Abstract
Objective
This study used real-world observational data to compare profiles of patients receiving different first-line treatment for hormone receptor positive (ER+), HER2 negative, metastatic breast cancer (MBC): CDK4/6 inhibitors (CDK4/6i) in combination with endocrine therapy (ET) versus ET alone.
Method
From a nationwide electronic health record-derived Flatiron Health de-identified database including 280 US cancer clinics, we identified patients with hormone receptor positive, HER2 negative, metastatic breast cancer receiving 1st -line therapy with ET alone or CDK4/6i plus ET between February 2015 and November 2021. Patient sociodemographic status, MBC treatment regimen and outcomes were the focus of this analysis. Patient characteristics were compared using t-tests and chi-square tests. Logistic regression analysis was performed to examine the association of patient characteristics with the likelihood of receiving 1st -line CDK4/6i plus ET vs. ET alone. Kaplan-Meier method and Cox proportional hazards were used to test the impact of 1st -line treatment regimen on real-world progression-free survival (PFS) and overall survival (OS). Baseline characteristics were balanced using inverse probability weighting (IPW).
Results
The study population included 3,917 patients receiving CDK4/6i plus ET (n = 2170) and ET alone (n = 1747) for their MBC. Compared to patients receiving ET alone, those receiving CDK4/6i plus ET were younger (mean age 66.8 vs. 68.6, p < 0.001), more likely to present with de novo MBC (p < 0.001), had better performance status (50.2% vs. 40.5% patients with ECOG value 0, p = 0.001) and lower number of comorbidities (29.7% vs. 26.6% ≥ 1 comorbidity, p < 0.001). Logistic regression revealed increased odds of CDK4/6i plus ET in individuals aged 50–64 (OR: 3.42, 95% CI [2.41, 4.86]) and 65–74 (OR: 3.18, 95% CI [1.68, 6.02]) versus those aged 18–49 years of age. Black individuals had lower odds of CDK4/6i plus ET (OR: 0.76, 95% CI [0.58, 1.00]) compared to White individuals. Other characteristics associated with lower odds of CDK4/6i plus ET included patients with stage III disease (OR: 0.69, 95% CI [0.52, 0.92]), lower performance status (OR: 0.50, 95% CI [0.40, 0.62]), and Medicare insurance (OR: 0.73, 95% CI [0.30, 1.78]) compared to those with commercial and Medicaid insurance. After IPW adjustment, use of CDK4/6i plus ET as 1st -line treatment was associated with significantly longer median PFS compared to ET alone (27 vs. 17 months; hazard ratio [HR] = 0.61, p < 0.001). Median OS was 52 months in the CDK4/6i plus ET group and was 42 months with ET alone (HR = 0.74, p < 0.001).
Conclusion
In this real-world database, disparities in receiving 1st -line CDK4/6 inhibitors were seen by age, diagnosis stage, baseline performance status, comorbidity, and insurance status. In adjusted analysis, the use of 1st -line CDK4/6i plus ET yielded better PFS and OS rates than ET alone. Further efforts are essential to enhance equitable use of and access to this crucial drug class.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01902-w.
Keywords: CDK4/6 inhibitors, Real-world evidence, Hormone receptor positive, Metastatic breast cancer, Treatment disparities
Introduction
Breast cancer is the second leading cause of cancer-related death in U.S. women [1]. Metastatic breast cancer (MBC), also known as stage IV or advanced breast cancer, is an incurable cancer that has spread beyond the breast and nearby lymph nodes [2]. About 30% of breast cancer survivors are eventually diagnosed with MBC, [3] and 6 to 10% of patients are diagnosed with de novo MBC [4]. Projections indicate there will be a substantial increase in MBC cases in the U.S. due to new treatments as patients survive longer with advanced stage breast cancer [5].
Among breast cancer subtypes, hormone receptor positive, HER2 negative (ER+) breast cancer is the most common [6]. Current guidelines for treatment of ER + MBC indicate that a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) in conjunction with endocrine therapy (ET) can be used [7, 8]. Currently, there are three FDA-approved CDK4/6i drugs: Palbociclib (Ibrance), Abemaciclib (Verzenio), Ribociclib (Kisqali).
Guideline recommendations are based on data from prospective randomized phase III trials comparing CDK4/6i plus ET to ET therapy alone for ER + MBC. The FDA first granted approval for CDK4/6i use in MBC in the United States in February 2015, based on pivotal results from three trials: PALOMA-1 and PALOMA-2 [9, 10], which examined the combination of Palbociclib with letrozole for postmenopausal women with advanced MBC, and PALOMA-3, which investigated Fulvestrant combination therapy for pre/postmenopausal women following ET [11–14]. Following the PALOMA trials, the MONALEESA trials examined Ribociclib [15–17], and later, the MONARCH trials studied Abemaciclib [18, 19]. Across these trials, the hazard ratio for progression-free survival (PFS) consistently falls around 0.5 (ranging from 0.46 to 0.59). With longer follow-up, we are also observing overall survival (OS) benefits, particularly in the first-line treatment setting [10, 20–23].
Evidence from both U.S [24–26]. and Non-U.S [27, 28]. real-world data focused on use of CDK4/6 inhibitors in treatment of ER + MBC typically mirrors efficacy results seen in controlled clinical trials [29–31]. However, these studies have limitations, including small sample size, the absence of control groups, narrow focus on specific CDK4/6 inhibitors and ET drugs [24–26], and relatively short patient follow-up durations [24, 29, 31]. This study provides U.S. based real-world evidence evaluating the effectiveness of combining a CDK4/6i with ET versus ET alone as a 1st -line treatment for ER + MBC, without limitation to a single CDK4/6i or ET agent. This study comprehensively examines all three CDK4/6 inhibitors—Palbociclib, Ribociclib, and Abemaciclib—along with various ET medications, including Tamoxifen, Anastrozole, Letrozole, Fulvestrant, and Exemestane, encompassing the simultaneous administration of multiple medications. Furthermore, using large scale real-world data, this study explores disparities in use of CDK4/6 inhibitors, based on patient characteristics.
Data and methods
Data
Data comes from the nationwide electronic health record (EHR)-derived Flatiron Health de-identified database. The database is a longitudinal database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction [24, 31]. During the study period, the de-identified data originated from approximately 280 US cancer clinics (~ 800 sites of care). The majority of patients in the dataset originate from community oncology settings; relative community/academic proportions may vary depending on the study cohort. The data are de-identified and subject to obligations to prevent re-identification and protect patient confidentiality. This study is approved by the University of Virginia’s Institutional Review Board (IRB-HSR 23533).
The study population included patients diagnosed with HR+/HER2- MBC, who had 3 months of follow-up after the date of metastatic diagnosis (Index date) beginning from 03 February 2015 to 02 November 2021, and who received either CDK4/6i plus ET (1st -line CDK4/6i) or ET alone (1st -line ET) as 1st -line treatment. Patients were excluded if they had a first structured activity (vital records, a medication administration, or a laboratory test/result) more than 90 days after the index date; received prior treatment with CDK4/6i (Palbociclib, Abemaciclib, Ribocliclib); received 1st -line therapy more than 30 days before the metastatic diagnosis date.
Variables and measurements
We included in the analyses the following demographic variables available in the Flatiron Health data: age, race/ethnicity, health insurance type (Commercial, Medicare, Medicaid, etc.) before the start of 1st -line therapy (and after the metastatic diagnosis date), and clinical characteristics, including ECOG performance status (PS), number of comorbidities, site of metastasis, stage of cancer at the initial diagnosis (I, II, III, IV), de novo versus relapse MBC, and the year of treatment initiation.
Outcomes
Progression-free survival (PFS) was characterized as the duration in months from curated data based on the commencement of the 1st -line treatment to either death or disease progression recorded in the patient’s medical record. Disease progression was determined based on evaluations by the treating clinician, utilizing radiology, pathology, clinical assessment, or laboratory findings. For patients who neither succumbed to the condition nor experienced disease progression, the endpoint was marked at the initiation date of the subsequent oncologist-defined, rule-based line of therapy for those receiving two or more lines of therapy, or at their final study period visit between February 2015 and November 2021 for patients with only one line of therapy.
Overall survival (OS) was defined as the duration in months from the start of 1st -line treatment to death as provided in the Flatiron dataset. Patients who did not die were censored at the last date of structured activity.
Statistical analysis
Two-sample statistical t-tests and chi-squared tests were conducted to examine differences in demographics and clinical characteristics between treatment groups. Logistic regression was used to examine the association of patient demographics, clinical characteristics, and practice type with the likelihood of receiving CDK4/6i plus ET.
The Kaplan-Meier method and 95% confidence intervals were employed to determine median values for the DFS and OS outcomes. Hazard ratios and 95% confidence intervals for outcomes were estimated through Cox proportional regression analyses. The Inverse Probability Weighting (IPW) method was applied to adjust for differences in baseline characteristics (age, race, stage at initial diagnosis, performance status, practice type, metastasis site, number of comorbidities, and health insurance coverage) between the two groups. Additionally, Propensity Score Matching (PSM) was utilized as a sensitivity analysis to assess the robustness of the IPW method [32, 33].
To address the problem of missing data, a new category for “missing” values was introduced as an additional level in categorical variables, such as race and ECOG value, when the reason for the unavailable data was unclear.
Results
Summary statistics
The study population included 3,917 participants undergoing 1st -line treatment for ER + MBC with CDK4/6i plus ET (n = 2,170) or ET alone (n = 1,747). Demographic and clinical characteristics, detailed in Table 1, exhibited differences (unadjusted) between the two groups. In contrast to those on ET alone, recipients of CDK4/6i plus ET were younger (mean age 66.8 vs. 68.6, p < 0.001), less likely to be uninsured or have undocumented health insurance status (7.8% vs. 8.2%, p < 0.001), had better performance status (50.2% vs. 40.5% with ECOG value 0, p < 0.001), lower number of comorbidities (29.7% vs. 26.6% with at least 1 comorbidity, p < 0.001) and were more likely to have been diagnosed with de novo MBC (35.9% vs. 28.5%, p-value < 0.001).
Table 1.
Patient characteristics by group of treatment- unadjusted analysis
| Group | |||
|---|---|---|---|
| ET alone N (%) |
ET plus CDK4/6i N (%) |
P-value | |
| N (%) | 1,747 (44.6%) | 2,170 (55.4%) | |
| Age (mean(SD)) | 68.6 (11.6) | 66.8 (9.7%) | < 0.001 |
| Age | |||
| 18–49 | 151 (8.6%) | 92 (4.2%) | < 0.001 |
| 50–64 | 385 (22.0%) | 762 (35.1%) | |
| 65–74 | 510 (29.2%) | 774 (35.7%) | |
| 75+ | 701 (40.1%) | 542 (25.0%) | |
| Race | |||
| White | 1,125 (64.4%) | 1,423 (65.6%) | 0.8 |
| Black | 157 (9.0%) | 183 (8.4%) | |
| Hispanic | 118 (6.8%) | 154 (7.1%) | |
| All other | 347 (19.9%) | 410 (18.9%) | |
| Practice Type | |||
| Community | 1,571 (89.9%) | 1,935 (89.2%) | 0.4 |
| Academic | 176 (10.1%) | 235 (10.8%) | |
| Stage at initial DX | |||
| I | 220 (12.6%) | 277 (12.8%) | < 0.001 |
| II | 473 (27.1%) | 615 (28.3%) | |
| III | 329 (18.8%) | 295 (13.6%) | |
| IV | 498 (28.5%) | 779 (35.9%) | |
| Not documented | 227 (13.0%) | 204 (9.4%) | |
| Performance Status | |||
| 0 | 587 (40.5%) | 959 (50.2%) | < 0.001 |
| 1 | 517 (35.7%) | 673 (35.2%) | |
| 2+ | 345 (23.8%) | 278 (14.6%) | |
| Metastasis site | |||
| Bone-Only | 569 (32.6%) | 644 (29.7%) | 0.1 |
| Non-Visceral | 405 (23.2%) | 522 (24.1%) | |
| Visceral | 773 (44.2%) | 1,004 (46.3%) | |
| Number of comorbidities | |||
| 0 | 1,079 (61.8%) | 1,320 (60.8%) | < 0.001 |
| 1 | 464 (26.6%) | 638 (29.4%) | |
| 2+ | 204 (11.7%) | 212 (9.8%) | |
| Health insurance type | |||
| Commercial/Medicaid | 36 (2.1%) | 49 (2.3%) | < 0.001 |
| Commercial | 209 (12.3%) | 384 (18.1%) | |
| Commercial/Other | 116 (6.9%) | 212 (10.0%) | |
| Medicaid only | 25 (1.5%) | 26 (1.2%) | |
| Medicare | 313 (18.5%) | 280 (13.2%) | |
| Medicare/Medicaid | 64 (3.8%) | 68 (3.2%) | |
| Medicare/other | 791 (46.7%) | 941 (44.3%) | |
| None | 139 (8.2%) | 166 (7.8%) | |
| ET backbone | |||
| Anastrozole | 561 (32.1%) | 211 (9.7%) | < 0.001 |
| Anastrozole, Fulvestrant | 34 (1.9%) | 6 (0.3%) | |
| Exemestane | 124 (7.1%) | 46 (2.1%) | |
| Exemestane, Fulvestrant | 6 (0.3%) | 6 (0.3%) | |
| Fulvestrant | 278 (15.9%) | 604 (27.8%) | |
| Letrozole | 499 (28.5%) | 1,277 (58.8%) | |
| Letrozole, Fulvestrant | 9 (0.5%) | 12 (0.6%) | |
| Tamoxifen | 232 (13.3%) | 7 (0.3%) | |
| Tamoxifen, Fulvestrant | 4 (0.2%) | 1 (0.0%) | |
| Backbone CDK4/6i | |||
| Abemaciclib | - | 178 (8.2%) | - |
| Abemaciclib, Ribociclib | - | 2 (0.1%) | |
| Palbociclib | - | 1,793 (82.6%) | |
| Palbociclib, Abemaciclib | - | 24 (1.1%) | |
| Palbociclib, Ribociclib | - | 7 (0.3%) | |
| Ribociclib | - | 166 (7.6%) | |
Most patients (32.1%) on ET alone were given Anastrozole as their ET backbone, while the majority of patients (58.8%) who received CDK4/6i plus ET were given Letrozole as their ET backbone (p-value < 0.001). Of those patients on CDK4/6i plus ET, 82.6% received Palbociclib, 8.2% were given Abemaciclib, and 7.6% received Ribociclib.
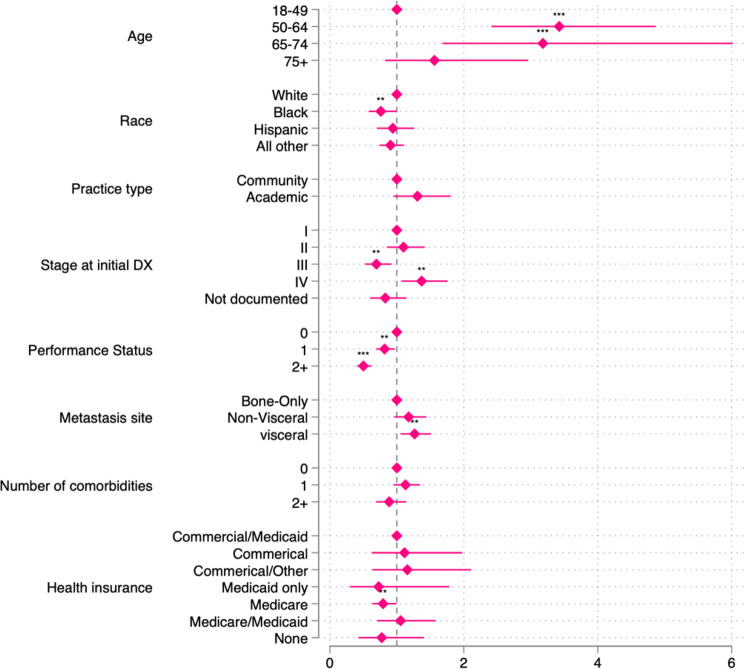
Figure 1 displays a forest plot demonstrating the odds of having received 1st -line CDK4/6i plus ET versus 1st -line ET alone by demographic and clinical characteristics. Notably, individuals aged 50–64 significantly more likely to receive CDK4/6i plus ET in comparison to the 18–49 age group (OR: 3.42, 95% CI [2.41, 4.86]). A similar pattern was observed in the 65–74 age group (OR: 3.18, 95% CI [1.68, 6.02]).
Fig. 1.
Adjusted odds ratios estimated by Logistic regression on likelihood of receiving CDK4/6i as first-line treatment. Note: The multiple logistic regression models were adjusted for the following variables: age, race, stage at initial diagnosis, performance status, practice type, metastasis site, number of comorbidities, and health insurance coverage. Reference group for each variable is situated on vertical line(x = 1). Asterisks indicate the significance level, with * denoting p < 0.05 and *** representing p < 0.001
Disparities related to race were evident, with Black individuals less likely to receive CDK4/6i plus ET compared to White individuals (OR: 0.76, 95% CI [0.58, 1.00]). Noteworthy distinctions were identified based on the cancer stage at initial diagnosis. Patients initially diagnosed with stage III disease were less likely to receive 1st -line CDK4/6i plus ET in comparison to those diagnosed at stage I (III: OR = 0.69, 95% CI [0.52, 0.92]). Conversely, patients diagnosed with de novo MBC (stage IV) were more likely to be prescribed CDK4/6i plus ET (OR = 1.37, 95% CI [1.07, 1.76]) compared to those initially diagnosed with stage I breast cancer.
Patients with poor PS were substantially less likely to receive CDK4/6i plus ET compared to those with a PS recorded as 0 (PS 1: OR = 0.82, 95% CI [0.69, 0.97]; PS 2+: OR = 0.50, 95% CI [0.40, 0.62]). Patients with visceral metastatic sites were more likely to receive 1st -line CDK4/6i plus ET compared to those with bone-only metastases (OR = 1.7, 95% CI [1.06, 1.51]).
A significant impact of insurance type was observed, with Medicare-only recipients having reduced odds (OR: 0.73, 95% CI [0.30, 1.78]) of receiving CDK4/6i compared to those with both commercial and Medicaid health insurance. The temporal trend indicated progressively higher odds of receiving CDK4/6i plus ET in later years, reaching substantial levels in 2021 (OR: 6.31, 95% CI [4.55, 8.77]) compared to 2014 (see supplemental material Table 1).
Survival analysis
Progression-free survival
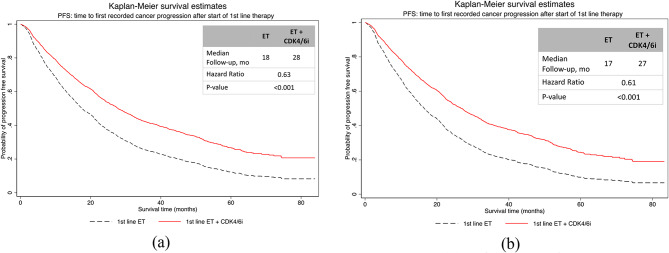
In the unadjusted analysis, the median time to first progression was 28 months (95% CI [26.4,30.5]) for 1st -line CDK4/6i plus ET and 17 months (95% CI [14.2,19.8]) for 1st -line ET alone (p < 0.001, Fig. 2(a)). After IPW adjustment, time to progression was 27 months (95% CI [26.8, 27.2]) among patients treated with 1st -line CDK4/6i plus ET compared with 17 months (95% CI [16.1, 17.9]), among patients treated 1st -line ET alone (hazard ratio, 0.61; p-value = 0.001; Fig. 2(b)). Sensitivity analysis using PSM method reports similar results.
Fig. 2.
Kaplan-Meier curves of real-world time to first progression (a) unadjusted analysis (b) IPW adjusted analysis
Overall survival
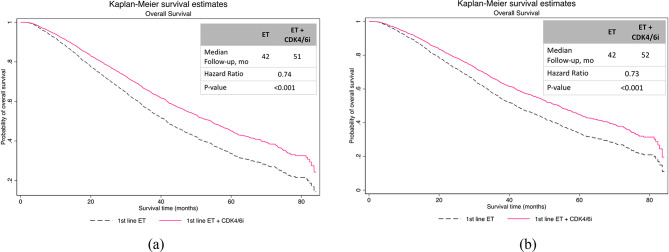
As shown in Fig. 2, the unadjusted analysis showed that the median OS was 51 months (95% CI [49.1,56.9]) for patients receiving 1st -line CDK4/6i plus ET versus 42 months (95% CI [39.1,44.4]) for patients receiving 1st -line ET alone (p < 0.001, Fig. 3a). Adjusted analysis revealed similar findings. In adjusted analysis, the median OS was 52 months (95% CI [28.6, not reached])) for patients who had 1st -line CDK4/6i plus ET versus 42 months (95% CI [22, 74]) in those who received 1st -line ET alone (hazard ratio, 0.73; p-value = 0.001; Fig. 3(b)).
Fig. 3.
Kaplan-Meier curves of real-world overall survival in (a) unadjusted analysis (b) IPW adjusted analysis
Discussion
This study, encompassing a significant cohort of patients undergoing 1st -line treatment, underscores substantial differences in demographic and clinical profiles between those receiving CDK4/6i plus ET and those receiving ET alone. The administration of CDK4/6i plus ET is influenced by various factors, including age, race/ethnicity, cancer stage, performance status, metastatic sites, insurance type, and temporal trends. To our knowledge, this study is the first to explore disparities in receipt of 1st -line CDK4/6i plus ET for treatment of ER + MBC across various patient clinical and non-clinical characteristics.
Importantly, receipt of CDK4/6i with 1st -line ET for ER + MBC translated to distinctly better outcomes, with longer progression-free survival and overall survival compared to those on 1st -line ET alone, even after adjusting for other relevant covariables. These results were aligned with the other real-world analyses [24, 34–37], as well as the clinical efficacy findings of these drugs [9–13, 19, 38].
This study has several strengths. First, the substantial size and widespread geographic distribution of the Flatiron Database contribute to the robustness and generalizability of the findings. The inclusion of diverse real-world data enhances the study’s applicability to a broad patient population. Another strength lies in the comprehensive approach of covering all types of drugs, rather than exclusively focusing on a specific CDK4/6i. This broader scope provides a more comprehensive understanding of the real-world utilization patterns across various CDK4/6i medications after FDA approval and wide clinical use of this drug class. This distinguishes the study from others that may have concentrated solely on the combination of a single CDK4/6i drug with a specific type ET, typically letrozole or Fulvestrant. The broader inclusivity in drug coverage enhances the study’s capacity to capture a more representative snapshot of contemporary clinical practices related to CDK4/6i therapies. Moreover, the real-world data incorporates physician judgment that allows patients receiving adjuvant endocrine agents to receive diverse 1st -line endocrine therapies based on individual clinical considerations, enhancing the study’s real-world applicability.
While this study demonstrates several strengths, it is essential to acknowledge certain limitations. The absence of randomization in treatment assignment, inherent in observational data, poses a challenge that necessitates careful consideration. In this study, the researchers employed rigorous statistical tools such as IPW and PSM to mitigate potential biases stemming from differences in baseline and clinical characteristics among patients and immortal-time bias. While PSM and IPW analyses were consistent, it is crucial to note that unobservable factors not captured in the data remain a limitation.
Conclusion
This study uncovers real-world disparities in the application of CDK4/6 inhibitors, revealing insights into contemporary clinical practices. Disparities in CDK4/6i use, spanning age, stage at diagnosis, baseline PS value, and comorbidities, highlight the complex nature of treatment decisions.
Moreover, this real-world evidence supports the use of CDK4/6 inhibitors with an aromatase inhibitor as a 1st -line treatment for HR+/HER2 − MBC. Ongoing research is crucial to refining our understanding of treatment patterns and their impact on patient outcomes in breast cancer management. Furthermore, efforts should focus on improving equitable access to CDK4/6 inhibitors, ensuring that patients benefit from evolving therapeutic options in HR+/HER2 − MBC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
A.P. conducted data analysis, study design, conceptualization, manuscript writing and editing. G.K. was involved in conceptualization, design and manuscript writing and editing. W.Y. was involved in conceptualization, data analysis design and manuscript editing. G.B. was involved with manuscript writing and editing. R.A. as the lead contributor contributed to project conceptualization, secured funding, as well as manuscript writing and editing.
Funding
This work/study was supported by the American Cancer Society/Flatiron Health, grant number “RWIA-21-123-01-RWIA”.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
This study was approved by the Institutional Review Board at the University of Virginia (IRB HSR 23533).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2019–2020. American Cancer Society, Inc; 2019.
- 2.National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. https://seer.cancer.gov/statfacts/html/breast.html
- 3.Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274(2):113–26. 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate Cancer — lessons in Cancer Dynamics. N Engl J Med. 2015;373(18):1685–7. 10.1056/NEJMp1510443. [DOI] [PubMed] [Google Scholar]
- 5.Gogate A, Wheeler SB, Reeder-Hayes KE, et al. Projecting the prevalence and costs of metastatic breast Cancer from 2015 through 2030. JNCI Cancer Spectr. 2021;5(4):pkab063. 10.1093/jncics/pkab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouabbi JA, Osborne CK, Schiff R, Rimawi MF. Management of hormone receptor–positive, human epidermal growth factor 2–negative metastatic breast cancer. Breast Cancer Res Treat. 2021;190(2):189–201. 10.1007/s10549-021-06383-5. [DOI] [PubMed] [Google Scholar]
- 7.Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor–positive metastatic breast Cancer: American Society of Clinical Oncology Guideline. JCO. 2016;34(25):3069–103. 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F, Senkus E, Costa A, et al. 4th ESO–ESMO International Consensus guidelines for advanced breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57. 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Boer K, Bondarenko I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2 – advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183(2):419–28. 10.1007/s10549-020-05755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loibl S, Turner NC, Ro J, et al. Palbociclib Combined with Fulvestrant in Premenopausal women with advanced breast Cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22(9):1028–38. 10.1634/theoncologist.2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda N, Inoue K, Nakamura R, et al. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24(3):262–73. 10.1007/s10147-018-1359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner NC, Slamon DJ, Ro J, et al. Overall survival with Palbociclib and fulvestrant in advanced breast Cancer. N Engl J Med. 2018;379(20):1926–36. 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Rugo HS, Im SA, et al. Overall survival with Palbociclib and Fulvestrant in Women with HR+/HER2 – ABC: updated exploratory analyses of PALOMA-3, a Double-blind, phase III randomized study. Clin Cancer Res. 2022;28(16):3433–42. 10.1158/1078-0432.CCR-22-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line therapy for HR-Positive, advanced breast Cancer. N Engl J Med. 2016;375(18):1738–48. 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 16.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–15. 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone Receptor–Positive, human epidermal growth factor receptor 2–Negative advanced breast Cancer: MONALEESA-3. JCO. 2018;36(24):2465–72. 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 18.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast Cancer. JCO. 2017;35(32):3638–46. 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 19.Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer. 2019;5(1):5. 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with Ribociclib plus Letrozole in Advanced breast Cancer. N Engl J Med. 2022;386(10):942–50. 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 21.Im SA, Lu YS, Bardia A, et al. Overall survival with Ribociclib plus endocrine therapy in breast Cancer. N Engl J Med. 2019;381(4):307–16. 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 22.Sledge GW, Frenzel M. Analysis of overall Survival Benefit of Abemaciclib Plus Fulvestrant in hormone Receptor–Positive, ERBB2-Negative breast Cancer—reply. JAMA Oncol. 2020;6(7):1122. 10.1001/jamaoncol.2020.1518. [DOI] [PubMed] [Google Scholar]
- 23.Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–29. 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMichele A, Cristofanilli M, Brufsky A, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2 – metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. 2021;23(1):37. 10.1186/s13058-021-01409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugo HS, Brufsky A, Liu X, et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2 – metastatic breast cancer. npj Breast Cancer. 2022;8(1):114. 10.1038/s41523-022-00479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuyun Carter G, Sheffield KM, Gossai A, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2 – metastatic breast cancer. Curr Med Res Opin. 2021;37(7):1179–87. 10.1080/03007995.2021.1923468. [DOI] [PubMed] [Google Scholar]
- 27.Gehrchen ML, Berg T, Garly R, et al. Real-world effectiveness of CDK 4/6 inhibitors in estrogen-positive metastatic breast cancer. BJC Rep. 2024;2(1):44. 10.1038/s44276-024-00070-w. [Google Scholar]
- 28.Cejuela M, Gil-Torralvo A, Castilla MÁ, et al. Abemaciclib, Palbociclib, and Ribociclib in Real-World Data: a direct comparison of First-Line treatment for endocrine-receptor-positive metastatic breast Cancer. IJMS. 2023;24(10):8488. 10.3390/ijms24108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varella L, Eziokwu AS, Jia X, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 2019;176(2):429–34. 10.1007/s10549-019-05176-1. [DOI] [PubMed] [Google Scholar]
- 30.Xi J, Oza A, Thomas S, et al. Retrospective Analysis of Treatment Patterns and effectiveness of Palbociclib and subsequent regimens in metastatic breast Cancer. J Natl Compr Canc Netw. 2019;17(2):141–7. 10.6004/jnccn.2018.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bui TBV, Burgers DM, Agterof MJ, Van De Garde EM. Real-world effectiveness of Palbociclib Versus Clinical Trial results in patients with Advanced/Metastatic breast Cancer that progressed on previous endocrine therapy. Breast Cancer (Auckl). 2019;13:117822341882323. 10.1177/1178223418823238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. The use of propensity score methods with survival or time-to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–58. 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiology Drug. 2006;15(5):291–303. 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 34.Rugo HS, Liu X, Li B, et al. Real-world treatment patterns for palbociclib plus an aromatase inhibitor, or an aromatase inhibitor alone, for patients with metastatic breast cancer in the Flatiron database. Intl J Cancer. 2024;154(4):701–11. 10.1002/ijc.34748. [DOI] [PubMed] [Google Scholar]
- 35.Goyal RK, Chen H, Abughosh SM, Holmes HM, Candrilli SD, Johnson ML. Overall survival associated with CDK4/6 inhibitors in patients with HR+/HER2– metastatic breast cancer in the United States: A SEER-Medicare population‐based study. Cancer. 2023;129(7):1051–63. 10.1002/cncr.34675. [DOI] [PubMed] [Google Scholar]
- 36.Rugo HS, Liu X, Li B, McRoy L, Layman RM, Brufsky A. Real-world comparative effectiveness of palbociclib plus letrozole versus letrozole in older patients with metastatic breast cancer. Breast. 2023;69:375–81. 10.1016/j.breast.2023.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha MJ, Singareeka Raghavendra A, Kettner NM, et al. Palbociclib plus endocrine therapy significantly enhances overall survival of HR +/ HER2 – metastatic breast cancer patients compared to endocrine therapy alone in the second-line setting: a large institutional study. Intl J Cancer. 2022;150(12):2025–37. 10.1002/ijc.33959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–7. 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.