Abstract
Background
Rib fractures are one of the most common traumatic injuries and may result in significant morbidity and mortality. Despite growing evidence, technological advances and increasing acceptance, surgical stabilization of rib fractures (SSRF) remains not uniformly considered in trauma centers. Indications, contraindications, appropriate timing, surgical approaches and utilized implants are part of an ongoing debate. The present position paper, which is endorsed by the World Society of Emergency Surgery (WSES), and supported by the Chest Wall Injury Society, aims to provide a review of the literature investigating the use of SSRF in rib fracture management to develop graded position statements, providing an updated guide and reference for SSRF.
Methods
This position paper was developed according to the WSES methodology. A steering committee performed the literature review and drafted the position paper. An international panel of experts then critically revised the manuscript and discussed it in detail, to develop a consensus on the position statements.
Results
A total of 287 studies (systematic reviews, randomized clinical trial, prospective and retrospective comparative studies, case series, original articles) have been selected from an initial pool of 9928 studies. Thirty-nine graded position statements were put forward to address eight crucial aspects of SSRF: surgical indications, contraindications, optimal timing of surgery, preoperative imaging evaluation, rib fracture sites for surgical fixation, management of concurrent thoracic injuries, surgical approach, stabilization methods and material selection.
Conclusion
This consensus document addresses the key focus questions on surgical treatment of rib fractures. The expert recommendations clarify current evidences on SSRF indications, timing, operative planning, approaches and techniques, with the aim to guide clinicians in optimizing the management of rib fractures, to improve patient outcomes and direct future research.
Keywords: Rib fractures, Surgical stabilization of rib fractures (SSRF), Flail chest, Multiple rib fractures, Thoracic/chest trauma injury, Rib fixation, Operative reduction and internal fixation (ORIF), Minimally invasive plating osteosynthesis (MIPO), Video-assisted thoracoscopy surgery (VATS), Consensus, Guidelines
Background
The majority of trauma patients endure a thoracic injury; of these, rib fractures are the most common ones [1, 2]. Approximately one third of middle-aged or elderly trauma patients sustain rib fractures, mainly following high-energy blunt trauma. Rib fractures are slightly less prevalent in younger patients, who are more prone to injuries in intrathoracic organs [3].
Patients sustaining thoracic trauma carry increased risk of mortality and morbidity, which correlates with the presence of flail chest, multiple rib fractures, as well as the presence of intrathoracic injuries [4]. The underlying lung contusion can lead to respiratory compromise, but splinting, shallow breathing, and poor cough, all due to pain, results in both atelectasis and secretion accumulation, ultimately leading to respiratory failure [1, 5].
Standard treatment of severe chest wall injuries includes nonoperative management (NOM) via multimodal analgesia, pulmonary hygiene, chest physiotherapy, pleural drainage as needed, and in severe cases, intubation and mechanical ventilation [6]. However, especially in the presence of flail chest or multiple and displaced rib fractures, the duration of mechanical ventilation may be prolonged, with increased rates of pneumonia, sepsis, tracheostomy, barotrauma and protracted intensive care unit (ICU) stay [7–9]. Furthermore, morbidity, health care resource utilization and hospital costs, remain significantly high regardless of the rib fracture pattern [10]. Even isolated rib fractures can be associated with functional impairment, chronic pain, significant loss of work days and suboptimal quality-of-life (QoL) [11–14].
Given the prevalence and related costs, an increasing interest in improving outcomes of rib fracture patients through surgical stabilization of rib fractures (SSRF) has occurred. Despite recognizing the potential benefits of surgical fixation in appropriate cases, operative management was described as an underused treatment at the beginning of the last decade [15].
Since then, several studies have refined the potential indications and contraindications of SSRF, accompanied by a progressive increase in the surgical experience and SSRF techniques including muscle sparing, minimally-invasive and intra-thoracic approaches in dedicated centers. Technological advances in hardware design and imaging evaluation of rib fractures have also contributed to improvements in preoperative planning [16–18].
Project rationale and design
This position paper is supported by the World Society of Emergency Surgery (WSES) and the Chest Wall Injury Society (CWIS) and aims to provide a systematic review of the literature investigating the surgical management of rib fractures in the emergency setting, to develop position statements based on the currently best available evidence and practice. For this purpose, the organizing committee constituted a steering committee, that had the task of drafting the present position paper, and an international expert panel composed of experts who were asked to revise the manuscript and position statements critically. The position paper was developed according to the WSES methodology [19]. We shall present the systematic review of the literature and provide the derived statements upon which a consensus was reached, specifying the quality of the supporting evidence and suggesting future research directions.
Purpose and use of these guidelines
These guidelines are evidence-based, with the grades of recommendation based on the evidence. They do not represent the standard of practice, but are suggested plans of care, based on best available evidence and a consensus of experts. They do not exclude other approaches as being within a standard of practice. The treating clinician should determine the most appropriate action, after taking into account conditions at the relevant medical institution (staff levels, experience, equipment, etc.) and the characteristics of the individual patient. The responsibility for the management and outcome rests with the engaging practitioners, and not the consensus group. Furthermore, we recognize that the SSRF literature is continually evolving, and these guidelines represent the state of the art at the current time, subject to modifications as new research becomes available.
Methods
Review questions, search strategy, and selection criteria
The systematic review of the literature was performed following the Cochrane Collaboration specific protocol [20], and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. Studies on SSRF in the management of rib fractures following chest trauma were retrieved from the following databases on January 2, 2024: MEDLINE (through PubMed), Embase, and the Cochrane Library.
The focus questions were the following:
What are the indications for SSRF?
What are the contraindications to SSRF?
What is the optimal timing of SSRF?
What is the optimal preoperative imaging evaluation?
Which ribs fracture sites are amenable for SSRF?
How to manage concurrent intrathoracic and chest wall injuries?
Which are the effective surgical approaches and techniques?
Which stabilization methods and materials are used?
A specific research query was formulated for each database, using the following keywords and MeSH terms: traumatic rib fractures; surgical stabilization of rib fractures (SSRF); non operative management (NOM); flail chest; multiple rib fractures; thoracic/chest trauma injury; rib fixation; rib plating; chest wall stabilization; operative reduction and internal fixation (ORIF); minimally invasive plating osteosynthesis (MIPO); video-assisted thoracoscopy surgery (VATS).Terms were variously combined, with the use of the Boolean operators “AND” and “OR”. An effort was made to account for plurals, synonyms and acronyms. The research was limited to studies published in English.
According to the PICOS format, the following items were used as selection criteria for articles emerging from the literature search:
P, population: adult trauma patients with rib fractures with/without flail chest, with/without sternum involvement, requiring surgery in emergent/urgent settings.
I, intervention: clearly reported surgical treatment performed (preoperative imaging, indications, criteria adopted for adequate stabilization, timing, approach, materials, technique).
C, comparison: operative versus nonoperative management
O, outcomes: postoperative outcomes, morbidity, mortality.
S, study design: systematic review of randomized clinical trial (RCT), RCT, systematic review of cohort studies, individual cohort studies, systematic review of case–control studies, individual case–control studies, case series, guidelines/consensus, expert opinion/survey, original articles and case report. The process of screening, selection, and coding of studies in this systematic review was supported by the use of Rayyan (http://rayyan.qcri.org), an AI Powered web-Tool for Systematic Literature Reviews that uses tagging and filtering to code and organize references.
Four reviewers (GS, RB, KH and BT) screened the list of articles. All records were reviewed for relevance concerning the title and abstract to ensure the quality and relevance of the literature included in the review. Records were removed when both reviewers excluded them. Otherwise, the disagreement was resolved via a discussion/ intervention of a tiebreaker (FC). Reviewers then performed an independent full-text analysis, which allowed them to include or exclude the preselected article.
Data extraction and synthesis
Data extraction and synthesis were performed by filling an electronic spreadsheet, which included the following items: first author’s name, year of publication, scientific journal, study type (or study design), number of patients included, disease requiring surgical intervention, type of surgical intervention, surgical approach, operative and postoperative surgical outcomes, cost analysis data (when available). The risk of bias in the selected studies was assessed by using validated systems according to the study design [22–24].
Results
Literature search and selection
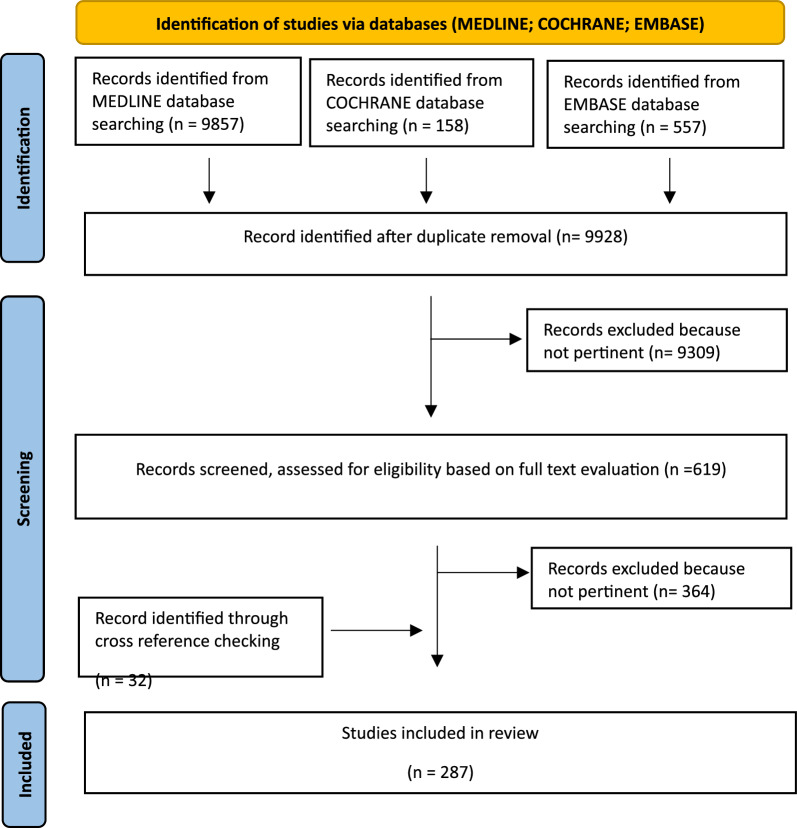
The initial search yielded 10.572 results (PubMed 9857, Cochrane 158, Embase 557). After removing duplicates, 9928 articles were screened for eligibility based on title and abstract, and 619 articles were retrieved for a full-text evaluation. After excluding 364 non-pertinent articles, a total of 287 studies were finally included in the review, including 32 articles identified through cross-reference checking (Fig. 1).
Fig. 1.
Shows PRISMA flow chart of Review paper
Position statements
Following a comprehensive literature review and the summary of current scientific evidence of SSRF, for each of the focus question, the following position statements (PS) were created. For each PS, the supporting literature, the level of evidence, and the strength of the consensus are reported. The level of evidence (LoE) is classified according to the GRADE system (https://training.cochrane.org/introduction-grade). For each statement, the consensus was assessed through a web survey open to all members of the steering committee and panel of experts and to the members of the Board of Governors of the WSES. If a statement reached < 75% of agreement, it was rediscussed, modified, and resubmitted to the experts’ vote until a consensus was reached.
SSRF indications
-
PS 1.1
SSRF should be considered in all flail chest (FC) patients (LoE IIa, Grade B).
-
PS 1.2SSRF should be considered in non-FC patients with rib fractures, in the presence of:
- Multiple (≥ 3) ipsilateral severely displaced rib fractures (LoE IIb, Grade B);
- Multiple (≥ 3) ipsilateral displaced, rib fractures in ribs 3–10 in combination with respiratory failure despite mechanical ventilation or weaning failure or at least two pulmonary derangements in non-ventilated patients despite loco-regional anesthesia and multimodal analgesia: (i) respiratory rate > 20 breaths per minute, (ii) incentive spirometry < 50% predicted, (iii) numeric pain score (NPS) > 5/10, and iv) poor cough (LoE IIb, Grade B)
- A chest wall deformity that significantly affects lung function (i.e. implosion chest wall injuries, “Stoved-in Chest”) or that shows mechanical instability on palpation (LoE IIb, Grade B);
- A flail segment, especially if antero-lateral and with some degree of displacement (LoE IIIa, Grade B);
- Severe pain non-responsive to other treatments (LoE IV, Grade C);
-
PS 1.3
SSRF is optimal in dedicated centers with a multidisciplinary team, developing and optimizing protocols addressing NOM and SSRF for rib fractures patients (LoE V, Grade D);
Flail chest (FC)
A flail chest (FC) is a clinical finding, characterized by the paradoxical movement of a flail segment with respirations, whereas a flail segment is a radiographic finding, defined as a contiguous segment of 3 or more consecutive ribs fractured at 2 or more places. This distinction was recently defined and not universally known and adopted among all clinicians, in both the research and clinical practice and these has led to misunderstandings about optimal patient management [25, 26].
Trauma patients with FC have an increased risk of respiratory failure and mortality [27]. The treatment of FC has evolved over the past half-century, with early strategies using chest wall traction and external stabilization methods, later abandoned in favor of internal pneumatic splinting using positive pressure mechanical ventilation [28]. Several authors subsequentially reported excellent results of SSRF, that has been practiced sporadically for decades and almost exclusively in patients with flail chest [29]. Randomized clinical trials (RCT) comparing SSRF with non-operative management (NOM) [30–32] found lower incidence of tracheostomies, pneumonia, respiratory failure and shorter mechanical ventilation and intensive care unit (ICU) stay in FC patients undergoing SSRF. Furthermore, significantly less restrictive impairments of pulmonary function were reported in the operative groups at one [30] and two months of follow-up avoiding chest wall deformity [31]. Moreover, the RCT by Tanaka et al. [30], documented a lower total medical expense per patient treated operatively and a faster return to work. Persistent chest tightness, thoracic cage pain, and dyspnea on effort were significantly more frequent complaints in patients in the internal pneumatic stabilization group at twelve months of follow-up.
Despite the added cost of surgery, Bhatnagar et al. showed that SSRF remained the most cost-effective for FC patients. The reduction in pneumonia rates, ventilator days and hospital length of stay resulted in an overall reduction in cost and improved effectiveness compared with internal pneumatic stabilization [33].
The improvement in pulmonary function tests and lower thoracic deformity and pain were confirmed in FC patients undergoing SSRF in some prospective studies [34, 35] and in an RCT by Liu et al. [36], which demonstrated lower morbidity and decreased risk of acute respiratory distress syndrome (ARDS) respect to non-operative strategies. However, in the presence of concomitant severe pulmonary contusion (PC) a shorter mechanical ventilator time and ICU stay were not observed in the operative group. SSRF for FC appeared to be most beneficial in patients with anterolateral FC and respiratory failure without severe PC, in patients with PC and persistent chest wall instability or weaning failure and non-intubated patients with deteriorating pulmonary function [37].
Four metanalyses [38–41] have demonstrated benefits of SSRF in reducing the duration of mechanical ventilation, pneumonia/ tracheostomy rates, ICU stay and costs compared to NOM. Mortality rates and total hospital stay were also significantly lower in the surgical groups, besides other outcomes mentioned above, in some metanalysis, case–control and prospective cohort studies [42–44], confirming that SSRF in FC patients results in improved clinical outcomes and is a cost-effective intervention. The latest guidelines and consensus papers regarding surgical treatment of traumatic rib fractures state that SSRF should be performed in patients with FC [45–49].
Non-FC rib fractures
The vast majority of patients with severe chest wall injuries do not have a FC. Accordingly, limiting SSRF to FC patients will address only the great minority of severe chest wall injuries. The number and displacement of fractures are recognized predictors of mortality and pulmonary complications, such as pneumonia, ARDS and need for tracheostomy in thoracic trauma patients, independent of the presence of FC [50–53]. Evidence regarding the effectiveness of SSRF in patients with some non-FC rib fractures has progressively emerged. However, definitions and injury classification are unclear and inconsistent between clinical trials. The heterogeneity of inclusion criteria and taxonomy of rib fractures, especially regarding the degree of displacement, makes it difficult to draw conclusions [25].
An interdisciplinary collaboration between the Chest Wall Injury Society (CWIS) and the American Society of Emergency Radiology (ASER) recently addressed chest wall injury nomenclature. Non-displaced ribs were defined as having ≥ 90% cross-sectional overlap, offset rib fractures < 90% cross-sectional overlap, and displaced rib fractures have no cross-sectional overlap. The term “cross-sectional overlap” is easy to understand and provides better correlation of the degree of displacement accounting for both the cortical and cancellous bone on axial and multiplanar reformats of the chest CT [26].
Considering the available evidence, which is presented below, we think that the following rib fracture displacement classification can further improve communication between health care providers and help to direct future research on chest wall injuries:
Non-displaced: > 90% cross-sectional overlap
Offset: 50–90% cross-sectional overlap,
Displaced: > 0 to < 50% cross-sectional overlap
Severely displaced: no cross-sectional overlap or overlapping ribs
In a recent retrospective analysis [54] and two prospective studies comparing SSRF to NOM in patients with non-FC chest wall injuries [55, 56], the authors concluded that rib fixation did not appear to provide benefits in short and long-term outcomes (QoL or pain up to 6 months). However, the only inclusion criteria in the first prospective cohort study was the presence of “three or more ipsilateral rib fractures”. No degree of displacement or baseline pulmonary physiologic derangements or pain were required for inclusion in the study and these variables were not even reported in the results. Regarding the second prospective study, approximately half of the patients enrolled had displaced fractures, and had a median of one fracture with an unreported degree of displacement. These studies underscore the importance of patient selection for SSRF.
Pieracci et al. included a wide variety of fracture patterns, with the most common being three or more severely displaced fracture (defined as bicortical displacement), in a 2-year prospective controlled clinical evaluation of chest trauma patients [57]. SSRF, as compared with the best medical management, was independently associated with a 76% decreased likelihood of respiratory failure and an 82% decreased likelihood of tracheostomy as well as 5-day decreased duration of mechanical ventilation and significantly improved spirometry readings among extubated patients. Additional inclusion criteria of the study were the presence of a flail segment, ≥ 30% volume loss of the affected hemithorax, or severe pain/respiratory failure despite optimal medical management.
An RCT by Wu et al. [58] including trauma patients with at least three displaced rib fractures (defined as frame fracture dislocation > 50%) confirmed the benefits of SSRF, compared to NOM, in decreasing acute chest pain, reducing the duration of mechanical ventilation, lowering the incidence of pneumonia, shortening the ICU and total hospital length of stay, also alleviating the forward chest wall discomfort.
In a recent multicenter prospective RCT, Denghan et al. [59], randomly assigned 207 patients with unstable chest wall injuries, defined by the presence of: ≥ 3 fractures with a severe displacement (defined as > 100%) or overriding (by minimum 15 mm each) or protrusion into lung parenchyma, to undergo operative or nonoperative management. Additionally, patients with a flail segment (with at least 3 of the rib fractures involved with some degree of displacement.), or ≥ 25% hemithorax volume loss were also considered as having unstable chest wall injuries and were included in the study. SSRF was associated with decreased mortality (0% vs. 6%; p = 0.01), and improvement in ventilator-free days and decreased length of hospitalization in the subgroup of patients who were undergoing mechanical ventilation at the time of randomization. However, only small benefits from surgical intervention were documented in non-ventilated patients, which constitute a much larger cohort of patients seen in trauma centers. Evidence regarding the benefits of surgical intervention in non–ventilated patients without FC were not described. The authors did not report the results of their analysis on meaningful outcomes in these patients such as pleural space complications, chronic pain and disability. Additionally, no mention was made about detail of the surgical approach and the time from injury to fixation. Of note, in the majority of patients, pelvic fixation plates were utilized for stabilization of fractured ribs, a surgical option that has been abandoned in SSRF.
However, Pieracci et al. [60], in a multicenter prospective clinical trial including non-FC, non-ventilator dependent trauma patients with ≥ 3 displaced rib fractures, found that SSRF, compared to NOM, was associated with lower pleural space complication rates, lower pain scores/respiratory disability and improved quality of life at two week follow up. The patients who were included had at least 3 ipsilateral, but not necessarily consecutive, displaced fractures of ribs 3–10 (displacement ≥ 50% of rib width measured on axial CT chest imaging) without flail segment (defined as ≥ 2 consecutive ribs each fractured in ≥ 2 places on CT chest). In addition, at least 2 of the following pulmonary derangements measured after initiation of loco-regional anesthesia were present: (i) respiratory rate > 20 breaths per minute, (ii) incentive spirometry < 50% predicted, (iii) numeric pain score (NPS) > 5/10, and (iv) poor cough. Therefore, these patients with persistent pain despite medical treatment and respiratory impairment should be considered for SSRF. The use of pain severity score as a criterion for fixation remains a matter of debate due to its subjectivity and variability from patient to patient [61], but rib fracture long-term morbidity is well documented in patients with chronic pain, deformity, respiratory compromise, reduced QoL for up to 2 years postinjury and associated poor return to work rate [14, 62].
Marasco et al. in a recent RCT [63] compared SSRF and NOM in non–ventilated trauma patients with at least 3 painful or displaced rib fractures. The authors were unable to document improvements in pain or QoL at 3 and 6 months after SSRF. However, return-to-work rates improved between 3 and 6 months, favoring the operative group. Other case series and retrospective studies have shown improvements in long term pain and QoL of patients who underwent SSRF [64–67].
In a prospective single center study involving 118 patients that had almost exclusively two or three rib fractures, Khandelwal et al. [68] stratified the management of all patients based on pain severity. Patients with mild to moderate pain received NOM, while patients with severe pain underwent SSRF. The result showed that SSRF reduced pain to a greater extent and shortened the time of disability and the time to return to work.
Several systematic reviews and meta-analyses have attempted to quantify the benefits of multiple rib fractures fixation compared to NOM. Most of these meta-analyses also examined a significant number of patients with flail injuries [69–75], but in many cases it was unclear whether it was a radiological or clinical flail, making results difficult to interpret. However, all meta-analyses found decreased pneumonia rates, reduced ICU length of stay, decreased duration of mechanical ventilation [69, 71–75], lower total hospital days [69, 71–73], decreased mortality [69–71, 73, 74], decreased tracheostomy rate [69–74], less chest wall deformity [69, 72] and less dyspnea [69] following SSRF.
A meta-analysis by Wijffels et al. [76] excluding studies in which the proportion of patients with FC or flail segment was > 50%, found a significant reduction in pneumonia rates, mortality, and hospital length of stay in patients who underwent SSRF compared to NOM, even without effects on the duration of mechanical ventilation and ICU stay.
A recent metanalysis included only studies focused on the management of patients with non-FC fracture patterns in the Asian population [77]. He et al. found that patients who underwent SSRF had a shorter duration of mechanical ventilation, ICU and hospital length of stay and lower risk of atelectasis and pneumonia with better pain scores at 4 weeks, although no clear mortality benefit was reported.
There are a number of retrospective and prospective observational studies that also support other indications for SSRF, including symptomatic non-union [78–81] and thoracotomy for other reasons such as retained hemothorax [82, 83].
Despite the advantages of SSRF and its growing popularity, it remains not uniformly considered in trauma centers [84]. The relationship between center-specific SSRF volume and patient-level outcomes has been debated due to contradictory evidence [85, 86]. Recently Tilman et al. [87] found that center-specific chest wall stabilization (CWS) volume is associated with better in-hospital patient outcomes. An optimal cut point of 12.5 procedures annually was used to define high- and low-volume centers. Patients treated at high-volume centers experienced significantly lower rates of in-hospital mortality, deep venous thrombosis with shorter lengths of stay. Centers that frequently perform CWS have adopted protocols for chest wall injury that focus on pulmonary and pain control, as well as implementing institutional policies, which have been associated with improved outcomes [88, 89]. These findings support efforts to establish chest wall injury centers of excellence [90] lay the foundation to improve patient outcomes.
-
2.
SSRF contraindications
-
PS 2.1
Patients who are hemodynamically unstable should not undergo SSRF (LoE V, Grade D).
-
PS 2.2
Traumatic brain injury (TBI) is not an absolute contraindication to SSRF. Patients with moderate to severe TBI, in presence of some prospect for neurological recovery, may benefit from the SSRF protective effects on pneumonia development and weaning from the ventilator, need for tracheostomy tube and mortality. Patients with TBI should be evaluated for SSRF involving a multidisciplinary team on an individual basis (LoE IIIb, Grade B).
-
PS 2.3
Lower spinal injury resulting in paraplegia should not be considered a contraindication to SSRF; patients may benefit from SSRF given that they still have intact sensation in the chest wall and likely did not need tracheostomy (LoE V, Grade D).
-
PS 2.4
Unstable fractures of the spine should be addressed before SSRF. SSRF before prone spine surgery should be considered only in unstable chest wall cases, in which the neurological status is stable, to decrease the risk of intraoperative complications. (LoE IV, Grade C).
-
PS 2.5
Advanced age should not be considered an absolute contraindication to SSRF. Elderly patients should be carefully assessed for SSRF on an individual basis (LoE IIIb, Grade B).
-
PS 2.6
Patient with significant cardiopulmonary comorbidities, anticoagulation use/uncorrected coagulopathy, active malignancy or other terminal illness should be carefully assessed for SSRF on an individual basis (LoE IV, Grade C).
-
PS 2.7
Patients with empyema or history of prior chest radiation should be carefully assessed for SSRF on an individual basis (LoE V, Grade D).
-
PS 2.8
Pulmonary contusion (PC) should not be considered a contraindication to SSRF. Patients with PC should be evaluated for SSRF on an individual basis (LoE IIIb, Grade B).
Hemodynamic instability
Consensus documents and guidelines state that patients who are hemodynamically unstable should not undergo SSRF [46, 47, 91, 92]. The 2020 CWIS Guideline states that recent shock is not a contraindication to the procedure, and patients with unstable chest wall injuries and on low and unchanging vasopressors may benefit from SSRF if it facilitates weaning of pain medications and sedation [47].
Traumatic brain injury (TBI)/spinal injury
Approximately one out of five patients with multiple rib fractures have concurrent TBI [93]. The presence of both injuries is associated with poor outcomes, including longer mechanical ventilation, prolonged ICU stay and increased risk of pneumonia, which is one of the strongest independent predictors of in-hospital mortality in polytrauma patients [94, 95]. TBI, irrespective of patient underlying thoracic injury, has historically been an exclusion criterion among various studies investigating SSRF outcomes. Patients with moderate TBI (GCS of 9–12) were the least likely to be recommended for SSRF, regardless of abnormal pulmonary variables, as shown by a survey among thoracic, orthopedic, and trauma surgeons [96]. The possible protective effects of SSRF on pneumonia development and earlier weaning from the ventilator were recognized in selected patients with mild (GCS > 13–15) or moderate TBI. However, TBI has been considered a contraindication to SSRF by several guidelines, especially in severe cases (GCS ≤ 8) [47, 91, 92].
Prins et al. compared outcomes of patients with rib fractures and moderate to severe TBI undergoing SSRF to those managed nonoperatively and found that SSRF was associated with a lower risk of pneumonia and 30-day mortality [97] A post hoc subgroup analysis found that patients with TBI who underwent SSRF for non-FC fracture pattern and for flail chest, respectively had a reduced pneumonia risk and a shorter ICU stay [98]. Freitag et al. [99], in a retrospective single-center cohort analysis of patients with moderate to severe TBI and chest wall injuries, found a shorter ICU stay and mechanical ventilation time in those who underwent SSRF compared to NOM. In a recent large propensity-matched analysis in patient with moderate to severe TBI, SSRF was associated with reduced mortality [100]. In all these studies, SSRF was reported as a safe procedure.
Patients with high spinal injury (i.e. quadriplegia) may not experience symptomatic relief from SSRF, such as pain control and need for tracheostomy. Contrarily, lower spinal injury resulting (i.e. paraplegia) may benefit from SSRF given that they still have intact sensation to the chest wall and likely did not need tracheostomy [47].
Vertebral and pelvic fractures
Management of associated vertebral fractures depends on their stability: unstable fractures of the spine should be addressed before SSRF is attempted. Pennington et al. described the ventral aspect of a thoracolumbar sacral orthosis to place the patient prone and successfully complete spinal fixation in the setting of flail chest physiology [101]. Prone positioning in case of unstable chest wall can increase the risk of intraoperative cardio-pulmonary complications with an increased intrathoracic pressure, ultimately compromising venous return, increasing intraoperative blood loss, and reducing cardiac output. Therefore, SSRF before prone spine surgery appeared reasonable for cases in which the neurological status is stable [47, 91, 102]. SSRF should be considered in select cases of FC prior to stabilization of the spinal column in the prone position. Further research is necessary to identify patients who are at highest risk of not tolerating tolerate prone surgery. Rib fixation was also found to be safe in patients with complex pelvic fractures requiring non-urgent surgery [103]. In order to maximize efficiency and minimize exposure to anesthesia, both spine surgery and repair of posterior rib fractures may be conducted at the same time in the prone position.
Age and comorbidities
Other conditions were considered contraindications to SSRF, such as minor or advanced age, significant comorbidities (cardiopulmonary, active malignancy other terminal illness and uncorrected coagulopathy) and the presence of empyema (due to the risk of hardware infection) or an history of chest wall radiation (due to the risk of hardware failure). However, there is little evidence regarding the real impact of the above factors on the outcome of patients undergoing SSRF [47].
Most studies not not support SSRF in patients < 18 years old as fractures should heal well as the patient grows and plates may need to be taken out within 3 months, to allow continued bone growth. Food and Drug Administration (FDA) approval for most plating systems excludes pediatric patients [47].
Advanced age is associated with higher morbidity and mortality in patients with chest wall trauma and rib fractures, albeit with slightly different age thresholds [104–111]. Some studies have suggested that elderly patients are at higher risk for post-operative morbidity from SSRF. However, there have been several studies suggesting that the elderly population may benefit more from SSRF as compared to their younger counterparts, considering that they are less likely to tolerate rib fractures and their clinical condition is likely to deteriorate faster. Some retrospective comparative studies [112–117] and a systematic review [118] report that SSRF in the elderly may be a safe procedure leading to a reduction in mortality compared to NOM, although it appears to be associated with longer hospitalization times, suggesting that standard indications for SSRF may be applicable in the elderly population. There is little data regarding the impact of other elements, such as comorbidities and anticoagulation use, on the outcome of patients undergoing SSRF, but some studies suggest that the presence of a single factor or a pulmonary comorbidity alone may not justify withholding SSRF in appropriate cases [112, 119].
Pulmonary contusions (PC)
A concomitant PC is present in about one out of five patients with ≥ 2 rib fractures [6] and it is a risk factor associated with ICU admission, infectious pulmonary complications and increased resource utilization [107, 120, 121]. Questions have been raised regarding the potential benefits of SSRF as compared to NOM of patients with FC or non-FC rib fractures associated with PC [122].
Several studies found that patients with mild to moderate PC who underwent SSRF had a significantly lower risk of respiratory failure and tracheotomy, decreased duration of mechanical ventilation, improved respiratory function, reduced pain and shorter hospital length of stay, as compared to NOM. The presence of severe PC (Blunt Pulmonary Contusion 18 score > = 7) appeared not to be associated with worse outcomes after SSRF but benefits from surgical treatment were not clearly demonstrated [123–126] However, a recent analysis showed shorter hospital stays and lower morbidity rates in patients undergoing early SSRF for multiple rib fractures and minor to major PC, suggesting that the early implementation of SSRF could be beneficial regardless of the severity of PC [127].
Considering the high number and complexity of the variables to be considered in defining the indication of SSRF, a multidisciplinary and tailored approach is recommended.
-
3.
Optimal timing
-
PS 3.1
SSRF should be performed within 48–72 h from the traumatic injury (LoE Ib, Grade A).
-
PS 3.2
In case of concomitant conditions contraindicating early SSRF, it should be performed as soon as possible, within 3–7 days after injury (LoE IIb, Grade B).
The timing to perform SSRF after chest wall trauma with rib fractures is crucial. Many studies of SSRF in which a benefit was not shown reserved the operation for “late failures,” including up to two weeks after injury, introducing selection bias. Advantages of early SSRF include minimizing the incidence of unfavorable outcomes such as prolonged mechanical ventilation or pneumonia and reducing the technical complexity of the surgical procedure (by mitigating factors such as inflammation, severe hematoma, clotted hemothorax, empyema, rigidity with chest wall deformities, and early callous formation).
Despite the growing evidence supporting early SSRF, defined as occurring within 72 h of the initial injury, or late SSRF, if performed beyond 72 h, the optimal timing remains debatable. In some of the RCTs demonstrating benefits of SSRF on NOM in FC patients, the surgical procedures were performed within 2 days [30] and 5 days [32] from traumatic injury.
The multicenter prospective clinical trial reported by Pieracci et al. compared SSRF to NOM for non-ventilator dependent trauma patients with non-flail displaced rib fractures. Lower morbidity rate and a decreased pain were reported in the surgical group, undergoing SSRF within 72 h from admission [60].
Single-center, retrospective series confirmed various benefits of SSRF compared to NOM when performed within 48 h [128] and 72 h [129–131] from injury, such as shorter duration of mechanical ventilation, shorter ICU and hospital stay, decreased risk of tracheostomies and lower medical costs.
A subgroup analysis of a recent meta-analysis by Sawyer et al. [74] supports benefits of early SSRF compared to late SSRF in terms of duration of mechanical ventilation and rates of pneumonia and need for tracheostomy.
A multicenter retrospective trial by Pieracci et al. divided enrolled patients into subgroups based on the time elapsed from initial injury to surgery. Late SSRF (3–10 days from admission) was associated with longer operative times and increased likelihood of prolonged mechanical ventilation, increased pneumonia rates and tracheostomy need [132].
In a recent RCT, 403 multiple rib fracture patients were randomly assigned to receive early (≤ 48 h) or delayed SSRF (> 48 h) [133]. Early SSRF decreased the overall hospital and ICU length of stay, duration of mechanical ventilation and the hospitalization costs. The timing of SSRF did not influence surgical procedure time, intraoperative blood loss, 30-day mortality and the rate of surgical site infection. However, early SSRF was associated with decreased levels of inflammatory cytokine and infection markers.
Also Owattanapanich et al. in a large retrospective study found that timing of fixation did not affect mortality, but early fixation within 72 h was associated with a reduced need for prolonged mechanical ventilation [134]. Data extracted from Japanese [135] and German [136] registers confirmed the benefits on in-hospital outcomes of patients who underwent early SSRF compared to late surgical procedure. The data from the German registry showed that despite most patients were not treated according to the current recommendations and incurred in a delay in the timing of surgery of at least 2 days, a significant lower mortality rate was observed compared to NOM.
In FC patients with concomitant serious injuries (i.e. severe TBI, abdominal injury, severe pelvic fracture and hemorrhage), Gao et al. [137] adopted the principle of damage control surgery. Emergently they used simple suspension/traction to minimize the impact of the floating chest segment in interfering with breathing, dealt with fatal injuries, addressed hemodynamic instability and volume resuscitation. Subsequently they performed SSRF, achieving good results.
Early SSRF seems to be safe and effective in subsets of patients. In obese patients [138], when compared with late SSRF, surgical fixation (performed within 72 h) decreases the need for prolonged mechanical ventilation and ICU stay. In geriatric patients [114, 139] SSRF was found to be associated with better in-hospital outcomes. Leasia et al. recently pushed the limits of early surgery even further, describing a group of patients who underwent surgery within 24 h of injury [140].
It is important to note that, in cases of severe chest wall injury, early, or even medium-term surgery (3–7 days from injury) may not be possible. Often times other injuries take priority, or patients and/or their surrogate decision makers are undecided or unavailable. There are no data to suggest that late surgery (i.e. 7–14 days from injury) confers a benefit over NOM; this is an area that requires further research [141] and until these data become available, this decision should be made on a case-by-case basis.
-
4.
Preoperative imaging evaluation
-
PS 4.1
Chest CT examination of rib fractures should be performed before SSRF. Ideally a 3D-CT reconstruction is included for surgical planning. (LoE IIa, Grade B).
-
PS 4.2
The application of 3D printing technology to pre-contouring plates, may be considered if available, especially when a minimally invasive approach is to be attempted (LoE IV, Grade C).
Although CXR and ultrasound play an essential role in the first assessment of trauma patients, major chest wall trauma needs to be evaluated with a CT scan, given its superior accuracy in diagnosing chest bone fractures, lung contusion, pneumothorax, mediastinal hematoma, and pneumomediastinum. CT scan is the imaging test of choice before SSRF, because it allows gathering of information on rib fracture number, location and displacement magnitude. [142, 143].
Some authors advocate the routine use of 3D reconstruction as an important tool for the preoperative planning of SSRF. In a retrospective analysis by Pulley et al. [144] the surgical plan of majority of the patients were changed with the addition of the information provided by the 3D CT. Ultrasound may be helpful for the intraoperative localization of rib fractures, allowing for smaller incisions and shorter operative time [145]. However, this data must be confirmed by further studies and there are no published studies comparing CXR/ultrasound to CT scan. Other tools such as a radiopaque film applied to the patient’s skin have been used for fracture localization and operative planning [146]. Some authors have reported the use of 3D printing from 3D CT reconstruction, with the aim to simulate the patient’s rib cage and determine the length and curve of the titanium plate before surgery. This technique is not ideal for emergency conditions because it is time consuming (takes at least 5–6 h), although it can allow for individualized management and for reduced operative time and difficulty [147–149]. In a retrospective study by Chen et al. of patients undergoing SSRF, the 3D printing technology was used in one third to create preoperatively a personalized design plate and predict incision length. There was no significant difference in age, body mass index, injury-severity score, number of rib fractures and fixed plates between patients with and without 3D printing for SSRF. Patients in whom preoperative 3D printing technology was applied, had statistically significant association with shorter operative time per fixed rib (p < 0.001), and a smaller incision length (p < 0.001) [149].
At a minimum a CT scan is required prior to SSRF. The other modalities, including 3D reconstruction, may add value in certain cases but their absence should neither delay nor preclude surgery.
-
5.
Rib fractures sites for SSRF
-
PS 5.1
SSRF should be considered for fractures in ribs 2–10. Repair of ribs 1, 11, and 12 does not confer additional benefits in terms of either chest wall stability or pain control and may be considered only in highly selected circumstances, such as marked displacement, thoracic or abdominal organs impalement/damage/herniation or marked chest wall deformity, vascular impingement or localized refractory pain (LoE V, Grade D).
-
PS 5.2
During preoperative image analysis, the surgeon should determine the rib fracture types and locations (anterior /lateral/ posterior), indicating the anatomical landmarks used (LoE V, Grade D).
-
PS 5.3
Fractures within 2.5 cm of the costal cartilage may be repaired by spanning plates to the sternum outer cortex if possible, or alternatively by fixation to the cartilage (LoE IV, Grade C).
-
PS 5.4
Fractures abutting the transverse process of the vertebral body may be repaired if the surgeon is able to obtain reliable fixation on the proximal fracture fragment. There is no absolute distance cutoff for this decision (LoE V Grade D).
-
PS 5.5
In patients with multiple fractures series (e.g. flail segment or FC), both fracture lines should be stabilized, wherever possible (LoE IV, Grade C).
-
PS 5.6
In patients with non-FC rib fracture patterns, all displaced ribs should be stabilized, whenever possible (LoE IV, Grade C).
Rib fracture sites for fixation
Knowledge and understanding of the taxonomy of rib fractures is fundamental for thoracic trauma patient prognosis, SSRF risk benefit analysis, and preoperative planning, to establish the most appropriate operative approach and technique for each case. We have yet to fully examine the importance of correctly defining the individual fracture displacement. In general, the type of individual fractures may be further characterized as: “simple”, defined as a single fracture line across the rib, with no fragmentation or comminution; “wedge” when the fracture has a second fracture line, which does not span the whole width of the rib, creating a single fragment that may be termed a butterfly fragment; and “complex” that has at least two fractures lines, with one or more fragments which span the width of the rib [25].
Beyond the number and degree of displacement, the anatomical location of rib fractures affects prognosis of chest trauma patients [150]. Among the scoring systems for the assessment of ribs fracture patients, the RibScore includes some anatomical criteria not previously considered and it was found to be the most predictive of adverse outcomes [151, 152]. Rib fractures are enumerated and divided into three zones: anterior, lateral and posterior. Because severe, bilateral fracture patterns involve many individual fractures, the routine completion of a standard preoperative planning sheet, that indicates the location of the fractures on each side, can assist the surgeon in preoperative SSRF planning and in the choice of surgical approach and technique [153, 154].
The first rib is located deeper than the other ribs, being crossed anteriorly by the subclavian vessels and nerves, which make surgical exposure more difficult and riskier as compared to the remaining ribs. Furthermore, the first rib contributes minimally to respiratory mechanics so unless it is significantly displaced or causing damage to blood vessels or nerves, SSRF is not recommended. The second rib may be considered for repair, particularly when fractured in an anterior or anterolateral location. Lower ribs, such as the 11th and 12th ribs are floating and likewise are not critical to respiration. Surgical repair (and the necessary tissue trauma to achieve it) likely does not improve pain levels and has to be considered only in cases of marked displacement, that might result in thoracic or abdominal organ impalement/damage or herniation, or marked chest wall deformity. Except for the cases mentioned above, SSRF should be considered for all remaining ribs, and ribs 3 to 8 are the most commonly plated [46–48, 60, 91, 92, 155], considering that fractures of ribs 6–8 strongly contribute to decreased thoracic volumes and are the most straightforward to expose without muscle division [156].
There is no universally accepted nomenclature to describe the fractures sites along the ribs. The general concept of three anatomic sections (anterior, lateral, and posterior) is known to radiologists and surgeons. The CWIS proposed nomenclature divided by the axillary lines: anterior (anterior to the anterior axillary line), lateral (between the anterior and posterior axillary lines), and posterior (posterior to the posterior axillary line) and these descriptions were accepted by consensus amongst participants and interobservers [25, 157, 158]. Anterior/Posterior axillary lines are coronal lines respectively marked by the anterior/posterior axillary fold. However, muscle markings do not run in vertical lines and may be difficult to see on CT scans, particularly at the lower chest. With no consensus found in anatomic texts for the precise definition of anterior and posterior axillary lines, the CWIS explored their views and boundary of using either vertical lines from a fixed point, muscle border lines, angles from the mid-thoracic point or equal-sized sectors to define the sector boundaries (Table 1).
Table 1.
Shows landmarks for axillary lines according to the CWIS method
| Type of anatomical landmark | Anterior axillary line (Anterior/Lateral Sector boundary) | Posterior axillary line (Lateral/Posterior Sector boundary) |
|---|---|---|
| vertical lines from a fixed point | vertical line from the intersection of the posterior border of Pectoralis Major and the 2nd rib | Vertical line through the tip of the scapula |
| muscle border lines | posterior border of Pectoralis Major | anterior border of Latissimus Dorsi |
| angles from the mid-thoracic point | 60 degree angle | 120 degree angle |
| equal-sized sectors to define the sector boundaries | 1/3rds of the circumference between the costochondral junction and the costotransverse joint | 2/3rds of the circumference between the costochondral junction and the costotransverse joint |
However, the exact borders of each region remain undefined, being not be applicable to all ribs, and therefore a uniform consensus was not feasible. Other classifications have also been proposed and the location has been associated with factors such as progressive offset of fractures [159–161]. At present, some version of anterior/lateral/posterior may continue to be used, but standardization is very much needed.
Selection of rib fractures for repair involves characterization of the fracture itself (i.e. degree of displacement, angulation, and bone loss) and consideration of the rib number and fracture location on the rib, as it relates to surrounding structures (e.g., costal cartilage anteriorly and transverse process posteriorly).
Current rib repair systems perform best when there is adequate rib length on both sides of a fracture line to securely anchor fixation screws and ensure adequate stability. In most cases, at least 2.5 cm of healthy rib is required to achieve adequate fixation. This issue most commonly arises when treating posterior fractures that abut the transverse process. Very anterior fractures are also challenging [46, 48, 91].
Costal cartilage fractures are a special type of fractures. The exact point of fracture has to be described as “Costal cartilage” if it refers to the cartilage itself, “Costo- chondral junction” if it refers to the transition between the rib and the cartilage, or “Chondro- sternal junction” which refers to the transition between the cartilage and sternum. The term “costochondral” should not be used in isolation, as it remains unclear. Finally, precise reporting language should specify if a cartilage fracture involves the short segment of cartilage associated with a single rib versus the shared cartilage segments of the 8th through 10th ribs [25]. Costal cartilage fractures are also possible candidates for surgical fixation. Ultrasound or MRI may be able to detect these injuries more effectively than a traditional CT scan [162, 163]. Current fixation systems are neither designed nor ideally suited for placement into cartilage. Several anecdotal reports of successful fixation of cartilage using plates, wires, and suture are available; however, these represent off label uses of FDA-approved fixation systems [91]. Medial fixation to the sternum has also been performed with plates spanning across to the sternum and additional screws placed into the cartilage to reduce the fracture [164]. Although larger and more robust studies are needed, some smaller case series have demonstrated the feasibility of this procedure with favorable outcomes and limited complications [165, 166]. Fokin et al. recommended to use a long plate to span chondral fracture and secure the plate medially to the sternum and laterally to the osseous part of the rib, thereby avoiding putting screws through the cartilage [92].
Posterior fractures within 2.5 cm of the transverse process of the vertebral body have not traditionally been candidates for fixation [46], although some studies have described a technique with plates spanning onto the transverse process [167]. In these cases, Fokin et al. performed plating only if at least 2 plate holes can be positioned on the neck of the rib, which requires approximately 20–25 mm of space between the head and the tubercle [92].
All these anatomic locations, as will be described later, also dictate incision placement [168] and may require different operative techniques or devices to reach locations such the subscapular region [169, 170].
Management of segmental fractures and multiple fracture series
Opinions regarding which fractures, and how many fractures should be stabilized have differed in the case of FC injury. Although fixing one fracture per rib, converting the flail segment to a ‘simple’ rib fracture appeared to be sufficient to stabilize the chest wall [30, 31], there are concerns regarding the fate of the non-fixed fractures in the flail segment. There is some evidence indicating that the fractures that are not fixed may continue to move, leading to interval displacement, and even increasing the risk of the fixated fracture failing [171]; however clinical correlation of impact on function or QoL in this scenario has not yet been demonstrated.
Pending further data, in cases of FC or displaced segmental fractures, the stabilization of all fractures of each rib involved is recommended whenever possible. For this purpose, the use of one long plate instead of two short plates may improve fixation through reduced implant stiffness [92].
There are no studies comparing a strategy of fixation of sequential ribs vs. an “every other” approach. Theoretically, repairing all ribs that can be readily accessed through the index incision will provide optimal stability, pain reduction, and healing, avoiding the risk of subsequent displacement, deformity and recurrent pain in cases of non-union. Pending further data, the stabilization of all displaced ribs that are accessible through the main incision is recommended whenever possible; in this regard it may be preferable to repair all fractures within the exposed surgical field.
Selective plating can address most severe fracture patterns if there are a limited number of fractures that are not severely displaced, especially if they are difficult to access or if the condition of the patient deteriorates and the surgical procedure must be abbreviated or aborted [92].
-
6.
Management of concurrent intra-thoracic and chest wall injuries
-
PS 6.1
There are insufficient data to recommend the routine use of thoracoscopy to evaluate the pleural space during SSRF (LoE V, Grade D).
-
PS 6.2
The use of VATS should be considered at the time of SSRF when intrathoracic organ injury is suspected or if significant hemothorax or pneumothorax is detected preoperatively, regardless of chest tube drainage (LoE IIIb, Grade B).
-
PS 6.3
Significant hemothorax and/or pneumothorax present at the time of SSRF should be drained as part of the operation, via either the SSRF incision or VATS-assisted (LoE IV, Grade C).
-
PS 6.4
A chest tube should be placed if the pleural space is noted to be violated at the time of SSRF (LoE V, Grade D).
-
PS 6.5
Significant chest wall muscle defect with lung herniation or at risk of future hernias formation should be considered for primary closure or pedicled myocutaneous flap and/or mesh repair depending on defect size, surgical site characteristics, surgeon’s individual experiences and material availability (LoE IV, Grade C).
-
PS 6.6
Bone grafting may be considered in the presence of gaps > 10 mm, based on experience and availability, using alternatively autologous or non-autologous grafts (LoE V, Grade D).
Rib fractures in the setting of blunt or penetrating chest trauma rarely happen in isolation. Patients with high- energy mechanisms of injury frequently present with other intrathoracic injuries such as PC, hemothorax, and pneumothorax, chest wall rib and muscle defects, and sternal fractures. SSRF may represents an opportunity to address non rib fracture-related pathology under general anesthesia and in a sterile environment.
Pleural space and lung injuries
Lung and pleural space injuries are common in the setting of rib fractures [48]. Some series in patients undergoing video-assisted thoracoscopic surgery (VATS) for thoracoscopic or open SSRF have found that more than 70% had a concurrent retained hemothorax [82, 172–174]. In addition to hemothorax evacuation, VATS can also assist in fracture identification, localization and reduction, chest drain placement and analgesia [175]. Furthermore, some series found that about 20% of patients undergoing VATS for internal rib fixation had an underlying intra-thoracic or diaphragm injury that required repair [172, 176]. Other injuries, such as stomach or lung lacerations requiring further surgical intervention have also been described [177]. Small series suggested that resection of punctured lung parenchyma at the time of diagnosis may favorably impact postoperative outcomes [178]. Despite these theoretical advantages, there are no data demonstrating a statistically significant greater number of occult intrathoracic injuries identified with the routine use of VATS, when compared to selective VATS [82, 179]. Therefore, the routine addition of thoracoscopy to evaluate the pleural space during SSRF in not supported and should be performed at the surgeon’s discretion [46].
The routine evacuation of hemothorax following blunt trauma remains controversial. Blunt compared to penetrating etiology of hemothorax is considered less likely to result in infection, although retained hemothorax following blunt chest trauma is an established risk factor for empyema [180]. In many cases, the pleural space has already been violated secondary to the trauma or during fracture reduction and fixation and a chest tube should be placed at the time of SSRF. By contrast, it is not necessary to place a chest tube if the pleural cavity remains intact and there is no significant hemothorax or pneumothorax [46, 48]. There are no data suggesting the superiority of any tube size or type. Routine chest lavage through a chest drain at the time of SSRF was performed by Majercik et al. [181], leading to decreased likelihood of retained hemothorax and empyema, compared to medical management. However, it is unclear if this benefit resulted from the pleural irrigation and drainage specifically and/or the rib fixation procedure.
Management of muscle loss
Injury to the intercostal muscles can result in pain and impaired pulmonary mechanics; although the degree of muscle damage rarely results in lung herniation [182–184]. Traumatic rib cage hernias are most commonly caused by blunt mechanisms. A higher number of rib fractures does not necessarily lead to a larger hernia size, but the majority of traumatic lung hernias occur from defects in the intercostal muscles (70% of cases) [185]. Lung herniation is a rare sequelae of rib fractures but one series found trapped lung or diaphragm in 10% of rib fixation patients [186] and surgical intervention is mandatory to repair these injuries and restore normal anatomy and prevent strangulation. However, there is a paucity of literature directly addressing management of significant intercostal muscle loss in the setting of SSRF [187]. The literature suggests that some form of repair is necessary when the defect is large and there is concern for possible lung herniation [188]. Primary closure, through pericostal fixation of adjacent ribs with absorbable sutures, should be attempted to close or minimize small intercostal defects [182]. A pedicled myocutaneous flap and/or mesh construct should be used to cover larger defects that preclude primary closure [46, 48, 189]. The mesh can be non-absorbable (polypropylene; Prolene, Marlex, and expanded polytetrafluoroethylene; ePTFE, Gore-Tex) or absorbable (polyglactin; Vircyl). Complications, such as fistula formation, seroma, and infection, have been reported with the use of meshes [190] and a contaminated surgical field may justify the preference for using a biological mesh. Until now, no data has demonstrated the superiority of absorbable versus non absorbable or synthetic versus biological mesh repair. Most lung hernia repairs are performed via thoracotomy, although there are several case reports demonstrating successful management using VATS [191–195]. Owing to the difficulty of repairing the bony defect of the chest wall from inside of the thorax, most thoracoscopically managed cases are of a hybrid nature, and the important procedure of repair/reconstruction is done under direct visualization, through a mini-thoracotomy with thoracoscopy guidance. Dual-layered material (e.g. Gore-Tex patch fixed with multiple spiral tacks) can be used for intrapleural fixation, to provide strength to the repair, and to prevent pleural adhesion [196]. However long-term implications of meshes have not been well studied and along with the benefits, further studies should collect data on possible long-term complications [197].
Management of bone loss
Anatomic reduction is a basic tenet of orthopedic surgery that should apply to SSRF. It is not recommended to leave a gap between fracture fragments because the implant will eventually fail in the absence of primary bone healing. Detached rib fragments should be returned to their native anatomic position and fixed to the plate. If this is not possible or following resection of callus/pseudoarthrosis in cases of chronic nonunion, alternative methods of reconstruction must be employed. Small gaps (< 10 mm) may usually be managed with anatomic reduction. The management of larger gaps in this way may result in increased tension for a single fixation device [46, 48] but there are no studies analyzing the use of multiple complementary fixation systems in the presence of larger gaps.
Options for bone loss replacement are two: autologous and non-autologous. Autologous grafts are most commonly harvested from either the ipsilateral iliac crest or 12th rib as these sites are frequently easier to expose in standard lateral decubitus positioning for SSRF [198–200]. No literature to date has demonstrated superiority in autologous versus non-autologous rib grafting. Frequently, non-autologous is favored due to the complications associated with the procurement of autologous grafts. Regardless of technique employed all grafting should strive for four elements: structural integrity, osteoinduction, osteoconduction, and osteointegration [201]. Prior, on a tray table outside of the wound, the graft is predrilled and attached to the plate, then the plate-bone assembly is fixed to the dorsal and ventral end of the fractured rib [92].
Additional fractures of the chest wall
Additional bony injuries of the thorax are common in case of rib fractures. Sternal fractures in combination with rib fractures can further worsen pulmonary function. Although uncommon, plate fixation of sternal fractures in combination with rib fixation may be considered for displaced or unstable fractures with the patient in a supine position, eventually prior to the SSRF, if a different patient positioning is necessary. Various techniques have been described, but more data is needed on this topic [92, 202, 203]. For upper rib fractures with indications for SSRF, the concomitant fixation of ipsilateral displaced clavicular fractures has to be considered, because their displacement may increase significantly and nonunion has been described when SSRF was not accompanied by clavicular fracture repair [92, 204]. In patient presenting a surgically-indicated scapula fracture and multiple rib fractures, the simultaneous surgical fixation of both fractures has to be considered [205].
-
7.
Surgical approach and technique
-
PS 7.1
When choosing the surgical approach and technique the surgeon should evaluate the rib fracture types and locations, the patient's medical and surgical history and the presence of associated chest wall or intrathoracic injuries, considering their own experience and confidence with each of the different approaches (LoE V, Grade D).
-
PS 7.2
The surgical incision for SSRF should be selected according to the rib fracture anatomical pattern and the underlying chest wall structure; whenever possible, muscle-sparring techniques should be utilized (LoE IIIb, Grade B).
-
PS 7.3
Minimally invasive plate osteosynthesis (MIPO) can be considered for patients with a localized chest wall area affected by single rib fractures, if dedicated instruments are available, especially in the context of a research study (LoE IIIB, Grade B).
-
PS 7.4
The use of VATS should be considered when a better localization of rib fractures site is needed, to refine the planned incision for ORIF/MIPO approaches or to perform reduction and fixation under direct visualization, especially with a poor operating field (LoE IIIb, Grade B).
-
PS 7.5
There are insufficient data to recommend VATS for intrathoracic rib fixation. However, it can be considered, if adequate expertise and equipment are available especially in the context of a research study (LoE IIIb, Grade B).
-
PS 7.6
A percutaneous approach for intramedullary fixation can be considered for simple, non-comminuted and easily reducible posterior but not paravertebral fractures, if adequate expertise and equipment are available (LoE IV, Grade C).
Understanding fracture anatomy through pre-operative CT is imperative to adequately plan surgery. Fiber-optic bronchoscopy can be performed in the operating room to evacuate any mucus plugs or for suspected large airway injuries. Operative approaches can be broadly divided into open, thoracoscopic and percutaneous. The patient rib fracture’s anatomical pattern and associated chest wall/ intrathoracic injuries, as well as the surgeon’s experience and confidence, are crucial in the choice of the surgical approach [206, 207].
Open approach—open reduction and internal fixation (ORIF)
To adequately plan the incision, the surgeon has to bear in mind that his/her goals are to provide adequate exposure for an effective fixation and minimizing morbidity (from both muscle division and scapula retraction) [46]. One concept to understand is that incisions are best made along the borders of muscles (e.g., latissimus dorsi, trapezius) as opposed to directly over the fracture; this approach will minimize muscle division and allow for retraction of the muscles out of the way. Anatomical landmarks such as sternum, suprasternal notch, xiphoid, spinous processes, mid-clavicular or axillary lines, and inferior scapular angle (dependent on arm position) can be marked to help with the orientation and length of the optimal incision. Creating subcutaneous flaps, superficial to the fascia, can allow to identify muscle group edges, prior to blunt dissection. Muscle-splitting technique alongside and between the muscle fibers, without fiber transection, have been used and complemented by muscle retraction to obtain open muscle-sparring exposures [7, 16, 46, 91, 92, 154, 155, 168, 208].
The exposure to each anterior, lateral or posterior areas for SSRF may be accomplished with minimal muscle division or scapular retraction. A retrospective case study showed that the internal fixation of rib fractures using muscle-sparing technique is associated with the recovery of shoulder function and strength [209].
Anterior fracture sites may be exposed with the patient in the supine position and the ipsilateral arm either suspended or out lateral on arm boards. Supine position with both arms suspended allows for bilateral anterior fracture repair without patient’s repositioning. The exposure to anterior sectors of ribs 4 to 6 can be provided through an oblique incision along the infra-mammary fold and the development of a sub-pectoral flap utilizing muscle sparing techniques. The pectoralis minor muscle may be lifted from its costal attachments using a blunt elevator and a sub-pectoral plane should be bluntly dissected with care to avoid injury to the intercostobrachial, median pectoral, medial pectoral, and long thoracic nerves. [7, 16, 46, 154, 155, 168, 207, 208]. To reach high anterior fractures, right-angled tools can be utilized, such as a right-angled powered drill and right-angled powered screwdriver and the exposure can be assisted by a retractor system [16]. Fractures of the second rib, although they very rarely require stabilization, may require a plate to be anchored medially to the sternum, through a separate transverse incision just above the rib and extend it to the sternum in order to preserve the pectoralis major muscle, by elevating the muscle from the sternal side, instead of dissecting through the pectoralis muscle [92]. Anterior fractures of the third rib may be approached through a small horizontal incision directly over the fracture, with splitting of the fibers of the pectoralis major and minor muscles, while care is taken to avoid injury to associated nerves [91].
The exposure of lateral, anterolateral or posterolateral rib fractures requires lateral decubitus positioning. If there are no lumbar or thoracic spine injuries, the operating room table can be flexed to approximately 10–15° on a beanbag positioned beneath the patient. The ipsilateral arm can be draped and placed on a padded overhead arm-board at 90° of abduction and toward the head. Including the ipsilateral arm in the sterilized surgical field to allow its movement during the procedure, can help to increase the surgical exposure by changing the scapular position. The contralateral arm is positioned on a padded horizontal arm-board with an axillary roll. If bilateral SSRF is attempted, the surgical procedure should start from the more severely injured side. Some authors prefer to perform SSRF on the contralateral side 24–48 h after the first procedure but bilateral procedure under the same anesthesia may be preferable in the presence of multiple bilateral displaced rib fractures and concomitant sternal fracture [7, 16, 46, 92]. The incision is tailored to the fracture pattern in a “line of best fit” to the fractures, usually as a vertical axillary or lateral incision along the anterior border of the latissimus dorsi. Lateral fractures of ribs 3–8 can be accessed with a longitudinal incision, placed along the anterior border of the latissimus dorsi muscle. A flap is then raised underneath the muscle, that may be retracted posteriorly to expose the serratus anterior muscle branches, which can be split, to access fracture sites. Care must be taken to avoid injury to the long thoracic nerve, which lies superficially on the serratus anterior muscle and descends down alongside its outer border in proximity to the anterior and mid axillary lines, to prevent scapular winging [7, 16, 46, 154, 155, 168, 207, 208]. This neurological deficit may also be caused by the trauma itself, but often it may be difficult to detect because of trauma severity and patient’s recumbency. Alternatively, a lateral curvilinear skin incision has been considered as the main approach to allows access to the majority of rib fractures that are located from the mid-clavicular line to the vertebral border of the scapula. Indeed, a “reversed lazy-S” extension of the incision can allow additional exposure of the posterior upper ribs and/or lower anterior ribs. Subcutaneous flaps can be developed so that the fascia continues encompassing the muscles. The subsequent blunt dissection between muscles can allow for retraction of the serratus anterior cephalad, the latissimus dorsi caudally, and the pectoralis major superiorly [92].
Posterior fractures in proximity to the transverse process and in sub-scapular location are typically the most difficult to repair. These fractures may be approached with the patient in the prone position and the ipsilateral arm supported on a table, that is lowered in abduction approximately 20 cm relative to the operating table to allows lateralization of the scapula and facilitates exposure. A parascapular longitudinal incision between the medial border of the scapula and the spine can allow to access to the triangle of auscultation (bounded by the trapezius muscle superiorly and medially, the medial border of the scapula laterally and the latissimus dorsi muscle inferiorly that is relatively free of muscle). The floor of the triangle is formed by the sixth and seventh ribs and the rhomboid major. The posterior sector of ribs 2–8 can be exposed, raising both sub-trapezial and sub-latissimus flaps and further developing this plane in a blunt fashion, with use of finger dissection, starting from underneath the inferior angle of the scapula and extending cephalad by dissecting the scapulothoracic bursa, to allow the use of a scapular retractor. The erector spinae muscle can be retracted medially and elevated to expose the neck of the rib and it is fundamental to allow enough space for the positioning of instrumentation and to place the contoured plate over the curved neck of the rib. As described by some authors, trapezius and then the rhomboids can be divided or taken down from their attachment to the scapula to allows dislocation of the scapula laterally and access to the rib fractures located under the scapula body, but excessive damage to the rhomboid muscle should be avoided because it may result in scapular winging. Alternatively, fractures at the limit of surgical exposure can be reached with either a right-angle screwdriver system or with the addition of a secondary incision [7, 16, 46, 92, 154, 155, 168, 207, 208]. Care must be taken to not overbend the correct curvature of the metal implants in order to avoid metal fatigue and fractures and to ensure that rib prostheses are as flush as possible, because contact between the scapula and any prosthesis on the outer cortex of the rib may be painful [155].
Patients with multiple fracture series or FC typically have a combination of either anterior and lateral fractures, or lateral and posterior fractures. Each fracture sector may be approached through two of the incisions described above. Frequently, fractures of more than five contiguous ribs, multiple medial subscapular fractures, or a combination of both lateral and posterior fractures may be best approached using a standard posterolateral thoracotomy incision [16] Another typical fracture pattern is the anterior FC, due to anterior bilateral fractures series, that may be effectively exposed via bilateral inframammary incisions [154].
Fracture fragments need to be exposed 2.5 cm on either side and the periosteum left on the bone for proper reduction and fixation; any additional exposure or unnecessarily strip off the intercostal attachments or the periosteum and can lead to devascularization. Proper reduction and countertraction can be accomplished by using a variety of clamps packaged with dedicated rib fracture sets. The reduction of the most displaced rib fracture may help to reduce the adjacent fractures [91, 92].
A penetrating towel clamp may be used applying gentle upward pressure on the fracture segments. Alternatively, the ‘‘Double right angle’’ technique can be useful for holding the fracture fragments in reduction for subsequent fixation. Two right-angle clamps can be inserted above both rib fracture broken ends, superiorly and inferiorly to achieve reduction with gentle pressure, and can be left in place to assist with countertraction against which both the drill and screwdriver will operate at the time of fixation [154].
When concerns remain in reducing overlapping ribs or rib fragments protruding into the pleura, putting a finger into the pleural space, through a small incision in the intercostal muscle, can allow for palpation of the rib fracture ends assisting in their reduction. This is also useful to identify if the fracture is in the rib above or below your position, as it may not be immediately evident in patients with a lot of chest wall tissue covering their ribs [155].
Once surgical exposure has been obtained, various contemporary rib stabilization systems can be used, as described later.
If a pleural rupture is found during SSRF and preoperative imaging has shown pleural effusion, the surgeon can clear it with a suction device through the pleural rupture. An exploratory thoracoscopy or an extension of the pleural rupture may be performed to better detect damage of visceral organs or to completely remove blood and blood clots in the thoracic cavity, that should be drained with a chest tube [46, 48].
Minimally invasive plate osteosynthesis (MIPO)
Several options exist to obtain fixation of fractures at the limit of surgical exposure. The first involves making a stab incision for the introduction of a trocar in the soft tissue to function as an accessory port, through which both a drill guide and screwdriver may be used, with the clamp lifting up the soft tissue creating a working window, enabling visualization of the plate on the rib. The second involves the use of a right-angle drill and screwdriver system in combination with upright plate holding and reduction clamps; it may be particularly useful for repairing subscapular fractures. Specific drill guides with four different angles may allow perpendicular drilling in the center of the hole [16, 170, 207].
With the advent of these dedicated minimally invasive surgical instruments, the interest in a minimally invasive approach for rib fracture fixation has grown. To further reduce the surgical trauma associated with chest wall stabilization, minimally invasive plate osteosynthesis (MIPO) involves the use of smaller incisions that are more focused on the fracture pattern to minimize soft tissue injury and better preserve blood supply [16]. After the division of the subcutaneous tissue and muscle fibers in a muscle-sparing manner, a cavity is created between the chest wall and the overlying soft tissue, enabling the placement of a wound retractor (e.g. Alexis O-ring). After placing the inner ring between the chest wall and the soft tissue, the retractor is tensioned by rolling itself on the outer ring, creating an outward retracting force and forming a window through which the procedure can be performed. Furthermore, the rubber seal between the inner and outer ring has a hemostatic feature, creating a dry operating field.
The location of the incision is crucial, since a certain number of rib fractures must be fixed through the same small incision. Furthermore, in the preoperative plan, the CT images are crucial for determining the rib thickness because working through small incisions makes it challenging for the surgeon to determine the appropriate screw length. Another challenge is the contouring of the plate through small incisions. If surgeons are not familiar with MIPO, these aforementioned concerns will translate into a longer operation time. All surgeons starting with the MIPO technique must go through a learning process [210].
Li et al. conducted a prospective cohort study comparing the efficacy of MIPO with NOM in one-hundred chest trauma patients suffering from non-FC rib fractures, with no significant differences in pain index (8 vs. 8; p > 0.05) or pulmonary function (VC: 31.0% vs. 26.5%; FEV1: 29.9% vs. 26.7%; PEF: 15.2% vs. 12.0%; p > 0.05) at the time of admission. Before discharge, patients undergoing MIPO had significant lower pain index (3 vs. 6), improved VC (42.1% vs. 35.3%) and FEV1 (4.2% vs. 35.9%) than patients treated non-operatively. Long-term follow-up showed that duration of pain, time required for the patient to regain the ability to engage in daily self-care, mental labor, moderate-to-severe physical labor, and duration of chest discomfort in the MIPO group, were significantly improved than in the conservative treatment group. Patients included had at least 3 consecutive rib fractures (an average of 5 in the MIPO group and 4 in the NOM group), with some degree of displacement and each rib fracture was a single fracture. Therefore, patients included in the study had a relatively localized area of the chest wall affected by single rib fractures. The described 5–7 cm length incisions were made at the lateral margin of the pectoralis major and the anterior margin of the latissimus dorsi (for anterior rib fractures), from the lower edge of scapula inferior angle to the medial edge through the auscultation triangle (for posterior fractures) or an anterior lateral incision (for lateral rib fractures). Thoracoscopy has shown to be important for planning the incision and observing the procedure, especially in cases of poor operating field, being used in one out of three cases [211]. Some authors advise placing a postoperative negative pressure drainage to drain the subcutaneous and muscular space fluid [212].
Video assisted thoracoscopic surgery (VATS) approach
Thoracoscopy has revolutionized the practice of thoracic surgery due to smaller incisions, improved visualization of intra-thoracic structures, less postoperative pain, and quicker return to work. VATS has shown its usefulness in assisting SSRF in various ways in the context of chest wall trauma patients with rib fractures: to aid in the evacuation of retained hemothorax, to guide in chest drain or loco-regional anesthetic catheters placement or for the identification and repair of associated intra-thoracic injuries [173–181, 186]. A 30° thoracoscope through a midaxillary incision in the fifth or ninth intercostal space can directly examine the type and status of fractures from multiple viewpoints, which allows a better selection of the ribs that need to be fixed, minimizing the size of the skin incision. VATS was effective to refine the planned incision for extrathoracic plating, allowing to a better identification and localization of fracture sites also for minimally invasive incision approaches and MIPO [169, 173, 174, 213–215].
Indeed, rib fractures can also be reduced and plated on the inner rib cortex under direct visualization. VATS-assisted intrathoracic rib plating offers several theoretical advantages over extrathoracic rib plating, improving visualization of rib fractures (particularly for sub-scapular and very posterior fractures), minimizing overlying muscles and nerves and intrapleural structures injuries and eliminating the need for scapular retraction. Furthermore, an increased margin for posterior fractures can be obtained with intrathoracic plating, eliminating discomfort due to palpable plates and contact with scapula during shoulder movement [16, 17].
Patient selection is relevant and the surgeon’s first cases should be of relative technical ease (i.e., lateral fractures of ribs 6–8). The surgery is performed under general anesthesia and requires lung isolation. Because neither hilar nor lung parenchymal dissection is required, a bronchial blocker in conjunction with low pressure pleural space insufflation can be adequate, but the patient has to be able to tolerate single lung ventilation. A lateral decubitus position provides the most comprehensive exposure to the inner chest wall, except for isolated very anterior or posterior fracture series, in which case a supine and prone position respectively may be considered. The table can be flexed at the patient’s ipsilateral anterior superior iliac spine to widen the rib spaces and allow for a better camera maneuverability. A low pressure (e.g., 6–10 mmHg) pleural space insufflation also aids in both lung deflation and mediastinal shifting, further widening the surgical field. Prior to addressing the rib fractures, a thorough exploration of the pleural cavity has to be conducted in order to identify any additional pathology [17, 216]. Following SSRF, an intercostal nerve block or cryoablation can be performed. The cryoablation probe can be applied to the inferior edge of the rib, 2–4 cm lateral to the spine in order to avoid iatrogenic injury to the sympathetic chain. Through axonotmesis, it seems to provide an extended pain relief and cutaneous sensations are gradually restored over 2–6 months as a result of nerve regeneration along the remaining perineural structures. Cryoablation of intercostal nerves below the tenth rib should be avoided because it may result in temporary bulging of the upper lateral abdominal wall. Cryogenic nerve block can also be performed during thoracotomy, although all areas may be reached through VATS [92].
At the end of the last decade, Pieracci et al. firstly described the completely thoracoscopic SSRF, that refers to intra-thoracic reduction, drilling, and plate placement to the inner cortex of the rib under thoracoscopic visualization [17]. Four incisions were required for the camera, to position and hold the plate and to operate both the drill and screwdriver, ensuring the triangulation of instruments. The fractures were noted and exposed by opening the underlying pleura using cautery, after identifying the precise location of the ribs and avoiding stripping of the periosteum. The next task was reduction and fixation, which was the most challenging step of the operation. Pieracci et al. described the use of a conventional extrathoracic plating systems for an intrathoracic approach. The fracture was reduced by using a stab incision in the overlying skin and passing a braided suture around each rib segment with a port site closure device. Next the plate was manually contoured, introduced into the thoracic cavity and positioned across the fracture line with a Kelly clamp. Fixation was achieved using a 90-degree screwdriver to secure the plate to the intrathoracic cortex of the rib. The maneuver described for reduction cannot take place for sub-scapular fractures, where internal reduction may be accomplished using two laparoscopic Kittner (peanut) blunt dissectors [217].
Some case reports and case series were subsequently published, describing different material and techniques to perform VATS with intrathoracic plating, which is increasingly pursued by leading SSRF groups [218–220].
A commercially available dedicated intrathoracic plate-based system was approved for use by the FDA (RibFix Advantage). This system uses a less invasive approach to fixate a plate to the undersurface of the rib with long wires threaded through the rib and out of the chest via a thoraco-port through which the plate can be pulled into the chest, that will be explained later. This system has been used effectively in a retrospective series of patients undergoing intrathoracic rib plating [221].
In a recent prospective observational single-center study, patients with similar injury severity who underwent intrathoracic plating had a decreased length of stay (10 days vs. 8 days, p = 0.04) and operative time (279 min vs. 188 min, p < 0.001) in comparison to patients who underwent extrathoracic plating, after adjusting for numbers of ribs fixed. Despite extrathoracic plating, patients more commonly received epidural anesthesia (56% vs. 24%, p < 0.001) and intercostal nerve block (56% vs. 29%, p = 0.01) compared with intrathoracic plating. There was no difference in median morphine equivalents between cohorts [222].
Despite technological advances and encouraging results, VATS with intrathoracic plating technique has not been widely adopted due to a lack of experience and wide availability of dedicated fixation system. Most of the commercially available plates are pre-contoured to mimic the outer rib cortex. Standardizing the indications for thoracoscopic SSRF and communicating surgical tips, particularly regarding surgical equipment, would be beneficial to expand the use of this evolving technique. The importance of VATS technique is instead well recognized when damage to intrathoracic organs is suspected, or better to identify rib fracture sites for planned focused incision and to observe rib fracture reduction and fixation especially in cases of poor operating field.
Some surgeons have employed a hybrid approach to SSRF, using smaller incisions and videoscopes and insufflation in the extra thoracic space. Merchant and Onugha described a technique of elevating sub-muscular, extra-thoracic flaps using a balloon dilator, followed by insufflation of this space and rib fractures repair under camera visualization, but remaining extra-thoracic [223].
Percutaneous approach
Rib fractures are typically stabilized with osteosynthesis plates. However, an intramedullary approach can be considered for simple, non-comminuted and easily reducible fractures of the posterior area that are not paravertebral [224, 225]. ORIF requires access to the rib surface over the entire plate length, that in some cases may be largely restricted by the scapula and latissimus dorsi. If a safe and adequate fixation is not expected to be possible even with MIPO instruments, intramedullary splints can be advanced through the medullary canal to reach stabilization with a percutaneous minimally invasive approach [155]. Over the past 50 years, percutaneous SSRF has evolved from the use of Kirschner wires to intramedullary splints, current employing trocars which are derivatives of laparoscopic instruments [224, 226, 227]. These splints have only one point of fixation, with no distal fixation and they seem to achieve stability by providing a stiff ‘rod’ within the intramedullary canal [155].
Based on the rib fracture pattern and location, these splints can be implanted through a posterior or a lateral approach, in which case the point of fixation on the rib is anterolateral with the splint pointing posteriorly. A skin incision is made to expose the fractured rib on the medial side for 4–5 cm, to allow splint head placement, minimizing the dissection of the soft tissue on the lateral side of the fracture. A caliper can be used to select the appropriate screw length for subsequent fixation. The splint insertion hole can be drilled in the upper 2⁄3 of the rib, approximately at 30 mm from the fracture line, with a 5.5 mm drill bit introduced unicortically through a drill guide. The lateral fracture segment should be at least 5 cm long to accommodate the splint insertion length. A splint template allows the selection of the correct splint width, that is subsequently introduced into the intramedullary canal by a splint driver, holding the medial rib segment with a bone reduction forceps. The splint is fully inserted when the head of the splint rests flush on the outside of the rib, where an appropriate length screw should then be placed to fixation. The percutaneous technique of SSRF has not been compared directly to any other surgical approach and thus remains a matter of surgeon preference [46].
-
8.
Stabilization methods, materials and technical aspects
-
PS 8.1
SSRF can be performed with different stabilization methods using either plates and screws, claw shape plates or intramedullary splints, including a cortical fixation component (LoE V, Grade D).
-
PS 8.2
The type and location of rib fractures encountered in different clinical scenarios will determine the selection of the appropriate surgical stabilization method and materials, which depend on surgeon experience and confidence with each stabilization method and system (LoE V, Grade D).
-
PS 8.3
Despite most of the evidence supporting the use of SSRF coming from studies in which bicortical fixation was attempted, biomechanical studies found no difference in fixation stability and not enough evidence demonstrate whether bicortical or monocortical fixation is superior in SSRF (LoE V, Grade D).
-
PS 8.4
Anatomical plates and screws can obtain the fixation of severe comminuted rib fractures and multi-segment fracture, even in cases where the fractured ends are located next to the sternum or spine, where U-plates, claw-shape plates and intramedullary fixation may be poorly tolerated or not possible. Polymer cable cerclage can be considered to enhance plates fixation in fragmented, osteoporotic bone, longitudinal or oblique rib fractures and in cases in which the rib thickness is < 8 mm (LoE V, Grade D).
-
PS 8.5
Although extrathoracic plating has more consistent supporting evidence, intrathoracic plating fixation can be considered if adequate expertise and equipment are available, especially in the context of a research study (LoE IIIB, Grade B).
-
PS 8.6
Non-absorbable materials should be used. The use of absorbable materials can be considered in the context of a research study (LoE V, Grade D).
Stabilization methods and materials
The character and geometry of the human ribs is peculiar. All 12 ribs have a unique shape and curvature and thickness, that ranges from 8 to 12 mm, with a relatively thin cortex (1–2 mm). The lower tolerance to hold cortical screw than bone with a thicker cortex and the extensive daily usage of the thoracic rib cage for breathing, makes it essential for fixation materials to be rigid, yet flexible and adaptive. Biomechanical material design should ideally display the following characteristics: histocompatibility, negligible rejection reactions, plasticity, malleability, tensile strength, and elasticity [16]. Fractures may often be oblique or even comminuted and adequate exposure of the fracture site may be difficult, further complicating the challenge of a reliable repair.
SSRF has failed to achieve routine use in many hospitals because of lack of awareness of the evidence for rib fixation and knowledge of appropriate technique [15]. Moreover, the lack of specific rib fixation prostheses has led to the use of alternatives such as plates applied with sutures and cerclage wires, Kirshner wires or other off label prostheses which have been associated with hardware failures [7, 155]. Nonetheless, several effective stabilization systems have been developed and applied for use in clinical practice and has addressed this deficiency in recent years.
Regardless of the system used, rib stabilization methods have historically been divided into cortical and intramedullary stabilization methods [7, 228]. To our knowledge, no RCT or prospective observational study comparing the two different stabilization methods has been published, therefore no evidence supports the use of one particular method over the others. Biomechanical studies conducted using post-mortem human subjects highlight differences in stiffness and load to failure, but these results are not necessarily related to clinical outcomes [229, 230]. However, it should be noted that current intramedullary stabilization systems still include a bicortical fixation component.
Extrathoracic plate fixation
The currently most used rib stabilization systems involve the use of plates with integral cortical fixation. All currently available systems involve securing plates on to the outer cortex of the rib with the exception of one (RibFix Advantage), which we will analyze later, in which this is planned to be on the internal cortex.
The plates can obtain cortical fixation through bicortical clamping mechanisms, through the use of screws, or through a combination of both cortical fixation components.
Most systems use titanium bicortical locking screw plates, and provide sets of surgical instruments that can allow for various abilities to clamp and manipulate the ribs, including MIPO, when the incisions have not easily extended over the fracture site or for costal cartilage fractures and fractures near the spine.
Screw fixation may be monocortical or bicortical according to its depth. Monocortical fixation can theoretically avoid complications such as potential pleural injury and thoracic organ damage, but only one of the currently available rib stabilization systems provides a monocortical fixation through a convergent biaxal fixation using single length, drill-free locking screws. Although a study on biomechanics of monocortical and bicortical plates screw fixation for rib fractures in a cadaveric model, found no difference in stability [231], at present, most of the evidence supports bicortical plates screw fixation [32, 36, 57, 66, 82, 225] or claw-shaped plates fixation spanning both outer and inner cortices [30, 232–234].
Bicortical plates and screw fixation technique and materials
If plate and bicortical screw fixation is attempted, after the exposure and approximation of broken rib segments, the use of depth gauges and calipers may assist in selecting the proper screw length and drill bit, adding the rib thickness measured to the plate (approximately 1.5–2 with current low-profile plates). A small incision or an existing access in the intercostal space at the superior border of the rib can allow the insertion of the caliper, with care to avoid damage to the nerve and vessel bundle at the inferior border of the rib. Rib thickness measurements on the preoperative CT scan are fundamental near the spine and high under the scapula, because these locations are not accessible by all calipers. Some authors have reported adding a polymer cable cerclage in cases where the rib thickness is < 8 mm [92]. Cutting and contouring of a bending plate template to a length that allows placement of screws on each side of the fracture can be useful for the selection of the best matching precontoured plate. Many systems include bendable templates to match the counter of the ribs. These templates are particularly helpful in hard-to-reach areas. The plate may then be bend to match the template on the back table. Screw plate fixation was traditionally and appropriately used for severe comminuted rib fractures, multi-segment fracture of single ribs or FC, being more conducive to the recovery of the thoracic shape, even in cases where the fractured ends are located next to the sternum or spine. Before the advent of the precontoured titanium plates, some studies have reported screw loosening and pullout with the previously employed steel plates, which were difficult to prepare during the operation to fit the rib contour [8, 34, 122].
The anatomically contoured nature of most of contemporary plating systems facilitates direct reduction of the rib fragments to the plate and minimize the need for templating and bending. This can lead to a reduction of surgical procedure complexity and time, especially when stabilization of multiple rib fractures or FC is required. Anatomical plates are thin, manufactured from titanium and can be long enough to allow bridging fixation of multiple fractures, enabling a strong yet low-profile that can flex with respiration and can reduce the risk of hardware or fixation failure secondary to repetitive loading. Furthermore, the plates low profile may prevent hardware irritation to minimize the need for removal after the fractures heal [235].
Complex rib geometry with variable curvature, which is particularly increased in the posterior rib segment, requires additional plate contouring to ensure an appropriate apposition [92]. If necessary, the plate can be cut to the desired length, using the plate cutter. Systems include various approaches to distinguishing plate types including presenting different colors to distinguish either the left or right side, different lengths, in-situ plate benders and templates. Universal plates are also included, which can be bent to particular encountered fractures. During contouring, it is necessary to avoid sharp bends, reverse bends, or bending the implant at a screw holes, when possible, avoid also notching or scratching the implant. These factors may produce internal stresses which may become the focal point for eventual implant breakage. These standard plates are mostly based on anterolateral fractures. Particular care is required with the anterior tight curvature of the costal cartilages. Furthermore, fixation of most prostheses into cartilage is under debate and fixation spanning to the sternum outer cortex may be more prudent in some cases. This can be done using some of new plates and screws fixation systems [154, 155]. A similar trouble occurs for posterior rib fractures near the costal tubercle, where the rib makes a near 90-degree turn and plate bending becomes fundamental to properly position the plate over the rib fragments. Pieracci et al. proposed to drill first the most distal hole relative to the fracture fragment, leaving the screw somewhat loose to achieve partial fixation of the plate to the fracture fragment, while still allowing for manipulation of the plate over the other fracture fragment to ensure proper alignment [154].
Plates should be positioned on the upper two-thirds of the rib to minimize risk of injury to the neurovascular bundle, verifying that the contour of the plate matches the rib. The plate holding forceps, inserted from the rib superior border, should hold the plate, preferably at its two ends on the rib, to allow fixation to begin close to the fracture site [91, 92, 154, 155].
Different types of screws are available with new fixation systems. Traditional screws require the creation of a drill hole before the application. They are non-self-drilling but self-tapping, locking or non-locking screw. Non-locking screws are available to ensure the plate sits flush with the bone for temporary fixation, but their replacement with locking screws is recommended [92]. The drill guide should be threated into the plate to facilitate the hole drilling at a right angle to the bone, although in certain cases (e.g., subscapular fractures) holes might be drilled without the use of a guide. In these cases, some systems offer a trocar instrumentation or a 90° Screwdriver. Alternatively, care must be taken to position the drill bit at a right angle to the rib [154]. To avoid the risk of injury to underlying organs or soft tissue, an appropriate drill bit can be selected to match the locking screw length for the achievement of bicortical purchase, that is noted with the drill by two separate ‘‘gives’’ in resistance, representing the superior and inferior cortices. In case of oblique fracture lines, care must be taken to achieve fixation to a portion of rib with intact cortices, which may require additional exposure of fracture fragments [154]. Irrigation during drilling, which should never exceed 1800 rpm, helps to avoid bone thermal necrosis and increased hole diameter that may lead to unstable fixation. It may not be necessary to drill the lower cortex entirely, into which the screw tip can be deepened later. Then, a depth gauge can be introduced through the plate to confirm the screw length determined and the appropriate locking screw that should be inserted through the plate and tightened until secure. Preferably, the insertion of the second screw must be carried out on the opposite side of the fracture, and that of the subsequent ones in the same way in a centrifugal direction with respect to the fracture site. Subsequently, irrigation and suction can help for removal of debris potentially generated during the implantation. In addition to screw fixation of the plates onto the bone, Fokin et al. mentioned the use of polymer cable cerclage to enhance fixation of the plates to the ribs in fragmented, osteoporotic bone and/or longitudinal or oblique rib fractures [92].
As an alternative to self-tapping screws, self-drilling locking and non-locking screws have a pointed and cutting tip that enables the surgeon to fixate plates or intramedullary splints in position, without drilling a pilot hole. A screw guide to engage the plate can be available for some of the fixation system mentioned. Incorrect screw selection, especially when self-drilling screws are used, may result in the protrusion of screws that are too long beyond the inner cortex and consequently can potentially cause lung parenchyma injury. Appropriate measurements during the surgical procedure become crucial. Iatrogenic longitudinal rib fracture may occur when the bone is cracked during screw insertion. To avoid this, predrilling with the appropriate bit size and self-tapping screws must be used. A long plate supplemented with a polymer cerclage has been proposed for its treatment [92].
U-plates
These plates are a titanium plate with a U shape at either end designed to sit over the rib, allowing for both embracing and screw bicortical fixation. A traditional primary guide, acting as a clamp, aids in fracture reduction once compressed onto the plate and the rib, at the same time allowing to rib thickness measurement for screw sizing. A 90° low-profile guide by dedicated drill and screwdriver can be used when minimally invasive fixation is attempted. The primary locking screws at either end go through the anterior U plate then through rib, then lock into the posterior part of the U plate, making a strong construct. Intermediate screws are also placed through the plate which lies between the two U shaped ends locking the plate to the rib. The U- shaped ends of the plates come in different sizes to accommodate different ribs thickness and the plates can be bent to further contour them to the rib [155].
In a simulation of an unstable rib fractures with a small bony gap of 5 mm, U-plate fixation was more durable than standard anterior bicortical plates and screw fixation [236] and another biomechanical study showed that the plates were stronger in the bending moment loading of repaired ribs, possibly due to the U-shape structure supporting both the inner and outer cortices [237]. However, these results are not necessarily related to clinical outcomes and require further investigation. Furthermore, their use may not be possible or poorly tolerated in cases where the fractured ends are located next to the sternum or spine [155].
Claw-shape plates
The use of shape memory alloy embracing fixators, encircling devices or claw-shaped bone plates for SSRF, simplifies plate stabilization without screws and may have been easier to perform [238]. Judet strut is a bendable plate that grasps the rib with tongs both superiorly and inferiorly without transfixing screws being suitable for spanning simple, comminuted, or spiroid single fractures [30, 122, 239, 240]. However, the fixation of these plates around the inferior margin of the rib has the potential to crimp the intercostal neurovascular bundle with subsequent chronic pain, as happened with the previous use of a variety of malleable flat plates cerclaged to the anterior surface with wires [241–244]. It makes them not suitable for costal cartilage fractures or fractures near the spine [238, 245, 246].
Newer generation titanium rib plates with multiple claws are now available and are secondarily tightened to the fractured rib with forceps. Clips must be firmly applied to the superior and inferior border of the rib to ensure that there is no slippage [155]. These systems provide for the possibility of building bridge systems, which could be potentially useful to fill bone fractures large gaps. The implant bridge relies on crimping clips onto the rib on either side of the fracture, with a connecting titanium bar shortened to the appropriate length and contoured to the rib, that has to be subsequently crimped to the clips [247].
Despite potential advantages and disadvantages of encircling fixation and plate and screw fixation, no clinical evidence has demonstrated the superiority of one system to the other [248]. However, both outer cortical plating with bicortical screw fixation [32, 34, 57, 249, 250] and claw-type plates with embracing fixation [35, 169, 246], when compared to NOM in clinical situations with indications for SSRF, have demonstrated patient benefits.
Intrathoracic plate fixation
Although some of the SSRF systems approved by the FDA do not specify where to place the plate (outer cortex vs. inner cortex), plates contours are mostly located on the outer cortex and studies related to structural strength, metal fatigue, plate flexibility and screw strength are mostly conducted with plates placed on the outer cortex [229–231]. However, the rib inner cortex is more than a third thicker than the outer cortex, and the density of the inner cortex is twice of the outer cortex [230, 251]; some anatomical and biomechanical studies have suggested that intrathoracic plating could provide higher construct stiffness and firmer fixation [252, 253].
A commercially available dedicated intrathoracic plate-based system, approved for use by the FDA, consists of curved bridges with adjustable posts that are secured to the ribs with washers and locking caps. Rib fractures can be identified through a VATS approach and a small external overlying incision can allow their external visualization and drilling for placement of the adjustable posts. Two drill holes can be performed through a drill guide placed in the desired position of broken ends with the pin of the instrument sitting against the superior aspects of the fractured rib. Two guide long tubes can be inserted through the drill guide and rib holes, and threaded out of the chest via the thoraco-port. Then guide cables can be inserted through guide tubes to replace them and to allow the insertion of the bridge construct through the port incision into the thoracic cavity, with posts in collapsed position. This passage and the progressive adhesion of the construct to the internal cortex, while reducing the fracture, is accomplished gently pulling on cables at the fractured site level, reducing the need for additional reduction instruments. Washers and locking caps are placed through the external incision to secure the intrathoracic bridge construct; then locking posts can be cut.
As we have previously reported, fixation on the internal cortex has been described successfully using system designed for outer cortex positioning, or different fixation systems, of which further future development is expected to reduce the complexity of the procedure and allow to expand the use of this evolving technique [217–221].
In a recent observational study, patients who underwent intrathoracic plating had a decreased length of stay and operative time in comparison to patient who underwent extrathoracic plating with similar injury severity [222], but future prospective multicenter research is needed to confirm these findings, to collect and compare long-term outcomes and eventually lead to further adoption of this minimally invasive technique.
Intramedullary splint fixation
Intramedullary fixation can be evaluated for the stabilization of simple fractures, or flail segments, where access for plating is limited, especially in the posterior sector but not paravertebral, reducing the need to elevate the latissimus dorsi and allowing fixation with less surgical dissection. However, its use is limited for patients with anterior rib fractures or comminuted fractures, narrow ribs, and small bone marrow cavities [224, 225]. The main advantage of the intramedullary fixation is that it causes relatively small surgical trauma as it makes a relatively small incision, and avoids peeling of the periosteum. At the same time, it is easy to displace the broken ends of the fracture and the needle itself, making the operation relatively complex with long operative time. Therefore, it has been less often used in clinical practice and it is best tried after gaining some familiarity with the rib fixation procedure [155].
Intramedullary fixation of rib fracture has traditionally been achieved with Kirschner wires. that follow the canal shape upon insertion, whereas plates require intraoperative contouring to match the rib surface [31, 254–256]. In the past, some authors described intramedullary implants as more durable and better tolerated than plates, which remain above the rib surface and can cause persistent discomfort or are more prone to screw loosening and plate pull-off, especially in the presence of osteoporotic bone [256, 257]. However, the thin and circular cross section of the Kirschner wire provides poor rotational stability at the fracture site and makes it prone to longitudinal splitting of osteoporotic ribs, loss of fixation, dislodgement and migration which may cause discomfort and penetration into the soft tissue, requiring removal [227].
Intramedullary fixation has subsequently been achieved with titanium alloy intramedullary splints with a rectangular cross-section for provision of rotational stability and flexible fixation of the fracture fragments. The splint front section was tapered to reduce the insertion force, and the splint tip was sloped to guide the splint along the medullary canal without penetrating the lateral cortex. Furthermore, these splints have a small extramedullary segment to aid the insertion and allow fixation with a single bicortical locking screw to prevent implant migration. The presence of only one point of fixation with no splint distal fixation, would provide no counterforce to prevent rib fracture distraction, but they seem to achieve stability by friction within the intramedullary canal. These splints were designed with a long intramedullary segment for stabilization of a single fracture. In case of multiple fractures of a single rib, splints may be combined with an additional splint or plate as long as implants do not structurally interfere or overlap. However, if a rib has several fractures that are accessible for plating, spanning multiple fractures with a single plate may remain advantageous over the use of multiple splints [155]. Tarng et al. have investigated the application of intramedullary splints with the help of VATS vs. NOM in a retrospective case–control study including 65 blunt chest trauma multiple rib fracture patients resulting in acute respiratory failure. Only twelve patients received intramedullary fixation, which however was associated with a shorter ICU length of stay, ventilator dependency time and total length of stay [258]. The placement of intramedullary splints has also been successfully applied in non-comparative trials [225, 259, 260]. The use of anatomically contoured titanium plates and splints can simplify the procedure of flail chest fixation. Marasco et al. reported the use of a combination of plates and splints in their practice to allows the greatest possible combination of fixation options to fix these complex chest wall injuries [260]. In contemporary practice, most surgeons experienced with SSRF do not routinely use splints; rather, their use is reserved for extenuating circumstances.
Absorbable plates and splint
Some studies reported the use of absorbable materials for SSRF, such as absorbable plates, splints and nails, mostly made of polylactic acid polymer, which have been attempted with the aim of eliminating the concern for residual substances in the body [32, 259, 261, 262]. However, a risk of re-fracture was described, especially for posterior rib fractures, because the strength of the plate may be inadequate [8, 263, 264]. Furthermore, a recent RCT by Ashley et al. raised worries about an increased postoperative rib displacement rate in patients treated with absorbable plates [265]. These plates remain an area of ongoing research.
Discussion
Rib fractures are one of the most common traumatic injuries [1–3] and are frequently associated with complications and long term poor functional outcomes [1, 4–6, 27]. The present position paper defines current indications, contraindications, timing, and technical details of SSRF, as sought by some other past consensus statements and guidelines [7, 16, 45–48, 91, 92, 154, 155], considering the contributions of the most recent literature.
Data from the last decade show that SSRF was used in less than 1% of rib fractures patients [95], being practiced almost exclusively but sporadically in those with flail chest (FC) [29]. Several studies showed decreased mechanical ventilation time, risk of pneumonia, ICU/hospital length of stay, in FC patients undergoing SSRF, that resulted in an overall reduction in cost and improved effectiveness compared with internal pneumatic stabilization [30–49]. Although the presence of FC remains the most evidence-based indication for SSRF, further studies that minimize selection bias are expected and a careful evaluation on an individual basis is essential in any case.
Most chest wall injuries are treated with NOM. However, there has been an increasing interest in SSRF, considering that the presence of FC or multiple rib fracture series have proven to be a risk factor for prolonged mechanical ventilation in patient treated non-operatively. Prolonged mechanical ventilation may lead to pneumonia development, sepsis, tracheostomy, barotrauma and often requires a protracted ICU stay [7–9]. Rib fractures number and displacement are recognized predictors of pulmonary complication and mortality in thoracic trauma patients, even in the absence of FC [50–53]. Multiple authors have shown that SSRF, compared with conventional NOM, can improve outcomes of trauma patients with non-FC fracture patterns, especially in the presence of pulmonary physiologic derangements or relapsing pain [57–60, 63–83]. Instead, rib fractures are also responsible for long-term morbidity, functional impairment and suboptimal quality-of-life, with significant loss of workdays and chronic pain, even in patients with less severe fracture patterns [10–14, 62]. Evidence regarding non-FC patients remains limited by lack of prospective study design, small sample sizes, delayed (> 72 h from injury) fixation, old or unclear fixation system, unreported surgical technique, poor follow-up, lack of national audit/ international register for SSRF and unclear and inconsistent injury categorization among studies [25, 26]. In response to these inconsistencies, a set of recommendations was proposed to standardize reporting SSRF studies [266] and the proposed displacement classification can improve communication between providers to help in adequately direct future research.
The development and optimization of protocols addressing NOM and surgical interventions for rib fractures patients is mandatory, especially for centers that frequently perform chest wall stabilization. A recent analysis from the CWIS collaborative centers found that 7% of patients with rib fractures underwent SSRF in dedicated centers [3]. Efforts to expand access to SSRF based on clinical factors may be warranted, as it currently appears to be driven mostly by center or surgeon characteristics [267]. Further investigation should also explore the center-specific volume impact on patient-reported outcomes including pain and post-discharge quality of life.
Evidence has shown that certain conditions previously considered a contraindication to SSRF, such as traumatic brain/spinal cord injury [97–100] or the presence of pulmonary contusion [123–127] are no longer regarded as such and should be evaluated on an individual case basis. In addition, advanced age [104–118] and significant comorbidities [112, 119] should promote a careful multidisciplinary evaluation to promote proper patient selection.
The timing of surgery must be made considering patients’ overall clinical conditions [137]. However, early SSRF (within 72 h of injury) is supported by growing evidence; it appears to be more technically feasible, improving patient outcomes, health care resource utilization and cost [30, 60, 74, 114, 128–136, 138, 139].
Chest CT is a cornerstone in the preoperative planning, and 3D reconstruction may assist it effectively. The application of 3D printing technique is becoming more valuable [144, 148, 149]; however, prospective multi-institutional studies are needed to validate its feasibility. In order to further refine the surgical plan, intraoperative ultrasound rib fracture sites localization may be helpful, allowing for shorter incision and operative time [145]. Recent studies found that the use of artificial intelligence (AI) on CT scans can aid radiologists in interpreting images to improve rib fracture detection [268, 269]. This AI application is very promising but further studies are necessary to better define its accuracy and suitability in clinical practice.
Various rib fracture sites suitable for SSRF have been investigated, considering factors such as fracture location, displacement, and associated injuries, to define the appropriate surgical approach and technique [46–48, 60, 91, 92, 155, 156, 164–166, 171]. However, there are no studies comparing different strategies of fixation.
Strategies for the management of concurrent intrathoracic injuries are discussed, including surgical prioritization and coordination of interventions to optimize patient outcomes [46, 172–179]. In addition, several surgical techniques for the management of significant chest wall injuries have been described in the literature [187–197] but new studies are necessary to detect the most appropriate management in each case. Mesh may have a role for significant chest wall muscle defects repair [190–196]. However, they are commercial devices with often poor scientific evidences of efficacy and safety due to the unique regulatory practices that distinguish medical devices from medications. Thus, surgeons must continue to advocate for more stringent oversight and improved scientific evaluation to serve patients properly [197].
Different surgical approaches and techniques for SSRF, including open reduction and internal fixation (ORIF) [7, 16, 46, 91, 92, 154, 155, 168, 208, 209], minimally invasive/ thoracoscopic [7, 16, 91, 92, 169, 170, 173, 174, 207, 210–223, 270, 271], and percutaneous [46, 224, 225] approaches have been analyzed with intraoperative considerations, as well as in terms of efficacy and safety. New studies comparing different surgical approaches are awaited [222], as well as studies that may better explore the combination of fixation options to stabilize complex chest wall injuries cases [260].
SSRF is not used frequently in many hospitals due to a lack of awareness of the evidence for rib fixation and knowledge of appropriate technique, but also due to poor diffusion and availability of prostheses specifically designed for rib fixation [7, 15, 155]. Nonetheless, recently several repair systems have addressed this deficiency. The review evaluates various stabilization methods and materials used in SSRF, such as plates and screws [32, 36, 57, 66, 82, 225], claw shaped [30, 122, 232–234, 239–244] and U plates [236, 237] as well as intramedullary devices, considering biomechanical properties, surgical outcomes and concerns.
Despite the potential advantages and disadvantages of these different repair system [91, 92, 154, 155, 238, 245, 246] and some highlighted peculiarities in biochemical studies [230, 231], no clinical evidence had demonstrated the superiority of one system respect to the others [248] and future studies must be performed to compare the results obtained with distinct fixation systems in relation to the different fracture types. Regarding plate and screw fixation, studies related to implant strength, fatigue and flexibility are mostly conducted on systems placed on the outer cortex [229–231]. Despite a biomechanical [252, 253] and a recent clinical study [222] have suggested some potential benefits of intrathoracic plating, future prospective multicenter researches are needed to confirm these findings, to collect and compare long-term outcomes and eventually lead to wide its adoption.
Similar to any operation and especially operations that involve implantation, concerns for post-operative infection following SSRF are justified. Surgical site infection (SSI) after SSRF is rare but morbid [272] and it was not one of our areas of recommendation due to the paucity of current data on the topic. Infection rates reported by some studies vary from approximately 2 to 4% of procedures [273, 274]. Infection following SSRF can become symptomatic within one week to several months after the procedure It usually presents with erythema, induration and drainage at the incision site, but increased pain or evidence of a systemic infection might be the only presenting symptoms. Hardware infection is usually treated with antibiotics and/or hardware removal. However, insufficient evidence precludes the ability to recommend statements for the treatment of SSI or implant-related infection following SSRF and further studies should be performed to identify the optimal management strategy in this population [275]. Moreover, limited evidence exists to delineate risk of implant infection among patients undergoing SSRF with or without concomitant infectious processes and further studies should be performed [276].
A retrospective study that included over 1200 patients found hardware failure in 3% of the patients with an equal number of them asymptomatic as those who had ongoing pain or clicking [277]. Hardware failure most commonly presents in a delayed fashion, weeks to months following the operation with the most common cause being screw migration or plate [73, 278], but insufficient individual patient data precluded characterizing where and why hardware failures occur. Minimizing SSRF hardware failure requires concerted research agenda to expand on the paucity of existing evidence. Patients with symptomatic failure may benefit from hardware removal if the clinical symptoms do not resolve or greatly affect their quality of life. Regardless of hardware failure, concerns remain for chronic pain and irritation from the implant, which needs to be better investigated in the long term in future studies.
Pain management for patients with rib fractures was not specifically addressed in this guideline due to its complexity and ongoing research. Traditionally, intravenous patient-controlled analgesia with opioids, thoracic epidural anesthesia (TEA), and paravertebral blocks (PVB) have been used as primary management techniques for pain associated with rib fractures. These techniques, however, may be contraindicated or have limited application in certain patient populations, such as in anticoagulated patients and in patients with vertebral fractures [279]. Recently, ultrasound-guided myofascial plane blocks such as the erector spinae plane blocks (ESPB) block and the serratus anterior plane blocks (SAPB) have been used to provide less invasive alternative pain management strategies, with low incidences of adverse effects while exhibiting similar levels of analgesia [280, 281]. Peripheral nerve blocks have significant potential and may be preferred to neuraxial techniques in the future; however, further research is needed to clarify the effectiveness and weaknesses of different techniques, the use of which in different centers often depends on the preferences and experience of the team [282]. The identification of the optimal pain management strategies in patients with rib fractures can allow to standardize their use during NOM in different centers and can contribute to improve patients’ selection for surgical treatment.
Furthermore, the optimal perioperative analgesic therapy when SSRF is attempted remains unclear, while suboptimal postoperative analgesia can adversely affect respiratory mechanics [279]. Postoperative analgesia regimens traditionally include TEA, acetaminophen, NSAIDs, gabapentinoids, and oral or intravenous opioid medications as necessary [283].
Surgeons can perform locoregional analgesia on the intercostal nerves under direct visualization tunneling analgesic catheter with continuous bupivacaine infusion into the subscapular space or trough thoracoscopic intercostal blocks with single-dose of liposomal bupivacaine [284]. Moreover, some studies described the simultaneous SSRF and intercostal nerve cryoablation as a safe and viable procedure, without immediate or long-term complications [285].
However, evaluating surgical analgesic techniques efficacy for rib fractures requires further study and preoperative regional anesthetic blocks are more frequently performed to provide analgesia to patients throughout the operation and into the postoperative period. Particularly, ESPB seems to provide an effective postoperative analgesia with an improved safety profile and relative ease of use compared to TEA [283]. Ultrasound-guided SAPB and intercostal nerve block are safe alternative regional blocks techniques that may confer analgesic benefits [286]. Potential recommendations regarding usage preferences of these alternative regional blocks techniques in rib fracture patients management awaits further studies, especially when SSRF is attempted.
Conclusion
This position paper provides a comprehensive overview of SSRF to address crucial key focus questions on surgical treatment of rib fractures. Expert recommendations clarify current evidence-based appropriate surgical indications, contraindications, optimal timing of surgery, preoperative imaging evaluation, rib fracture sites for surgical fixation, management of concurrent thoracic injuries, surgical approaches, stabilization methods and material selection. This review could help to guide clinicians in optimizing the management of rib fractures and improving patient outcomes, as well as to direct future research.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ASER
American Society of Emergency Radiology
- CWIS
Chest Wall Injury Society
- CWS
Chest wall stabilization
- ESPB
Erector spinae plane blocks
- FC
Flail chest
- FDA
Food and Drug Administration
- ICU
Intensive care unit
- LoE
Level of Evidence
- MIPO
Minimally invasive plating osteosynthesis
- NOM
Nonoperative management
- NPS
Numeric pain score
- ORIF
Operative reduction and internal fixation
- PVB
Paravertebral blocks
- PS
Position statement
- PC
Pulmonary contusion
- QoL
Quality of life
- RCT
Randomized clinical trial
- SAPB
Serratus anterior plane blocks
- SSI
Surgical site infection
- SSRF
Surgical stabilization of rib fractures
- TEA
Thoracic epidural anesthesia
- TBI
Traumatic brain injury
- VATS
Video-assisted thoracoscopy surgery
- WSES
World Society of Emergency Surgery
Author contributions
G.S., R.B., K.H., D.P., A.R. and B.T. performed the literature review. G.S. and R.B. wrote the main manuscript text. F.C. and F.P. are responsible for the manuscript conception and draft. All listed authors made important contributions to the conception and design of the work, have drafted the work, or substantively revised it; all authors reviewed and approved the final manuscript.
Funding
No authors received any funding resource.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lundin A, et al. Thoracic injuries in trauma patients: epidemiology and its influence on mortality. Scand J Trauma Resusc Emerg Med. 2022;30:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benhamed A, Ndiaye A, Emond M, Lieutaud T, Boucher V, Gossiome A, Laumon B, Gadegbeku B, Tazarourte K. Road traffic accident-related thoracic trauma: epidemiology, injury pattern, outcome, and impact on mortality-A multicenter observational study. PLoS ONE. 2022;17(5):e0268202. 10.1371/journal.pone.0268202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson EA, Wijffels MME, Kaye A, Forrester JD, Moutinho M, Majerick S, Bauman ZM, Janowak CF, Patel B, Wullschleger M, Clevenger L, Van Lieshout EMM, Tung J, Woodfall M, Hill TR, White TW, Doben AR. Incidence of surgical rib fixation at chest wall injury society collaborative centers and a guide for expected number of cases (CWIS-CC1). Eur J Trauma Emerg Surg. 2023. 10.1007/s00068-023-02343-4. [DOI] [PubMed] [Google Scholar]
- 4.Beshay M, Mertzlufft F, Kottkamp HW, et al. Analysis of risk factors in thoracic trauma patients with a comparison of a modern trauma centre: a mono-centre study. World J Emerg Surg. 2020;15:45. 10.1186/s13017-020-00324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Tennakoon L, You JG, Kaghazchi A, Forrester JD, Spain DA. Pulmonary contusions in patients with rib fractures: the need to better classify a common injury. Am J Surg. 2021;221(1):211–5. 10.1016/j.amjsurg.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Peuker F, Hoepelman RJ, Beeres FJP, Balogh ZJ, Beks RB, Sweet AAR, IJpma FFA, Lansink KWW, van Wageningen B, Tromp TN, Minervini F, van Veelen NM, Hoogendoorn JM, de Jong MB, van Baal M, Leenen LPH, Groenwold RHH, Houwert RM. Nonoperative treatment of multiple rib fractures, the results to beat: International multicenter prospective cohort study among 845 patients. J Trauma Acute Care Surg. 2023. 10.1097/TA.0000000000004183. [DOI] [PubMed] [Google Scholar]
- 7.Nirula R, Diaz JJ Jr, Trunkey DD, Mayberry JC. Rib fracture repair: indications, technical issues, and future directions. World J Surg. 2009;33(1):14–22. 10.1007/s00268-008-9770-y. [DOI] [PubMed] [Google Scholar]
- 8.Lafferty PM, Anavian J, Will RE, Cole PA. Operative treatment of chest wall injuries: indications, technique, and outcomes. J Bone Jt Surg Am. 2011;93(1):97–110. 10.2106/JBJS.I.00696. [DOI] [PubMed] [Google Scholar]
- 9.Simon B, Ebert J, Bokhari F, et al. Management of pulmonary contusion and flail chest: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S351–61. 10.1097/TA.0b013e31827019fd. [DOI] [PubMed] [Google Scholar]
- 10.Sarode AL, Ho VP, Pieracci FM, Moorman ML, Towe CW. The financial burden of rib fractures: national estimates 2007 to 2016. Injury. 2021;52:2180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caragounis EC, Olsen MF, Pazooki D, Granhed H. Surgical treatment of multiple rib fractures and flail chest in trauma: a one-year follow-up study. World J Emerg Surg. 2016;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J, Khan S, Sheira D, Hakes NA, Aboukhater L, Spain DA. Prospective study of long-term quality-of-life after rib fractures. Surgery. 2022;172(1):404–9. 10.1016/j.surg.2021.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Heindel P, Ordoobadi A, El Moheb M, Serventi-Gleeson J, Garvey S, Heyman A, Patel N, Sanchez S, Kaafarani HMA, Herrera-Escobar J, Salim A, Nehra D. Patient-reported outcomes 6 to 12 months after isolated rib fractures: A nontrivial injury pattern. J Trauma Acute Care Surg. 2022;92(2):277–86. 10.1097/TA.0000000000003451. [DOI] [PubMed] [Google Scholar]
- 14.Fabricant L, Ham B, Mullins R, Mayberry J. Prolonged pain and disability are common after rib fractures. Am J Surg. 2013;205:511–5. [DOI] [PubMed] [Google Scholar]
- 15.Mayberry JC, Ham LB, Schipper PH, Ellis TJ, Mullins RJ. Surveyed opinion of American trauma, orthopedic, and thoracic surgeons on rib and sternal fracture repair. J Trauma. 2009;66(3):875–9. 10.1097/TA.0b013e318190c3d3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Song L, Ning S, Xie H, Li N, Wang Y. Recent advances in rib fracture fixation. J Thorac Dis. 2019;11:S1070–7. 10.21037/jtd.2019.04.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieracci FM. Completely thoracoscopic surgical stabilization of rib fractures: can it be done and is it worth it? J Thorac Dis. 2019;11:S1061–9. 10.21037/jtd.2019.01.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaban Y, Frank M, Schubl S, Sakae C, Bagga A, Hegazi M, Gross R, Doben A, Nahmias J. The history of surgical stabilization of rib fractures (SSRF). Surg Pract Sci. 2022;10:100084. 10.1016/j.sipas.2022.100084. [Google Scholar]
- 19.Bala M, Kashuk J, Moore EE, Catena F, Leppaniemi A, Ansaloni L, et al. Establishing position papers by the WSES. World J Emerg Surg. 2018. 10.1186/s13017-018-0163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. In: Cochrane handbook for systematic reviews of interventions. Cochrane Book Series. Hoboken: Wiley; 2008. [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–3. 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‑Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‑analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 25.Edwards JG, Clarke P, Pieracci FM, Bemelman M, Black EA, Doben A, et al. Taxonomy of multiple rib fractures: results of the chest wall injury society international consensus survey. J Trauma Acute Care Surg. 2020;88(2):e40–5. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen J, Archer-Arroyo K, Gross JA, Steenburg SD, Sliker CW, Meyer CH, Nummela MT, Pieracci FM, Kaye AJ. Improved chest wall trauma taxonomy: an interdisciplinary CWIS and ASER collaboration. Emerg Radiol. 2023;30(5):637–45. 10.1007/s10140-023-02171-4. [DOI] [PubMed] [Google Scholar]
- 27.Alanwer KM, Refat AM, Negm EM. Impact of flail chest injury on morbidity and outcome: ten years’ experience at a tertiary care hospital in a developing country. BMC Anesthesiol. 2023;23:229. 10.1186/s12871-023-02185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson TR, McElvenny RT, Head JR. Chest wall stabilization by soft tissue traction: a new method. JAMA. 1954;156:768–9. [DOI] [PubMed] [Google Scholar]
- 29.Bemelman M, Poeze M, Blokhuis TJ, Leenen LP. Historic overview of treatment techniques for rib fractures and flail chest. Eur J Trauma Emerg Surg. 2010;36(5):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka H, Yukioka T, Yamaguti Y, Shimizu S, Goto H, Matsuda H, Shimazaki S. Surgical stabilization of internal pneumatic stabilization? A prospective randomized study of management of severe flail chest patients. J Trauma. 2002;52:727–32. 10.1097/00005373-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Granetzny A, Abd El-Aal M, Emam E, Shalaby A, Boseila A. Surgical versus conservative treatment of flail chest. Evaluation of the pulmonary status. Interact Cardiovasc Thorac Surg. 2005;4:583–7. 10.1510/icvts.2005.111807. [DOI] [PubMed] [Google Scholar]
- 32.Marasco SF, Davies AR, Cooper J, Varma D, Bennett V, Nevill R, Lee G, Bailey M, Fitzgerald M. Prospective randomized controlled trial of operative rib fixation in traumatic flail chest. J Am Coll Surg. 2013;216:924–32. 10.1016/j.jamcollsurg.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Bhatnagar A, Mayberry J, Nirula R. Rib fracture fixation for flail chest: what is the benefit? J Am Coll Surg. 2012;215(2):201–5. 10.1016/j.jamcollsurg.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Lardinois D, Krueger T, Dusmet M, et al. Pulmonary function testing after operative stabilisation of the chest wall for flail chest. Eur J Cardiothorac Surg. 2001;20:496–501. [DOI] [PubMed] [Google Scholar]
- 35.Jayle CP, Allain G, Ingrand P, Laksiri L, Bonnin E, Hajj-Chahine J, Mimoz O, Corbi P. Flail chest in polytraumatized patients: surgical fixation using Stracos reduces ventilator time and hospital stay. BioMed Res Int. 2015. 10.1155/2015/624723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Liu P, Chen J, Xie J, Yang F, Liao Y. A randomized controlled trial of surgical rib fixation in polytrauma patients with flail chest. J Surg Res. 2019;242:223–30. 10.1016/j.jss.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Davignon K, Kwo J, Bigatello LM. Pathophysiology and management of the flail chest. Minerva Anestesiol. 2004;70:193–9. [PubMed] [Google Scholar]
- 38.Cataneo AJ, Cataneo DC, de Oliveira FH, Arruda KA, El Dib R, de Oliveira Carvalho PE. Surgical versus nonsurgical interventions for flail chest. Cochrane Database Syst Rev. 2015;2015(7):CD009919. 10.1002/14651858.CD009919.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coughlin TA, Ng JW, Rollins KE, Forward DP, Ollivere BJ. Management of rib fractures in traumatic flail chest: a meta-analysis of randomised controlled trials. Bone Jt J. 2016;98(8):1119–25. 10.1302/0301-620X.98B8.37282. [DOI] [PubMed] [Google Scholar]
- 40.Schuurmans J, Goslings JC, Schepers T. Operative management versus non-operative management of rib fractures in flail chest injuries: a systematic review. Eur J Trauma Emerg Surg. 2016;43:163–8. 10.1007/s00068-016-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apampa AA, Ali A, Kadir B, Ahmed Z. Safety and effectiveness of surgical fixation versus non-surgical methods for the treatment of flail chest in adult populations: a systematic review and meta-analysis. Eur J Trauma Emerg Surg. 2022;48(2):1025–34. 10.1007/s00068-021-01606-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slobogean GP, MacPherson CA, Sun T, Pelletier ME, Hameed SM. Surgical fixation vs nonoperative management of flail chest: a meta-analysis. J Am Coll Surg. 2013;216:302–11. 10.1016/j.jamcollsurg.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Leinicke JA, Elmore L, Freeman BD, Colditz GA. Operative management of rib fractures in the setting of flail chest: a systematic review and meta-analysis. Ann Surg. 2013;258(6):914–21. 10.1097/SLA.0b013e3182895bb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swart E, Laratta J, Slobogean G, Mehta S. Operative treatment of rib fractures in flail chest injuries: a meta-analysis and cost-effectiveness analysis. J Orthop Trauma. 2017;31:64–70. 10.1097/BOT.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 45.Kasotakis G, Hasenboehler EA, Streib EW, Patel N, Patel MB, Alarcon L, Bosarge PL, Love J, Haut ER, Como JJ. Operative fixation of rib fractures after blunt trauma: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:618–26. 10.1097/TA.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 46.Pieracci FM, Majercik S, Ali-Osman F, Ang D, Doben A, Edwards JG, French B, Gasparri M, Marasco S, Minshall C, Sarani B, Tisol W, VanBoerum DH, White TW. Consensus statement: surgical stabilization of rib fractures rib fracture colloquium clinical practice guidelines. Injury. 2017;48:307–21. 10.1016/j.injury.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Chest Wall Injury Society. Indications for surgical stabilization of rib fractures. 2020. https://cwisociety.org/wp-content/uploads/2020/05/CWIS-SSRF-Guideline-01102020.pdf
- 48.Kong LW, Huang GB, Yi YF, Du DY. The Chinese consensus for surgical treatment of traumatic rib fractures 2021 (C-STTRF 2021). Chin J Traumatol. 2021;24:3–319. 10.1016/j.cjtee.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulte K, Whitaker D, Attia R. In patients with acute flail chest does surgical rib fixation improve outcomes in terms of morbidity and mortality? Interact Cardiovasc Thorac Surg. 2017;23:314–9. 10.1093/icvts/ivw092. [DOI] [PubMed] [Google Scholar]
- 50.Chien CY, Chen YH, Han ST, Blaney GN, Huang TS, Chen KF. The number of displaced rib fractures is more predictive for complications in chest trauma patients. Scand J Trauma Resusc Emerg Med. 2017;25:19. 10.1186/s13049-017-0368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flagel BT, Luchette FA, Reed RL, et al. Half-a-dozen ribs:the breakpoint for mortality. Surgery. 2005;138:717–23. [DOI] [PubMed] [Google Scholar]
- 52.Gerakopoulos E, Walker L, Melling D, Scott S. Surgical management of multiple rib fractures reduces the hospital lenght of stay and the mortality rate in major trauma patients: a comparative study in a UK Maior Trauma Center. J Orthop Trauma. 2019;33(1):9–14. [DOI] [PubMed] [Google Scholar]
- 53.Senekjian L, Birkas Y, Buhavac M, et al. Stop flailing: the impact of bicortically displaced rib fractures on pulmonary outcomes in patients with chest trauma—an American Association for the Surgery of Trauma multi-institutional study. J Trauma Acute Care Surg. 2020;89(4):658–64. 10.1097/ta.0000000000002848. [DOI] [PubMed] [Google Scholar]
- 54.Marasco SF, Martin K, Niggemeyer L, Summerhayes R, Fitzgerald M, Bailey M. Impact of rib fixation on quality of life after major trauma with multiple rib fractures. Injury. 2019;50(1):119–24. [DOI] [PubMed] [Google Scholar]
- 55.Hoepelman RJ, Beeres FJP, Beks RB, et al. Non-operative treatment for multiple rib fractures after blunt thoracic trauma: a multicenter prospective cohort study. Eur J Trauma Emerg Surg. 2023;49(1):461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer DE, Harvin JA, Vincent L, et al. Randomized controlled trial of surgical rib fixation to nonoperative management in severe chest wall injury. Ann Surg. 2023;278(3):357–65. 10.1097/SLA.000000000005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pieracci FM, Lin Y, Rodil M, Synder M, Herbert B, Tran DK, Stoval RT, Johnson JL, Biffl WL, Barnett CC, Cothren-Burlew C, Fox C, Jurkovich GJ, Moore EE. A prospective, controlled clinical evaluation of surgical stabilization of severe rib fractures. J Trauma Acute Care Surg. 2016;80:187–94. 10.1097/TA.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 58.Wu WM, Yang Y, Gao ZL, Zhao TC, He WW. Which is better to multiple rib fractures, surgical treatment or conservative treatment? Int J Clin Exp Med. 2015;8:7930–6. [PMC free article] [PubMed] [Google Scholar]
- 59.Dehghan N, Nauth A, Schemitsch E, Vicente M, Jenkinson R, Kreder H, McKee M. Operative vs nonoperative treatment of acute unstable chest wall injuries: a randomized clinical trial. JAMA Surg. 2022;157:983–90. 10.1001/jamasurg.2022.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pieracci FM, Leasia K, Bauman Z, Eriksson EA, Lottenberg L, Majercik S, Powell L, Sarani B, Semon G, Thomas B, Zhao F, Dyke C, Doben AR. A multicenter, prospective, controlled clinical trial of surgical stabilization of rib fractures in patients with severe, nonflail fracture patterns (Chest Wall Injury Society NONFLAIL). J Trauma Acute Care Surg. 2020;88:249–57. 10.1097/TA.0000000000002559). [DOI] [PubMed] [Google Scholar]
- 61.de Moya M, Bramos T, Agarwal S, Fikry K, Janjua S. Pain as an indication for rib fixation: a bi-institutional pilot study. J Trauma. 2011;71:1750–4. [DOI] [PubMed] [Google Scholar]
- 62.Gordy S, Fabricant L, Ham B, Mullins R, Mayberry J. The contribution of rib fractures to chronic pain and disability. Am J Surg. 2014;207(5):659–63. [DOI] [PubMed] [Google Scholar]
- 63.Marasco SF, Balogh ZJ, Wullschleger ME, Hsu J, Patel B, Fitzgerald M, Martin K, Summerhayes R, Bailey M. Rib fixation in non-ventilator-dependent chest wall injuries: a prospective randomized trial. J Trauma Acute Care Surg. 2022;92:1047–53. 10.1097/TA.0000000000003549. [DOI] [PubMed] [Google Scholar]
- 64.Billè A, Okiror L, Campbell A, Simons J, Routledge T. Evaluation of longterm results and quality of life in patients who underwent rib fixation with titanium devices after trauma. Gen Thorac Cardiovasc Surg. 2013;61(6):345–9. [DOI] [PubMed] [Google Scholar]
- 65.Campbell N, Conaglen P, Martin K, Antippa P. Surgical stabilization of rib fractures using inion OTPS wraps—techniques and quality of life followup. J Trauma Acute Care Surg. 2009;67(3):596–601. [DOI] [PubMed] [Google Scholar]
- 66.Majercik S, Cannon Q, Granger SR, VanBoerum DH, White TW. Long-term patient outcomes after surgical stabilization of rib fractures. Am J Surg. 2014;208(1):88–92. [DOI] [PubMed] [Google Scholar]
- 67.Mayberry JC, Kroeker AD, Ham LB, Mullins RJ, Trunkey DD. Long-term morbidity, vain, and disability after repair of severe chest wall injuries. Am Surg. 2009;75(5):389–94. [PubMed] [Google Scholar]
- 68.Khandelwal G, Mathur RK, Shukla S, et al. A prospective single center study to assess the impact of surgical stabilization in patients with rib fracture. Int J Surg. 2011;9:478–81. 10.1016/j.ijsu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Liang YS, Yu KC, Wong CS, Kao Y, Tiong TY, Tam KW. Does surgery reduce the risk of complications among patients with multiple rib fractures? A meta-analysis. Clin Orthop Relat Res. 2018;477:193–205. 10.1097/CORR.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beks RB, Peek J, de Jong MB, Wessem KJP, Öner CF, Hietbrink F, Leenen LPH, Groenwold RHH, Houwert RM. Fixation of flail chest or multiple rib fractures: current evidence and how to proceed. A systematic review and meta-analysis. Eur J Trauma Emerg Surg. 2018;45:631–44. 10.1007/s00068-018-1020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X, Xiong K. Surgical management versus non-surgical management of rib fractures in chest trauma: a systematic review and meta-analysis. J Cardiothorac Surg. 2019;14:45. 10.1186/s13019-019-0865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Long R, Tian J, Wu S, Li Y, Yang X, Fei J. Clinical efficacy of surgical versus conservative treatment for multiple rib fractures: a meta-analysis of randomized controlled trials. Int J Surg. 2020;83:79–88. 10.1016/j.ijsu.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 73.Choi J, Gomez GI, Kaghazchi A, Borghi JA, Spain DA, Forrester JD. Surgical stabilization of rib fracture to mitigate pulmonary complication and mortality: a systematic review and Bayesian meta-analysis. J Am Coll Surg. 2021;232:211–9. 10.1016/j.jamcollsurg.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 74.Sawyer E, Wullschleger M, Muller N, Muller M. Surgical rib fixation of multiple rib fractures and flail chest: a systematic review and meta-analysis. J Surg Res. 2022;276:221–34. 10.1016/j.jss.2022.02.055. [DOI] [PubMed] [Google Scholar]
- 75.Craxford S, Owyang D, Marson B, Rowlins K, Coughlin T, Forward D, Ollivere B. Surgical management of rib fractures after blunt trauma: a systematic review and meta-analysis of randomised controlled trials. Ann R Coll Surg Engl. 2022;104:249–56. 10.1308/rcsann.2021.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wijffels MME, Prins JTH, Perpetua Alvino EJ, Van Lieshout EMM. Operative versus nonoperative treatment of multiple simple rib fractures: a systematic review and meta-analysis. Injury. 2020;51:2368–78. 10.1016/j.injury.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 77.He W, Yang Y, Salonga R, et al. Surgical stabilization of multiple rib fractures in an Asian population: a systematic review and meta-analysis. J Thorac Dis. 2023;15(9):4961–75. 10.21037/jtd-23-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Jong MB, Houwert RM, van Heerde S, de Steenwinkel M, Hietbrink F, Leenen LPH. Surgical treatment of rib fracture nonunion: a single center experience. Injury. 2018;49(3):599–603. 10.1016/j.injury.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Van Wijck SFM, Van Lieshout EMM, Prins JTH, Verhofstad MHJ, Van Huijstee PJ, Vermeulen J, Wijffels MME. Outcome after surgical stabilization of symptomatic rib fracture nonunion: a multicenter retrospective case series. Eur J Trauma Emerg Surg. 2022;48(4):2783–93. 10.1007/s00068-021-01867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeGenova DT, Miller KB, McClure TT, Schuette HB, French BG, Taylor BC. Operative fixation of rib fracture nonunions. Arch Orthop Trauma Surg. 2023;143(6):3047–54. 10.1007/s00402-022-04540-z. [DOI] [PubMed] [Google Scholar]
- 81.Bauman ZM, Khan H, Cavlovic L, Todd S, Cemaj S, Daubert T, Raposo-Hadley A, Matos M, Sheppard O, Berning B, Kamien A, Evans CH, Cantrell E. Better late than never-a single-center review of delayed rib fixation for symptomatic rib fractures and nonunions. J Trauma Acute Care Surg. 2023;95(6):880–4. 10.1097/TA.0000000000004136. [DOI] [PubMed] [Google Scholar]
- 82.Lin HL, Tarng YW, Wu TH, et al. The advantages of adding rib fixations during VATS for retained hemothorax in serious blunt chest trauma-A prospective cohort study. Int J Surg. 2019;65:13–8. 10.1016/j.ijsu.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 83.Green EA, Guidry C, Harris C, McGrew P, Schroll R, Hussein M, Toraih E, Kolls J, Duchesne J, Taghavi S. Surgical stabilization of traumatic rib fractures is associated with reduced readmissions and increased survival. Surgery. 2021;170(6):1838–48. 10.1016/j.surg.2021.05.032. [DOI] [PubMed] [Google Scholar]
- 84.Mullens CL, Seamon MJ, Shiroff A, Cannon JW, Kaplan LJ, Pascual JL, et al. A statewide assessment of rib fixation patterns reveals missed opportunities. J Surg Res. 2019;244:205–11. [DOI] [PubMed] [Google Scholar]
- 85.Kane ED, Jeremitsky E, Pieracci FM, Majercik S, Doben AR. Quantifying and exploring the recent national increase in surgical stabilization of rib fractures. J Trauma Acute Care Surg. 2017;83(6):1047–52. [DOI] [PubMed] [Google Scholar]
- 86.Rockne WY, Grigorian A, Christian A, Nahmias J, Lekawa M, Dolich M, et al. No difference in mortality between level I and level II trauma centers performing surgical stabilization of rib fracture. Am J Surg. 2021;221(5):1076–81. [DOI] [PubMed] [Google Scholar]
- 87.Tillman AC, Martin TJ, Lueckel SN, Kheirbek T. Lucky number 13: association between center-specific chest wall stabilization volumes and patient outcomes. J Trauma Acute Care Surg. 2022;93(6):774–80. 10.1097/TA.0000000000003764. [DOI] [PubMed] [Google Scholar]
- 88.Sahr SM, Webb ML, Renner CH, Sokol RK, Swegle JR. Implementation of a rib fracture triage protocol in elderly trauma patients. J Trauma Nurs. 2013;20(4):172–5 (quiz 6-7). [DOI] [PubMed] [Google Scholar]
- 89.Forrester JD, Bauman ZM, Doben AR, Eriksson EA. Chest wall injury centers-how we did it. J Thorac Dis. 2021;13(10):6104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eriksson EA, Waite A, Whitbeck SS, Bach JA, Bauman ZM, Cavlovic L, Dale K, DeVoe WB, Doben AR, Edwards JG, Forrester JD, Kaye AJ, Green J, Hsu J, Hufford A, Janowak C, Kartiko S, Moore EE, Patel B, Pieracci F, Sarani B, Schubl SD, Semon G, Thomas BW, Tung J, Van Lieshout EMM, White TW, Wijffels MME, Wullschleger ME, CWIS Collaborative Centers (corporate authorship). An initiative to assess and improve the resources and patient care processes used among Chest Wall Injury Society collaborative centers (CWIS-CC2). J Trauma Acute Care Surg. 2023. 10.1097/TA.0000000000004158. [DOI] [PubMed] [Google Scholar]
- 91.de Campos JRM, White TW. Chest wall stabilization in trauma patients: why, when, and how? J Thorac Dis. 2018;10:S951–62. 10.21037/jtd.2018.04.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fokin AA, Hus N, Wycech J, Rodriguez E, Puente I. Surgical stabilization of rib fractures: indications, techniques, and pitfalls. JBJS Essent Surg Tech. 2020;10:e0032. 10.2106/JBJS.ST.19.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofman M, Andruszkow H, Kobbe P, Poeze M, Hildebrand F. Incidence of post-traumatic pneumonia in poly-traumatized patients: identifying the role of traumatic brain injury and chest trauma. Eur J Trauma Emerg Surg. 2020;46(1):11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ziegler DW, Agarwal NN. The morbidity and mortality of rib fractures. J Trauma. 1994;37(6):975–9. [DOI] [PubMed] [Google Scholar]
- 95.Dehghan N, de Mestral C, McKee MD, Schemitsch EH, Nathens A. Flail chest injuries: a review of outcomes and treatment practices from the National Trauma Data Bank. J Trauma Acute Care Surg. 2014;76(2):462–8. [DOI] [PubMed] [Google Scholar]
- 96.Pieracci FM, Agarwal S, Doben A, Shiroff A, Lottenberg L, Whitbeck SA, White TW. Indications for surgical stabilization of rib fractures in patients without flail chest: surveyed opinions of members of the Chest Wall Injury Society. Int Orthop. 2018;42(2):401–8. 10.1007/s00264-017-3612-1. [DOI] [PubMed] [Google Scholar]
- 97.Prins JTH, Van Lieshout EMM, Ali-Osman F, Bauman ZM, Caragounis EC, Choi J, Christie DB 3rd, Cole PA, DeVoe WB, Doben AR, Eriksson EA, Forrester JD, Fraser DR, Gontarz B, Hardman C, Hyatt DG, Kaye AJ, Ko HJ, Leasia KN, Leon S, Marasco SF, McNickle AG, Nowack T, Ogunleye TD, Priya P, Richman AP, Schlanser V, Semon GR, Su YH, Verhofstad MHJ, Whitis J, Pieracci FM, Wijffels MME. Outcome after surgical stabilization of rib fractures versus nonoperative treatment in patients with multiple rib fractures and moderate to severe traumatic brain injury (CWIS-TBI). J Trauma Acute Care Surg. 2021;90(3):492–500. 10.1097/TA.0000000000002994 [DOI] [PubMed]
- 98.Prins JTH, Van Lieshout EMM, Ali-Osman F, Bauman ZM, Caragounis EC, Choi J, Christie DB 3rd, Cole PA, DeVoe WB, Doben AR, Eriksson EA, Forrester JD, Fraser DR, Gontarz B, Hardman C, Hyatt DG, Kaye AJ, Ko HJ, Leasia KN, Leon S, Marasco SF, McNickle AG, Nowack T, Ogunleye TD, Priya P, Richman AP, Schlanser V, Semon GR, Su YH, Verhofstad MHJ, Whitis J, Pieracci FM, Wijffels MME. Surgical stabilization versus nonoperative treatment for flail and non-flail rib fracture patterns in patients with traumatic brain injury. Eur J Trauma Emerg Surg. 2022;48(4):3327–38. 10.1007/s00068-022-01906-1 [DOI] [PMC free article] [PubMed]
- 99.Freitag P, Bechmann C, Eden L, Meffert R, Walles T. Surgical stabilization of serial rib fractures is advantageous in patients with relevant traumatic brain injury. Eur J Trauma Emerg Surg. 2022;48(4):3237–42. 10.1007/s00068-022-01886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lagazzi E, de Roulet A, Proaño-Zamudio JA, Argandykov D, Romijn AS, Abiad M, Rafaqat W, Hwabejire JO, Velmahos GC, Paranjape C. Is severe traumatic brain injury no longer a contraindication for surgical stabilization of rib fractures in patients with multiple rib fractures? A propensity-matched analysis. J Trauma Acute Care Surg. 2023;94(6):823–30. 10.1097/TA.0000000000003954. [DOI] [PubMed] [Google Scholar]
- 101.Pennington MW, Roche AM, Bransford RJ, Zhang F, Dagal A. A technique to allow prone positioning in the spine surgery patient with unstable spine fracture and flail segment rib fractures. A A Case Rep. 2016;7(1):2–4. [DOI] [PubMed] [Google Scholar]
- 102.Alvi MA, Kapurch JR, Ivanov DV, Kerezoudis P, Bydon M, Freedman BA. Does the coexistence of multiple segmental rib fractures in polytrauma patients presenting with “major” vertebral fracture affect care and acute outcomes? J Orthop Trauma. 2019;33:23–30. 10.1097/BOT.0000000000001316. [DOI] [PubMed] [Google Scholar]
- 103.Ladhani HA, Harrell KN, Burlew CC, van Wijck SFM, Smith EF, Coleman JR, et al. Early surgical stabilization of rib fractures is feasible in patients with non-urgent operative pelvic injuries. Am Surg. 2023;12:5813–20. [DOI] [PubMed] [Google Scholar]
- 104.Barry R, Thompson E. Outcomes after rib fractures in geriatric blunt trauma patients. Am J Surg. 2018;215:1020–3. 10.1016/j.amjsurg.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 105.Elmistekawy EM, Hammad AA. Isolated rib fractures in geriatric patients. Ann Thorac Med. 2007;2:166–8. 10.4103/1817-1737.36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Battle CE, Evans PA. Predictors of mortality in patients with flail chest: a systematic review. Emerg Med J EMJ. 2015;32:961–5. 10.1136/emermed-2015-204939. [DOI] [PubMed] [Google Scholar]
- 107.Marini CP, Petrone P, Soto-Sánchez A, García-Santos E, Stoller C, Verde J. Predictors of mortality in patients with rib fractures. Eur J Trauma Emerg Surg. 2021;47:1527–34. 10.1007/s00068-019-01183-5. [DOI] [PubMed] [Google Scholar]
- 108.McGuinness MJ, Ferguson LR, Watt I, Harmston C. Outcomes in patients with fractured ribs: middle aged at same risk of complications as the elderly. N Z Med J. 2021;134:38–45. [PubMed] [Google Scholar]
- 109.Stitzel JD, Kilgo PD, Weaver AA, Martin RS, Loftis KL, Meredith JW. Age thresholds for increased mortality of predominant crash induced thoracic injuries. Ann Adv Autom Med. 2010;54:41–50. [PMC free article] [PubMed] [Google Scholar]
- 110.Mvoula L, Skubic J, Weaver D, Betancourt-Garcia M. Morbidity and mortality after rib fracture in elderly patients (> 65 years old) compared to a younger cohort (≤ 65 years of age) at doctor hospital renaissance health. Cureus. 2022;14:e30941. 10.7759/cureus.30941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Battle C, Carter K, Newey L, Giamello JD, Melchio R, Hutchings H. Risk factors that predict mortality in patients with blunt chest wall trauma: an updated systematic review and meta-analysis. Emerg Med J. 2023;40(5):369–78. 10.1136/emermed-2021-212184. [DOI] [PubMed] [Google Scholar]
- 112.Cooper E, Wake E, Cho C, Wullschleger M, Patel B. Outcomes of rib fractures in the geriatric population: a 5-year retrospective, single-institution, Australian study. ANZ J Surg. 2021;91:1886–92. 10.1111/ans.17064. [DOI] [PubMed] [Google Scholar]
- 113.Agrafiotis AC, Bourlon L, Etienne H, Le Roux M, Mazzoni L, Giol M, Debrosse D, Assouad J. Is surgical rib fixation in patients aged more than 65 years old associated with worse outcomes compared to younger patients? A retrospective single-center study. Acta Chir Belg. 2022;122:35–40. 10.1080/00015458.2020.1846939. [DOI] [PubMed] [Google Scholar]
- 114.Pieracci FM, Leasia K, Hernandez MC, Kim B, Cantrell E, Bauman Z, Gardner S, Majercik S, White T, Dieffenbaugher S, Eriksson E, Barns M, Benjamin Christie D, Lasso ET, Schubl S, Sauaia A, Doben AR. Surgical stabilization of rib fractures in octogenarians and beyond-what are the outcomes? J Trauma Acute Care Surg. 2021;90:1014–21. 10.1097/TA.0000000000003140. [DOI] [PubMed] [Google Scholar]
- 115.Fitzgerald MT, Ashley DW, Abukhdeir H, Christie DB. Rib fracture fixation in the 65 years and older population: a paradigm shift in management strategy at a Level I trauma center. J Trauma Acute Care Surg. 2017;82:524–7. 10.1097/TA.0000000000001330. [DOI] [PubMed] [Google Scholar]
- 116.Christie DB, Nowack T, Drahos A, Ashley DW. Geriatric chest wall injury: is it time for a new sense of urgency? J Thorac Dis. 2019;11:S1029–33. 10.21037/jtd.2018.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wasfie T, Sowa M, White H, Nesheiwat Oms-Iv SN, Hille J, Hella J, Barber K, Shapiro B. Rib plating outcomes in elderly trauma patients with multiple rib fractures: a community hospital experience. Am Surg. 2023. 10.1177/00031348231161713. [DOI] [PubMed] [Google Scholar]
- 118.Hoepelman RJ, Beeres FJP, Heng M, Knobe M, Link BC, Minervini F, Babst R, Houwert RM, van de Wall BJM. Rib fractures in the elderly population: a systematic review. Arch Orthop Trauma Surg. 2023;143:887–93. 10.1007/s00402-022-04362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alvarado F, Kaban J, Chao E, Meltzer JA. Surgical stabilization of rib fractures in patients with pulmonary comorbidities. Injury. 2023;54(5):1287–91. 10.1016/j.injury.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 120.Wang L, Zhao Y, Wu W, He W, Yang Y, Wang D, Xu E, Huang H, Zhang D, Jin L, Jing B, Wang M, Jin Z, Daemen JHT, de Loos ER, Greiffenstein P, Bertoglio P, Molnar TF, Pieracci FM, China Chest Injury Research Society. Development and validation of a pulmonary complications prediction model based on the Yang’s index. J Thorac Dis. 2023;15(4):2213–23. 10.21037/jtd-23-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller C, Stolarski A, Ata A, et al. Impact of blunt pulmonary contusion in polytrauma patients with rib fractures. Am J Surg. 2019;218:51–5. 10.1016/j.amjsurg.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 122.Voggenreiter G, Neudeck F, Aufmkolk M, Obertacke U, Schmit-Neuerburg KP. Operative chest wall stabilization in flail chest–outcomes of patients with or without pulmonary contusion. J Am Coll Surg. 1998;187(2):130–8. [DOI] [PubMed] [Google Scholar]
- 123.Van Wijck SFM, Pieracci FM, Smith EF, Madden K, Moore EE, Wijffels MME, Werner NL. Rib fixation in patients with severe rib fractures and pulmonary contusions: is it safe? J Trauma Acute Care Surg. 2022;93:721–6. 10.1097/TA.0000000000003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang Y, Wang X, Teng L, Liu Y, Wang J, Zheng Z. Comparison of the effectiveness of surgical versus nonsurgical treatment for multiple rib fractures accompanied with pulmonary contusion. Ann Thorac Cardiovasc Surg. 2019;25:185–91. 10.5761/atcs.oa.18-00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taylor BC, Fowler TT, French BG, Dominguez N. Clinical outcomes of surgical stabilization of flail chest injury. J Am Acad Orthop Surg. 2016;24:575–80. 10.5435/JAAOS-D-15-00476. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Y, Tang X, Xie H, Wang RL. Comparison of surgical fixation and nonsurgical management of flail chest and pulmonary contusion. Am J Emerg Med. 2015;33:937–40. 10.1016/j.ajem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Lagazzi E, Rafaqat W, Argandykov D, de Roulet A, Abiad M, Proaño-Zamudio JA, Velmahos GC, Hwabejire JO, Paranjape C, Albutt KH. Timing matters: early versus late rib fixation in patients with multiple rib fractures and pulmonary contusion. Surgery. 2024;175(2):529–35. 10.1016/j.surg.2023.09.012. [DOI] [PubMed] [Google Scholar]
- 128.Iqbal HJ, Alsousou J, Shah S, Jayatilaka L, Scott S, Scott S, Melling D. Early surgical stabilization of complex chest wall injuries improves short-term patient outcomes. J Bone Joint Surg Am. 2018;100(15):1298–308. 10.2106/JBJS.17.01215. [DOI] [PubMed] [Google Scholar]
- 129.Çınar HU, Çelik B. Comparison of surgical stabilization time in patients with flail chest. Thorac Cardiovasc Surg. 2020;68(8):743–51. 10.1055/s-0040-1713661. [DOI] [PubMed] [Google Scholar]
- 130.Su YH, Yang SM, Huang CH, Ko HJ. Early versus late surgical stabilization of severe rib fractures in patients with respiratory failure: a retrospective study. PLoS ONE. 2019;14(4):e0216170. 10.1371/journal.pone.0216170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tran Z, Cho NY, De Polo N, Mukherjee K, Benharash P, Burruss S. Association of early rib plating on clinical and financial outcomes: a national analysis. Am Surg. 2023. 10.1177/00031348231211041. [DOI] [PubMed] [Google Scholar]
- 132.Pieracci FM, Coleman J, Ali-Osman F, Mangram A, Majercik S, White TW, Jeremitsky E, Doben AR. A multicenter evaluation of the optimal timing of surgical stabilization of rib fractures. J Trauma Acute Care Surg. 2018;84(1):1–10. 10.1097/TA.0000000000001729. [DOI] [PubMed] [Google Scholar]
- 133.Wang Z, Jia Y, Li M. The effectiveness of early surgical stabilization for multiple rib fractures: a multicenter randomized controlled trial. J Cardiothorac Surg. 2023;18(1):118. 10.1186/s13019-023-02203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Owattanapanich N, Lewis MR, Benjamin ER, Jakob DA, Demetriades D. Surgical rib fixation in isolated flail chest improves survival. Ann Thorac Surg. 2022;113(6):1859–65. 10.1016/j.athoracsur.2021.05.085. [DOI] [PubMed] [Google Scholar]
- 135.Otaka S, Aso S, Matsui H, Fushimi K, Yasunaga H. Effectiveness of surgical fixation for rib fractures in relation to its timing: a retrospective Japanese nationwide study. Eur J Trauma Emerg Surg. 2022;48(2):1501–8. 10.1007/s00068-020-01548-1. Erratum in: Eur J Trauma Emerg Surg. 2021. [DOI] [PMC free article] [PubMed]
- 136.Becker L, Schulz-Drost S, Spering C, Franke A, Dudda M, Kamp O, Lefering R, Matthes G, Bieler D, Committee on Emergency Medicine, Intensive Care, Trauma Management (Sektion NIS) of the German Trauma Society (DGU). Impact of time of surgery on the outcome after surgical stabilization of rib fractures in severely injured patients with severe chest trauma-a matched-pairs analysis of the German trauma registry. Front Surg. 2022;11(9):852097. 10.3389/fsurg.2022.852097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao JM, Du DY, Liu CP, et al. Damage control surgery for treatment of flail chest combined with multiple trauma. Chin J Trauma. 2013;29:343–7. [Google Scholar]
- 138.Dilday J, Chien CY, Lewis M, Emigh B, Benjamin ER, Demetriades D. Surgical rib fixation in obese patients with isolated flail chest improves outcomes: a matched cohort study. World J Surg. 2022;46(12):2890–9. 10.1007/s00268-022-06748-x. [DOI] [PubMed] [Google Scholar]
- 139.Chen Zhu R, de Roulet A, Ogami T, Khariton K. Rib fixation in geriatric trauma: mortality benefits for the most vulnerable patients. J Trauma Acute Care Surg. 2020;89(1):103–10. 10.1097/TA.0000000000002666. [DOI] [PubMed] [Google Scholar]
- 140.Leasia K, Prins JTH, Lawless R, Burlew CC, Moore EE, Platnick B, Karamsetty L, Pieracci FM. Door to door in 24: factors that allow surgical stabilization of rib fractures within 24 hours of admission. J Thorac Dis. 2023;15(11):5922–30. 10.21037/jtd-23-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bordes SJ, Greiffenstein P. Early surgical stabilization of rib fractures (SSRF) is better, but delayed SSRF is not worse. J Thorac Dis. 2023;15(12):6403–4. 10.21037/jtd-2023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Traub M, Stevenson M, McEvoy S, Briggs G, Lo SK, Leibman S, Joseph T. The use of chest computed tomography versus chest X-ray in patients with major blunt trauma. Injury. 2007;38(1):43–7. 10.1016/j.injury.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 143.Trupka A, Waydhas C, Hallfeldt KK, Nast-Kolb D, Pfeifer KJ, Schweiberer L. Value of thoracic computed tomography in the first assessment of severely injured patients with blunt chest trauma: results of a prospective study. J Trauma. 1997;43(3):405–11. 10.1097/00005373-199709000-00003. (discussion 411-2). [DOI] [PubMed] [Google Scholar]
- 144.Pulley BR, Taylor BC, Fowler TT, Dominguez N, Trinh TQ. Utility of three-dimensional computed tomography for the surgical management of rib fractures. J Trauma Acute Care Surg. 2015;78(3):530–4. 10.1097/TA.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 145.Martin TJ, Cao J, Benoit E, Kheirbek T. Optimizing surgical stabilization of rib fractures using intraoperative ultrasound localization. J Trauma Acute Care Surg. 2021;91(2):369–74. 10.1097/TA.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 146.Xia HG, Zhu DQ, Li J, Li X, Sun ZY, Zhu PZ, et al. Application of fracture body surface localization film combined with CT volume rendering in the minimally invasive rib fractures internal fixation. Eur Rev Med Pharmacol Sci. 2020;24(24):12948–54. [DOI] [PubMed] [Google Scholar]
- 147.Zhou XT, Zhang DS, Yang Y, Zhang GL, Xie ZX, Chen MH, Liang Z. Analysis of the advantages of 3D printing in the surgical treatment of multiple rib fractures: 5 cases report. J Cardiothorac Surg. 2019;14(1):105. 10.1186/s13019-019-0930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou X, Zhang D, Xie Z, Yang Y, Chen M, Liang Z, Zhang G, Li S. Application of 3D printing and framework internal fixation technology for high complex rib fractures. J Cardiothorac Surg. 2021;16(1):5. 10.1186/s13019-020-01377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen YY, Lin KH, Huang HK, Chang H, Lee SC, Huang TW. The beneficial application of preoperative 3D printing for surgical stabilization of rib fractures. PLoS ONE. 2018;13(10):e0204652. 10.1371/journal.pone.0204652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haines KL, Zens T, Warner-Hillard C, DeSouza E, Jung HS, Agarwal S. Rib fracture location should be evaluated when predicting morbidity and mortality in trauma patients. Am Surg. 2018;84(9):1462–5. [PubMed] [Google Scholar]
- 151.Chapman BC, Herbert B, Rodil M, Salotto J, Stovall RT, Biffl W, et al. RibScore: a novel radiographic score based on fracture pattern that predicts pneumonia, respiratory failure, and tracheostomy. J Trauma Acute Care Surg. 2016;80(1):95–101. [DOI] [PubMed] [Google Scholar]
- 152.Wycech J, Fokin AA, Puente I. Evaluation of patients with surgically stabilized rib fractures by different scoring systems. Eur J Trauma Emerg Surg. 2020;46(2):441–5. 10.1007/s00068-018-0999-3. [DOI] [PubMed] [Google Scholar]
- 153.Thomas CN, Lindquist TJ, Schroder LK, Cole PA. Rib fracture map in high-energy injuries. J Orthop Trauma. 2023;37(4):e165–9. [DOI] [PubMed] [Google Scholar]
- 154.Pieracci FM, Rodil M, Stovall RT, et al. Surgical stabilization of severe rib fractures. J Trauma Acute Care Surg. 2015;78:883–7. 10.1097/TA.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 155.Marasco S, Saxena P. Surgical rib fixation-technical aspects. Injury. 2015;46:929–32. 10.1016/j.injury.2014.12.021.57. [DOI] [PubMed] [Google Scholar]
- 156.Chen XK, Liu YJ, Guo FZ, Deng JX, Xiong J, Wang TB, et al. Assessment of thoracic volume changes after the collapse of lateral rib fractures based on chest computed tomography data: computer simulation and a multiple variable linear regression analysis. J Cardiothorac Surg. 2020;15(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clarke PTM, Simpson RB, Dorman JR, Hunt WJ, Edwards JG. Determining the clinical significance of the Chest Wall Injury Society taxonomy for multiple rib fractures. J Trauma Acute Care Surg. 2019;87(6):1282–8. [DOI] [PubMed] [Google Scholar]
- 158.Van Wijck SFM, Curran C, Sauaia A, Van Lieshout EMM, Whitbeck SS, Edwards JG, et al. Interobserver agreement for the Chest Wall Injury Society taxonomy of rib fractures using computed tomography images. J Trauma Acute Care Surg. 2022;93(6):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bemelman M, Baal MV, Raaijmakers C, Lansink K, Leenen L, Long W. An interobserver agreement study with a new classification for rib fractures. Chirurgia. 2019;114(3):352–8. [DOI] [PubMed] [Google Scholar]
- 160.Head W, Kumar N, Thomas C, Leon S, Dieffenbaugher S, Eriksson E. Are rib fractures stable? An analysis of progressive rib fracture offset in the acute trauma setting. J Trauma Acute Care Surg. 2021;91(6):917–22. [DOI] [PubMed] [Google Scholar]
- 161.Zhang D, Zhou X, Yang Y, Xie Z, Chen M, Liang Z, et al. Minimally invasive surgery rib fracture fixation based on location and anatomical landmarks. Eur J Trauma Emerg Surg. 2022;48(5):3613–22. [DOI] [PubMed] [Google Scholar]
- 162.Lee WS, Kim YH, Chee HK, Lee SA. Ultrasonographic evaluation of costal cartilage fractures unnoticed by the conventional radiographic study and multidetector computed tomography. Eur J Trauma Emerg Surg. 2012;38(1):37–42. [DOI] [PubMed] [Google Scholar]
- 163.Subhas N, Kline MJ, Moskal MJ, White LM, Recht MP. MRI evaluation of costal cartilage injuries. AJR Am J Roentgenol. 2008;191(1):129–32. [DOI] [PubMed] [Google Scholar]
- 164.Bonne SL, Turnbull IR, Southard RE. Technique for repair of fractures and separations involving the cartilaginous portions of the anterior chest wall. Chest. 2015;147(6):e199–204. [DOI] [PubMed] [Google Scholar]
- 165.Schuette HB, Taylor BC, Rutkowski P, Huber G, Mehta V. Cartilage plating in flail chest fixation. Injury. 2021;52(9):2560–4. [DOI] [PubMed] [Google Scholar]
- 166.Li Y, Zhao Y, Yang Y, Wu W, Guo X, Zhao T. Surgical treatment of costal cartilage fractures with titanium plate internal fixation. J Cardiothorac Surg. 2022;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schuette HB, Glazier MT, Triplet JJ, Taylor BC. Far posterior rib plating: Preliminary results of a retrospective case series. Injury. 2021;52(5):1133–7. [DOI] [PubMed] [Google Scholar]
- 168.Greiffenstein P, Tran MQ, Campeau L. Three common exposures of the chest wall for rib fixation: anatomical considerations. J Thorac Dis. 2019;11(Suppl 8):S1034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jian X, Lei W, Yuyang P, Yongdong X. A new instrument for surgical stabilization of multiple rib fractures. J Int Med Res. 2020;48(2):300060519877076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nickerson TP, Kim BD, Zielinski MD, Jenkins D, Schiller HJ. Use of a 90 degrees drill and screwdriver for rib fracture stabilization. World J Surg. 2015;39(3):789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marasco S, Liew S, Edwards E, Varma D, Summerhayes R. Analysis of bone healing in flail chest injury: do we need to fix both fractures per rib? J Trauma Acute Care Surg. 2014;77:452–8. [DOI] [PubMed] [Google Scholar]
- 172.Fraser SF, Tan C, Kuppusamy MK, Gukop P, Hunt IJ. The role of a video-assisted thoracic approach for rib fixation. Eur J Trauma Emerg Surg. 2017;43(2):185–90. 10.1007/s00068-016-0641-1. [DOI] [PubMed] [Google Scholar]
- 173.Schots JP, Vissers YL, Hulsewe KW, Meesters B, Hustinx PA, Pijnenburg A, et al. Addition of video-assisted thoracoscopic surgery to the treatment of flail chest. Ann Thorac Surg. 2017;103(3):940–4. [DOI] [PubMed] [Google Scholar]
- 174.van Gool MH, van Roozendaal LM, Vissers YLJ, van den Broek R, van Vugt R, Meesters B, et al. VATS-assisted surgical stabilization of rib fractures in flail chest: 1-year follow-up of 105 cases. Gen Thorac Cardiovasc Surg. 2022;70(11):985–92. [DOI] [PubMed] [Google Scholar]
- 175.Wu TH, Lin HL, Chou YP, Huang FD, Huang WY, Tarng YW. Facilitating ventilator weaning through rib fixation combined with video-assisted thoracoscopic surgery in severe blunt chest injury with acute respiratory failure. Crit Care. 2020;24(1):49. 10.1186/s13054-020-2755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Murfee JR, Pardue KE, Farley P, Polite NM, Mbaka MI, Bright AC, et al. Unexpected diaphragmatic hernia among patients undergoing video-assisted thoracic surgery for internal fixation of rib fractures. Am Surg. 2022;88(4):618–22. [DOI] [PubMed] [Google Scholar]
- 177.Wemeijer TM, Hogeboom W, Steenvoorde P, Withaar DS, de Groot R. Missed injuries in trauma patients: the value of a diagnostic thoracotomy or thoracoscopy during surgical stabilisation of rib fractures. Ir J Med Sci. 2022;191(3):1285–9. [DOI] [PubMed] [Google Scholar]
- 178.Chou YP, Kuo LC, Soo KM, Tarng YW, Chiang HI, Huang FD, Lin HL. The role of repairing lung lacerations during video-assisted thoracoscopic surgery evacuations for retained haemothorax caused by blunt chest trauma. Eur J Cardiothorac Surg. 2014;46:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bui JT, Browder SE, Wilson HK, Kindell DG, Ra JH, Haithcock BE, et al. Does routine uniportal thoracoscopy during rib fixation identify more injuries and impact outcomes? J Thorac Dis. 2020;12(10):5281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aguilar MM, Battistella FD, Owings JT, Su T. Posttraumatic empyema. Risk factor analysis. Arch Surg. 1997;132:647–50 (discussion 650-1). [DOI] [PubMed] [Google Scholar]
- 181.Majercik S, Vijayakumar S, Olsen G, Wilson E, Gardner S, Granger SR, Van Boerum DH, White TW. Surgical stabilization of severe rib fractures decreases incidence of retained hemothorax and empyema. Am J Surg. 2015;210:1112–6 (discussion 1116-7). [DOI] [PubMed] [Google Scholar]
- 182.Allen GS, Fischer RP. Traumatic lung herniation. Ann Thorac Surg. 1997;63:1455–6. [DOI] [PubMed] [Google Scholar]
- 183.Forty J, Wells FC. Traumatic intercostal pulmonary hernia. Ann Thorac Surg. 1990;49:670–1. [DOI] [PubMed] [Google Scholar]
- 184.Scullion DA, Negus R, Al-Kutoubi A. Case report: extrathoracic herniation of the lung with a review of the literature. Br J Radiol. 1994;67:94–6. [DOI] [PubMed] [Google Scholar]
- 185.Kuckelman J, Karmy-Jones R, Windell E, Izenberg S, David JS, Long W, et al. Traumatic thoracic rib cage hernias: operative management and proposal for a new anatomic-based grading system. Am J Surg. 2018;215(5):794–800. [DOI] [PubMed] [Google Scholar]
- 186.Su YH, Yang SM, Ko HJ. Diagnosis and management of a trapped lung or diaphragm by fractured ribs: analysis of patients undergoing rib fracture repair. BMC Surg. 2019;19(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ibrahim-Zada I, Bell MT, Campion EM, Pieracci FM, Truitt MS. Delayed presentation of pulmonary hernia following surgical stabilization of severe rib fractures. J Trauma Acute Care Surg. 2016;81(2):397–9. [DOI] [PubMed] [Google Scholar]
- 188.Seder CW, Allen MS, Nichols FC, Wigle DA, Shen KR, Deschamps C, Cassivi SD. Primary and prosthetic repair of acquired chest wall hernias: a 20-year experience. Ann Thorac Surg. 2014;98:484–9. [DOI] [PubMed] [Google Scholar]
- 189.Lanier ST, Wetterau M, Smith-Singares E, Bilfinger T, Vosswinkel J, Shapiro MJ, Dagum AB. Management of pulmonary hernia through a flail segment in closed thoracic trauma using open reduction, internal fixation and pectoralis major flap reconstruction: a case report. Can J Plast Surg. 2011;19(4):145–7. 10.1177/229255031101900408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fackeldey V, Junge K, Hinck D, et al. Repair of intercostal pulmonary herniation. Hernia. 2003;7:215–7. 10.1007/s10029-003-0135-z. [DOI] [PubMed] [Google Scholar]
- 191.Reardon MJ, Fabré J, Reardon PR, et al. Video-assisted repair of a traumatic intercostal pulmonary hernia. Ann Thorac Surg. 1998;65:1155–7. 10.1016/S0003-4975(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 192.Van Den Bossche MR, Leman G, Ballaux KE, et al. Video-assisted thoracoscopic repair of an intercostal pulmonary hernia. Surg Endosc. 1999;13:166–7. 10.1007/s004649900931. [DOI] [PubMed] [Google Scholar]
- 193.Fiscon V, Portale G, Frigo F, et al. Thoracoscopic repair of lung herniation following minimally invasive cardiothoracic surgery. Chir Ital. 2009;61:261–3. [PubMed] [Google Scholar]
- 194.Khalil MW, Masala N, Waller DA, et al. Surgical repair of post-traumatic lung hernia using a video-assisted open technique. Interact Cardiovasc Thorac Surg. 2008;7:506–7. 10.1510/icvts.2007.168658. [DOI] [PubMed] [Google Scholar]
- 195.Hebra A, Cina R, Streck C. Video-assisted thoracoscopic repair of a lung hernia in a child. J Laparoendosc Adv Surg Tech. 2011;21:763–5. 10.1089/lap.2011.0147. [DOI] [PubMed] [Google Scholar]
- 196.Chiang TY, Yin MF, Yang SM, Chen KC. Thoracoscopic management of incarcerated lung herniation after blunt chest trauma: a case report and literature review. J Thorac Dis. 2017;9(3):E253–7. 10.21037/jtd.2017.03.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kirkpatrick AW, Coccolini F, Tolonen M, Minor S, Catena F, Celotti A, Gois E Jr, Perrone G, Novelli G, Garulli G, et al. Are surgeons going to be left holding the bag? Incisional hernia repair and intra-peritoneal non-absorbable mesh implant complications. J Clin Med. 2024;13(4):1005. 10.3390/jcm13041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gauger EM, Hill BW, Lafferty PM, Cole PA. Outcomes after operative management of symptomatic rib nonunion. J Orthop Trauma. 2015;29:283–9. [DOI] [PubMed] [Google Scholar]
- 199.Bille A, Okiror L, Karenovics W, Routledge T. Experience with titanium devices for rib fixation and coverage of chest wall defects. Interact Cardiovasc Thorac Surg. 2012;15:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sawan TG, Nickerson TP, Thiels CA, Aho JM, Cross WW, Schiller HJ, Kim BD. Load sharing, not load bearing plates: lessons learned from failure of rib fracture stabilization. Am Surg. 2016;82:E15–7. [PubMed] [Google Scholar]
- 201.Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. [DOI] [PubMed] [Google Scholar]
- 202.Choi J, Khan S, Syed M, Tennakoon L, Forrester JD. Early national landscape of surgical stabilization of sternal fractures. World J Surg. 2021;45(6):1692–7. [DOI] [PubMed] [Google Scholar]
- 203.Klei DS, de Jong MB, Öner FC, Leenen LPH, van Wessem KJP. Current treatment and outcomes of traumatic sternal fractures-a systematic review. Int Orthop. 2019;43(6):1455–64. 10.1007/s00264-018-3945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Langenbach A, Pinther M, Krinner S, Grupp S, Ekkernkamp A, Hennig FF, et al. Surgical stabilization of costoclavicular injuries—a combination of flail chest injuries and a clavicula fracture. Chirurgia. 2017;112(5):595–606. [DOI] [PubMed] [Google Scholar]
- 205.Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthopaed Surg. 2012;20(3):130–41. [DOI] [PubMed] [Google Scholar]
- 206.de Moya M, Nirula R, Biffl W. Rib fixation: who, what, when? Trauma Surg Acute Care Open. 2017;2:e000059. 10.1136/tsaco-2016-000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stopenski S, Binkley J, Schubl SD, Bauman ZM. Rib fracture management: a review of surgical stabilization, regional analgesia, and intercostal nerve cryoablation. Surg Pract Sci. 2022;10:100089. [Google Scholar]
- 208.Hasenboehler EA, Bernard AC, Bottiggi AJ, Moghadamian ES, Wright RD, Chang PK, et al. Treatment of traumatic flail chest with muscular sparing open reduction and internal fixation: description of a surgical technique. J Trauma. 2011;71:494–501. [DOI] [PubMed] [Google Scholar]
- 209.Taylor BC, Fowler TT, Reddy H, et al. Functional outcomes after muscle-sparing fixation of flail chest injuries. J Orthop Trauma. 2019;33:366–9. 10.1097/BOT.0000000000001456. [DOI] [PubMed] [Google Scholar]
- 210.Bemelman M, van Baal M, Yuan JZ, Leenen L. The role of minimally invasive plate osteosynthesis in rib fixation: a review. Korean J Thorac Cardiovasc Surg. 2016;49(1):1–8. 10.5090/kjtcs.2016.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li Y, Gao E, Yang Y, et al. Comparison of minimally invasive surgery for non-flail chest rib fractures: a prospective cohort study. J Thorac Dis. 2020;12:3706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao W, Chen Y, He W, et al. Nonintubated minimally invasive chest wall stabilization for multiple rib fractures: a prospective, single-arm study. World J Emerg Surg. 2020;15:53. 10.1186/s13017-020-00335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bae CM, Son SA, Lee YJ, Lee SC. Clinical outcomes of minimally invasive surgical stabilization of rib fractures using video-assisted thoracoscopic surgery. J Chest Surg. 2023. 10.5090/jcs.22.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Akil A, Ziegeler S, Reichelt J, et al. Rib osteosynthesis is a safe and effective treatment and leads to a significant reduction of trauma associated pain. Eur J Trauma Emerg Surg. 2019;45:623–30. 10.1007/s00068-018-01062-5. [DOI] [PubMed] [Google Scholar]
- 215.Diaz JJ, Azar FK. Minimally invasive chest wall stabilization: a novel surgical approach to video-assisted rib plating (VARP). Trauma Surg Acute Care Open. 2019;4:e000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bauman ZM, Loftus J, Raposo-Hadley A, Samuel S, Ernst W, Evans CH, Cemaj S, Kaye AJ. Surgical stabilization of rib fractures combined with intercostal nerve cryoablation proves to be more cost effective by reducing hospital length of stay and narcotics. Injury. 2021;52(5):1128–32. 10.1016/j.injury.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 217.Pieracci FM, Johnson JL, Stovall RT, Jurkovich GJ. Completely thoracoscopic, intra-pleural reduction and fixation of severe rib fractures. Trauma Case Rep. 2015;1(5–8):39–43. 10.1016/j.tcr.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bauman ZM, Beard R, Cemaj S. When less is more: a minimally invasive, intrathoracic approach to surgical stabilization of rib fractures. Trauma Case Rep. 2021;32:100452. 10.1016/j.tcr.2021.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang J, Hong Q, Mo X, Ma C. Complete video-assisted thoracoscopic surgery for rib fractures: series of 35 cases. Ann Thorac Surg. 2022;113:452–8. 10.1016/j.athoracsur.2021.01.065. [DOI] [PubMed] [Google Scholar]
- 220.Castater C, Hazen B, Davis C, Hoppe S, Butler C, Grant A, et al. Video-assisted thoracoscopic internal rib fixation. Am Surg. 2021;88:994. [DOI] [PubMed] [Google Scholar]
- 221.Zhang G, Shurtleff E, Falank C, et al. Thoracoscopic-assisted rib plating (TARP): initial single-center case series, including TARP in the super elderly, technical lessons learned, and proposed expanded indications. Trauma Surg Acute Care Open. 2022;7:e000943. 10.1136/tsaco-2022-000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tay-Lasso E, Alaniz L, Grant W, Hovis G, Frank M, Kincaid C, Brynn S, Pieracci FM, Nahmias J, Barrios C, Rockne W, Chin T, Swentek L, Schubl SD. Prospective single-center paradigm shift of surgical stabilization of rib fractures with decreased length of stay and operative time with an intrathoracic approach. J Trauma Acute Care Surg. 2023;94(4):567–72. 10.1097/TA.0000000000003811. [DOI] [PubMed] [Google Scholar]
- 223.Merchant NN, Onugha O. Extra-thoracic video-assisted thoracoscopic surgery rib plating and intra-thoracic VATS decortication of retained hemothorax. Surg Technol Int. 2018;33:251–4. [PubMed] [Google Scholar]
- 224.Helzel I, Long W, Fitzpatrick D, Madey S, Bottlang M. Evaluation of intramedullary rib splints for less-invasive stabilisation of rib fractures. Injury. 2009;40:1104–10. [DOI] [PubMed] [Google Scholar]
- 225.Bottlang M, Long WB, Phelan D, Fielder D, Madey SM. Surgical stabilization of flail chest injuries with MatrixRIB implants: a prospective observational study. Injury. 2013;44(2):232–8. 10.1016/j.injury.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 226.Sillar W. The crushed chest. J R Coll Surg Edinb. 1962;7:101–9. [PubMed] [Google Scholar]
- 227.Bottlang M, Helzel I, Long W, Fitzpatrick D, Madey S. Less-invasive stabilization of rib fractures by intramedullary fixation: a biomechanical evaluation. J Trauma. 2010;68(5):1218–24. 10.1097/TA.0b013e3181bb9df1. [DOI] [PubMed] [Google Scholar]
- 228.Fitzpatrick DC, Denard PJ, Phelan D, Long WB, Madey SM, Bottlang M. Operative stabilization of flail chest injuries: review of literature and fixation options. Eur J Trauma Emerg Surg. 2010;36(5):427–33. 10.1007/s00068-010-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Prins JTH, Van Wijck SFM, Leeflang SA, Kleinrensink GJ, Lottenberg L, de la Santa Barajas PM, Van Huijstee PJ, Vermeulen J, Verhofstad MHJ, Zadpoor AA, Wijffels MME, Van Lieshout EMM. Biomechanical characteristics of rib fracture fixation systems. Clin Biomech. 2023;102:105870. 10.1016/j.clinbiomech.2023.105870. [DOI] [PubMed] [Google Scholar]
- 230.Bottlang M, Walleser S, Noll M, Honold S, Madey SM, Fitzpatrick D, Long WB. Biomechanical rationale and evaluation of an implant system for rib fracture fixation. Eur J Trauma Emerg Surg. 2010;36(5):417–26. 10.1007/s00068-010-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Choke A, Wong YR, Joethy JV. Biomechanical comparison of monocortical and bicortical plate fixation for rib fractures in a cadaveric model using a locking plate system. J Thorac Dis. 2019;11:4966–71. 10.21037/jtd.2019.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu WM, Zhang JP, Jiang MY, et al. Internal fixation with claw-shaped rib plate for traumatic floating chest wall. Chin J Thorac Cardiovasc Surg. 2005;21:23. [Google Scholar]
- 233.Xu EW, Qiao GB, Peng XF, et al. Indications and surgical techniques of fixation of rib fractures with memory alloy osteosynthesis plates. Chin J Traumatol. 2012;28:533–6. [Google Scholar]
- 234.Liu Y, Yu Q, Zhu J, et al. Pure titanium rib plate for treatment of flail chest and multiple rib fractures. Chin J Traumatol. 2013;9:650–2. [Google Scholar]
- 235.Bottlang M, Helzel I, Long WB, Madey S. Anatomically contoured plates for fixation of rib fractures. J Trauma. 2010;68(3):611–5. [DOI] [PubMed] [Google Scholar]
- 236.Sales JR, Ellis TJ, Gillard J, et al. Biomechanical testing of a novel, minimally invasive rib fracture plating system. J Trauma. 2008;64:1270–4. 10.1097/TA.0b013e31804a7fd5. [DOI] [PubMed] [Google Scholar]
- 237.Oppizzi G, Xu D, Patel T, Diaz JJ, Zhang LQ. Open reduction internal fixation of rib fractures: a biomechanical comparison between the RibLoc U Plus® system and anterior plate in rib implants. Eur J Trauma Emerg Surg. 2023;49(1):383–91. 10.1007/s00068-022-02075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang Y, Dong LW, Wang J. Memory alloy embracing fixator in treatment of multiple fractured ribs and flail chest. World J Emerg Med. 2010;1(3):212–5. [PMC free article] [PubMed] [Google Scholar]
- 239.Judet R. Osteosynthese costale. Rev Chir Orthop Reparatrice Appar Mot. 1973;59(Suppl 1):334–5. [PubMed] [Google Scholar]
- 240.Menard A, Testart J, Philippe JM, Grise P. Treatment of flail chest with Judet’s struts. J Thorac Cardiovasc Surg. 1983;86:300–5. [PubMed] [Google Scholar]
- 241.Moore BP. Operative stabilization of nonpenetrating chest injuries. J Thorac Cardiovasc Surg. 1975;70:619–30. [PubMed] [Google Scholar]
- 242.Nirula R, Allen B, Layman R, Falimirski ME, Somberg LB. Rib fracture stabilization in patients sustaining blunt chest injury. Am Surg. 2006;72:307–9. [DOI] [PubMed] [Google Scholar]
- 243.Kroeker A, Hoke N, Peck E, Mullins R, Ham B, Mayberry J. Long-term morbidity, pain and disability following repair of severe chest wall injury. J Invest Med. 2008;56:210. [Google Scholar]
- 244.Slater MS, Mayberry JC, Trunkey DD. Operative stabilization of a flail chest six years after injury. Ann Thorac Surg. 2001;72:600–1. [DOI] [PubMed] [Google Scholar]
- 245.Xu FY, Wang N, Ren J, et al. Comparison of the therapeutic effect between anatomical plates and clawtype bone plates in fixation of multiple rib fractures. Chin J Emerg Med. 2019;28:232–5. [Google Scholar]
- 246.Zhang XF, Guo ZQ, Zhao CC, et al. Management of patients with flail chest by surgical fixation using claw-type titanium plate. J Cardiothoracic Surg. 2015;10:145. 10.1186/s13019-015-0363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Berthet J-P, Wihlm J-M, Canaud L, et al. The combination of polytetrafluoroethylene mesh and titanium rib implants: an innovative process for reconstructing large full thickness chest wall defects. Eur J Cardiothorac Surg. 2012;42(3):444–53. 10.1093/ejcts/ezs028. [DOI] [PubMed] [Google Scholar]
- 248.Divisi D, Mucilli F, Leonardo GD, et al. Plates versus struts versus an extracortical rib fixation in flail chest patients: two-center experience. Injury. 2021;52:235–42. [DOI] [PubMed] [Google Scholar]
- 249.Majercik S, Wilson E, Gardner S, Granger S, VanBoerum DH, White TW. In-hospital outcomes and costs of surgical stabilization versus nonoperative management of severe rib fractures. J Trauma Acute Care Surg. 2015;79(4):533–8. 10.1097/TA.0000000000000820. (discussion 538-9). [DOI] [PubMed] [Google Scholar]
- 250.Althausen PL, Shannon S, Watts C, et al. Early surgical stabilization of flail chest with locked plate fixation. J Orthop Trauma. 2011;25:641–7. [DOI] [PubMed] [Google Scholar]
- 251.Holcombe SA, Kang YS, Derstine BA, Wang SC, Agnew AM. Regional maps of rib cortical bone thickness and cross-sectional geometry. J Anat. 2019;235(5):883–91. 10.1111/joa.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Casha AR, Camilleri L, Manche A, et al. Internal Rib structure can be predicted using mathematical models: an anatomic study comparing the chest to a shell dome with application to understanding fractures. Clin Anat. 2015;28:1008–16. 10.1002/ca.22614. [DOI] [PubMed] [Google Scholar]
- 253.Mischler D, Schopper C, Gasparri M, Schulz-Drost S, Brace M, Gueorguiev B. Is intrathoracic rib plate fixation advantageous over extrathoracic plating? A biomechanical cadaveric study. J Trauma Acute Care Surg. 2022;92(3):574–80. 10.1097/TA.0000000000003443. [DOI] [PubMed] [Google Scholar]
- 254.Samarrai AR. Costosynthetic stabilization of massive chest wall instability. Int Surg. 1990;75:231–3. [PubMed] [Google Scholar]
- 255.Ahmed Z, Mohyuddin Z. Management of flail chest injury: internal fixation versus endotracheal intubation and ventilation. J Thorac Cardiovasc Surg. 1995;110:1676–80. [DOI] [PubMed] [Google Scholar]
- 256.Engel C, Krieg JC, Madey SM, Long WB, Bottlang M. Operative chest wall fixation with osteosynthesis plates. J Trauma. 2005;58:181–6. [DOI] [PubMed] [Google Scholar]
- 257.Mouton W, Lardinois D, Furrer M, Regli B, Ris HB. Long-term follow-up of patients with operative stabilisation of a flail chest. Thorac Cardiovasc Surg. 1997;45:242–4. [DOI] [PubMed] [Google Scholar]
- 258.Tarng YW, Liu YY, Huang FD, Lin HL, Wu TC, Chou YP. The surgical stabilization of multiple rib fractures using titanium elastic nail in blunt chest trauma with acute respiratory failure. Surg Endosc. 2016;30(1):388–95. 10.1007/s00464-015-4207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chai X, Lin Q, Ruan Z, Zheng J, Zhou J, Zhang J. The clinical application of absorbable intramedullary nail and claw plate on treating multiple rib fractures. Minerva Chir. 2013;68(4):415–20. [PubMed] [Google Scholar]
- 260.Marasco S, Quayle M, Summerhayes R, Šutalo ID, Liovic P. An assessment of outcomes with intramedullary fixation of fractured ribs. J Cardiothorac Surg. 2016;11(1):126. 10.1186/s13019-016-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Morodomi Y, Okamoto T, Tagawa T, Shoji F, Katsura M, Fujishita T, Fujiyoshi T, Akahoshi T, Yasuda M, Maehara Y. A novel method of using bioabsorbable materials for the surgical repair of flail chest. J Trauma Acute Care Surg. 2016;81(5):984–7. 10.1097/TA.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 262.Mayberry JC, Terhes JT, Ellis TJ, Wanek S, Mullins RJ. Absorbable plates for rib fracture repair: preliminary experience. J Trauma. 2003;55(5):835–9. 10.1097/01.TA.0000090037.72142.33. [DOI] [PubMed] [Google Scholar]
- 263.Marasco SF, Sutalo ID, Bui AV. Mode of failure of rib fixation with absorbable plates: a clinical and numerical modeling study. J Trauma. 2010;68:1225–33. 10.1097/TA.0b013e3181d27cab. [DOI] [PubMed] [Google Scholar]
- 264.Marasco SF, Liovic P, Šutalo ID. Structural integrity of intramedullary rib fixation using a single bioresorbable screw. J Trauma Acute Care Surg. 2012;73(3):668–73. 10.1097/TA.0b013e3182569f75. [DOI] [PubMed] [Google Scholar]
- 265.Ashley DW, Christie DB 3rd, Long EL, Adiga R, Johns TJ, Fabico-Dulin J, Montgomery A. Prospective randomized trial of metal versus resorbable plates in surgical stabilization of rib fractures. J Trauma Acute Care Surg. 2022;93(2):147–56. 10.1097/TA.0000000000003642. [DOI] [PubMed] [Google Scholar]
- 266.Pieracci FM, Schubl S, Gasparri M, Delaplain P, Kirsch J, Towe C, White TW, Whitbeck S, Doben AR. The chest wall injury society recommendations for reporting studies of surgical stabilization of rib fractures. Injury. 2021;52:1241–50. 10.1016/j.injury.2021.02.032. [DOI] [PubMed] [Google Scholar]
- 267.Parra KT, Badiee J, Calvo RY, Rooney A, Krzyzaniak A, Bansal V, Martin MJ. The where, when, and why of surgical rib fixation: utilization patterns, outcomes, and readmissions. Am J Surg. 2022;224(2):780–5. 10.1016/j.amjsurg.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 268.Niiya A, Murakami K, Kobayashi R, Sekimoto A, Saeki M, Toyofuku K, Kato M, Shinjo H, Ito Y, Takei M, Murata C, Ohgiya Y. Development of an artificial intelligence-assisted computed tomography diagnosis technology for rib fracture and evaluation of its clinical usefulness. Sci Rep. 2022;12(1):8363. 10.1038/s41598-022-12453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.van den Broek MCL, Buijs JH, Schmitz LFM, Wijffels MME. Diagnostic performance of artificial intelligence in rib fracture detection: systematic review and meta-analysis. Surgeries. 2024;5(1):24–36. 10.3390/surgeries5010005. [Google Scholar]
- 270.Ke S, Duan H, Cai Y, Kang J, Feng Z. Thoracoscopy-assisted minimally invasive surgical stabilization of the anterolateral flail chest using Nuss bars. Ann Thorac Surg. 2014;97(6):2179–82. 10.1016/j.athoracsur.2013.08.066. [DOI] [PubMed] [Google Scholar]
- 271.Nakagawa T, Masuda R, Yamada S, Iwazaki M. Minimally invasive surgery for anterior flail chest injury in the acute phase: series of 10 cases. Gen Thorac Cardiovasc Surg. 2023;71(7):403–8. 10.1007/s11748-023-01908-9. [DOI] [PubMed] [Google Scholar]
- 272.Prins JTH, Leasia K, Dull MB, Lawless RA, Platnick KB, Werner NL, Wijffels MME, Moore EE, Pieracci FM. Surgical site infection after surgical stabilization of rib fractures: rare but morbid. Surg Infect. 2022;23(1):5–11. 10.1089/sur.2021.165. [DOI] [PubMed] [Google Scholar]
- 273.Junker MS, Kurjatko A, Hernandez MC, Heller SF, Kim BD, Schiller HJ. Salvage of rib stabilization hardware with antibiotic beads. Am J Surg. 2019;218(5):869–75. 10.1016/j.amjsurg.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 274.Thiels CA, Aho JM, Naik ND, et al. Infected hardware after surgical stabilization of rib fractures: outcomes and management experience. J Trauma Acute Care Surg. 2016;80(5):819–23. 10.1097/TA.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Forrester JD, Faliks B, Cardarelli C, Choudhry MS, Patel B, Ricaurte D, Sarani B, Sfakianos M, Kartiko S, Huston JM. Surgical Infection Society-Chest Wall Injury Society recommendations for management of surgical site or implant-related infections after surgical stabilization of traumatic rib or sternal fractures. Surg Infect. 2023;24(5):414–24. 10.1089/sur.2023.086. [DOI] [PubMed] [Google Scholar]
- 276.Forrester JD, Bukur M, Dvorak JE, Faliks B, Hindin D, Kartiko S, Kheirbek T, Lin L, Manasa M, Martin TJ, Miskimins R, Patel B, Pieracci FM, Ritter KA, Schubl SD, Tung J, Huston JM. Surgical Infection Society: chest wall injury society recommendations for antibiotic use during surgical stabilization of traumatic rib or sternal fractures to reduce risk of implant infection. Surg Infect. 2022;23(4):321–31. 10.1089/sur.2022.025. [DOI] [PubMed] [Google Scholar]
- 277.Sarani B, Allen R, Pieracci FM, Doben AR, Eriksson E, Bauman ZM, Gupta P, Semon G, Greiffenstein P, Chapman AJ, Kim BD, Lottenberg L, Gardner S, Marasco S, White T. Characteristics of hardware failure in patients undergoing surgical stabilization of rib fractures: a Chest Wall Injury Society multicenter study. J Trauma Acute Care Surg. 2019;87(6):1277–81. 10.1097/TA.0000000000002373. [DOI] [PubMed] [Google Scholar]
- 278.Choi J, Kaghazchi A, Sun B, Woodward A, Forrester JD. Systematic review and meta-analysis of hardware failure in surgical stabilization of rib fractures: who, what, when, where, and why? J Surg Res. 2021;268:190–8. 10.1016/j.jss.2021.06.054. [DOI] [PubMed] [Google Scholar]
- 279.Thiruvenkatarajan V, Cruz Eng H, Adhikary SD. An update on regional analgesia for rib fractures. Curr Opin Anaesthesiol. 2018;31(5):601–7. 10.1097/ACO.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 280.Syal R, Mohammed S, Kumar R, Jain N, Bhatia P. Continuous erector spinae plane block for analgesia and better pulmonary functions in patients with multiple rib fractures: a prospective descriptive study. Braz J Anesthesiol. 2024;74(1):744289. 10.1016/j.bjane.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jiang M, Peri V, Ou Yang B, Chang J, Hacking D. Erector spinae plane block as an analgesic intervention in acute rib fractures: a scoping review. Local Reg Anesth. 2023;12(16):81–90. 10.2147/LRA.S414056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Koushik SS, Bui A, Slinchenkova K, Badwal A, Lee C, Noss BO, Raghavan J, Viswanath O, Shaparin N. Analgesic techniques for rib fractures-a comprehensive review article. Curr Pain Headache Rep. 2023;27(11):747–55. 10.1007/s11916-023-01172-9. [DOI] [PubMed] [Google Scholar]
- 283.Bethlahmy JM, Hanst BA, Giafaglione SM, Elia JM. Perioperative considerations for patients undergoing surgical stabilization of rib fractures: a narrative review. J Clin Anesth. 2023;91:111275. 10.1016/j.jclinane.2023.111275. [DOI] [PubMed] [Google Scholar]
- 284.Leasia KN, Ciarallo C, Prins JTH, Preslaski C, Perkins-Pride E, Hardin K, Cralley A, Burlew CC, Coleman JJ, Cohen MJ, Lawless R, Platnick KB, Moore EE, Pieracci FM. A randomized clinical trial of single dose liposomal bupivacaine versus indwelling analgesic catheter in patients undergoing surgical stabilization of rib fractures. J Trauma Acute Care Surg. 2021;91(5):872–8. 10.1097/TA.0000000000003264. [DOI] [PubMed] [Google Scholar]
- 285.Choi J, Min JG, Jopling JK, Meshkin S, Bessoff KE, Forrester JD. Intercostal nerve cryoablation during surgical stabilization of rib fractures. J Trauma Acute Care Surg. 2021;91(6):976–80. 10.1097/TA.0000000000003391. [DOI] [PubMed] [Google Scholar]
- 286.Guerra-Londono CE, Privorotskiy A, Cozowicz C, Hicklen RS, Memtsoudis SG, Mariano ER, Cata JP. Assessment of intercostal nerve block analgesia for thoracic surgery: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(11):e2133394. 10.1001/jamanetworkopen.2021.33394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.