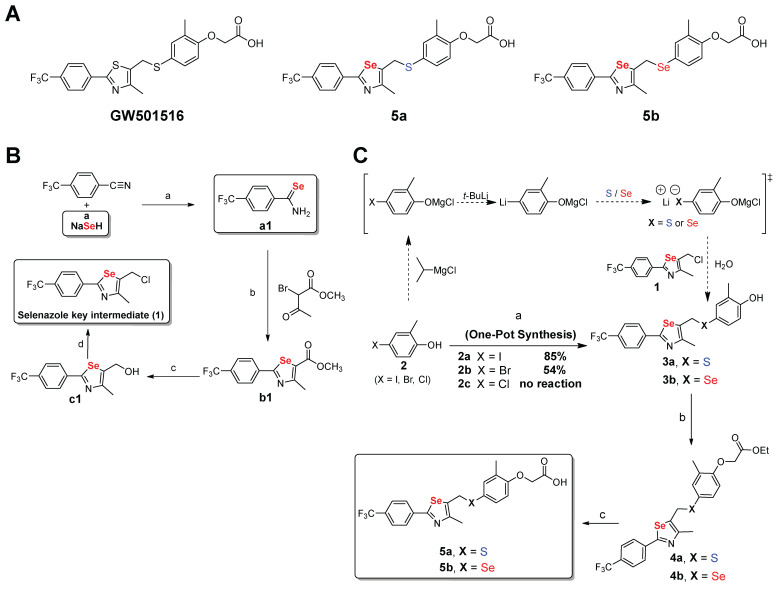
Figure 1.
Synthesis of novel PPARδ agonists containing one selenazole moiety. (A) Structures of GW501516 and PPARδ agonists with a selenazole core (5a and 5b). (B) Synthesis of key selenazole intermediate. Reagents and conditions: (a) The reaction between HCl 2 M and pyridine/ethanol occurred for 1 h at a temperature varying from room temperature (r.t.) to 100 oC (84%); (b) methyl 2-chloroacetoacetate and tetrahydrofuran (THF) reacted for 12 h at a temperature varying from r.t. to 80 oC (95%); (c) diisobutyl aluminum hydride (DIBAL-H) and dichloromethane (DCM) reacted for 1 h at a temperature varying from -78 to -10 oC (94%); and (d) triphenylphosphine (TPP), DCM, and N-chlorosuccinimide (NCS) reacted at r.t. for 10 h (90%). (C) Synthesis of selenium-containing PPARδ agonists. Reagents and conditions: (a) (i) Isopropylmagnesium chloride (1.0 equiv) and THF reacted at 0 oC for 10 min; (ii) t-butyllithium (2.0 equiv) was added at -78 oC for 0.5 h; (iii) sulfur powder (for 3a) or selenium powder (for 3b) and THF reacted at -78 to -20 oC for 1 h; (iv) selenazole key intermediate (1) and THF reacted at 0 oC for 0.5 h (85%); (b) Ethyl bromoacetate, K2CO3 and aq. acetone reacted at r.t. for 4 h (~95%); (c) (i) 2.0 M LiOH and aq. THF reacted at r.t. for 1 h; (ii) the product was acidified with 0.5 M NaHSO4 (90-92%).