Abstract
Canine parvovirus (CPV) enters and infects cells by a dynamin-dependent, clathrin-mediated endocytic pathway, and viral capsids colocalize with transferrin in perinuclear vesicles of cells shortly after entry (J. S. L. Parker and C. R. Parrish, J. Virol. 74:1919–1930, 2000). Here we report that CPV and feline panleukopenia virus (FPV), a closely related parvovirus, bind to the human and feline transferrin receptors (TfRs) and use these receptors to enter and infect cells. Capsids did not detectably bind or enter quail QT35 cells or a Chinese hamster ovary (CHO) cell-derived cell line that lacks any TfR (TRVb cells). However, capsids bound and were endocytosed into QT35 cells and CHO-derived TRVb-1 cells that expressed the human TfR. TRVb-1 cells or TRVb cells transiently expressing the feline TfR were susceptible to infection by CPV and FPV, but the parental TRVb cells were not. We screened a panel of feline-mouse hybrid cells for susceptibility to FPV infection and found that only those cells that possessed feline chromosome C2 were susceptible. The feline TfR gene (TRFC) also mapped to feline chromosome C2. These data indicate that cell susceptibility for these viruses is determined by the TfR.
Canine parvovirus (CPV) and feline panleukopenia virus (FPV) are important pathogens of dogs and cats. CPV is a new virus of dogs that first appeared in 1978, having arisen as a variant of a virus that infected cats or a related carnivore (31). CPV and FPV are over 99% identical in DNA sequence, but they differ in host range (29, 30). Both viruses can infect feline and mink cells in tissue culture, but only CPV can efficiently infect cultured canine cells (30). FPV infection of dogs is restricted to certain cells of the bone marrow and thymus (30). The molecular determinants of CPV host range have been mapped to three regions on the surface of the capsid structure. Single amino acid changes in these regions lead to loss of the ability of CPV to infect canine, but not feline, cells (8, 19). Mutation of residues Asn93→Asp and Asn323→Asp in the VP2 capsid protein of FPV to the corresponding amino acids found in the VP2 protein of CPV allows that mutant to infect dog cells (8). The surface location of these host range determinants suggests that host range may be determined by the ability to bind a cell surface receptor or other cellular ligand (1).
During natural infections, CPV and FPV infect actively dividing cells of the lymphopoietic system and the crypt cells of the intestine (reviewed in reference 22). Initial virus replication occurs in the oropharyngeal lymphoid tissue, and the virus then spreads hematogenously to other lymphoid organs and the intestine. Autonomous parvoviruses (including CPV and FPV) can replicate only in mitotically active cells during the S phase of the cell cycle (9), and so the target organs in vivo are those that contain actively dividing cell populations.
The pathway of viral entry into cells is only partially characterized. Both CPV and FPV can bind sialic acid on the surface of some host cells. However, binding sialic acid does not appear important for viral infection, as mutants unable to bind sialic acid retain infectivity (3). CPV capsids bound a 40- to 42-kDa protein when overlaid on protein blots prepared from canine cell lysates (5), but that protein has not been further characterized. CPV and FPV can infect feline and mink cells, indicating that they likely share a common receptor and infection pathway in those cells. The process of capsid uptake involves clathrin-mediated endocytosis, and in feline or mink cells, capsids colocalize with transferrin in a perinuclear compartment (20). Once endocytosed into cells, capsids appear to penetrate only slowly into the cytoplasm. Anticapsid antibodies injected into the cytoplasm of cells prevent virus infection even 6 h after virus inoculation, suggesting that capsids still remain within endocytic compartments several hours after uptake (32).
We report that CPV and FPV bind to the human and feline transferrin receptors (TfRs) and use those receptors to enter and infect cells. Microinjected or exogenously added antibodies against the TfR prevented viral infection of cells. CPV and FPV did not bind, enter, or infect TfR-negative Chinese hamster ovary cells (TRVb cells), but they did bind, enter, and infect TRVb-1 cells which express the human TfR and TRVb cells transfected with the cDNA of the feline TfR. In feline-mouse hybrid cells, the feline TfR gene locus (TFRC) was mapped to feline chromosome C2 along with susceptibility to FPV infection.
MATERIALS AND METHODS
Cells and viruses.
Feline CRFK and Norden Laboratories feline kidney (NLFK) cells were grown in a 1:1 mixture of McCoy's 5A and Leibovitz L15 media with 5% fetal bovine serum. Quail QT35 cells were grown in M-24 medium with 4% chicken serum. HeLa cells were grown in Dulbecco's modified Eagle medium with 10% fetal bovine serum. Chinese hamster ovary-derived TRVb and TRVb-1 cells (16) were grown in Ham's F-12 medium with 10% fetal bovine serum, TRVb-1 cells being grown in the presence of 400 μg of Geneticin per ml.
CPV-d and FPV-b isolates were derived from infectious plasmid clones by transfection of NLFK cells (21), and the viruses were passaged fewer than four times before use in these studies. To prepare capsids, viruses were propagated in NLFK cells; the capsids were concentrated by polyethylene glycol precipitation; and then full capsids were purified in sucrose gradients, dialyzed against 20 mM Tris HCl (pH 7.5), and stored at 4°C.
Infection assays and antibody treatments.
For all infections, a multiplicity of infection of ∼1 was used. Infected cells were detected by immunostaining with a monoclonal antibody (MAb) against the nonstructural protein 1 (NS1) (36), followed by a goat anti-mouse immunoglobulin G (IgG)-Alexa 488 secondary conjugate (Molecular Probes, Eugene, Oreg.), or Texas Red-conjugated anti-NS1 IgG was used directly. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and then permeabilized with 0.1% Triton X-100 in PBS with 1% bovine serum albumin (permeabilization buffer). All antibody incubations were carried out in permeabilization buffer for 45 min at room temperature.
To examine the effect of a polyclonal anti-human TfR on infection by CPV and FPV, HeLa or TRVb-1 cells were inoculated with CPV or FPV at a multiplicity of infection of 1, with titers being determined by 50% tissue culture infective dose assay in NLFK cells (19). A 1:500 dilution of sheep anti-human TfR (33) was added to the cells either at the time of virus inoculation or 2 h later and remained in the culture throughout the incubation. After 18 h of incubation at 37°C, the cells were fixed and permeabilized; infected cells were detected by staining with Texas Red–anti-NS1. The percentage of cells infected was compared to the average infection of untreated cells.
To define the role of the feline TfR in virus infection and entry, antibody H68.4 (Zymed Laboratories Inc., South San Francisco, Calif.) against the cytoplasmic domain of the TfR (35) (anti-TfR-cyt) was injected into the cytoplasm of cells at various times around the time of virus inoculation. The H68.4 antibody was prepared against the human TfR and recognizes a peptide common to many species of TfRs, including the feline and canine receptors. Antibody 12CA5 IgG against an influenza virus hemagglutinin epitope was injected into control cells. The IgG antibodies were dialyzed against PBS, and then ∼4 pl of antibody at 3 mg/ml was injected into CRFK cells either 2 h before or 4 or 7 h after inoculation with virus. The cells were then incubated for 24 h before fixing and staining for the viral NS1 protein as described above. To examine effects on capsid uptake, cells injected with anti-TfR-cyt or antihemagglutinin IgG were incubated for 2 h at 37°C and then incubated with 20 μg of CPV full capsids per ml in Dulbecco's modified Eagle medium with 1% bovine serum albumin for 2 h at 37°C. The cells were then washed in PBS, fixed in 4% paraformaldehyde in PBS, and permeabilized. The injected IgG was detected with goat anti-mouse IgG-Alexa 594 (Molecular Probes), while virus was detected with a rabbit polyclonal serum against intact CPV capsids followed by a goat anti-rabbit IgG-Alexa 488 conjugate (Molecular Probes).
Cloning and expression of the TfR genes.
cDNA was prepared from feline liver mRNA and used in a PCR to amplify the TfR gene in two fragments using the Access reverse transcriptase PCR system (Promega, Madison, Wis.). Gene-specific primer pairs ([i] 5′-TCTAGATTAAAACTCATTGTCAATATCCC-3′ and 5′-CAGAAAAGGTTGCAAATGC-3′; [ii] 5′-ATATGGGTCACCTGTTCCCAGATGGGCAT-3′ and 5′-CTCGAGGCCGCCACGGTGTGGCAGTTCAGAATGATGGAT-3′) were used in PCR. The intact cDNA was then cloned into the vector pcDNA3.1(−) (Invitrogen, San Diego, Calif.) for expression. The gene sequence was determined using automated sequencing.
The human TfR gene was expressed from the plasmid pCB6-HuTfR (24). TRVb or QT35 cells seeded at a density of 2.5 × 102 cells per cm2 in 9.6-cm2 wells containing glass coverslips were transfected with 2 μg of DNA using 6 μl of Lipofectamine (Life Technologies, Inc., Bethesda, Md.) according to the manufacturer's directions. Twenty-four hours after transfection, the cells were washed and incubated in Dulbecco's modified Eagle medium with 1% bovine serum albumin for 30 min at 37°C. The cells were then incubated with 20 μg of purified full virus per ml and 50 μg of Alexa 594-labeled human transferrin (Molecular Probes) per ml for 2 h at 37°C. Following virus and transferrin uptake, the cells were fixed and permeabilized as described above. Capsids were detected with anticapsid MAb A3B10, followed by a goat anti-mouse IgG-Alexa 488 conjugate (Molecular Probes). Coverslips were mounted on slides with ProLong antifade agent (Molecular Probes), and confocal sections were collected with an Olympus Fluoview scanning confocal microscope using 480-nm and 594-nm cutoff filters. Images were further processed in Adobe Photoshop to enhance contrast and brightness. For flow cytometry, the cells were suspended by treatment with nonenzymatic dissociation reagent (Sigma, St. Louis, Mo.) and then fixed, permeabilized, and stained for TfR expression with anti-TfR-cyt followed by goat anti-mouse–R-phycoerythrin conjugate (Jackson Immunoresearch, Rockville, Md.) and stained for cell-associated capsids with a rabbit anti-CPV antibody followed by goat anti-rabbit–Alexa 488 conjugate (Molecular Probes). Isotype or prebleed controls and singly stained samples were used to compensate for bleed-through fluorescence in each channel. Data were collected with a Becton Dickinson (San Jose, Calif.) FACScalibur flow cytometer and analyzed using CellQuest software (Becton Dickinson).
Expression of the feline TfR in TRVb cells was detected by uptake of iron-loaded canine transferrin. Uptake assays were the same as described above for human transferrin, but internalized canine transferrin was detected with a rabbit polyclonal anti-canine transferrin antibody (kind gift of G. Apodaca, University of Pittsburgh) followed by a goat anti-rabbit–Alexa 594 conjugate. These assays allowed us to monitor both TfR expression and the degree of virus infection.
Mapping virus susceptibility and the TfR gene in mouse-feline hybrid cells.
Mouse × cat somatic hybrid cell lines that contain segregated feline chromosomes in different combinations (18) were inoculated with FPV at a multiplicity of infection of 0.1. Forty-eight hours later, low-molecular-weight DNA recovered from cells was tested for viral replicative-form DNA and single-stranded DNA by Southern blot analysis (8). The discordance between the presence of each feline chromosome and the cell susceptibility to FPV infection was calculated.
Radiation hybrid chromosomal mapping of the feline TfR gene (TFRC) was performed as described by Murphy et al. (17). Primers specific for the feline TfR gene were prepared (5′-CTGGCTCTCACACTCTGTCA-3′ and 5′-CCCAAATGTCACCAGAGAGG-3′). DNA prepared from 93 clones of a feline-mouse 5,000-rad-radiation hybrid cell panel was tested using PCR for the presence or absence of the feline TfR gene. The results obtained allowed the gene to be positioned onto the C2 framework as described previously (17). The corresponding human chromosome 3 and chromosome 21 conserved segments were derived from the Genebridge-4-based maps in the Gene Map '99 radiation hybrid map database (www.ncbi.nlm.nih.gov/genemap).
Nucleotide sequence accession number.
The nucleotide sequence of the TfR gene was deposited in GenBank under accession no. AF276984.
RESULTS
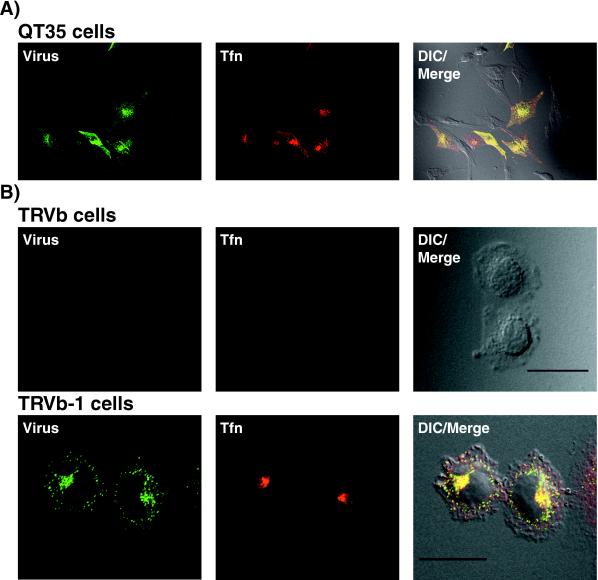
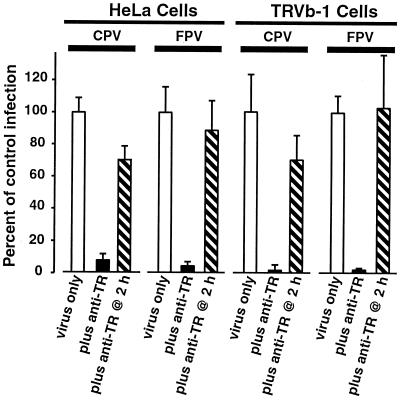
In preliminary studies, we observed that canine and mink cells transiently overexpressing the human TfR appeared to bind and endocytose more CPV capsids than did control plasmid-transfected cells or nontransfected cells (results not shown). Therefore, to examine the role of the TfR in virus binding and uptake, we expressed the human TfR or a control plasmid in quail QT35 cells. CPV capsids did not detectably bind to or enter nontransfected QT35 cells or cells transfected with a control plasmid, but they were bound to and taken up to high levels in QT35 cells expressing the human TfR (Fig. 1A). In addition, CPV capsids did not bind or enter TRVb cells, which do not express endogenous TfR, but they efficiently bound to TRVb-1 cells that stably expressed the human TfR (16) (Fig. 1B), as well as to human HeLa cells (data not shown). The TRVb cells and both the nontransfected and human TfR-expressing QT35 cells were not susceptible to infection by CPV or FPV (data not shown). In contrast, the human TfR-expressing TRVb-1 cells and HeLa cells were susceptible to infection by both CPV and FPV (Fig. 2). Infection of TRVb-1 and HeLa cells was blocked when antibodies against the human TfR were added to those cells at the time of virus inoculation but not when the antibodies were added 2 h later (Fig. 2).
FIG. 1.
CPV binds and enters cells expressing the human TfR. Cells were incubated with 20 μg of CPV capsids per ml and 50 μg of Alexa 594-conjugated human transferrin per ml for 2 h at 37°C and then washed in PBS, fixed, and immunostained for the presence of capsids. Differential interference contrast (DIC) images (right), transferrin (Tfn) uptake (center), and capsid uptake (left) are shown. (A) Uptake of CPV capsids and transferrin in QT35 quail cells transfected with pCB6-HuTfR (∼50% of cells transfected). Scale bar, 50 μm. (B) Uptake of CPV capsids and transferrin into TRVb cells (which have no endogenous TfR expression) or into TRVb-1 cells (expressing the human TfR). Scale bar, 20 μm.
FIG. 2.
Treatment of HeLa and TRVb-1 cells with polyclonal anti-human TfR inhibits infection by CPV and FPV. HeLa or TRVb-1 cells were inoculated with CPV or FPV (multiplicity of infection of 1 as assayed on NLFK cells). A 1:500 dilution of sheep anti-human TfR (anti-TR) (13) was added to the cells either at the time of virus inoculation or 2 h later, and then that was left in the culture throughout the experiment. After 18 h of incubation, cells were fixed and permeabilized, and then the infected cells were stained with a Texas Red-conjugated MAb raised against NS1. The percentage of infected cells was normalized to the average infection of untreated cells. The averages and standard deviations of the percentages of infected control (quadruplicate wells, minimum of 1,000 cells counted per well) are shown. TRVb cells, which do not express the TfR, were not susceptible to CPV or FPV infection (results not shown).
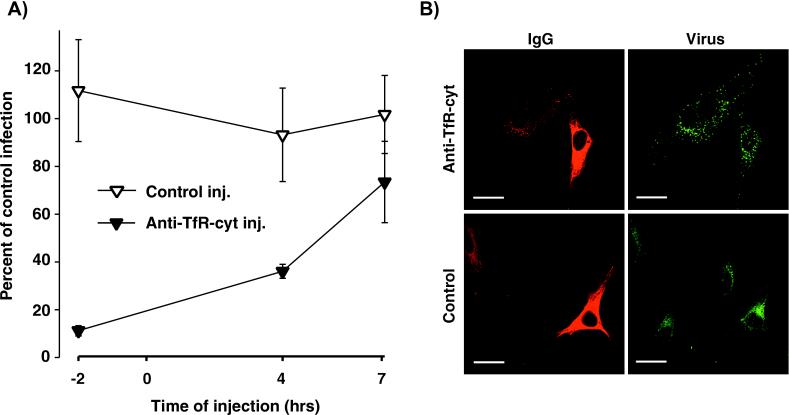
The anti-human TfR polyclonal serum did not recognize the feline TfR. We therefore examined the effects of microinjection of an antibody which recognized the cytoplasmic tail of the feline TfR (anti-TfR-cyt) on CPV infection and uptake. Injecting anti-TfR-cyt into the cytoplasm of feline CRFK cells 2 h before virus inoculation rendered them almost completely resistant to CPV infection (Fig. 3A). In addition, the injected cells were significantly less susceptible when the antibody was injected at 4 but not 7 h after virus inoculation (Fig. 3A). The anti-TfR-cyt injected 2 h prior to the virus inoculation did not prevent viral endocytosis, but the virus-containing vesicles in the anti-TfR-cyt-injected cells were larger and more widely dispersed within the cytoplasm than were virus-containing vesicles in noninjected cells (Fig. 3B). Injection of a control IgG had no effect on either virus infection or the size and distribution of virus-containing vesicles compared to those for noninjected cells (Fig. 3).
FIG. 3.
Cytoplasmic injection of an antibody against the cytoplasmic domain of the TfR (anti-TfR-cyt) inhibits CPV infection in feline cells. (A) Cells were injected with anti-TfR-cyt (Anti-TfR-cyt inj.) or a control IgG against the influenza virus hemagglutinin epitope (Control inj.) 2 h before virus inoculation or 4 or 7 h after inoculation and then incubated for a total infection time of 18 h. Cells were fixed and stained for the viral NS1 protein as a marker of infection. The percentages of the IgG-injected cells that became infected were compared to those of the noninjected cells in the same experiment. (B) Intracytoplasmic injection of anti-TfR-cyt antibodies but not of control IgG changes the morphology and distribution of virus-containing endosomes in cells. Cells were injected 2 h prior to adding CPV capsids (20 μg/ml) and then incubated for 1 h at 37°C. The cells were then fixed and permeabilized, and the injected antibody was detected with goat anti-mouse IgG-Alexa 594 conjugate (red). Capsids were detected with a Cy-2-conjugated MAb against CPV (green). Scale bar, 20 μm.
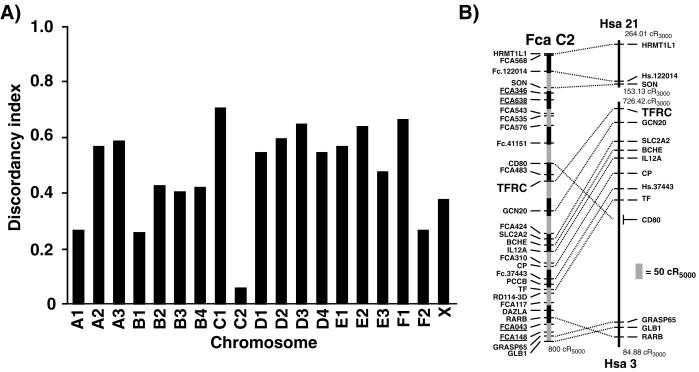
We inoculated feline-mouse hybrid cells that contain different known combinations of feline chromosomes with FPV and examined the cells for susceptibility to infection. The presence of feline chromosome C2 in a hybrid cell correlated most closely with susceptibility to infection (Fig. 4A). TFRC was mapped to a conserved centromeric region of feline chromosome C2 by analysis of the DNA from a 5,000-rad-radiation hybrid panel of 93 clones (Fig. 4B).
FIG. 4.
(A) Feline chromosome C2 confers susceptibility to FPV infection on feline-mouse hybrid cells. Members of a panel of feline-mouse somatic cell hybrids with known feline karyotypes were inoculated with FPV, and the presence of replicating viral DNA was assayed 48 h later. The discordancy between the presence of each feline chromosome and the susceptibility of the hybrid cells to virus infection is shown. (B) Radiation hybrid mapping of the feline TfR gene (TFRC) in a panel of feline-mouse radiation hybrids. The corresponding human chromosome 3 and 21 segments aligned to the right are derived from the Genebridge-4-based maps in the Gene Map '99 radiation hybrid map database (www.ncbi.nlm.nih.gov/genemap). Numbers at the ends of each human conserved segment represent map positions of the most distal markers. A scale bar is shown to the lower right (cR, centiray).
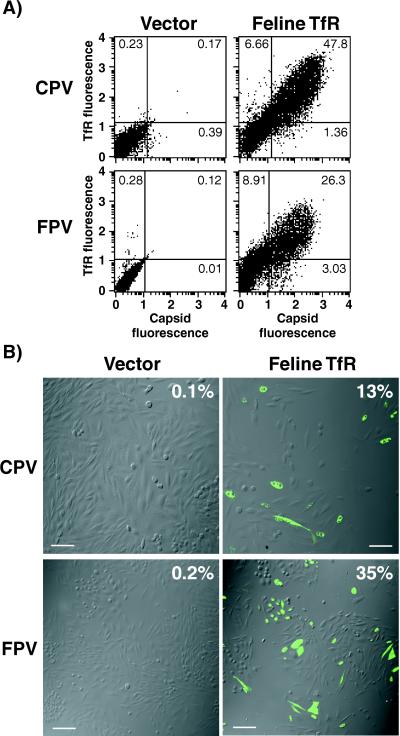
We cloned the feline TfR gene by reverse transcriptase PCR. The translated sequence of the feline TfR cDNA was 80 and 76% identical to the sequences of the human and Chinese hamster TfRs, respectively. CPV and FPV capsids bound efficiently to TRVb cells expressing the feline TfR but not to cells transfected with a control plasmid (Fig. 5A). To determine if the feline TfR would confer susceptibility on TRVb cells, we inoculated feline TfR-transfected or vector control-transfected TRVb cells with CPV or FPV. A high percentage of the feline TfR-expressing cells became infected, while few infected cells were observed among cells transfected with a control plasmid (Fig. 5B). We did not detect any infected cells when nontransfected cells were inoculated.
FIG. 5.
Feline TfR confers virus binding and CPV and FPV susceptibility on TRVb cells. (A) TRVb cells were transfected with a plasmid expressing the feline TfR or with a control plasmid containing no insert (Vector). Twenty-four hours after transfection, the cells were washed in Dulbecco's modified Eagle medium containing 1% bovine serum albumin and then incubated for 2 h at 37°C with 20 μg of full CPV or FPV capsids per ml in the same medium. Cells were then harvested, fixed, and stained for virus antigen and for the TfR using anti-TfR-cyt. Cells were analyzed by flow cytometry, and numbers in each quadrant indicate the percentages of cells in that category. (B) TRVb cells that were transfected as described above were inoculated with CPV or FPV at a multiplicity of infection of 1. Representative panels stained for infected cells are shown. Expression of the TfR was determined by the binding of canine transferrin, and virus infection was detected by staining for NS1 expression. The percentage of the feline TfR-expressing TRVb cells that became infected with CPV or FPV is shown at the top right of each panel. Scale bar, 50 μm.
DISCUSSION
Our finding that CPV and FPV use the TfR to attach to and infect cells is consistent with the current knowledge of the cellular infection pathway and of the pathogenesis of these viruses. We have previously shown that CPV capsids enter cells by a dynamin-dependent, clathrin-mediated endocytic process and that they colocalize with human transferrin in perinuclear vesicles of mink lung cells that express the human TfR (20). In light of the findings reported here, our previous observation of increased binding of CPV capsids to mink lung cells overexpressing the Lys44→Ala (K44A) mutant of dynamin I can be explained by increased numbers of TfRs on the cell surface, as has been reported for the insulin receptor GLUT4 in cells overexpressing dynamin K44A (12).
The TfR is a 90-kDa type II membrane protein that is expressed as a homodimer with an ∼65- to 70-residue N-terminal cytoplasmic tail, a 20-residue transmembrane sequence, and an extracellular sequence of about 680 residues (27). Structures of the ectodomain show that it consists of apical, helical, and protease-like domains arranged in a “butterfly” configuration (14).
The TfR is constitutively endocytosed from the plasma membrane by clathrin-mediated endocytosis (13, 34). The natural function of the TfR is the cellular uptake of ferric iron bound to transferrin. The TfR-transferrin complex is delivered to the low-pH environment of the early endosome where ferric iron is released from transferrin. The iron-free transferrin remains bound to the receptor at low pH within endosomes until the complex is recycled to the cell surface, where the transferrin is released from the receptor at neutral pH, allowing iron-loaded transferrin to bind. As actively dividing cells require more iron than do nondividing cells, they express greater numbers of TfRs on their cell surface (26). Autonomous parvoviruses can replicate only in mitotically active cells during the S phase of cell cycle; they cannot induce cells to enter the cell cycle (9). Therefore, by binding the TfR, CPV and FPV can preferentially target actively dividing cells that will support viral DNA replication. High levels of TfR are found on small intestinal crypt cells and on actively dividing lymphoid cells, and both of these cell types are major targets for virus replication (2, 7, 15, 25).
Natural infection by CPV and FPV occurs via the oropharyngeal route, and initial viral replication occurs in the local oropharyngeal lymphoid tissue. Thereafter, these viruses spread to other organs hematogenously (reviewed in reference 22). The TfR is expressed mainly on the basolateral surface of polarized epithelial cells (10), and a previous study noted that CPV binds preferentially to the basolateral surface of polarized canine MDCK cells (4). Thus, the expression pattern of the TfR in epithelial cells correlates with the polarized binding of CPV and the hematogenous spread of these viruses to the intestinal epithelium during infection (22).
CPV and FPV did not bind, enter, or infect TfR-deficient TRVb cells. However, CPV and FPV did bind, enter, and infect TRVb-1 or HeLa cells, which express the human TfR on their surface, and infection of those cells was blocked by addition of a polyclonal antiserum against the human TfR at the time of virus inoculation. The TRVb and TRVb-1 cells are genetically identical except for the expression of the human TfR in TRVb-1 cells (16). Therefore, the TRVb cells possess all of the factors required for successful virus replication except the viral receptor. Control of virus host range and susceptibility at the level of the cell surface receptor has been reported for many viruses, including adenoviruses (6), coronaviruses (11), and picornaviruses (6). Although CPV and FPV bound and entered QT35 cells expressing the human TfR, they did not infect those cells (unpublished data), indicating that other factors required for the later stages of infection of mammalian cells differ in those avian cells.
MAb H68.4 (anti-TfR-cyt) binds the cytoplasmic tail of the feline TfR (35). When added to permeabilized cells, the H68.4 antibody blocked endocytosis but did not prevent formation of deep invaginations of the cell membrane that contained the TfR (23). In our studies, the microinjected anti-TfR-cyt antibodies efficiently blocked cell infection by CPV (Fig. 3A). Cells injected with anti-TfR-cyt still endocytosed capsids, but both the morphology and location of the virus-containing vesicles within the cytoplasm differed from those seen for noninjected or control-injected cells (Fig. 3B). The anti-TfR-cyt may interfere with the normal trafficking of viral capsids by masking portions of the cytoplasmic tail of the TfR that are required for trafficking or by cross-linking the receptors. Another possibility is that anti-TfR-cyt antibody binding to the cytoplasmic tail disrupts virus-receptor interactions required for infection which occur after the capsid has been removed from the cell surface and has entered the early endosome.
The animal host range of CPV and FPV appears to be naturally restricted to carnivores. Although the human TfR is used by CPV and FPV to infect TRVb-1 cells and human HeLa cells, there is no evidence that humans are infected by these viruses, and it is likely that many other host or viral factors prevent CPV or FPV from efficiently infecting and spreading between humans. The TfR appears to be a primary determinant of cell susceptibility to CPV and FPV infection of cat cells. Our preliminary studies show that the feline TfR can transfer FPV susceptibility to dog cells, indicating that the block to canine cell infection by FPV is likely due to the specific lack of a functional receptor. We are currently examining whether differences in the feline and canine TfRs determine virus susceptibility and the host range of these viruses.
ACKNOWLEDGMENTS
S. C. Harrison, P. J. Bjorkmann, C. A. Enns, I. Mellman, and T. E. McGraw generously provided reagents and cell lines. We are grateful to J. F. Collawn for helpful discussions.
This work was supported by grants AI28385 and AI33468 from the National Institute of Allergy and Infectious Diseases to C.R.P. J.S.L.P. is supported by National Research Service Award F32 AI10134.
REFERENCES
- 1.Agbandje M, Parrish C R, Rossmann M G. The structure of parvoviruses. Semin Virol. 1995;6:299–309. [Google Scholar]
- 2.Anderson G J, Powell L W, Halliday J W. Transferrin receptor distribution and regulation in the rat small intestine. Effect of iron stores and erythropoiesis. Gastroenterology. 1990;98:576–585. doi: 10.1016/0016-5085(90)90276-7. [DOI] [PubMed] [Google Scholar]
- 3.Barbis D P, Chang S-F, Parrish C R. Mutations adjacent to the dimple of the canine parvovirus capsid structure affect sialic acid binding. Virology. 1992;191:301–308. doi: 10.1016/0042-6822(92)90192-r. [DOI] [PubMed] [Google Scholar]
- 4.Basak S, Compans R W. Polarized entry of canine parvovirus in an epithelial cell line. J Virol. 1989;63:3164–3167. doi: 10.1128/jvi.63.7.3164-3167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basak S, Turner H, Parr S. Identification of a 40- to 42-kDa attachment polypeptide for canine parvovirus in A72 cells. Virology. 1994;205:7–16. doi: 10.1006/viro.1994.1614. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan L N, Gerhardt E M. Transferrin receptor gene is hyperexpressed and transcriptionally regulated in differentiating erythroid cells. J Biol Chem. 1992;267:8254–8259. [PubMed] [Google Scholar]
- 8.Chang S-F, Sgro J-Y, Parrish C R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 10.Fuller S D, Simons K. Transferrin receptor polarity and recycling accuracy in “tight” and “leaky” strains of Madin-Darby canine kidney cells. J Cell Biol. 1986;103:1767–1779. doi: 10.1083/jcb.103.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensley L E, Holmes K V, Beauchemin N, Baric R S. Virus-receptor interactions and interspecies transfer of a mouse hepatitis virus. Adv Exp Med Biol. 1998;440:33–41. doi: 10.1007/978-1-4615-5331-1_5. [DOI] [PubMed] [Google Scholar]
- 12.Kao A W, Ceresa B P, Santeler S R, Pessin J E. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J Biol Chem. 1998;273:25450–25457. doi: 10.1074/jbc.273.39.25450. [DOI] [PubMed] [Google Scholar]
- 13.Larrick J W, Enns C, Raubitschek A, Weintraub H. Receptor-mediated endocytosis of human transferrin and its cell surface receptor. J Cell Physiol. 1985;124:283–287. doi: 10.1002/jcp.1041240217. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence C M, Ray S, Babyonyshev M, Galluser R, Borhani D W, Harrison S C. Crystal structure of the ectodomain of human transferrin receptor. Science. 1999;286:779–782. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- 15.Levine D S, Woods J W. Immunolocalization of transferrin and transferrin receptor in mouse small intestinal absorptive cells. J Histochem Cytochem. 1990;38:851–858. doi: 10.1177/38.6.2186090. [DOI] [PubMed] [Google Scholar]
- 16.McGraw T E, Greenfield L, Maxfield F R. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy W J, Sun S, Chen Z, Yuhki N, Hirschmann D, Menotti-Raymond M, O'Brien S J. A radiation hybrid map of the cat genome: implications for comparative mapping. Genome Res. 2000;10:691–702. doi: 10.1101/gr.10.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien S J, Nash W G. Genetic mapping in mammals: chromosome map of domestic cat. Science. 1982;216:257–265. doi: 10.1126/science.7063884. [DOI] [PubMed] [Google Scholar]
- 19.Parker J S L, Parrish C R. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J Virol. 1997;71:9214–9222. doi: 10.1128/jvi.71.12.9214-9222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker J S L, Parrish C R. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J Virol. 2000;74:1919–1930. doi: 10.1128/jvi.74.4.1919-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrish C R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991;183:195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- 22.Parrish C R. Pathogenesis of feline panleukopenia virus and canine parvovirus. Baillière's Clin Haematol. 1995;8:57–71. doi: 10.1016/S0950-3536(05)80232-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid S L, Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol. 1991;114:869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheff D R, Daro E A, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testa U, Pelosi E, Peschle C. The transferrin receptor. Crit Rev Oncog. 1993;4:241–276. [PubMed] [Google Scholar]
- 26.Trowbridge I S, Omary M B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci USA. 1981;78:3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trowbridge I S, Shackelford D A. Structure and function of transferrin receptors and their relationship to cell growth. Biochem Soc Symp. 1986;51:117–129. [PubMed] [Google Scholar]
- 28.Truyen U, Agbandje M, Parrish C R. Characterization of the feline host range and a specific epitope of feline panleukopenia virus. Virology. 1994;200:494–503. doi: 10.1006/viro.1994.1212. [DOI] [PubMed] [Google Scholar]
- 29.Truyen U, Gruenberg A, Chang S-F, Obermaier B, Veijalainen P, Parrish C R. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truyen U, Parrish C R. Canine and feline host ranges of canine parvovirus and feline panleukopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. J Virol. 1992;66:5399–5408. doi: 10.1128/jvi.66.9.5399-5408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truyen U, Parrish C R. The evolution and control of parvovirus host ranges. Semin Virol. 1995;6:311–317. [Google Scholar]
- 32.Vihinen-Ranta M, Yuan W, Parrish C R. Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport. J Virol. 2000;74:4853–4859. doi: 10.1128/jvi.74.10.4853-4859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren R A, Green F A, Enns C A. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J Biol Chem. 1997;272:2116–2121. doi: 10.1074/jbc.272.4.2116. [DOI] [PubMed] [Google Scholar]
- 34.Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985;100:633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White S, Miller K, Hopkins C, Trowbridge I S. Monoclonal antibodies against defined epitopes of the human transferrin receptor cytoplasmic tail. Biochim Biophys Acta. 1992;1136:28–34. doi: 10.1016/0167-4889(92)90081-l. [DOI] [PubMed] [Google Scholar]
- 36.Yeung D E, Brown G W, Tam P, Russnak R H, Wilson G, Clark-Lewis I, Astell C R. Monoclonal antibodies to the major nonstructural nuclear protein of minute virus of mice. Virology. 1991;181:35–45. doi: 10.1016/0042-6822(91)90467-p. [DOI] [PubMed] [Google Scholar]