold type was used for the p value that were statistically significant.
aExcluding associated causes (activity plus infection).
bAt baseline.
cIncrease on per 1 unit.
dPrevious at entry to the cohort
ehighest dose; Ref: reference group.
In terms of infections, the variables associated with the first hospitalization that maintained statistical significance in the multivariable analysis were: previous hospitalizations (before entry into the cohort) due to SLE (OR 2.52(1.59–4.02); p < 0.0001) and the presence of arthritis at baseline (OR 2.09(1.08–4.03); p = 0.0279).
Discussion
This study describes the causes of the first hospitalization in lupus patients, their associated factors and highlights the importance of achieving remission as well as of AM use as being protective factors of the first hospitalization.
In relation to the causes, disease activity was the most frequent cause followed by infections, as has been described in several studies.1,2,17–20 The active organs that required hospitalizations were renal, followed by hematological and serosal involvements; these finding are similar to those from other studies, particularly in terms of renal involvement,7,8,17,18 but differ from the Hopkins Lupus Cohort data, where neurological involvement was the most frequent cause of hospitalization. 20 This difference could be due to limitations in determining SLE as the cause of the neurological manifestations in these patients. On the other hand, and in concordance with other studies,1,2,17,18,21 the most common infection sites that led to hospitalizations were respiratory and cutaneous.
Hospitalized patients had a shorter time to diagnosis, were younger, and had a lower SES. These sociodemographic variables have already been described in the GLADEL cohort as factors of a worse disease course.12,22–26 In relation to the clinical variables, hospitalized patients presented a higher frequency of renal, serological and serosal involvement, higher SLEDAI and SDI at baseline and at the end of the follow-up which is similar to other studies.19,20,23 They use, however, less AM. The beneficial effect of AM has been extensively studied and GLADEL27–31 has demonstrated the impact of AM use on different outcomes, such as the onset of renal compromise and associated accrual damage.
In relation to the disease state, hospitalized patients were less likely to have achieved LDAS and remission at the end of follow-up. The treat-to-target (T2T) strategy for SLE has been proposed by experts to optimize the outcomes of these patients, such as damage accumulation, survival and use of health resources. Previous studies have shown that achieving remission or LDAS is associated with lower rates of damage accrual and of mortality.4,10,11
Factors associated with the first hospitalization due to activity were lower SES, SLEDAI, and SDI at baseline. In the same way, the Lupus Canadian cohort and the Hopkins Lupus Cohort suggested hospitalizations were associated with disease activity and comorbidities, among other variables.19,20
This study, as well as several other publications, highlight that AM use reduces the risk of hospitalizations.6,17 This effect of AM adds to others such as reducing disease activity, preventing lupus flares, lowering damage accrual, and improving survival. Despite the low percentage of AM use in this study, its benefits were demonstrated.
The most relevant risk factor for the first hospitalization due to infections was a history of previous hospitalizations for lupus before cohort entry. This may be associated with the use of a more aggressive treatment, with the consequent occurrence of an infection secondary to immunosuppression.
In the multivariable Cox regression analysis, independent predictors of the first hospitalization regardless of the cause were: lower SES, renal and serosal involvement. Conversely, AM use and having reached remission prior to the hospitalization were protective factors.4,6,17 These observations are relevant to the management of patients with lupus; that despite presenting an unfavorable socioeconomic condition and a severe disease, AM use and the attempt made by physicians to achieve remission will have a favorable impact in the prognosis of our patients.
This study has strengths and limitations. Within the first, it is a Latin American multicenter study that reflects the different factors involved in the hospitalizations of our patients. GLADEL represents a diverse patient population from various countries and ethnicities, providing a good representation of SLE patients, the natural history of the disease, treatment burden, and use of medical resource utilization (including hospitalizations) in the real life. The main limitations were some missing data, not having precise data about the hospitalizations, as well as the fact, that the therapeutic strategies used do not reflect current practices, in which T2T is prioritized; a mean follow-up time of only 5 years is also highlighted.
In conclusion, the first hospitalization in LA patients with diagnosis of lupus were associated with socioeconomic disadvantages and renal and serosal involvement. Conversely, reaching remission and AM use are goals that must be prioritized. Likewise, new studies are necessary since the introduction of new therapies for lupus and the changes in the paradigms in the management of these patients.
Acknowledgements
To members of Grupo Latino Americano De Estudio del Lupus (GLADEL), Study Group of PANLAR, for their participation.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AO, US, EA and FZ are Janssen Pharmaceutical Companies employees.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This manuscript received financial support for their publication from Janssen Pharmaceutical Companies.
Contributorship: All of the listed authors have contributed to collecting data and reviewing the manuscript.
Ethical statement
Ethical approval
This study involves human participants and was approved by an Ethics Committee(s) or Institutional Board(s). GLADEL is an observational, multiethnic, longitudinal inception cohort study started in 1997 and constituted by patients from 34 centers from nine Latin American countries under local institutional review boards’ regulations and the Declaration of Helsinki’s guidelines. Each center has autonomy for the inclusion of patients and the consent of the participants.
Patient consent for publication
The manuscript is sufficiently anonymised in line with our anonymisation policy and checklist.
ORCID iDs
Rosana Quintana https://orcid.org/0000-0003-0643-2755
Manuel F Ugarte-Gil https://orcid.org/0000-0003-1728-1999
Rosa Serrano-Morales https://orcid.org/0000-0002-9109-6276
Cristina Drenkard https://orcid.org/0000-0002-6832-7291
Loreto Massardo https://orcid.org/0000-0002-4790-1258
Graciela S Alarcon https://orcid.org/0000-0001-5190-9175
Data availability statement
To be considered upon request.
References
- 1.Dhital R, Pandey RK, Poudel DR, et al. All-cause hospitalizations and mortality in systemic lupus erythematosus in the US: results from a national inpatient database. Rheumatol Int 2020; 40(3): 393–397. [DOI] [PubMed] [Google Scholar]
- 2.Duffy KN, Duffy CM, Gladman DD. Infection and disease activity in systemic lupus erythematosus: a review of hospitalized patients. J Rheumatol 1991; 18(8): 1180–1184. [PubMed] [Google Scholar]
- 3.Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltim) 1993; 72(2): 113–124. [PubMed] [Google Scholar]
- 4.Reátegui-Sokolova C, Rodríguez-Bellido Z, Gamboa-Cárdenas RV, et al. Remission and low disease activity state prevent hospitalizations in systemic lupus erythematosus patients. Lupus 2019; 28(11): 1344–1349. [DOI] [PubMed] [Google Scholar]
- 5.Nangit A, Lin C, Ishimori ML, et al. Causes and predictors of early hospital readmission in systemic lupus erythematosus. J Rheumatol 2018; 45(7): 929–933. [DOI] [PubMed] [Google Scholar]
- 6.Assunção H, Rodrigues M, Prata AR, et al. Predictors of hospitalization in patients with systemic lupus erythematosus: a 10-year cohort study. Clin Rheumatol 2022; 41(10): 2977–2986. [DOI] [PubMed] [Google Scholar]
- 7.Aldarmaki R, Al Khogali HI, Al Dhanhani AM. Hospitalization in patients with systemic lupus erythematosus at Tawam Hospital, United Arab Emirates (UAE): rates, causes, and factors associated with length of stay. Lupus 2021; 30(5): 845–851. [DOI] [PubMed] [Google Scholar]
- 8.Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ, et al. Remission and Low Disease Activity Status (LDAS) protect lupus patients from damage occurrence: data from a multiethnic, multinational Latin American Lupus Cohort (GLADEL). Ann Rheum Dis 2017; 76(12): 2071–2074. [DOI] [PubMed] [Google Scholar]
- 9.Reátegui-Sokolova C, Ugarte-Gil MF, Harvey GB, et al. Predictors of renal damage in systemic lupus erythematous patients: data from a multiethnic, multinational Latin American lupus cohort (GLADEL). RMD Open 2020; 6(3): e001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarcón GS, Ugarte-Gil MF, Pons-Estel G, et al. Remission and low disease activity state (LDAS) are protective of intermediate and long-term outcomes in SLE patients. Results from LUMINA (LXXVIII), a multiethnic, multicenter US cohort. Lupus 2019; 28(3): 423–426. [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Magder LS. Comparison of remission and lupus low disease activity state in damage prevention in a United States systemic lupus erythematosus cohort. Arthritis Rheumatol 2018; 70(11): 1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics. Medicine (Baltim) 2004; 83(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25(11): 1271–1277. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35(6): 630–640. [DOI] [PubMed] [Google Scholar]
- 15.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39(3): 363–369. [DOI] [PubMed] [Google Scholar]
- 16.van Vollenhoven R, Voskuyl A, Bertsias G, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017; 76(3): 554–561. [DOI] [PubMed] [Google Scholar]
- 17.Rosa GPD, Ortega MF, Teixeira A, et al. Causes and factors related to hospitalizations in patients with systemic lupus erythematosus: analysis of a 20-year period (1995-2015) from a single referral centre in Catalonia. Lupus 2019; 28(9): 1158–1166. [DOI] [PubMed] [Google Scholar]
- 18.Alhassan N, Almetri T, Abualsoud S, et al. Causes of hospitalization for systemic lupus erythematosus in Saudi arabia compared with the global setting: a retrospective single-center observational study. Cureus 2021; 13(10): e18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Dhillon N, Pope J. All-cause hospitalizations in systemic lupus erythematosus from a large Canadian referral centre. Rheumatology 2013; 52(5): 905–909. [DOI] [PubMed] [Google Scholar]
- 20.Petri M, Genovese M. Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins Lupus Cohort. J Rheumatol 1992; 19(10): 1559–1565. [PubMed] [Google Scholar]
- 21.Ko T, Koelmeyer R, Li N, et al. A Predictors of infection requiring hospitalization in patients with systemic lupus erythematosus: a time-to-event analysis. Semin Arthritis Rheum 2022; 57: 152099. [DOI] [PubMed] [Google Scholar]
- 22.Pimentel-Quiroz VR, Ugarte-Gil MF, Pons-Estel GJ, et al. Factors predictive of high disease activity early in the course of SLE in patients from a Latin-American cohort. Semin Arthritis Rheum 2017; 47(2): 199–203. [DOI] [PubMed] [Google Scholar]
- 23.Ugarte-Gil MF, Silvestre AM, Pons-Estel BA. Access to an optimal treatment. Current situation. Clin Rheumatol 2015; 34(Suppl 1): S59–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons-Estel GJ, Catoggio LJ, Cardiel MH, et al. Lupus in Latin-American patients: lessons from the GLADEL cohort. Lupus 2015; 24(6): 536–545. [DOI] [PubMed] [Google Scholar]
- 25.Pons-Estel BA, Bonfa E, Soriano ER, et al. First Latin American clinical practice guidelines for the treatment of systemic lupus erythematosus: Latin American Group for the Study of Lupus (GLADEL, Grupo Latino Americano de Estudio del Lupus)-Pan-American League of Associations of Rheumatology (PANLAR). Ann Rheum Dis 2018; 77(11): 1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González LA, Pons-Estel GJ, Toloza SMA, et al. Understanding risk factors for poor outcomes in a multiethnic longitudinal cohort: the LUMINA (lupus in minorities: nature vs. Nurture) experience (LUMINA LXXXII). Rheum Dis Clin North Am 2021; 47(1): 55–64. [DOI] [PubMed] [Google Scholar]
- 27.Pons-Estel GJ, Alarcón GS, McGwin G, Jr, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61(6): 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pons-Estel GJ, Alarcón GS, González LA, et al. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res 2010; 62(3): 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García MA, Alarcón GS, Boggio G, et al. Primary cardiac disease in systemic lupus erythematosus patients: protective and risk factors--data from a multi-ethnic Latin American cohort. Rheumatology 2014; 53(8): 1431–1438. [DOI] [PubMed] [Google Scholar]
- 30.Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Anti-malarials exert a protective effect while Mestizo patients are at increased risk of developing SLE renal disease: data from a Latin-American cohort. Rheumatology 2012; 51(7): 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu K, Gladman DD, Su J, et al. Hospitalizations in patients with systemic lupus erythematosus in an academic health science center. J Rheumatol 2017; 44(8): 1173–1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
To be considered upon request.