Abstract
Muscone is the main chemical ingredient in Musk which is main crude drug in Tongqiaohuoxue decoction (TQHXD), and TQHXD has a protective effect on damaged neurons, so we hypothesize that muscone can alter blood–brain barrier (BBB) permeability via the modulation of P-glycoprotein (P-gp) and matrix metalloproteinase-9 (MMP-9) expression. In this study, astrocytes (AC) and human umbilical vein endothelial cells (ECV304) were co-cultured to simulate the BBB model in vitro. Leak testing, transmembrane resistance experiments, and BBB-specific enzyme testing were used to test whether the model was successful. Different concentrations of muscone permeating the BBB were detected by gas chromatography (GC). The change of the transendothelial electrical resistance (TEER) on the BBB in vitro after treating with muscone was detected by Millicell-ERS. The protein expression of P-gp, MMP-9 in normal, and oxygen/glucose deprivation (OGD) BBB model was determined by western blotting to inquire that the mechanism of muscone penetrates the BBB model in vitro. The results show that muscone was detected in the lower medium of the BBB model by GC; the values of TEER were no significant difference before and after muscone (8 μM) was added to the BBB model; the expression of P-gp significantly decreased after the BBB model treatment with muscone (4, 8, and 16 μM) for 24 h; the expression of P-gp and MMP-9 in different concentrations of muscone groups had different degrees of reduction compared with the BBB in the state of OGD. In conclusion, muscone could permeate the BBB model, and it was associated with the inhibition of P-gp and MMP-9 expression. An understanding of the mechanisms of muscone across the BBB is crucial to the development of therapeutic modalities for cerebral vascular diseases.
Keywords: Blood–brain barrier (BBB), Muscone, Tight junction, Matrix metalloproteinase-9 (MMP-9), P-glycoprotein (P-gp)
Introduction
Tongqiaohuoxue decoction (TQHXD) is a classic prescription for cerebral vascular diseases created by Qingren Wang, a famous doctor in Qing Dynasty. In our previous study, the serum of rats containing TQHXD could reduce the neuronal damage caused by excitatory amino acid, and also had a protective effect on damaged neurons (Wang et al. 2012c). Muscone is the main chemical ingredient in Musk which is main crude drug in TQHXD. Our study showed that muscone could specifically combined with nerve cells using nerve cells bio-chromatography. Muscone could ameliorate cellular morphology of pheochromocytoma (PC12) cells damaged by glutamate, promote cell proliferation obviously, reduce the release of lactate dehydrogenase (LDH), and decrease the rate of apoptosis. Thus, it had a protective effect on impaired PC12 cells (Wang et al. 2012b). Muscone could also be detected in the cerebrospinal fluid of rabbits after receiving intragastric administration of TQHXD twice a day for 5 days (Wang et al. 2012a).
The blood–brain barrier (BBB) separates the central nervous system (CNS) from systemic blood circulation by physical, metabolic, and transport barriers and is formed by complex interactions between several types of cells, including cerebral endothelial cells and perivascular elements (Reese and Karnovsky 1967; Ribatti et al. 2006). Tight junction (TJ) between cerebral microvascular endothelial cells is the important structure and functional base of BBB, which allows BBB to maintain a constant and optimal microenvironment for neurons and protects CNS from exogenous toxicants (Liu et al. 2012). But on the other hand, this structure also constitutes the most redoubtable obstacle for drug delivery for the treatment of brain disorders. Because of the fact that Traditional Chinese medicine (TCM) treating nervous system disease, which is closely related to chemical components of TCM penetrating the BBB, the BBB plays an important role in the pathophysiology of CNS disorders such as hypoxia, hypoxia–ischemia, and stroke. Although muscone has been reported to offer neuroprotection against cerebral ischemia injury, its impact on BBB is not clear. The objective of this investigation is to evaluate the effects of muscone post-treatment on BBB integrity and its mechanism of action, focusing on modulation of matrix metalloproteinase-9 (MMP-9) and P-glycoprotein (P-gp). P-gp, widely expressed in murine and human brain, is an important drug pumps limiting substrates across BBB as well as involved in the neuropathology. Therefore, we hypothesize drug pump such as P-gp may be involved in the transport of muscone across the BBB. The ability to modulate this active regulation might be useful for efficient CNS drug delivery and the treatment of cerebral vasogenic edema and inflammation following stroke. Discovery of mechanisms that determine brain permeation of muscone will undoubtedly enable more efficient analgesia and an improved utility of these compounds as potential therapeutics.
In this research, the BBB model in vitro was established to explore how the muscone penetrated the BBB, and whether it was related to the expression of P-gp and MMP-9. The primary cultured astrocytes (AC) and ECV304 cells were co-cultured to simulate the BBB model in vitro (Hurst and Fritz 1996). Leak testing, transmembrane resistance experiment, and specific enzyme of BBB were used to validate the success of the BBB model in vitro. Different concentrations of muscone were added to the BBB model. Gas chromatography (GC) was used to test whether the muscone can penetrate the BBB. Millicell-ERS was used to detect the change of transendothelial electrical resistance (TEER) of the BBB model after treating with muscone. The expression of P-gp, MMP-9 in normal state, and oxygen/glucose deprivation (OGD) state had been tested by western blotting to inquire the mechanism of muscone penetrating through the BBB.
Materials and Methods
Animals
Neonatal Sprague–Dawley (SD) rats (1–3 days) of either sex were purchased from Experimental animal center of Anhui Medical University (Hefei, SCXK 2011-002, China), and they were obtained to culture primary astrocytes (AC). All experiments were approved by the institutional Animal Care and Use Committee of the Anhui university of Chinese medicine.
The Primary Culture and Identification of AC
Cerebral astrocytes were isolated from the neonatal SD rats as previously described (McCarthy and de Vellis 1980), with minor modifications as listed below. Briefly, they were killed by rapid decapitation, the cerebral cortices were removed and separated from meninges and basal ganglia, and tissue was dissociated with 0.25 % tryptase at 37 °C and terminated by Dulbecco’s modified Eagle’s medium (DMEM, GIBCO Corporation, USA) supplemented with 10 % fetal bovine serum (FBS) and penicillin/streptomycin. After centrifugation at 1500 r/m for 5 min, the cell pellets were resuspended and seeded on poly-lysine-coated flask. The cultures were maintained at 37 °C in a humidified 5 % CO2/95 % air atmosphere. Culture medium was replaced 24 h later and then changed every 2–3 days. Before experiments, astrocytes were replated on poly-lysine-coated 6- or 24-well plates. Immunocytochemistry showed that 98 % of the cells stained positively for the astrocytic marker glial fibrillary acid protein (GFAP) (Sun et al. 2008).
ECV304 Cells Culture
ECV304 cell lines were purchased from American Type Culture Collection (ATCC). The cells were cultured in DMEM containing 10 % FBS, penicillin and streptomycin (100 U/mL; 100 µg/mL), and l-glutamine (2 mM). All percentage concentrations are v/v, unless otherwise stated. Cells were maintained at 37 °C in a humidified atmosphere consisting of 5 % (v/v) CO2. Culture media were replaced every 2–3 days, and cells passaged at least once a week (Youdim et al. 2003; Wang et al. 2014).
ECV304 Cells and AC were Co-cultured to Simulate the BBB Model In Vitro
The in vitro BBB model was established by co-culturing ECV304 and AC. The astrocytes were seeded at a density of 1 × 107 cells/mL onto the undersurface of precoated (1 % matrigel) transwell membrane inserts (12 mm diameter with 1 μm pore size and 1.12 cm2 surface areas; Millipore, USA). After the inserts were incubated in an upside down position for 12 h, they were returned to the upright position and incubated until 80 % of confluency was reached. The ECV304 were seeded inside the insert at a density of 1 × 105 cells/mL. The procedure is shown in Fig. 1a (Hurst and Fritz 1996; Dobbie et al. 1999; Nakagawa et al. 2009; Neuhaus et al. 2011; Scism et al. 1999; Youdim et al. 2003).
Fig. 1.
Establishment of in vitro BBB model. a ECV304 cells co-cultured with AC in plug-in petri dishes. The in vitro BBB model consisted of co-cultured AC and ECV304 cells. ECV304 were placed on permeable polyester transwell insert membranes with pore sizes of 3 μm on 24-well plates, and AC were placed on the bottom of the wells. The ECV304 cells are seen growing on the permeable membrane, which represents a cerebral vessel, while the astrocytes are growing on the bottom of the well and represent the brain parenchyma surrounded by medium, which represents the cerebrospinal fluid. The TEER measurement will be adjusted when the Millicell-ERS2 instrument was inserted into 70 % ethanol for 15 min, and dry 15 s in the air, and washed by sterilized electrolyte solution. Short and long sides of electrode were inserted into the inside and outside of medium of Transwell little, respectively. Electrode was vertical to the surface. Then, the value was read and recorded. b–e Morphological changes of primary cultured AC by fluorescence microscope, original magnification ×100. Astrocytes can be identified by the expression of GFAP and their specific morphology in culture. Primary astrocytes appeared polygonal with long cell processes resembling astrocytic endfeet (b the third day, c the seventh day); GFAP was strongly positively immunostained (green fluorescence) in the cytoplasm of primary astrocytes (d the third day, e the seventh day)
Identification of the BBB Model
The BBB permeability was assayed by measuring the TEER across the cell monolayer. TEER represents the impedance required to pass through the barrier structure and is widely recognized as one of the most accurate and sensitive indicators of BBB integrity (Rutten et al. 1987; Sill et al. 1992). A decrease in TEER reflects an increase in permeability and a loss of barrier function. In this study, TEER of the BBB model was recorded in PBS at 2, 4, 8, 12, 16, 20, 24, 48, 72, and 96 h using the Millicell-ERS (Millipore, USA). The resistance value was multiplied by the surface area of the insert (1.12 cm2) and expressed as Ω cm2. The TEER of each sample was corrected for background resistance without cells and reported as Ω cm2.
When the value of TEER was stable, the medium was added to the inside of transwell and made sure that the liquid level difference between the inside and outside of transwell was more than 0.5 cm. The test was positive if the liquid level difference could maintain for 4 h, and the BBB was basically formed (Kuchler-Bopp et al. 1999).
Enzymatic activities of gamma-glutamyltranspeptidase (γ-GT) and alkaline phosphatase (ALP) were taken as indicators for the expression of the BBB phenotype (Meyer et al. 1991; Brust et al. 1994; Meyer et al. 1990). They were measured in monolayer ECV304 cells and co-cultured cells. Briefly, the adherent ECV304 cells on the plug-in cell culture dishes polyester film were washed twice with ice-cold phosphate-buffered saline (PBS), and the cells were scraped off the flask. After centrifugation (5000 r/m, 5 min, 4 °C), the supernatant was discarded and the pellets were resuspended in 150 µL lysis buffer (Beyotime Institute of Biotechnology, China). During 30 min incubation on ice, the lysates were resuspended several times. After centrifugation (12,000 r/m, 30 min, 4 °C), the activity of γ-GT was measured in 50 μL aliquots of the supernatants. The protein content of the cultured cells was determined using the BCA protein assay kit (Beyotime Institute of Biotechnology, China). The protein of co-culture model of cells was extracted in the same way. To compare the differential expression of γ-GT and ALP between the normal BBB model and ECV304 monolayer cells, the γ-GT Kit (Yuanye Biotechnology, China) and the ALP assay kit (Jiancheng Biological Institute, China) were used to detect the related protein (Poetsch et al. 2010; Reichel et al. 2003; Tóth et al. 2011; Joó 1996).
Screening Safe Concentration of Muscone on ECV304 and Primary Cultures of AC
The safe concentration of muscone was examined by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Muscone-treated groups were post-treated with various concentrations of muscone (1, 2, 4, 8, and 16 μM). MTT was added to a final concentration of 0.5 mg/mL for 4 h before the end of the experiment. The supernatant was removed, and 150 mL of DMSO was added for 20 min. The MTT optical density values were measured on a microplate reader at 490 nm wavelengths (Lu et al. 2010; Adriana et al. 2011).
Gas Chromatography
The muscone was obtained from Koeman Manchester Medical Technology Development Corporation, China (purity >98 %). Muscone standard solutions were prepared by diluting the muscone with physiological saline to a concentration of 1 mg/mL.
The BBB models were randomly divided into control group, muscone treatment groups which were set to different concentrations of 4, 8, and 16 μM. The corresponding concentration of the muscone was added to the inner layer of the BBB model, and the outer medium was collected after 24 h. The amount of three times methanol was added to the collected medium, oscillated for 60 s, and centrifuged at 10,000 r/m for 10 min. The supernatant was placed in −80 °C refrigerator overnight and evaporated in the freeze-drying machine next day completely. The dried residue was then dissolved in 1 mL of methanol and filtered through a 0.22-μm membrane (Parran et al. 2005).
Chromatographic condition was as follows: Gas chromatography (GC) analysis of muscone was carried out on a GC with flame ionization detection (FID; GC2010, Shimadzu, Kyoto, Japan). A CP-Sil 24 CB (50 % phenyl, 50 % dimethylsiloxane) capillary column, WCOT fused silica, 30 m × 0.32 mm id with 0.25 μm stationary film thickness (Chrompack, Middelburg, The Netherlands) was used for the GC analysis. Chromatographic separations were performed with an initial oven temperature of 120 °C, followed by heating at 40 °C/min to 260 °C, held for 15 min. The injection port and the FID temperatures were maintained at 260 °C. The injector was operated in the split mode with a split ratio of 1:20. Helium (99.999 % pure) was used as carrier gas with a flow rate of 1.5 mL/min. 1 µL of the sample was injected into the GC (Paik and Kim 2004).
The Effects of Muscone on the Opening of Tight Junction of BBB Model In Vitro
The muscone (8 μM) was added to the inside of the transwell after co-culture for 2 days. The values of TEER were measured to observe the impacts of muscone on the opening of TJ of BBB model in vitro before the muscone (8 μM) was added to the transwell and after the muscone (8 μM) was added to the transwell for 5 min, 10 min, 30 min, 60 min, 2 h, 4 h, 8 h, and 24 h.
The Effects of Muscone on the Expression of P-gp in BBB In Vitro
Western blotting was used to detect the distribution of P-gp in BBB model in vitro. The protein was extracted from AC group, ECV304 cells group, and the BBB model group. The expression of P-gp was compared among three groups to determine the distribution of P-gp. The cells were washed twice with ice-cold PBS and were scraped off the flask. After centrifugation (5000 r/m, 5 min, 4 °C), the supernatant was discarded and the pellets were resuspended in 150 µL lysis buffer. During 30 min incubation on ice, the lysates were resuspended several times. After centrifugation (12,000 r/m, 30 min, 4 °C), the result was measured in 50 μL aliquots of the supernatants. The protein content of the cultured cells was determined using the BCA protein assay kit. Proteins lysates were electrophoresed through a 15 % sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and electrically transferred to a nitrocellulose membrane. This membrane was overnight incubated at 4 °C in tris-(hydroxymethyl)-aminomethane-buffered saline (TBS) containing 5 % milk and detected with the primary rabbit polyclonal antibody against P-gp (1:200; Santa Cruz Biotechnology, USA). After washing with TBST, membranes were incubated with the secondary antibodies (Santa Cruz, 1:1000) for 1 h at room temperature. Western blots were developed with the ECL chemiluminescence system and were captured on autoradiographic films. Films were scanned, and densitometric analysis of the bands was performed with Labworks 4.6 Analysis System. The assay was repeated in triplicate. Muscone groups were treated with 4, 8, and 16 µM muscone, respectively. The expression of P-gp in BBB model was also detected by western blotting.
The Expression of P-gp, MMP-9 in OGD BBB Model
To mimic ischemic condition in vitro, cells were exposed to OGD as described previously (Furuichi et al. 2005). In brief, the cells were deprived of O2 and glucose by changing the culture medium to a glucose-free ‘‘ionic shift’’ solution (ISS) with a pH 6.55, containing NaCl (39 mM), nagluconate (11 mM), K-gluconate (65 mM), NMDG-Cl (38 mM), NaH2PO4 (1 mM), CaCl2 (0.13 mM), MgCl2 (1.5 mM), and Bis–Tris (10.5 mM) as previously described (Ming et al. 2006). The coverslips were placed in an anaerobic chamber (Thermo Forma Scientific 3131) in an atmosphere of 10 % H2, 5 % CO2, and 85 % N2. After washing the cells twice with deoxygenated ISS, they were incubated in the anaerobic chamber for 1 h. Subsequently, the cells were removed from the anaerobic environment, the ISS was replaced with serum-free medium containing 5 mM glucose, and cultures were placed in an incubator with 95 % air (20 % O2)/5 % CO2.
Control experiments were performed with cells maintained under identical conditions before, during, and after OGD except that during the sham OGD they were maintained in serum-free medium that contained 5 mM glucose. Control and treated cells were treated identically except that they were not exposed to OGD. To observe the effect of muscone post-treatment, cells were post-treated with various concentrations of muscone (4, 8, and 16 µM) after a 1-h period of OGD followed by 24 h of reoxygenation. Inhibitor groups were post-treated with signal inhibitor for P-gp (verapamil) and MMP-9 (minocycline). P-gp and MMP-9 expressions were detected by western blotting as previously described (Wang et al. 2007).
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Data were analyzed by one-way ANOVA. Differences between experimental groups were determined by Fisher’s LSD post-test. Significance was assigned at P < 0.05.
Results
Identification of AC by GFAP Immunofluorescence Staining
Astrocytes characterized by GFAP immunostaining were polygonal with long cell processes, and green fluorescence could clearly emerge in most of the cytoplasm and irregular projections. Immunocytochemical labeling showed that the astrocytes expressed the GFAP astrocytic marker. Following passage, >98 % of astrocytes were GFAP positive (Fig. 1b–e).
Identification of BBB Model In Vitro
The permeability to ions and low molecular weight molecules was assessed daily by measuring the TEER. The TEER increased after cultured for 20 h (Fig. 2a). Then, the TEER was significantly greater in co-cultures than in monocultures that were grown for the same time period. The TEER reached a plateau after co-cultured for 72 and 96 h. Arrived at 72 h, the two groups of TEER values moved into a stable state, so 72 h was the final time point for the co-cultured model used for all the other experiments. Our results are consistent with those of a related study (Perrière et al. 2005), suggesting that co-culture plays an important role in the formation of tight BBB.
Fig. 2.
Identification of BBB model in vitro. a The permeability of the BBB to small ions was assessed by measuring the TEER. The electrical resistance across the endothelial cell layer was calculated using a volt-ohm meter. TEER was calculated by subtracting the electrical resistance of the model from that of a blank transwell membrane. Data are presented as mean ± SD (n = 6) *P < 0.05, **P < 0.01 versus monolayer ECV304 group. b Transwell culture medium of medial and lateral liquid level difference were ≥0.5 cm, after 4 h suggesting that the cultured cells had formed dense barrier. c Detection of specific enzyme of BBB. Enzyme activities of γ-GT and ALP were determined in monolayer ECV304 cells and co-cultured cells with diagnostic assay kits. All values presented are mean ± SD (n = 6). *P < 0.05, **P < 0.01 versus monolayer ECV304 group
The liquid level difference between the donor compartment and acceptor compartment was greater than 0.5 cm. The leak testing showed that 0.5 cm liquid medium levels were maintained in the in vitro BBB model after 4 h, which showed that the model was established (Fig. 2b).
The content of ALP in the monolayer ECV304 cells was (7.083 ± 0.463) King units/100 mL, the content of ALP in co-cultured cells was (16.771 ± 0.110) King units/100 mL, and there were significant difference between monolayer ECV304 cells and the co-culture system. The standard curve of γ-GT was y = 0.253x + 0.017, R 2 = 0.9985. After calculation, the content of γ-GT in monolayer cells was (35.57 ± 2.60) U/L, and the content of γ-GT in co-cultured cells was (44.27 ± 0.42) U/L, and there were significant difference between monolayer ECV304 cells and ECV304 cells co-cultured with AC. The content of ALP and γ-GT in co-cultured cells significantly increased compared with monolayer cells (Fig. 2c).
Screening Safe Concentration of Muscone on ECV304 and Primary Cultures of AC
Compared to the control group, muscone (1–16 μM) increased cell viability in ECV304 and primary cultures of AC. As shown in Fig. 3, combined with the results of MTT activity, incubation of ECV304 and primary cultures of AC with 8 μM muscone for 24 h in DMEM/F12 medium shows the best cell viability than other doses of 1, 2, 4, and 16 μΜ muscone. Therefore, in the following experiments, we used muscone (4, 8, and 16 μM) as standard treatment. The safe concentration of muscone is 1–16 μΜ.
Fig. 3.
Effects of muscone on primary cultured astrocytes (AC) and ECV304. a Cell viability of ECV304 was measured by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl-tetrazolium bromide assay. Data were normalized by control as 100 % (n = 6). b Cell viability of AC was measured by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl-tetrazolium bromide assay. Data were normalized by control as 100 %. All values presented are mean ± SD (n = 6). *P < 0.05, **P < 0.01
Detection of Muscone Penetrating the BBB Model In Vitro by GC
The results showed that the retention time of the sample of muscone was the same as the muscone standard solution, and the concentrations of 4, 8, and 16 μΜ muscone could reach the outside of transwell from the inside of transwell. It illustrated that muscone could penetrate the BBB (Fig. 4).
Fig. 4.
Muscone penetrated the BBB in vitro by gas chromatography. Different concentrations of muscone permeating the BBB were detected by GC. The retention time of the sample of muscone was the same as the muscone standard solution, which illustrated that muscone could penetrate the BBB model. a Methanol, b the BBB model without treatment, c the muscone standard solution, d 4 μM muscone, e 8 μM muscone, f 16 μM muscone
The Effects of Muscone on the Opening of Tight Junction in the BBB
There were no significant differences of TEER values after the muscone (8 μM) was added to the transwell for 0 min, 5 min, 10 min, 30 min, 60 min, 2 h, 4 h, 8 h, and 24 h (Fig. 5).
Fig. 5.
The effects of muscone on the opening of tight junction in the BBB model in vitro. TEER across the in vitro BBB model was measured using a Millicell-ERS system. The BBB was treated with muscone (8 μM) for 0 min, 5 min, 10 min, 30 min, 60 min, 2 h, 4 h, 8 h, and 24 h followed by replacement of fresh media prior to recording of TEER. TEER measurements (Ω cm2) are expressed as mean ± SD of three separate experiments done in triplicate. *P < 0.05, **P < 0.01 versus the untreated control
The Effects of Muscone on the Expression of P-gp on the BBB In Vitro
The results showed that P-gp expression was detected in astrocytes, ECV304, and BBB. The expression of P-gp was mainly in ECV304 cells on the BBB (Fig. 6a). Different concentrations of muscone (4, 8, and 16 μM) inhibited the expression of P-gp compared with control group (Fig. 6b).
Fig. 6.
The effects of muscone on the expression of P-gp on the BBB in vitro. a P-gp expression in BBB, ECV304, and astrocytes was determined by standard Western blot analysis protocols (n = 3 in each group). Intensities of P-gp bands were normalized to β-actin. *P < 0.05, **P < 0.01 versus BBB model group. b Quantification of P-gp expression in BBB model (n = 3 in each group). **P < 0.01 versus BBB model group. # P < 0.05 versus muscone (4 μM) group. △△ P < 0.01 versus muscone (8 μM) group
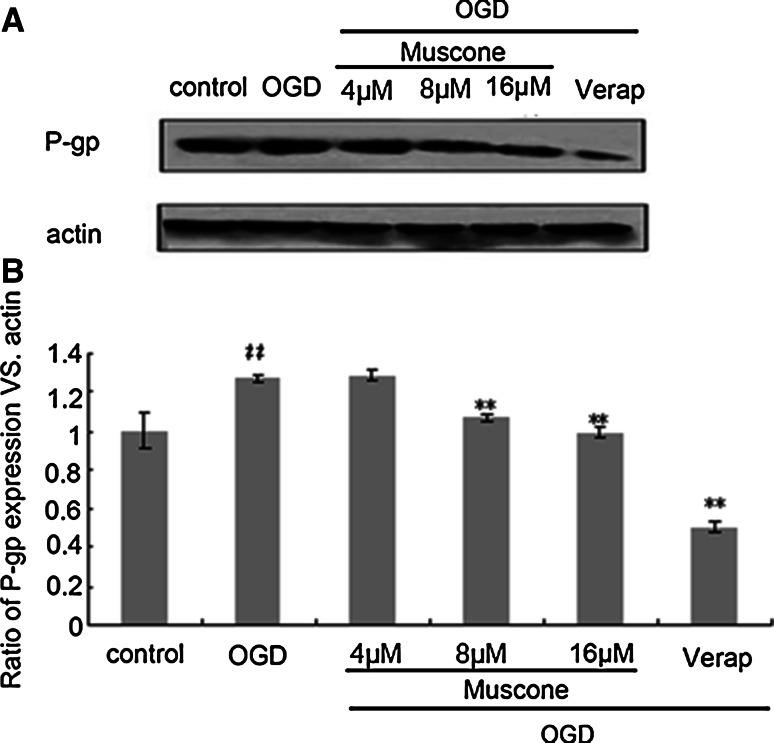
Muscone Down-Regulated the Expression of P-gp, MMP-9 in OGD BBB Model
The results showed that the expression of P-gp and MMP-9 increased in model group induced by OGD. After muscone treatment, the expression of P-gp and MMP-9 significantly decreased compared with the model group, and the expression of P-gp was the lowest in P-gp inhibitor (verapamil) group (Figs. 7, 8). This illustrated that muscone could reduce the expression of P-gp and MMP-9 in the state of OGD.
Fig. 7.
The effects of Muscone on P-gp expression in oxygen/glucose deprivation BBB model. Representative Western blots and semiquantitative analyses of P-gp levels in BBB model. Quantification of P-gp expression in groups (n = 3 in each group). Intensities of P-gp bands were normalized to β-actin. ## P < 0.01 versus control group, **P < 0.01 versus OGD group
Fig. 8.
The effects of Muscone on MMP-9 expression in the oxygen/glucose deprivation BBB model. Representative Western blots and semiquantitative analyses of MMP-9 levels in BBB model. Quantification of MMP-9 expression in groups (n = 3 in each group). Intensities of MMP-9 bands were normalized to β-actin. ## P < 0.01 versus control group, **P < 0.01 versus OGD group
Discussion
The BBB is a barrier system between the blood and brain tissue, and as the interface of the CNS and blood, BBB plays important physiological functions. In pathological cases, BBB can prevent drugs into the brain; the injury of the BBB is also the main reason of the brain-derived diseases; and the structure and function of the BBB is related to the occurrence of cerebral ischemia (Daneman and Barres 2005).
In vitro models of the BBB started to emerge in the early 1990s as potential new research tools complementary to in vivo and human studies in basic, translational, and clinical/pharmaceutical research bearing several aspects of desirable advantages: cost effective, simplified working environment, and versatility (Naik and Cucullo 2012). Now in vitro reconstituted BBB models have played an important role in studying various aspects of the BBB, including the structural and functional organization of barrier complex and the barrier regulation mechanisms, especially extensively used for in vitro prediction of the BBB permeability of drugs. Co-culture of brain microvascular endothelial cells (BMEC) and AC is a classical method to establish the BBB model in vitro (Daneman and Barres 2005). But it has been reported that the ECV304 can exhibit the characteristics which is similar to the blood–brain barrier such as glucose transporter, the leucine transporter system as well as P-gp, and adhesion molecule expression characteristics (Bankstahl et al. 2008; West and Mealey 2007). The ECV304 cell line is a useful model system expressing a robust endothelial phenotype, and when co-cultured with astrocytic glia shows upregulation of a number of features characteristic of the BBB in vivo, including increased tight junctional organization and elevated TEER (Tan et al. 2001). These cell lines bear many advantages over primary cultures, which include being easy to grow and retain their differentiating properties even after repetitive passages, reducing cost and labor (Naik and Cucullo 2012). Muscone is the main chemical constitution in Musk, and it can penetrate the BBB. But how the muscone penetrated the BBB and whether it was related to the expression of P-gp and MMP-9 were still unknown.
In this study, the mechanical separation and enzymatic digestion were used to isolate AC from newborn rats. ECV304 cells were selected to co-culture with AC to establish the BBB model in vitro. The success of the establishment of the BBB model was characterized by the increased value of TEER, γ-GT, and ALP. These results demonstrate that this in vitro BBB system mimics a physiologically relevant situation and may therefore provide a tool for studying the effects of biological fluids such as serum and cerebrospinal fluid from patients with neurological disorders underlying a BBB alteration in disease pathogenesis.
Gas chromatography, invented by Martin and Synge in 1941, was first applied for the separation of a series of fatty acids in 1951 (Martin and Synge 1941; James and Martin 1952). Gas chromatography is also an effective method to study the active composition of Chinese herbal medicine distributing through the blood–brain barrier into the brain (Chen et al. 2004; Liu et al. 2014). In our study, a peak of muscone was detected in different concentrations of muscone groups by GC, indicating that muscone could penetrate the BBB.
The BBB cells containing TJs serve to restrict and control the movement of substances between the systemic circulation and brain extracellular fluid and are characterized by a high TEER and low permeability (Butt et al. 1990; Crone and Olesen 1982). A TEER measurement system has been integrated into a microfluidic device to provide real-time monitoring of polarized monolayer formation and simultaneous evaluation of cellular permeability. A decrease in TEER reflects an increase in permeability and a loss of barrier function. In this study, following muscone exposure, the TEER values of in vitro BBB model were relatively stable. There was no significantly difference between the exposed groups and the control group, so muscone may not affect the opening of TJ in the BBB, and it may regulate levels of some proteins such as P-gp and MMP-9.
Transport across the BBB may depend on efflux transporters expressed by brain endothelial cells such as P-gp. This efflux protein actively pump compounds out of the endothelial cells and back into the blood, reducing the exposure of the CNS to delivered drugs. In many tissues, the expression of P-gp plays an important role in drug uptake, intracellular distribution, metabolism, and excretion (Bauer et al. 2012). The drug efflux transporter P-gp is an active component of the BBB operating as an ATP-driven efflux pump, controlling the movement of a variety of structurally diverse compounds across the BBB (Youdim et al. 2003). The activity of P-gp can accelerate drug efflux, reduce the accumulation of the drug in the brain, and prevent harmful substances into the brain which can cause more damage to the CNS. Previous studies have found that P-gp was mainly concentrated in the cortex of microvascular endothelial cells and the cavity membrane surface and its outer membrane cells (Virgintino et al. 2002; Zhang et al. 2004). In the present study, the expression of P-gp was mainly in endothelial cells and not astrocytes on the BBB model in vitro, which was consistent with their studies. Muscone inhibited the expression of P-gp under normal and OGD conditions, leading to an increase in the brain concentration of muscone. The data also suggest that muscone could penetrate the BBB which is due to the P-gp transport system in the brain. The present study also generates important information for clinical application of muscone and further structural transformation for rational drug design to avoid P-gp-mediated drug efflux.
Matrix metalloproteinase enzyme proteins (MMPs) are the most important proteases which can degradate extracellular matrix and the specific serum markers after brain damage, especially MMP-9. MMP-9 can damage the blood–brain barrier, promote the formation of brain edema, and induce the death of neurons after traumatic brain injury. The expression level of MMP-9 may be used as indicators to determine the development and prognosis of traumatic brain injury (Zhao et al. 2006). TJs between the specialized endothelial cells that maintain the BBB’s integrity consist of integral membrane proteins (occludin, claudins, and junctional adhesion molecules (JAMs)) as well as cytoplasmic scaffolding proteins (zonula occludens (ZO) proteins) and actin cytoskeleton-associated proteins. Disruption of these TJs has been positively associated with MMP-9 activity, and the TJ proteins claudin-5 and occludin have been shown to be degraded by MMP-9 through cleavage of portions of their extracellular domains (Asahi et al. 2001; Chen et al. 2009). Here, we showed that muscone treatment (4, 8, and 16 µM) decreases BBB permeability under OGD condition. This effect may be due in part to suppress MMP-9-mediated degradation of the TJ proteins. These results present a novel use for muscone and may reveal a promising new treatment for cerebral ischemia patients.
Further studies will be needed to clarify the mechanisms of how the muscone penetrates the in vitro BBB model, such as up-regulating/down-regulating hypoxia inducible factor-1 alpha (HIF-1α). We could assess the effects of muscone post-treatment on the expression level of HIF-1α. HIF-1α is a transcription factor composed of an oxygen-regulated subunit, HIF-1α, and a constitutively expressed subunit, HIF-1b. HIF-1 activity is determined by the availability and activity of the HIF-1α subunit (Nagle and Zhou 2006). HIF-1 exerts its activity through proteins encoded by its downstream genes such as vascular endothelial growth factor (VEGF), erythropoietin, and glucose transporter (Lee et al. 2004). In addition, VEGF, a downstream product of HIF-1, can induce MMP-9 activation (Valable et al. 2005). Therefore, HIF-1α is closely associated with BBB integrity. We would show that muscone post-treatment reduced the early expression of HIF-1α leading to decreased expression of MMPs, aquaporin-4 (AQP-4), VEGF, and consequently alter BBB permeability. Additionally, identification and characterization of intracellular signaling pathways such as reactive oxygen species-sensitive pathways (Lochhead et al. 2010, 2012) and protein trafficking mechanisms (Ronaldson and Davis 2013; Hawkins and Davis 2005) that can regulate functional expression/activity of uptake or efflux transporters (P-gp) provide an additional approach for pharmacological modulation/control of drug transporter systems in an effort to deliver therapeutics (muscone) to the CNS. Further studies will also aim to examine other BBB markers expression in vitro, such as occludin, claudin-5, AQP-4, and ZO-1, demonstrating effects of muscone altering or not altering the expression of these BBB markers to strengthen our conclusions.
In conclusion, muscone reduced the ability of P-gp efflux and inhibited the MMP-9 on the decomposition of the basal lamina of the BBB. Muscone can alter the permeability of BBB model in vitro, which is related to reduce the expression of P-gp and MMP-9.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81374005) and Outstanding Youth Science and Technology Fund of Anhui Province (Grant No. 10040606Y17).
Compliance with Ethical Standards
On behalf of, and having obtained permission from all the authors, I declare that (a) the material has not been published in whole or in part elsewhere; (b) the paper is not currently being considered for publication elsewhere; (c) all authors have been personally and actively involved in substantive work leading to the report, and will hold themselves jointly and individually responsible for its content; and (d) all protocols, including surgical procedures and animal use, were approved by the institutional Animal Care and Use Committee of the Anhui university of Chinese medicine and conformed to the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. I testify to the accuracy of the above on behalf of all the authors.
Conflict of interest
None.
References
- Adriana G, Adriana M, Chad S, Villaverde N, Priestap HA, Tonn CE, Lopez LA, Barbieri MA (2011) The effect of dehydroleucodine in adipocyte differentiation. Eur J Pharmacol 671:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz M (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankstahl JP, Kuntner C, Abrahim A, Karch R, Stanek J, Wanek T, Wadsak W, Kletter K, Müller M, Löscher W, Langer O (2008) Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with(R)-11C-verapamil and PET. J Nuci Med 49:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer F, Wanek T, Mairinger S, Stanek J, Sauberer M, Kuntner C, Parveen Z, Chiba P, Müller M, Langer O, Erker T (2012) Interaction of HM30181 with P-glycoprotein at the murine blood-brain barrier assessed with positron emission tomography. Eur J Pharmacol 696:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust P, Bech A, Kretzschmar R, Bergmann R (1994) Developmental changes of enzymes involved in peptide degradation in isolated rat brain microvessels. Peptides 15:1085–1088 [DOI] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ (1990) Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 429:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WK, Huang YF, Wang HD (2004) An experimental study on distribution of musk into the brain through blood brain barrier. Zhong Xi Yi Jie He Xue Bao 2:288–291 [DOI] [PubMed] [Google Scholar]
- Chen F, Ohashi N, Li W, Eckman C, Nguyen JH (2009) Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 50:1914–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Olesen SP (1982) Electrical resistance of brain microvascular endothelium. Brain Res 241:49–55 [DOI] [PubMed] [Google Scholar]
- Daneman R, Barres BA (2005) The blood-brain barrier lessons from moody flies. Cell 123:9–12 [DOI] [PubMed] [Google Scholar]
- Dobbie MS, Hurst RD, Klein NJ, Surtees RA (1999) Up-regulation of intercellular adhesion molecule-1 expression on human endothelial cells by tumour necrosis factor-α in an in vitro model of the blood-brain barrier. Brain Res 830:330–336 [DOI] [PubMed] [Google Scholar]
- Furuichi T, Liu W, Shi H, Miyake M, Liu KJ (2005) Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res 79:816–824 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57:173–185 [DOI] [PubMed] [Google Scholar]
- Hurst RD, Fritz IB (1996) Properties of an immortalized vascular endothelial glioma cell co-cultured model of the blood-brain barrier. Physiology 167:81–88 [DOI] [PubMed] [Google Scholar]
- James AT, Martin AJ (1952) Gas-liquid partition chromatography; the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem J 50:679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joó F (1996) Endothelial cells of the brain and other organ systems: some similarities and differences. Prog Neurobiol 48:255–273 [DOI] [PubMed] [Google Scholar]
- Kuchler-Bopp S, Delaunoy JP, Artault JC, Zaepfel M, Dietrich JB (1999) Astrocytes induce several blood-brain barrier properties in non-neural endothelial cells. NeuroReport 10:1347–1353 [DOI] [PubMed] [Google Scholar]
- Lee J, Bae S, Jeong J, Kim S, Kim K (2004) Hypoxia-inducible factor (HIF-1) alpha: its protein stability and biological functions. Exp Mol Med 36:1–12 [DOI] [PubMed] [Google Scholar]
- Liu WY, Wang ZB, Zhang LC, Wei X, Li L (2012) Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 18:609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wang KM, Xiao X, Lun LJ, Zhang S (2014) Determination of the content of residual solvents in muscone by head-space gas chromatography. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 36:606–609 [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, Demarco KM, Nametz N (2010) Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30:1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, Finch JD, Demarco KM, Quigley CE (2012) Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol 302:H582–H593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai Y, Katakowski M, Chopp M, To SS (2010) Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol 641:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AJ, Synge RL (1941) A new form of chromatogram employing two liquid phases: a theory of chromatography. 2. Application to the micro-determination of the higher monoamino-acids in proteins. Biochem J 35:1358–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Mischeck U, Veyhl M, Henzel K, Galla HJ (1990) Blood-brain barrier characteristic enzymatic properties in cultured brain capillary endothelial cells. Brain Res 514:305–309 [DOI] [PubMed] [Google Scholar]
- Meyer J, Rauh J, Galla HJ (1991) The susceptibility of cerebral endothelial cells to astroglial induction of blood-brain barrier enzymes depends on their proliferative state. J Neurochem 57:1971–1977 [DOI] [PubMed] [Google Scholar]
- Ming Y, Zhang H, Long L, Wang F, Chen J (2006) Modulation of Ca2+ signals by phosphatidylinositol-linked novel D1 dopamine receptor in hippocampal neurons. J Neurochem 98:1316–1323 [DOI] [PubMed] [Google Scholar]
- Nagle DG, Zhou Y (2006) Natural product-derived small molecule activators of hypoxia-inducible factor-1 (HIF-1). Curr Pharm Des 12:2673–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P, Cucullo L (2012) In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci 101:1337–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M (2009) A new blood-brain barrier model using primary rat brain endothelial cells, pericytes, and astrocytes. Neurochem Int 54:253–263 [DOI] [PubMed] [Google Scholar]
- Neuhaus W, Freidl M, Szkokan P, Berger M, Wirth M, Winkler J, Gabor F, Pifl C, Noe CR (2011) Effects of NMDA receptor modulators on a blood-brain barrier in vitro model. Brain Res 1394:49–61 [DOI] [PubMed] [Google Scholar]
- Paik MJ, Kim KR (2004) Solid-phase extraction of L-muscone from aqueous samples with Amberlite XAD-4 for gas chromatographic assay. Arch Pharm Res 27:539–543 [DOI] [PubMed] [Google Scholar]
- Parran DK, Magnin G, Li W, Jortner BS, Ehrich M (2005) Chlorpyrifos alters functional integrity and structure of an in vitro BBB model: co-cultures of bovine endothelial cells and neonatal rat astrocytes. NeuroToxicology 26:77–88 [DOI] [PubMed] [Google Scholar]
- Perrière N, Demeuse P, Garcia E (2005) Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem 93:279–289 [DOI] [PubMed] [Google Scholar]
- Poetsch V, Bennani-Baiti B, Neuhaus W, Muchitsch EM, Noe CR (2010) Serum-derived immunoglobulins alter amyloid beta transport across a blood-brain barrier in vitro model. Pharmazie 65:267–273 [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel A, Begley DJ, Abbott NJ (2003) An overview of in vitro techniques for blood-brain barrier studies. Methods Mol Med 89:307–324 [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, Artico M (2006) Development of the blood-brain barrier: a historical point of view. Anat Rec 289B:3–8 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP (2013) Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev 65:291–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten MJ, Hoover RT, Karnovsky MJ (1987) Electrical resistance and macromolecular permeability of brain endothelial monolayercultures. Brain Res 425:301–310 [DOI] [PubMed] [Google Scholar]
- Scism JL, Laska DA, Horn JW, Gimple JL, Pratt SE, Shepard RL, Dantzig AH, Wrighton SA (1999) Evaluation of an in vitro coculture model for the blood-brain barrier: comparison of human umbilical vein endothelial cells (ECV304) and rat glioma cells (C6) from two commercial sources. In Vitro Cell Dev Biol Anim 35:580–592 [DOI] [PubMed] [Google Scholar]
- Sill HW, Butler C, Hollis TM (1992) Albumin permeability and electrical resistance as means of assessing endothelial monolayer integrity in vitro. J Tissue Cult Methods 14:253–258 [Google Scholar]
- Sun XL, Zeng XN, Zhou F, Dai CP, Ding JH, Hu G (2008) KATP channel openers facilitate glutamate uptake by GluTs in rat primary cultured astrocytes. Neuropsychopharmacology 33:1336–1342 [DOI] [PubMed] [Google Scholar]
- Tan KH, Dobbie MS, Felix RA, Barrand MA, Hurst RD (2001) A comparison of the induction of immortalized endothelial cell impermeability by astrocytes. Neurochemistry 12:1329–1334 [DOI] [PubMed] [Google Scholar]
- Tóth A, Veszelka S, Nakagawa S, Niwa M, Deli MA (2011) Patented in vitro blood-brain barrier models in CNS drug discovery. Recent Pat CNS Drug Discov 6:107–118 [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E (2005) VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab 25:1491–1504 [DOI] [PubMed] [Google Scholar]
- Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, Roncali L, Bertossi M (2002) Expression of P-glycoprotein in human cerebral cortex microvessels. Histochem Cytochem 50:1671–1676 [DOI] [PubMed] [Google Scholar]
- Wang YQ, Tan ZW, Guang P (2007) Establishment of neurons glucose oxygen deprivation model in vitro and the cellular injuries. Sichuan J Anat 15:2–4 [Google Scholar]
- Wang N, Chang GJ, Jin QZ, He Q, Liu JS, Peng DY (2012a) Analysis of muscone from serum and cerebrospinal fluid of rabbits after intragastric administration of tongqiao huoxue granules. Chin Med Mater 35:107–112 [PubMed] [Google Scholar]
- Wang N, Deng Y, Wei W, Song LH, Wang Y (2012b) Serum containing Tongqiaohuoxue decoction suppresses glutamate-induced PC12 cells injury. Neural Regen Res 7:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Deng Y, He Q (2012c) Tongqiao huoxue granules containing serum has protective effect of PC12 cells induced by glutamate. Chin Mater Med 35:1309–1310 [Google Scholar]
- Wang LF, Li X, Gao YB, Wang SM, Zhao L, Dong J, Yao BW, Xu XP, Chang GM, Zhou HM, Hu XJ, Peng RY (2014) Activation of VEGF/Flk-1-ERK pathway induced blood-brain barrier injury after microwave exposure. Mol Neurobiol. doi:10.1007/s12035-014-8848-9 [DOI] [PubMed] [Google Scholar]
- West CL, Mealey KL (2007) Assessment of antiepileptie drugs as substrates for canine P-glycoprotein. Am J Vet Res 68:1106–1110 [DOI] [PubMed] [Google Scholar]
- Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (2003) Interaction between flavonoids and the blood–brain barrier: in vitro studies. J Neurochem 85:180–192 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuetz JD, Elmquist WF, Miller DW (2004) Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther 311:449–455 [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12:441–445 [DOI] [PubMed] [Google Scholar]