Abstract
Background
Despite the application of various tools for the control of vectors of Plasmodium falciparum, malaria remains the major killer disease in sub-Saharan Africa accounting for up to 90% of deaths due to the disease. Due to limitations of the useage of chemical insecticides such as resistance, negative impact on the environment and to nontarget organisms, the World Health Organization (WHO) requires that affected countries find alternative vector control tools. This study evaluated the effectiveness of ( +)-usnic acid (UA) as an insecticide through oral administration to male and female Anopheles gambiae as an alternative or additional active ingredient to be used in toxic sugar bait.
Methods
( +)-usnic acid was diluted using acetone at 5, 10, and 15 mg/ml concentrations in three replicates. A 5 ml mixture of 2% food dye and 10% sugar using chlorine-free water mixed with the dilutions of the ( +)-usnic acid and negative control was made containing 2% food dye and 10% sugar solution. The preparations were soaked on a ball of cotton wool and placed over the net of a cup. 5 male and 5 non-blood-fed female newly hatched starved An. gambiae Kisumu strain were introduced together into a cup and monitored for knockdown and mortalities after 4, 24 48, and 72 h. The data were analysed using a multiple linear regression model using the lm function, a base R function and a posthoc test were conducted on the significant main effects and interaction terms using the emmeans function from the emmeans R package. All analyses were performed in RStudio using base R (version 4.3.3).
Results
There was high mortality of both male and female An. gambiae after ingestion of the toxic sugar bait. 15 mg/ml usnic acid caused the highest mortality (50%) within the first 4 h compared to 5 and 10 mg/ml ( +)-UA. There was a decline in the mortality rate with increased exposure time from 24 to 72 h, however, there was a significant difference in mortality at 5, 10 and 15 mg/ml. Acute toxicity was associated with ingestion of 15 mg/ml after 24 h. 72 h post-mortality was lower in all concentrations than in the control. High mortality was observed among females over the first 4 h (60%) compared to males (40%) due to higher feeding rate of the toxic agent. The proportion of dead males and females was equal after 24 h while after 48 h, the proportion of dead males was high.There was a significantly lower mortality rate after 72 h for both males and females (0 to 13.3%). Compared to all the treatments, high mortality of males was observed.
Conclusions
The results of this study indicate that ( +)-UA when administered as oral sugar bait to An. gambiae has insecticidal properties and is a suitable ingredient to be used as a toxic agent in the novel attractive toxic sugar bait for the control of malaria vectors. ( +)-UA may be an alternative active ingredient as toxic bait in the effort to reduce and eliminate the transmission of Plasmodium falciparum in Africa.
Keywords: Mosquitoes, Vector, Lichen secondary metabolites, Bioactive substances, Usnic acid, Enantiomer, Kenya, East Africa
Background
Malaria is still a burden in African regions and accounts for 95% of malaria-related deaths (580,000 annually) where approximately 80% are children. Approximately 249 million cases of malaria were reported in the world in 2022 in 85 countries [1]. Anopheles gambiae is the main vector of Plasmodium falciparum that causes malaria in sub-Saharan Africa due to its high abundance and longevity, strong preference to feed on human blood and high vectorial capacity [2–5].
The most effective methods used to control the spread of malaria are to prevent mosquito bites or reduce their population [6]. To control mosquitoes, methods such as reducing or eliminating their breeding sites, using natural predators, genetically modifying mosquitoes and applying pathogenic microorganisms such as entomopathogenic fungi (e.g., Lagenidium, Coelomomyces and Culicinomyces) or bacteria (e.g., Bacillus thuringiensis) have been used [7]. Despite the continuous application of these methods, the challenge to eliminate malaria through vector control still exists due to resistance of the target mosquitoes to the chemical agents, human and mosquito behaviour factors and climate change [8–14]. The possibility of transmission of infectious agents by the vector between humans and non-human hosts also underlines the importance of eliminating malaria. The spread of other species of mosquitoes to other new geographical areas, such as Anopheles stephensi in Africa, can be a major challenge to control malaria [15, 16]. However, some lessons can be learned from countries that have eliminated malaria in sub-Saharan Africa to attain a malaria-free continent status [17].
Therefore, there is a need for new mosquito control strategies to overcome the limitations of the existing vector control methods and other indirect factors that hinder the expected goal of eliminating malaria. Novel mosquito control methods are needed to address the limitations of current intervention strategies in eliminating malaria [18, 19]. One such potential method is the Attractive Toxic Sugar Bait (ATSB), which uses sugar bait to lure and kill insects, a method with origins that dates back to 77 CE [20]. Toxic sugar baits (TSB) with arsenic and boric acid as a toxic agent was used to kill termites and mosquitoes, respectively [21, 22]. The concept has been used successfully to control mosquitoes and other insects of medical importance based on the sugar-seeking natural behaviour observed among mosquitoes sandflies and blackflies [23–27]. However, arsenic is an exceptionally toxic element that causes serious health problems to humans in case of long-term application and accumulation similarly to other heavy metals [28].
Both males and females require sugar as the main source of energy, however, males exclusively feed on sugar and the females occasionally seek sugar during their lifetime and only seek blood from animals to obtain protein for egg production [29]. Anopheles gambiae naturally feeds on preferred sugar sources of glucose, fructose and gulose which contributes to its fitness and survival for both males and females. Similar results on preference for types of sugar have been observed in a baseline study in Kenya on the sugar-feeding patterns in mosquitoes [30, 31].
The concept behind toxic sugar bait is to lure both male and female mosquitoes that exhibits a natural feeding behaviour of sugar. Toxic agents are added to the sugar, and when mosquitoes consume them, they are poisoned. Boric acid, dinotefuran and ivermectin are among the common synthetic insecticide chemicals used in sugar bait which have shown promising results in killing mosquitoes [32–37]. Although the chemicals used have been demonstrated to be potential oral insecticides, they have a limitation of being toxic to the environment and non-target organisms especially when used outdoor [38]. Non-toxic plant and microbial-based products such as microencapsulated garlic oil, eugenol, spinsyns, erythritol, B. thuringiensis var. israelensis (Bti) and sodium ascorbate have been used as toxic agents in sugar bait and shown to be safe [39–43]. The use of plant-based toxic agents has been emphasized by Rezende et al. [38].
Lichens are composed of one or more photosynthetic partners, fungi and other indeterminate numbers of microscopic organisms that live together [44]. However, this definition is limited since it is based on limited knowledge about the role of other microorganisms on the lichen [45]. They are known to produce over 1000 unique secondary metabolites. The amount of usnic acid produced by lichens varies and factors such as the algal partner and season have been shown to have a significant relationship [46, 47]. These metabolites exhibit various biological activities that include insecticidal properties [48–51]. Various lichen secondary metabolites have been used against insects' larval and adult stages and shown to be potential insecticide [52].
Usnic acid exhibits biological properties and it is a potential candidate to be explored as a potential insecticide to be used as an oral insecticide in a toxic sugar bait (TSB) [53–56]. Usnic acid (UA) is a chiral molecule and it is known to occur in two forms in nature: ( +)-usnic acid and (−)-usnic acid, the two isomers may exhibit different biological activities, hence their efficacy as insecticide need to be further investigated and determined [57, 58].
There is limited knowledge and studies on the potential of ( +)-UA as an oral insecticide against An. gambiae, therefore, this study aimed to determine the effectiveness of different concentrations of ( +)-UA on both male and female adult stages of An. gambiae by performing laboratory bioassay experiments for various lengths of time and concentrations.
Methods
This study aimed to determine the killing ability of the 3 concentrations of ( +)-usnic acid (5, 10 and 15 mg/ml) mixed with 10% sugar solution and 2% food dye as an oral insecticide. Insecticidal property was determined by measuring the knockdown effect of male and female An. gambiae at 4, 24, 48, and 72 h.
Mosquitoes
The mosquito, Anopheles gambiae Kisumu strain, used in this study was reared at Kenya Medical Research Institute Insectary (KEMRI). Its susceptibility to pyrethroids is known and has been used for bioassay studies as a control in insecticide resistance and other bioassay studies [59].
The conditions of the insectary were maintained at 270C and relative humidity of 78% ± 10, and 12:12 L/D. Adult females were fed on bovine blood by use of a membrane feeding machine. Males and females were fed on a 10% sugar solution ad libitum.
( +)-usnic acid
The active compound used in the experiments in this study was ( +)-usnic (IUPAC name: 2,6-diacetyl-7,9-dihydroxy-8,9b-dimethyl-1,3(2H,9bH)- dibenzo-furandione (Fig. 1). It is more soluble in acetone than in water [60]. It is a natural compound produced by various taxa of lichens that belong e.g., to the genera Cladonia, Usnea, Lecanora, Ramalina, Parmelia and Evernia that are widely distributed all over the world [54, 61–63]. The ( +)-usnic acid was supplied from Phytolab where purity was certified [64].
Fig. 1.
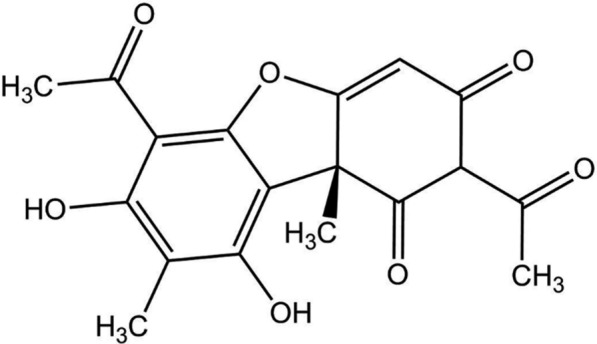
Molecular structure of ( +)-usnic acid
Preparation of the usnic acid bait in sugar solutions
A stock solution of usnic acid was prepared by dissolving 500 mg of usnic acid powder in 10 ml of acetone and labelled as 50 mg/ml usnic acid (UA). This was diluted to 5 mg/ml, 10 mg/ml, and 15 mg/ml ( +)-usnic acid solution in acetone and for each of the concentrations, 10% sugar solution and 2% food dye solution were added. The negative control was prepared by mixing 10% sugar solution and 2% food dye.
Bioassay to determine susceptibility
Bioassay experiments were performed according to Allan et al. [65] and Stewart et al. [66] with a slight modification that did not affect the outcome of the results. Newly hatched 5 males and 5 non-blood-fed females that were starved before the experiment (only water was provided) were aspirated using an aspirator and gently blown together in paper coffee cups. The opening of the cups was secured with an insecticide-free net. They were allowed to acclimatize for one hour due to the shock caused by aspiration and being in the new environment. Each coffee cup was labelled according to the concentration of the ( +)-UA and a negative control.
Each concentration of the usnic acid was introduced on a ball of cotton wool and placed on top of the net of each cup. The temperature and humidity of the bioassay room were recorded at the time of the start of the experiment. The knockdown effect was observed after 4, 24, 48, and 72 h. The knocked down and dead or moribund mosquitoes were aspirated out and their sex was determined. Their abdominal status was also determined by observation using a light microscope. A coloured and extended abdomen was used as a basis to confirm that the toxic agent was ingested.
The data was analysed using R program
The data was analysed using a multiple linear regression model approach that included main effects for Concentration (Conc.), Sex, and Time, as well as interaction terms of Conc.*Time, Conc.*Sex, and Time*Sex. A Posthoc test was then conducted on the significant main effects and interaction terms using the emmeans function from the emmeans R package. All analyses were performed in RStudio using base R (version 4.3.3).
The specific code for the model used was
Model <—lm(TotalDeaths ~ Conc + Time + Sex + Conc*Time + Conc*Sex + Time*Sex, group_data).
Code for the Posthoc analysis
lsmeans < -emmeans(model, ~ Conc)
lsmeans_df <—as.data.frame(lsmeans)
lsmeansT < -emmeans(model, ~ Time)
lsmeansT_df <—as.data.frame(lsmeansT)
lsmeansCT < -emmeans(model, ~ Conc:Time)
lsmeansCT_df <—as.data.frame(lsmeansCT)
lsmeans_df; lsmeansT_df; lsmeansCT_df
Results
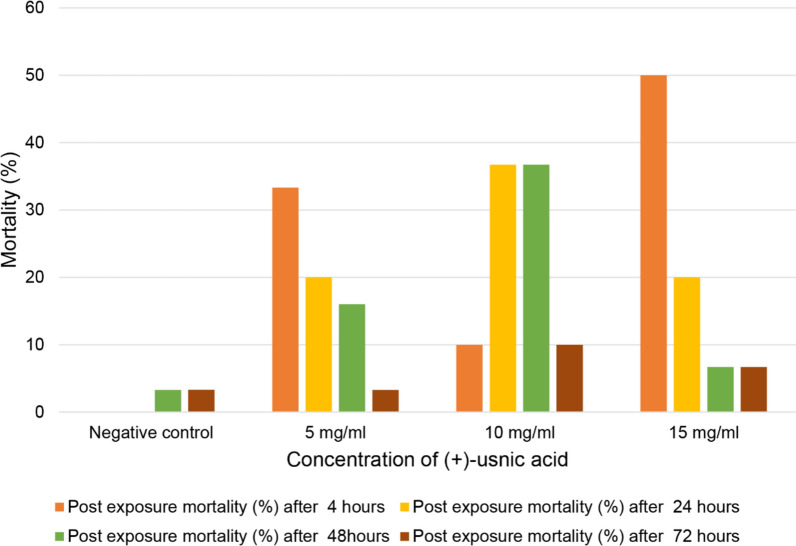
The total mortality of both male and female mosquitoes as a percentage was high after ingestion of the ( +)-UA toxic sugar bait (TSB). Higher mortality (50%) was observed at 15 mg/ml compared to 5 and 10 mg/ml within the first 4 h, however, the mortality rate declined over the next 24, 48 and 72 h (Fig. 2). This indicates that the extension of the exposure time has no significant difference in the mortality but there is a considerable difference when mortality was compared to the concentration of 5,10, and 15 mg/ml. Despite the decrease in mortality after 4 h of exposure, lower concentrations (5 and 10 mg/ml) caused mortality higher than 15 mg/ml after 24 h, this indicates that 15 mg/ml ( +)-UA caused higher acute toxicity. At 10 mg/ml, mortality was lower (10%) for the first 4 h and sustained at the same rate (36.7%) for the next 24 and 48 h (Fig. 2). In all the treatments, increased higher mortalities were observed for the first 4, 24 and 48 h. After 72 h, lower mortalities were observed compared to the control and the treatments.
Fig. 2.
Post-exposure mortalities (%) of Anopheles gambiae after 4, 24, 48, and 72 h in different concentrations of ( +)-UA
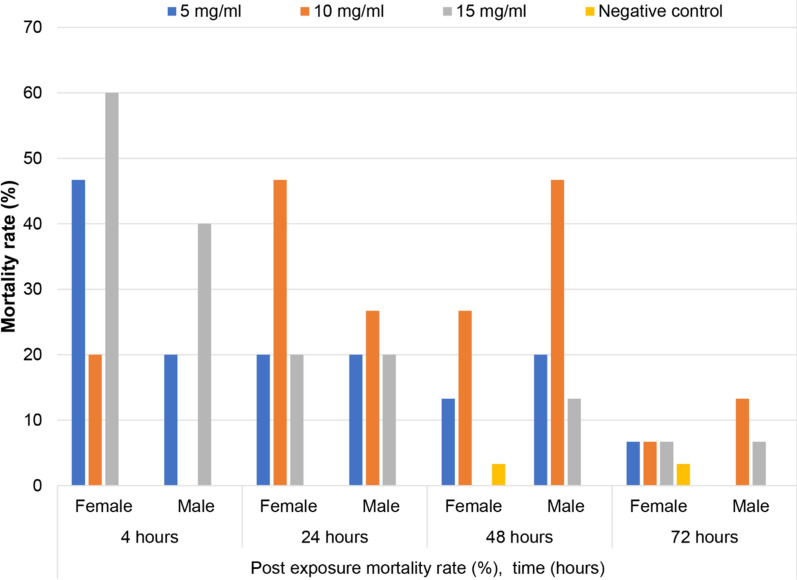
To determine the individual mortalities of both male and female target mosquitoes, the mortality percentage was considered between 4 to 72 h. Higher mortality of females was observed at 4 h (60%) while the males were 40% (Fig. 3). This indicates a higher proportion of intake of ( +)-UA toxic sugar bait for both males and females. Also, both males and females exhibited a considerable susceptibility to the ( +)-UA poison that was in the sugar as a result of a higher feeding rate. There was a high mortality rate at 5 mg/ml and 10 mg/ml for the first 4 h with equal proportions of death rate for both males and females. After 24 h, there were equal mortality rates at 5 mg/ml and 10 mg/ml for both males and females and a relatively higher death rate at 10 mg/ml for males after 48 h. After 72 h, there were significantly lower mortality rates for both males and females (0 to 13.3%). Higher mortality of males was observed after 48 h compared to females in all three treatments (Fig. 3).
Fig. 3.
Mortality (%) of both males and females Anopheles. gambiae after oral ingestion of ( +)-UA
Discussion
The sugar-seeking behaviour of mosquitoes has been demonstrated in many studies including recovery of specific types of sugar from plant sources and specific plant types[30, 31, 67]. This natural behaviour observed among male and female mosquitoes can be utilized as a point of vulnerability for their control by adding poison to the sugar bait. The success of this ATSB has been shown to reduce the mosquito population, biting rates, vectorial capacity and malaria prevalence. In Mali, there was approximately a 57% reduction in mosquito catch, an 89% reduction in entomological inoculation and a 30% reduction in malaria prevalence based on epidemiological modelling prediction on the ATSBs [25, 26, 33, 34, 68, 69].
In this study susceptibility of An. gambiae after oral administration of ( +)-UA was observed at different concentrations, there was high mortality among female mosquitoes the first 4 h (60%) (Fig. 3), although intake of the sugar bait was also high among males(40%). This indicates that both males and females readily took the sugar bait, hence the toxic agent is suitable as an ingredient of the TSBs against newly hatched male and female An. gambiae mosquitoes investigated in this study. When the total mortalities for male and female mosquitoes were evaluated, 50% mortality was observed after ingestion of 15 mg/ml ( +)-UA toxic sugar bait after the 4 h. Compared with other studies, 4% boric acid resulted in 100% mortality of Aedes aegypti and An. stephensi within 24 h in laboratory conditions. Studies by Allan et al. however confirmed a variation in susceptibility among males and females where female Culex quinquefasciatus was less susceptible [65, 70].
The application of deltamethrin as a toxic agent in ATSB demonstrated a significant toxicity effect on Ae. aegypti, with mortality rates ranging from 8.33% to 97.44% within 24 h of exposure [71]. A study to evaluate the effect of ATSB against Ae. aegypti by use of boric acid indicates that there was no significant difference in the proportion of uptake of the ATSB for both males and females and the mortality decreased after 24 h. However, females engorged more than males [72].
When Bti was used as an active ingredient in ATSB, mortality rates were higher after 48 h, 97% for Ae. aegypti, 98% for Aedes albopictus, and 100% for Cx. quinquefasciatus. This indicates that Bti has an optimum mortality effect after 24 h compared to synthetic insecticides when used as an oral poison [43]. However, a study to determine the toxicity of nano-formulated oral sugar bait on An. gambiae indicates that the use of nano-ATSB cypermethrin demonstrated a high and prolonged efficacy after 72 h of delayed mortality assessment [73]. Therefore, it implies that the application of nanotechnology using the various active ingredients that have insecticidal potential as an insecticide in ATSB may provide the desired prolonged efficacy and safety to the non-target arthropods. Also exposure to field collected Ae. albopictus to boric acid as ATSB for 48 h and later determination of delayed mortality of 93.3–100.0% for up to 7 days resulted in total mortality of both males and females [74]. The use of naturally occurring sugar (erythritol) as a toxic agent with or without another toxicant has been shown to be effective in increasing the mortality of adult Ae. aegypti by 90% within 72 h when mixed with other toxic agents like boric acid, Bti and spinosyn [41].
This study confirmed that the 10% sugar was capable of inducing feeding and sustaining uptake of the toxic agent as observed in the control experiments. This has also been confirmed in other studies to determine the impact of sugar concentrations on engorgement to the target mosquitoes where mosquitoes were more engorged regardless of the sugar concentration between 10 to 70%. However, high concentrations of sugar resulted in a high number of engorged Ae. aegypti [75].
This study also confirms that in the event that mosquitoes are knocked down faster, within the first 4 to 72 h, it would have the advantage of killing the mosquitoes before they can transmit the infectious agent. This claim agrees with a similar study where bacterial secondary metabolites (spinosyns) have been used as toxicants in TSBs. The impact has been shown to reduce vectorial capacity when the mean knockdown time of mosquitoes is lower [42].
Based on the promising results of the current new mosquito control method (ATSB), this study aimed at exploring the potential of ( +)-UA as oral toxic sugar bait to kill both male and female An. gambiae. It provides the knowledge that increasing concentrations of ( +)-UA acid have the potential to kill target mosquitoes from 4 to 72 h post-exposure and the degree of susceptibility of both male and female mosquitoes under laboratory conditions. The susceptibility of laboratory-reared An. gambiae to ( +)-UA as an oral insecticide via sugar bait has not been evaluated. This study demonstrated its promising potential as an effective oral insecticide.
Limitations
The experiments in this study were conducted under laboratory conditions, thus it did not use field-collected or resistant mosquitoes for bioassay experiments. Furthermore, the effects of ( +)-UA on non-target organisms were not determined. No further mortality recordings were performed after 72 h of exposure. No other sources of meal were provided to determine feeding preferences among male and female mosquitoes.
Conclusions
Laboratory experiment on the susceptibility of An. gambiae to 5, 10 and 15 mg/ml ( +)-UA when administered as oral sugar bait has first been demonstrated by this study. Mortality for both males and females is higher in the first 4 h of exposure and continues up to 72 h. Therefore, this study has confirmed that ( +)- UA can be used as an ingredient in the novel attractive toxic sugar bait for the control of malaria vectors in the current effort to search for new tools to reduce malaria transmission in African countries.
Acknowledgements
The authors wish to thank Johnson Onguka for insectary maintenance, and Joan Wanjiku and Diana Wagura for technical help. Abigael Onyango for quality control, and Polo Brian for corresponding with the ethics review team. National Commission for Science, Technology and Innovation for the permission to carry out this study.
Abbreviations
- ATSBs
Attractive toxic sugar baits
- Bti
Bacillus thuringiensis Var. israelensis
- IUPAC
International Union of Pure and Applied Chemistry
- SERU
Scientific and Ethics Review Unit
- TSBs
Toxic sugar Bait
- UA
Usnic acid
- WHO
World Health Organization
- NACOSTI
National Commission for Science, Technology and Innovation
Author contributions
The study protocol and laboratory bioassay procedures experiments were designed by A.M. who wrote the manuscript, E.O. supervised the experiments and guidance in drafting the study protocol, E.F. initiated the research concept, supervised the research and revised the manuscript, I.N. guided in the analytical work of the active ingredients, L.R. analysed the data. J.J. guided the bioassay experiments. All authors read and approved the final manuscript.
Funding
Laboratory work, purchase of ( +)-usnic acid and the cost of travelling were funded by Stipendium Hungaricum Scholarship (2020–2024) support. The procurement of reagents was funded by the National Research Development and Innovation Fund, grant number NKFI K 124341.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the scientific and ethics review unit of KEMRI protocol number SERU04-06–423/4610.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Fact sheet about malaria . Geneva: World Health Organization; 2023. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 19 Apr 2024.
- 2.Bass C, Williamson MS, Wilding CS, Donnelly MJ, Field LM. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malar J. 2007;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akpan GE, Adepoju KA, Oladosu OR, Adelabu SA. Dominant malaria vector species in Nigeria: modelling potential distribution of Anopheles gambiae sensu lato and its siblings with MaxEnt. PLoS ONE. 2018;13: e0204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiebe A, Longbottom J, Gleave K, Shearer FM, Sinka ME, Massey NC, et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar J. 2017;16:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tizifa TA, Kabaghe AN, McCann RS, van den Berg H, Van Vugt M, Phiri KS. Prevention efforts for malaria. Curr Trop Med Rep. 2018;5:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karunaratne SHPP, Surendran SN. Mosquito control: a review on the past, present and future strategies. J Natl Sci Found Sri Lanka. 2022;50:277. [Google Scholar]
- 8.Karunaratne P, De Silva P, Weeraratne T, Surendran N. Insecticide resistance in mosquitoes: development, mechanisms and monitoring. Ceylon J Sci. 2018;47:299. [Google Scholar]
- 9.Owuor KO, Machani MG, Mukabana WR, Munga SO, Yan G, Ochomo E, et al. Insecticide resistance status of indoor and outdoor resting malaria vectors in a highland and lowland site in Western Kenya. PLoS ONE. 2021;16: e0240771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machani MG, Ochomo E, Amimo F, Kosgei J, Munga S, Zhou G, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: implication on malaria vector control measures. PLoS ONE. 2020;15: e0224718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odero JI, Abong’o B, Moshi V, Ekodir S, Harvey SA, Ochomo E, et al. Early morning anopheline mosquito biting, a potential driver of malaria transmission in Busia County, western Kenya. Malar J. 2024;23:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kripa PK, Thanzeen PS, Jaganathasamy N, Ravishankaran S, Anvikar AR, Eapen A. Impact of climate change on temperature variations and extrinsic incubation period of malaria parasites in Chennai, India: implications for its disease transmission potential. Parasit Vectors. 2024;17:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nganga PN, Aduogo P, Mutero CM. Long lasting insecticidal mosquito nets (LLINs) ownership, use and coverage following mass distribution campaign in Lake Victoria basin, Western Kenya. BMC Public Health. 2021;21:1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay SW, Thomas MB, Kleinschmidt I. Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob Health. 2021;9:e1325–31. [DOI] [PubMed] [Google Scholar]
- 15.Antinori S, Bonazzetti C, Giacomelli A, Corbellino M, Galli M, Parravicini C, et al. Non-human primate and human malaria: past, present and future. J Travel Med. 2021;28:taab036. [DOI] [PubMed] [Google Scholar]
- 16.Ochomo E, Milanoi S, Abongo B, Onyango B, Muchoki M, Omoke D, et al. Molecular surveillance leads to the first detection of Anopheles stephensi in Kenya. Res Sq. 2023;139:39. [Google Scholar]
- 17.Badmos AO, Alaran AJ, Adebisi YA, Bouaddi O, Onibon Z, Dada A, et al. What sub-Saharan African countries can learn from malaria elimination in China. Trop Med Health. 2021;49:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sougoufara S, Ottih EC, Tripet F. The need for new vector control approaches targeting outdoor biting anopheline malaria vector communities. Parasit Vector. 2020;13:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015. [Google Scholar]
- 20.Sippy R, Rivera GE, Sanchez V, Heras F, Morejón B, Beltrán E, et al. Ingested insecticide to control Aedes aegypti: developing a novel dried attractive toxic sugar bait device for intra-domiciliary control. Parasit Vectors. 2020;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esenther GR, Beal RH. Attractant-mirex bait suppresses activity of reticulitermes spp. J Econom Entomol. 1974;67:85–8. [Google Scholar]
- 22.Xue RD, Barnard DR. Boric acid bait kills adult mosquitoes (Diptera: Culicidae). J Econom Entomol. 2003;96:1559–62. [DOI] [PubMed] [Google Scholar]
- 23.Lewis DJ, Domoney CR. Sugar meals in Phlebotominae and Simuliidae (Diptera). Proc R Entomol Soc Lond. 1966;41:175–9. [Google Scholar]
- 24.Müller G, Junnila A, Schlein Y. Effective control of adult Culex pipiens by spraying an attractive toxic sugar bait solution in the vegetation near larval habitats. J Med Entomol. 2010;47:63–6. [DOI] [PubMed] [Google Scholar]
- 25.Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–74. [DOI] [PubMed] [Google Scholar]
- 27.Burgin SG, Hunter FF. Sugar-meal sources used by female black flies (Diptera: Simuliidae): a four-habitat study. Can J Zool. 1997;75:1066–72. [Google Scholar]
- 28.Ganie SY, Javaid D, Hajam YA, Reshi MS. Arsenic toxicity: sources, pathophysiology and mechanism. Toxicol Res. 2024;13:tfad111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone CM, Hamilton IM, Foster WA. A survival and reproduction trade-off is resolved in accordance with resource availability by virgin female mosquitoes. Anim Behav. 2011;81:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, et al. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J. 2007;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omondi S, Kosgei J, Agumba S, Polo B, Yalla N, Moshi V, et al. Natural sugar feeding rates of Anopheles mosquitoes collected by different methods in western Kenya. Sci Rep. 2022;12:20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Sharma A, Samal RR, Kumar M, Verma V, Sagar RK, et al. Laboratory evaluation of the efficacy of deltamethrin-laced attractive toxic sugar bait formulation on Anopheles stephensi. Malar J. 2023;22:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qualls WA, Müller GC, Traore SF, Traore MM, Arheart KL, Doumbia S, et al. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar J. 2015;14:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diarra RA, Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar J. 2021;20:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenywa FC, Kambagha A, Saddler A, Maia MF. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar J. 2017;16:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revay EE, Schlein Y, Tsabari O, Kravchenko V, Qualls W, De-Xue R, et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015;150:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser KJ, Mwandigha L, Traore SF, Traore MM, Doumbia S, Junnila A, et al. Estimating the potential impact of attractive targeted sugar baits (ATSBs) as a new vector control tool for Plasmodium falciparum malaria. Malar J. 2021;20:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezende-Teixeira P, Dusi RG, Jimenez PC, Espindola LS, Costa-Lotufo LV. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ Pollut. 2022;300: 118983. [DOI] [PubMed] [Google Scholar]
- 39.Junnila A, Revay EE, Müller GC, Kravchenko V, Qualls WA, de Xue R, et al. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015;152:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDermott EG, Morris EK, Garver LS. Sodium ascorbate as a potential toxicant in attractive sugar baits for control of adult mosquitoes (Diptera: Culicidae) and sand flies (Diptera: Psychodidae). J Med Entomol. 2019;56:1359–67. [DOI] [PubMed] [Google Scholar]
- 41.Baker KA, White GS, Faraji A, Bibbs CS. Enhancing toxic sugar meals against Aedes aegypti (Diptera: Culicidae) by adulterating with erythritol in combination with other active ingredients. J Med Entomol. 2023;60:833–6. [DOI] [PubMed] [Google Scholar]
- 42.Alomar AA, Alto BW, Walker ED. Spinosyns delivered in sugar meals to Aedes aegypti and Aedes albopictus (Diptera: Culicidae): acute toxicity and subacute effects on survival, fecundity, and fertility. J Med Entomol. 2022;59:623–30. [DOI] [PubMed] [Google Scholar]
- 43.Davis J, Bibbs CS, Müller GC, Xue RD. Evaluation of Bacillus thuringiensis israelensis as toxic sugar bait against adult Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes. J Vector Ecol. 2021;46:30–3. [DOI] [PubMed] [Google Scholar]
- 44.Hawksworth DL, Grube M. Lichens redefined as complex ecosystems. New Phytol. 2020;227:1281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders WB. The disadvantages of current proposals to redefine lichens. New Phytol. 2024;241:969–71. [DOI] [PubMed] [Google Scholar]
- 46.Farkas E, Xu M, Muhoro A, Szabó K, Lengyel A, Heiðmarsson S, et al. The algal partnership is associated with quantitative variation of lichen specific metabolites in Cladonia foliacea from Central and Southern Europe. Symbiosis. 2024;92:1–17. [Google Scholar]
- 47.Farkas E, Biró B, Szabó K, Veres K, Csintalan Z, Engel R. The amount of lichen secondary metabolites in Cladonia foliacea (Cladoniaceae, lichenised Ascomycota). Acta Bot Hung. 2020;62:33–48. [Google Scholar]
- 48.Muhoro AM, Farkas EÉ. Insecticidal and antiprotozoal properties of lichen secondary metabolites on insect vectors and their transmitted protozoal diseases to humans. Diversity. 2021;13:342. [Google Scholar]
- 49.Molnár K, Farkas E. Current results on biological activities of lichen secondary metabolites: a review. Z Naturfr C J Biosci. 2010;65:157–73. [DOI] [PubMed] [Google Scholar]
- 50.Stocker-Wörgötter E. Metabolic diversity of lichen-forming ascomycetous fungi: culturing, polyketide and shikimate metabolites production, and PKS genes. Nat Prod Rep. 2008;25:188–200. [DOI] [PubMed] [Google Scholar]
- 51.Emsen B, Yildirim E, Aslan A, Anar M, Ercisli S. Insecticidal effect of the extracts of Cladoniafoliacea (Huds.) Willd. and Flavoparmeliacaperata (L.) Hale against adults of the grain weevil, Sitophilusgranarius (L.) (Coleoptera Curculionidae). Egypt J Pest Control. 2012;22:145–9. [Google Scholar]
- 52.Koc S, Tufan Cetin O, Candan M, Turk A, Cetin H. Larvicidal activity of lichen secondary metabolites atranorin and (–)-usnic acid against the yellow fever mosquito Aedesaegypti. Fresenius Environ Bull. 2021;30:11938–41. [Google Scholar]
- 53.Araújo AAS, de Melo MGD, Rabelo TK, Nunes PS, Santos SL, Serafini MR, et al. Review of the biological properties and toxicity of usnic acid. Nat Prod Res. 2015;2(29):2167–80. [DOI] [PubMed] [Google Scholar]
- 54.Cocchietto M, Skert N, Nimis PL, Sava G. A review on usnic acid, an interesting natural compound. Naturwissenschaften. 2002;89:137–46. [DOI] [PubMed] [Google Scholar]
- 55.Vinayaka KS, Krishnamurthy YL, Prashith Kekuda TR, Praveen Kumar SV, Sudharshan SJ, Chinmaya A. Larvicidal and wormicidal efficacy of methanolic extracts of five macrolichens collected from Bhadra wildlife sanctuary. Biomedicine (India). 2009;29:327–31. [Google Scholar]
- 56.Galanty A, Paśko P, Podolak I. Enantioselective activity of usnic acid: a comprehensive review and future perspectives. Phytochem Rev. 2019;18:527–48. [Google Scholar]
- 57.Kinoshita Y, Yamamoto Y, Yoshimura I, Kurokawa T, Huneck S. Distribution of optical isomers of usnic and isousnic acids analyzed by high performance liquid chromatography. J Hattori Bot Lab. 1997;83:173–8. [Google Scholar]
- 58.Oxborough R, N’Guessan R, Kitau J, Ngufor C, Malone D, Mosha F, et al. The activity of the pyrrole insecticide chlorfenapyr in mosquito bioassay: towards a more rational testing and screening of non-neurotoxic insecticides for malaria vector control. Malar J. 2015;14:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munywoki DN, Kokwaro ED, Mwangangi JM, Muturi EJ, Mbogo CM. Insecticide resistance status in Anophelesgambiae (s.l.) in coastal Kenya. Parasit Vectors. 2021;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin JQ, Rao Y, Bian XL, Zeng AG, Yang GD. Solubility of (+)-usnic acid in water, ethanol, acetone, ethyl acetate and n-hexane. J Solut Chem. 2013;42:1018–27. [Google Scholar]
- 61.Calcott MJ, Ackerley DF, Knight A, Keyzers RA, Owen JG. Secondary metabolism in the lichen symbiosis. Chem Soc Rev. 2018;47:1730–60. [DOI] [PubMed] [Google Scholar]
- 62.Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. London: Yale University Press; 2001. p. 828. [Google Scholar]
- 63.Smith CW. The Lichens of Great Britain and Ireland. London: British Lichen Society; 2009. p. 1064. [Google Scholar]
- 64.(+)-Usnic acid phyproof® reference substance, PhytoLab. 2024. https://phyproof.phytolab.com/en/reference-substances/details/usnic-acid-89818. Accessed 7 Apr 2024.
- 65.Allan SA. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. J Vector Ecol. 2011;36:59–67. [DOI] [PubMed] [Google Scholar]
- 66.Stewart ZP, Oxborough RM, Tungu PK, Kirby MJ, Rowland MW, Irish SR. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS ONE. 2013;8: e84168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brantjes NBM, Leemans JA. Silene otites (Caryophyllaceae) pollinated by nocturnal Lepidoptera and mosquitoes. Acta Bot Neerl. 1976;25:281–95. [Google Scholar]
- 68.Traore M, Junnila A, Sekou FT, Doumbia S, Revay E, Kravchenko V, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar J. 2020;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar J. 2012;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar G, Sharma A, Dhiman R. Laboratory evaluation of the efficacy of boric acid containing toxic sugar baits against Anophelesculicifacies, An. stephensi and Aedesaegypti mosquitoes. J Vector Borne Dis. 2022;59:52. [DOI] [PubMed] [Google Scholar]
- 71.Kumar S, Sharma A, Samal RR, Verma V, Sagar RK, Singh SP, et al. Development of deltamethrin-laced attractive toxic sugar bait to control Aedes aegypti (Linnaeus) population. J Trop Med. 2024;2024:6966205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barbosa DS, Rodrigues MMS, Silva AAE. Evaluation of attractive toxic sugar baits (ATSB) against Aedes aegypti (Diptera: Culicidae) in laboratory. Trop Biomed. 2019;36:578–86. [PubMed] [Google Scholar]
- 73.Farhan M, Zhao C, Akhtar S, Ahmad I, Jilong P, Zhang S. Assessment of nano-formulated conventional insecticide-treated sugar baits on mosquito control and the effect on non-target aphidophagous Coccinella septempunctata. Insects. 2024;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiu MC, Neoh KB, Hwang SY. The effect of attractive toxic sugar bait on the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) in community farms in Northern Taiwan. Acta Trop. 2024;250: 107102. [DOI] [PubMed] [Google Scholar]
- 75.Dias ACA, Teixeira AV, Lima Bezerra F, Andriolo A, de Silva AA. Sugar bait composition containing ivermectin affect engorgement and mortality of the mosquito Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2023;60:159–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.