Abstract
Background
Drought stress is a significant abiotic stressor that hinders growth, development, and crop yield in soybeans. Strigolactones (SLs) positively regulate plant resistance to drought stress. However, the impact of foliar application of SLs having different concentrations on soybean growth and metabolic pathways related to osmoregulation remains unknown. Therefore, to clarify the impact of SLs on soybean root growth and cellular osmoregulation under drought stress, we initially identified optimal concentrations and assessed key leaf and root indices. Furthermore, we conducted transcriptomic and metabolic analyses to identify differential metabolites and up-regulated genes.
Results
The results demonstrated that drought stress had a significant impact on soybean biomass, root length, root surface area, water content and photosynthetic parameters. However, when SLs were applied through foliar application at appropriate concentrations, the accumulation of ABA and soluble protein increased, which enhanced drought tolerance of soybean seedlings by regulating osmotic balance, protecting membrane integrity, photosynthesis and activating ROS scavenging system. This also led to an increase in soybean root length, lateral root number and root surface area. Furthermore, the effects of different concentrations of SLs on soybean leaves and roots were found to be time-sensitive. However, the application of 0.5 µM SLs had the greatest beneficial impact on soybean growth and root morphogenesis under drought stress. A total of 368 differential metabolites were screened in drought and drought plus SLs treatments. The up-regulated genes were mainly involved in nitrogen compound utilization, and the down-regulated metabolic pathways were mainly involved in maintaining cellular osmoregulation and antioxidant defenses.
Conclusions
SLs enhance osmoregulation in soybean plants under drought stress by regulating key metabolic pathways including Arachidonic acid metabolism, Glycerophospholipid metabolism, Linoleic acid metabolism, and Flavone and flavonol biosynthesis. This study contributes to the theoretical understanding of improving soybean adaptability and survival in response to drought stress.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05663-8.
Keywords: Soybean, Drought, Strigolactones (SLs), Photosynthetic, Metabolomics
Introduction
With the gradual increase in global temperature, drought stress has emerged as a major concern in agricultural systems. Plants face challenges in obtaining sufficient water to meet their demands and ensure their survival [1]. In China, soybean (Glycine max L.) is highly important both as food and cash crop due to its abundance of vegetable protein and oil [2]. The yield of soybean is influenced primarily by the variety characteristics and the prevailing environmental conditions. Optimal varietal traits and favorable environmental factors contribute significantly to yield enhancement. However, drought can inhibit plant growth and development, leading to internal metabolic disorders that result in elevated levels of reactive oxygen species (ROS) in plants. These changes, in turn, affect membrane permeability and induce lipid peroxidation, ultimately impacting normal plant growth and development [3].
Improving soybean yield is currently the primary focus of researchers, who are exploring various technical means and measures. However, phytohormones play a crucial role in this process. Therefore, it is widely recognized that crops have developed a range of regulatory mechanisms at different levels such as morphological, physiological, biochemical, and molecular to adapt environments changes and ensure the species’ continuation, development, and prosperity. Among these intricate mechanisms, hormonal regulation is particularly significant. Phytohormones are involved in all stages of plant growth and development, including seed germination, nutrient growth, and reproductive growth. They help to maintain a harmonious relationship between plant growth, development, and environmental conditions [4]. To mitigate the toxicity caused by drought stress, various molecules have been utilized. Plants contain various growth regulators known as phytohormones, which contribute to diverse plant activities, pathways, and regulatory mechanisms at minimum concentrations [5]. In addition to the well-known growth hormones such as gibberellins, cytokinins, and ethylene, Strigolactones (SLs) have emerged as a new type of plant hormone [6, 7]. SLs, a small class of carotenoid-derived compounds, are considered rhizosphere signaling molecules and have been classified as a new class of phytohormones that regulate various processes in plants [8]. Recent studies have revealed several roles of SLs in plants and the rhizosphere, such as suppression of shoot branching by inhibiting the outgrowth of axillary buds, enhancement of symbiosis between plants and arbuscular mycorrhizal fungi (AMF) and development of root system architecture has been revealed [9–12]. It is revealed that in Arabidopsis, SLs act as positive regulator to abiotic stress tolerance in plant such as drought stress [13]. In addition to this, SLs respond to abiotic stresses by cross-interacting with hormonal signaling pathways. Whereas, SLs and abscisic acid (ABA) are involved in regulation of flavonoid synthesis to enhance drought tolerance [14]. Van et al. [13] demonstrated that SLs with ABA also act as a regulator of stomatal movement in leaves under water stress. Min et al. [15] demonstrated that SLs could enhance plant drought tolerance by regulating chlorophyll content and photosynthetic rate in grape seedlings. Another study showed that exogenous application of SLs reduced H2O2 and MDA levels in wheat under drought conditions. Which suggests that SLs can act as a scavenger of reactive oxygen species under drought stress, thereby reducing lipid peroxidation in wheat [16]. SLs do not only involve in oxidative reactions in plant cells, but also promote the production of osmoregulators in stressed cells to maintain homeostasis in vivo in the presence of environmental stresses, including salt, light, temperature, drought, nutrient deficiencies and heavy metals [17]. SLs also accelerate leaf senescence and regulate shoot branching and root architecture [18]. Furthermore, a lot of experiments have demonstrated the existence of direct or indirect interactions between SLs and ABA, which jointly regulate the abiotic stress response of plants [8]. Liu et al. observed an increase in stomatal conductance and a decrease in drought tolerance in plants with low concentrations of SLs. This was found due to the effect of ABA on the rate of stomatal closure [14]. Toh et al. demonstrated that the application of SLs under heat stress resulted in the downregulation of the NCED9 gene and a reduction in ABA levels in Arabidopsis seeds [19]. Soma et al. [20] revealed that the ABA-unresponsive SnRK2 protein kinase regulates mRNA degradation in plants under osmotic stress.
Osmoregulation, as an important physiological mechanism in response to drought stress, can be regulated by the interaction of various hormones. Although the involvement of various phytohormones, such as ABA, in the regulation of plant stress responses is well known. However, the internal mechanism of exogenous SLs on soybean osmosis regulation remains unclear. In our experiment we chose soybean crop due to its importance in term of human consumption, however, it is currently threatened by climate change and drought. Building on this background information, this paper aims to gain a better understanding to the effects of externally applied phytohormones, specifically SLs, on the growth and physiological aspects of soybean under normal and drought conditions. We comparatively analyzed key physiological indicators of leaf and root development at optimal concentrations to elucidate the physiological regulatory effects of SLs on soybean growth and root development during drought stress. Furthermore, we performed a comprehensive analysis of morphological and physiological indicators, along with transcriptome and metabolome data to explore the regulatory effects of SLs on soybeans under drought conditions. The findings of this study have significant implications for the practical use of exogenous SLs for enhancing crop drought tolerance, as well as providing insights into plant water use efficiency.
Materials and methods
Test material
Drought intolerant varieties soybean Suinong 26 was selected as the test. The variety was bred by the Heilongjiang Academy of Agricultural Sciences, unlimited podding habit, reproductive period about 120d. The variety met the National Soybean Variety Certification Standards with certification. Strigolactones (SLs) were purchased from Sigma-Aldrich with a relative molecular mass of 298.29 and a purity of > 99%. Molecular formula: C17H14O5; CAS:76974-79-3.
Test design
A trial was conducted in 2023 at the Biotechnology Center of Heilongjiang Bayi Agricultural University. The experiment was conducted by the pot test method, and black soil was used as the test soil (organic matter content, 2.79 mg·kg− 1; alkali hydrolyzable nitrogen, 91 mg·kg− 1; fast-acting phosphorus, 44 mg·kg− 1; fast-acting potassium, 120 mg·kg− 1, pH 6.8). The fertilizer amounts per pot were as follows: 1.84 g of urea, 1.54 g of calcium superphosphate, and 1.13 g of potassium sulfate. The soil was mixed with the pots and packed into a pot with a height of 33 cm and a diameter of 30 cm. To ensure proper drainage, three holes of 1 cm were drilled in the bottom of each pot, and a screen mesh was placed on top of the holes. Before sowing, it is important to measure the quality and moisture content of the soil in each pot. Additionally, the maximum water holding capacity should be determined using the ring knife method to accurately calculate the amount of water required for subsequent water control processes.
The soil water content was measured by weighing on day 0 when the soybeans reached the V1 stage, which is characterized by the full expansion of the first compound leaf in 90% of the plants in the population. For the normal water supply treatment, the soil water content was maintained at 80% of the field moisture capacity. In contrast, the drought treatment gradually reduced the soil water content by stopping the water supply, reaching 50% of the field moisture capacity. The exogenous SLs spraying commenced when the soil moisture content reached 50% of the field moisture capacity. The experiment consisted of 100 pots for each treatment. Whereas, the experiment comprised of five treatments: (1) Normal water supply treatment (CK), where the soil water content was maintained at 80% of the field water capacity. (2) Drought stress treatment (D), on the seventh day of the cessation of water supply, the soil water content was reduced to 50% of the field moisture capacity. (3) Spray SLs treatment (D + GR0.1) under drought stress, where a concentration of 0.1 µM SLs were sprayed when the soil moisture content reached 50% of the field moisture capacity. (4) Spray SLs treatment (D + GR0.5) under drought stress, where a concentration of 0.5 µM SLs were sprayed when the soil moisture content reached 50% of the field moisture capacity. (5) Drought stress spraying SLs treatment (D + GR1), where a concentration of 1 µM SLs were sprayed when the soil moisture content reached 50% of the field moisture capacity.
Test methods and analysis of parameters
Determination of plant morphology and biomass
Root samples were collected at 5, 10, 15, and 20 days after foliar spraying of SLs. The FGX-A root analysis system (Shijiazhuang Fansheng Technology Co., Ltd, Hebei, China) was used to scan the root system, and the morphological analysis software win-RHIZO was employed to analyze root length, area, volume, and number of lateral roots. Additionally, three plants (with three replicates) were randomly chosen for measuring plant height, stem thickness, and leaf area in each treatment. The roots were subsequently removed using filter paper and weighed on a balance.
Determination of relative water content (RWC), water potential, osmotic potential, saturation osmotic potential and osmotic adjustment ability
Leaf water potential was determined using a pressure bomb technique as described in method [21]. The saturation osmotic potential (Hs) was assessed with a dew point microvolt-meter (HR-33T, Wescor, USA), following the procedure detailed in Song [22]. The fully developed leaves were kept in a freezer at -20 ℃ for 12 h and the osmotic potential was measured after thawing.
The saturated permeability potential (Js) is calculated according to the following equation.
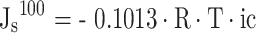 |
1 |
where: R is the gas constant, 0.008314, T is the Kjeldahl temperature, T = 273 + t, t is the room temperature in Celsius, ic is the osmotic molar concentration of the sample (Osm kg− 1 H2O).
The osmotic adjustment ability (OA) was calculated according to the following formula.
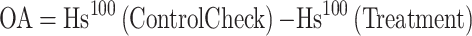 |
2 |
The relative water content (RWC) was assessed through the drying and weighing technique as outlined in method [23]. The functional leaves were detached and their fresh weight (W1) was recorded. These leaves were submerged in distilled water for 12 h, blotted to remove surface moisture, and then weighed to obtain the weight (BW). Subsequently, they were oven-dried at 80 ℃ to ascertain the dry weight (W2). The leaf water content was then calculated using the specified formula:
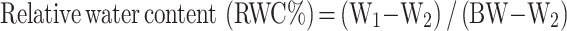 |
3 |
Determination of photosynthetic parameters
The Li-6400XT (LI-COR, Lincoln, USA) portable photosynthesis system was used to measure the net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr) and intercellular CO2 concentration (Ci) of the leaf from 9:00 to 11:00. Three leaf blades were selected per test treatment. Leaf chamber conditions were as follows: light intensity at 1,500 mol·mol− 1;CO2 concentration: 400 mol·mol− 1; temperature: 25 ℃; relative humidity: 60-70%, which enabled blades to adapt in the leaf chamber.
Relative chlorophyll content SPAD in soybean functional leaves was determined by atLEAF CHL chlorophyll analyzer (FT Green LLC, USA).
Determination of antioxidant enzymes
For the analysis of antioxidant enzymes, leaf samples (0.5 g) were processed by homogenization in 50 mM phosphate buffer (pH 7.0) containing 1% polyvinylpyrrolidone, followed by centrifugation at 15,000×g for 10 min at 4 ℃. The resultant supernatant was utilized to assess the activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD). To evaluate POD activity, 0.1 mL of the enzyme solution was combined with pyrogallol, phosphate buffer (pH 6.8), and 1% H2O2, with the absorbance changes monitored at 420 nm at 20-second intervals for 2 min. CAT activity was gauged by mixing the enzyme extract with a solution of phosphate buffer and H2O2, and the reaction was quantified using potassium permanganate post the addition of sulfuric acid [24]. The capability of SOD to obstruct the photochemical reduction of nitroblue tetrazolium (NBT) was quantified following the methodology proposed by Beauchamp and Fridovich [25].
Determination of endogenous hormones
Referring to the methods of Mostafa and Wang [26], IAA, ABA, standards were purchased from Sigma-Aldrich. All experiments were performed on an Agilent 1290 Promise LC system (Agilent T Technologies). In ESI negative/positive mode, the measurement was performed using triple quadrupole tandem mass spectrometry (triple quadrupole tandem mass spectrometry) and turbojet interface from Applied Biosystems USA.
Transcriptome and metabolome analysis
Transcriptome analysis
The leaves were picked on day 15 of treatment for transcriptome analysis. The NEB Next Ultra II RNA Library Prep Kit for Illumina (New England Biolabs Inc; Ipswich, Massachusetts, USA) was used in this study. Total RNA was isolated using the Trizol Reagent (Invitrogen Life Technologies), after which the concentration, quality and integrity were determined using a NanoDrop spectrophotometer (Thermo Scientific). Three micrograms of RNA were used as input material for the RNA sample preparations. The sequencing library was then sequenced on NovaSeq 6000 platform (Illumina) Shanghai Personal Biotechnology Cp. Ltd (https://docs.qiime2.org/2023.9/tutorials/).
Untargeted metabolomics analysis
Metabolites were analyzed from leaves picked on day 15, frozen, ground, and extracted with methanol/acetonitrile/water. After centrifugation, the supernatant was dried, re-dissolved in acetonitrile/water, and analyzed by UHPLC-Q-Exactive Orbitrap MS. Conditions included HILIC separation, and ESI settings were optimized for comprehensive metabolite profiling. Finally, the mass spectrometry data underwent the following bioinformatics analyses: KEGG pathway analysis (https://www.genescloud.cn).
Statistical analysis
Six replicates were analyzed for morphological and physiological indicators at each stage. Three replicates were analyzed for transcriptome and metabolome analysis. Significant differences in the data were analyzed via ANOVA and the Duncan’s method was used to compare the means for each variable (P < 0.05) based on the IBM SPSS Statistics (version 26.0; IBM Corporation, New York, NY, USA), while Origin 20.0 was employed for graph plotting. MS raw data were converted to MzXML using ProteoWizard MSConvert and processed in XCMS for peak detection and for peak grouping. CAMERA annotated isotopes and adducts, retaining variables with over 50% nonzero measurements in any group. Metabolites were identified by matching m/z values (< 10 ppm) and MS/MS spectra with a custom database.
Results
Effects of SLs on phenotype and morphological characteristics of soybean under drought stress
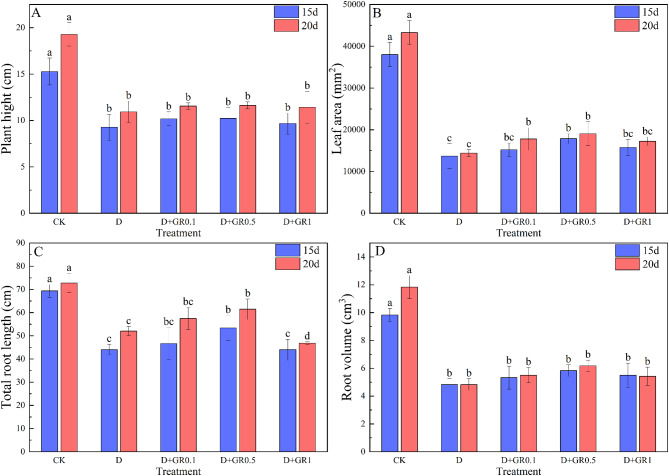
The effects of Strigolactones (SLs) on the phenotype and morphology of soybean plants at the seedling stage are presented in Fig. 1. Drought treatment alone significantly impacted plant growth. However, when a certain amount of SLs were sprayed, it improved the plant growth. Whereas, the influence of SLs at a concentration of 0.5 µM is relatively high among the different concentrations. The relevant morphological data in Fig. 1 shows that drought stress significantly reduced plant height, leaf area, root length, and root volume. Specifically, compared to the control group (CK), the reductions were 39.3% and 43.3%, 64.0% and 66.5%, 36.5% and 28.5%, 50.9% and 59.2% at 15 and 20 days, respectively. However, when SLs were sprayed, they mitigated decrease in some indices caused by drought treatment alone and improved them as compared to the drought treatment alone. Notably, the D + GR0.5 treatment showed a more significant effect than the other concentration treatments. The analysis results revealed that in the D + GR0.5 treatment, compared to the drought treatment (D), plant height, leaf area, root length, and root volume increased by 10.4%, 30.5%, 21.7%, 20.7% at 15 days and 6.4%, 31.9%, 18.2%, 27.7% at 20 days, in which the leaf area and total root length reached significant difference.
Fig. 1.
Effects of SLs on phenotype and morphological characteristics of soybean under drought stress. CK, control; D, drought stress; D + GR0.1, combined drought and 0.1 µM SLs; D + GR0.5, combined drought and 0.5 µM SLs; D + GR1, combined drought and 1 µM SLs. Vertical bars indicate the mean ± standard deviation (n = 6). Different lowercase letters indicate significant differences among treatments (P < 0.05)
Effects of SLs on relative water content (RWC) of soybean under drought stress
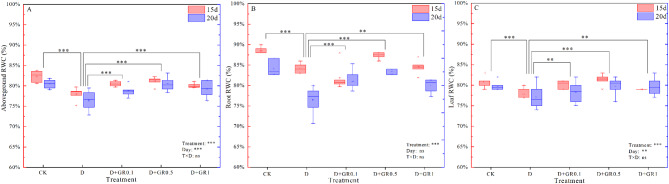
The effect of relative water content (RWC) of plants without different treatments is shown in Fig. 2. The application of a certain amount of SLs through foliar spraying resulted in a slight increase in leaf and root RWC. However, the RWC of above-ground, roots and leaves significantly decreased under drought stress. Nevertheless, when 0.5 µM SLs were sprayed on the leaves, a significant increase in leaf water content was observed even under drought stress, and root RWC was also significantly affected. Compared with the CK, drought stress caused decreases in the leaf, root, and total aboveground RWC of 5.2%, 4.9%, 4.8% after 15 days, and 6.6%, 3.9%, 3.0%, after 20 days, respectively. The application of SLs through spraying effectively improved these indices, with the most significant improvement observed at 0.5 µM, resulting in a 4.0% and 5.2% increase in aboveground RWC compared to the drought treatment after 15 days.
Fig. 2.
The relative water content in soybean under different treatments. Vertical bars indicate the mean ± standard deviation (n = 6). Different lowercase letters indicate significant differences among treatments (P < 0.05)
Effects of exogenous SLs on osmotic adjustment in soybean roots under drought stress
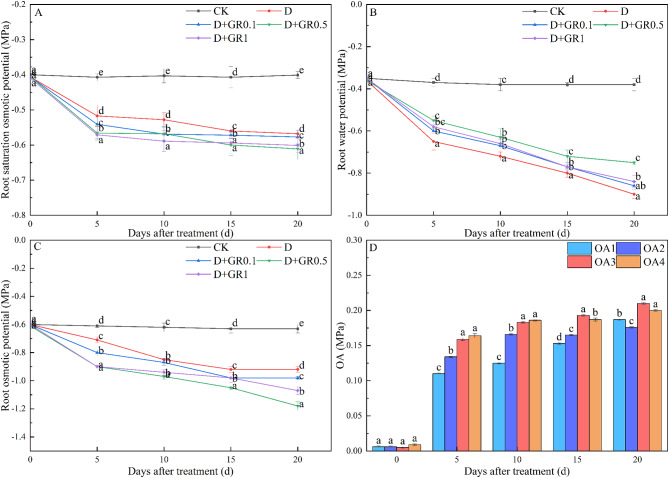
The trends of water potential, osmotic potential, saturation osmotic potential, and osmotic adjustment over time after foliar spraying of SLs under drought stress are depicted in Fig. 3. The indices remained stable over time under a normal water supply. However, the water potential significantly decreased on the fifth day of drought stress and continued to slowly decrease over time. The application of exogenous SLs via foliar spraying demonstrated a notable retardation of the decline in water potential under conditions of drought stress. In contrast, the change in water potential differed from that of osmotic potential and saturation osmotic potential. The roots exhibited the most pronounced saturating osmotic potential at a concentration of 1 µM during the first 10 days. In contrast, on the 15th day, the saturation osmotic potential was most pronounced at a lower concentration of 0.5 µM. Moreover, foliar spraying of SLs significantly decreased both the osmotic potential and the saturation osmotic potential under drought stress, with the most significant decrease observed at a concentration of 0.5 µM between 15 and 20 days. Furthermore, as the duration of drought stress increased, the saturated osmotic potential of the root system decreased, while the osmotic adjustment capacity increased (OA1). The saturated osmotic potential of the SLs subsequently decreased, with the most significant reduction occurring on the 5th day. After drought stress, plants treated with different concentrations of SLs exhibited varying levels of osmotic adjustment in the root system at different time points. OA4 reached its maximum value within the first 10 days, indicating that 1 µM SLs were more effective on the root system. Moreover, OA3 reached its maximum value after 10 days, suggesting that 0.5 µM SLs were more effective. This may be attributed to the timely effect of foliar spraying of SLs on the root system.
Fig. 3.
The effects of SLs on water potential (A), osmotic potential (B), saturation osmotic potential (C) and osmotic adjustment ability (D) in roots of soybean seedlings under drought stress. OA, The osmotic adjustment ability. Hs100, saturation osmotic potential. OA1, Hs100(CK)-Hs100(D); OA2, Hs100(CK)-Hs100(D + GR0.1); OA3, Hs100(CK)-Hs100(D + GR0.5); OA4, Hs100(CK)-Hs100(D + GR1). Vertical bars indicate the mean ± standard deviation (n = 6). Different lowercase letters indicate significant differences among treatments (P < 0.05)
Effects of exogenous SLs on photosynthetic characteristics in soybean under drought stress
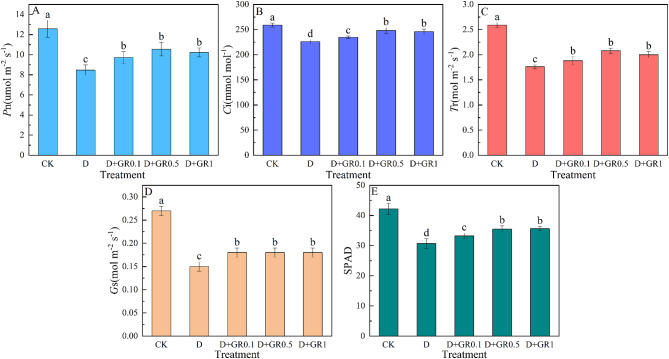
Photosynthesis is a critical process in primary metabolism and plays a crucial role in plant growth and development, especially under drought conditions. Significant reductions were observed in the physiological parameters of soybean leaves on the 15th day of drought stress (Fig. 4). Specifically, the Pn, Ci, Tr, Gs, and the SPAD decreased by 32.7%, 12.8%, 32.0%, 44.4%, and 27.3%, respectively. However, foliar sprays of SLs proved to be effective in helping soybean plants resist the decrease in photosynthetic indices caused by drought stress. Compared with the treatments without SLs (D), the treatments with different concentrations of SLs (D + GR0.1, D + GR0.5, and D + GR1) increased the Pn by 14.8%, 24.6%, and 20.7%, the Ci by 3.8%, 9.8%, and 8.5%, the Tr by 6.8%, 18.2%, and 13.6%, the Gs by 20.0%, 26.7%, and 31.3%, and the SPAD by 8.3%, 15.7%, and 16.1%, respectively. These results indicate that the application of exogenous SLs significantly improved the leaf photosynthetic parameters under drought conditions.
Fig. 4.
Effect of SLs on Pn (A), Ci (B), Tr (C), Gs (D), and SPAD (E) of soybean under drought stress. Pn, net photosynthetic rate; Ci, intercellular CO2 concentration; Tr, transpiration rate; Gs, stomatal conductance; SPAD, relative chlorophyll content. Vertical bars indicate the mean ± standard deviation (n = 6). Different lowercase letters indicate significant differences among treatments (P < 0.05)
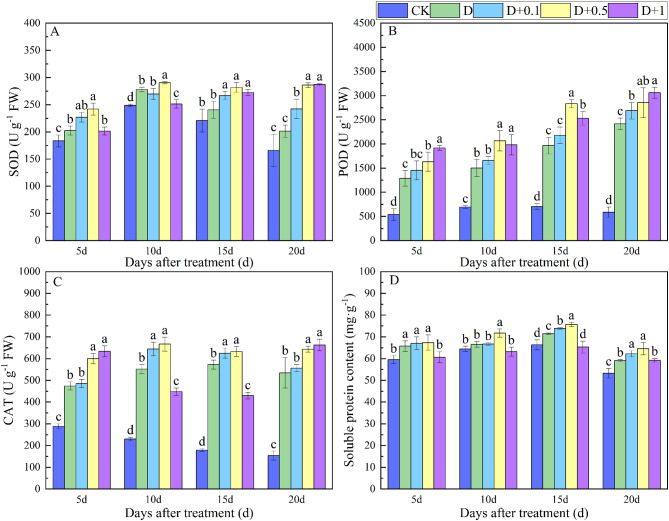
Effects of exogenous SLs on ROS in soybean under drought stress
As shown in Fig. 5-A, B and C, drought stress significantly increased the activities of SOD, POD, and CAT in soybean leaves. Compared with those in the CK treatment, the activities of SOD, POD and CAT in the leaves were increased by 8.9.%-21.3%, 116.0-311.3%,140.3-248.3% at 10, 15 and 20d after D treatment, respectively. The activities of CAT, SOD, and POD in soybean leaves were further promoted by the application of exogenous SLs. Among them, CAT, SOD and POD activities were increased by 17.1%, 41.9% and 43.9%, respectively, on day 15 and 18.0%, 10.5% and 20.3%, respectively, on day 20 of the D + 0.5GR treatment compared to the D treatment. Exogenous SLs at a concentration of 0.5 µM significantly increased the activity of antioxidant enzymes in soybean leaves under drought stress. This helped alleviate the oxidative damage caused by drought stress on plants and improved the drought tolerance of soybean. As shown in Fig. 5-D, drought stress resulted in a significant increase in the soluble protein content of soybean leaves, and compared with the CK treatment, the soluble protein content of leaves increased by 10.5%, 7.7%, and 10.9% at 5, 15, and 20d after the D treatment, respectively. Compared with the D treatment, the soluble protein content of leaves treated with D + GR0.5 increased significantly by 7.7%, 5.9% and 9.2% at 10, 15 and 20 d after treatment, respectively.
Fig. 5.
The contents of antioxidant enzyme (SOD, POD and CAT), and soluble protein in soybean leaves under different treatments. Vertical bars indicate the mean ± standard deviation (n = 6). Different lowercase letters indicate significant differences among treatments (P < 0.05)
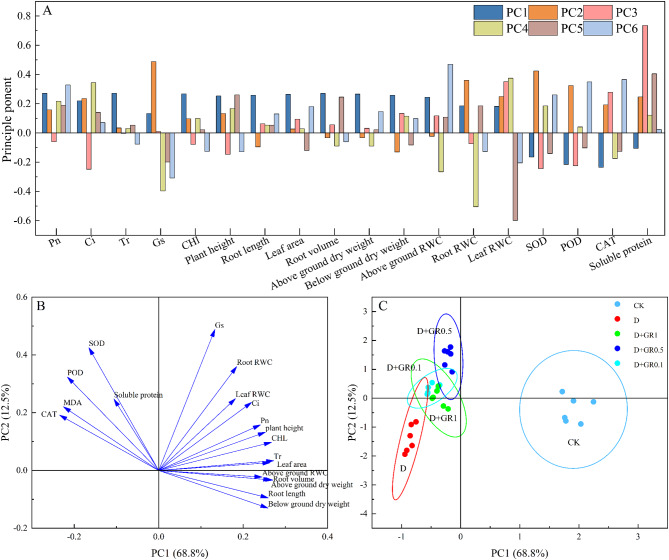
Principal component analysis (PCA)
To further investigate the differences caused by SLs treatments, soybean seedling physiological index data were subjected to principal component analysis (Fig. 6-A). The largest loadings in the 1st principal component were Pn, Tr and chlorophyll content, with loadings of 0.271, 0.272, and 0.267, respectively, and the eigenvectors reflected the photosynthetic capacity of soybean seedlings under drought stress. The largest loadings of the 2nd principal component were Gs and SOD, mainly reflecting the degree of oxidative stress on stomatal conductance of soybean seedlings. The largest loadings in the corresponding eigenvectors of the 3rd principal component were soluble protein content and SOD, with loadings of 0.736 and 0.351, reflecting the osmotic adjustment ability and the degree of antioxidant damage of soybean seedlings. The largest loadings of the fourth principal component corresponding to the eigenvector were leaf water content and Ci, with loadings of 0.375 and 0.344, reflecting the drought tolerance and adaptability of soybean leaves under drought stress. The largest loadings of the fifth principal component corresponding to the eigenvector were plant height and root volume, with loadings of 0.261 and 0.246, mainly reflecting the growth condition of soybean plants under drought stress. The largest loadings of the sixth principal component corresponding to the eigenvectors were POD and CAT, with loadings of 0.350 and 0.366, mainly reflecting the scavenging ability of reactive oxygen species in soybean seedlings under drought stress.
Fig. 6.
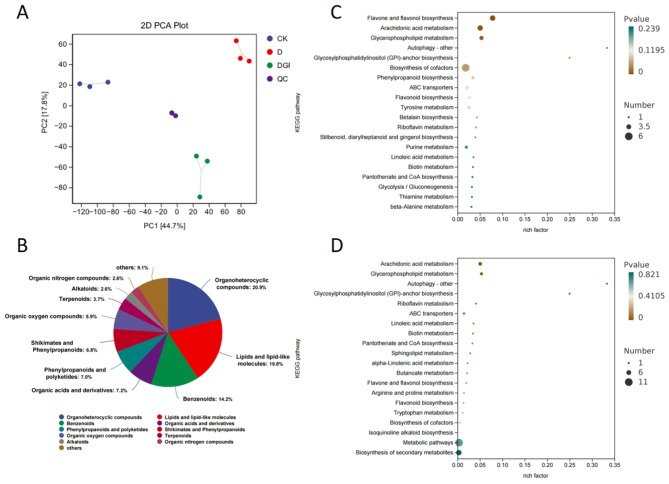
Soybean seedling physiological index data were subjected to principal component analysis. (A) The loading matrix of each indicator and its contribution for the effects of drought stress, exogenous SLs treatments. (B, C) Principal Component Analysis (PCA) of physiological indicators of soybean under exogenous SLs and drought stresses
The PCA plot reveals distinct distributions of the samples across different treatment groups (Fig. 6-C). All treatments exhibits a tight clustering, indicating high consistency within the group. The absence of clear outliers in the plot indicates that there are no anomalous samples that could potentially skew the analysis. The distances between samples within each group are relatively small, showing the higher degree of similarity. As can be seen from Fig. 6-B, photosynthesis, morphology, and water content were positively correlated with PC1 and PC2; SOD, POD, CAT, and soluble protein were negatively correlated with PC1 and positively correlated with PC2; Root length, Root volume, Above ground dry weight, Below ground dry weight, and Above ground dry weight were positively correlated. Root length, root volume, above ground dry weight, below ground dry weight, and above ground RWC were positively correlated with PC1 and negatively correlated with PC2. Taken together, the growth characteristics, water potential, antioxidant enzyme activities, photosynthetic characteristics and osmoregulatory substances of soybean can be used as comprehensive indexes for evaluating the drought stress resistance of soybean seedlings.
Differential metabolite screening, KEGG enrichment, and enrichment analysis
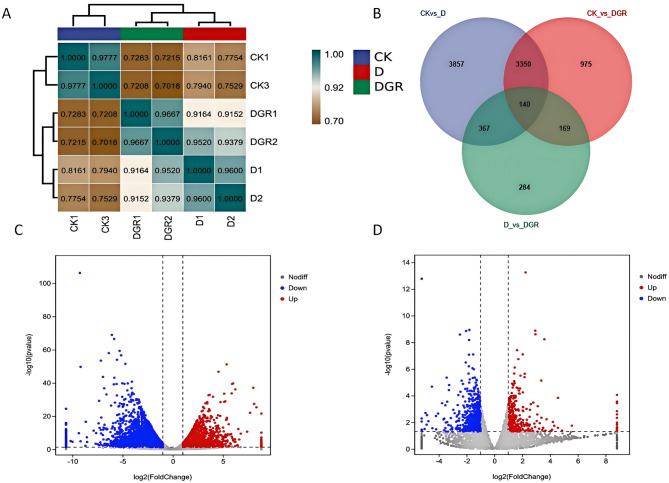
Sequencing quality evaluation and differential expression gene analysis
In this study, we investigated the regulatory mechanism of exogenous SLs on soybean drought stress at the gene level. We analyzed the transcriptome sequencing and bioinformatics data of soybean leaves subjected to different treatments: normal water supply (CK), drought stress (D), and drought stress with an optimal concentration of SLs treatment (D + GR0.5) (Fig. 7). The results demonstrated a high correlation between biological replicates for all treatments, indicating the reliability of the data (Fig. 7-A). By comparing the gene expression results, we observed that the differences in gene expression between the normal water supply and drought stress conditions were greater than those between normal water supply and drought stress with simultaneous SLs treatment (Fig. 7-B). This suggests that SLs have the potential to influence the drought tolerance of soybeans by modulating gene expression levels. To assess the impact of drought stress (D) on gene expression, we conducted a two-by-two comparison of transcript levels in soybean leaves under different conditions. These comparisons included normal water supply (CK) and drought stress (D), as well as simultaneous SLs treatment under drought stress (D + GR0.5) and drought stress (D). The selection of expressed genes was based on empirical values. Genes were included in the analysis if the average expression value FPKM (the number of bipartite sequences that can be aligned to an exon per one thousand bases per one million sequences on the ratio) was greater than 1 for all samples and the false discovery rate (FDR) was less than 0.05. As illustrated in Figs. 7-C and D and 2798 genes were found to be up-regulated and 4916 genes were identified as down-regulated in soybean leaves subjected to a normal water supply (CK), while 357 genes were observed to be up-regulated and 603 genes were identified as down-regulated in soybean leaves treated with SLs under drought stress (D + GR0.5), in comparison to the drought stress (D) treatment.
Fig. 7.
Sequencing quality evaluation and differential expression gene analysis. (A) Pearson correlation coefficients from all genes between each pair of samples of transcriptome. (B) The Venn diagram shows the overlapped differentially expressed genes between the CK / D and D / D + GR comparisons, the sum of the numbers in each circle represents the total number of differential genes for that comparison set, and the overlapping part of the circle indicates the differential genes shared between the two comparison sets. Volcano plots of differentially expressed genes in CK / D (C) and D / D + GR (D) comparisons, Grey dots represent genes without significant differential expression, red and blue dots denote significantly up-regulated and down-regulated genes respectively in the CK / D and D / D + GR comparisons
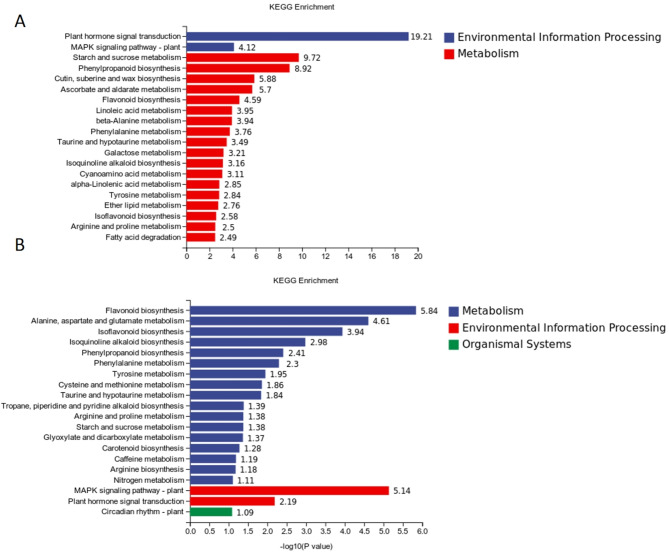
Enrichment analysis of differentially expressed genes in soybean leaves
KEGG enrichment analysis under drought stress and normal water conditions revealed that plant hormone signal transduction, MAPK signaling pathway-plant, and Starch and sucrose metabolism were more enriched than other pathways (Fig. 8). Additionally, most genes were found to be significantly down-regulated. Another comparative analysis was performed to assess the impact of exogenous SLs treatment and drought stress treatment on metabolic pathways. The findings revealed that Flavonoid biosynthesis, Alanine, aspartate and glutamate metabolism, Isoflavonoid biosynthesis, MAPK signaling pathway-plant, and Plant hormone signal transduction were the main pathways enriched. It was observed that the up-regulated genes were primarily involved in Alanine, aspartate and glutamate metabolism, which are crucial for protein synthesis and nitrogen metabolism. These genes play a pivotal role in the production and utilization of nitrogen compounds within plants. Further analysis revealed that several metabolic pathways were significantly down-regulated, including Isoflavonoid biosynthesis, Phenylpropanoid biosynthesis, Phenylalanine metabolism, Tyrosine metabolism, Cysteine and methionine metabolism, Taurine and hypotaurine metabolism, and Arginine and proline metabolism (Annex 1). These pathways play a crucial role in maintaining cellular osmoregulation and antioxidant defense. The down-regulation of genes in these pathways can lead to a decrease in the synthesis and accumulation of harmful substances, mitigating oxidative damage and preserving cellular homeostasis and function. These findings suggest that SLs have the potential to enhance osmoregulation and reduce reactive oxygen species production in soybean cells exposed to drought stress.
Fig. 8.
Kmeans figure of differential pathway. (A) KEGG differential metabolite pathway CK and D, (B) KEGG differential metabolite pathway D + DGR. Note: figure A and figure B ordinate is the name of the KEGG metabolic pathway, and the abscissa is the number of metabolites annotated to the pathway and their total number of metabolites annotated
Analysis of differential metabolites and KEGG in soybean leaves
To explore the impact of SLs on soybean leaf metabolites during drought conditions, we acquired dependable, high-quality metabolomic information utilizing mass spectrometry (Fig. 9-A). This data initially underwent Quality Control (QC) assessment. We conducted Principal Component Analysis (PCA) on the dataset, which included QC samples, to gain an initial insight into the general metabolic variances across different groups and the variability within each group. The PCA findings revealed distinct patterns of metabolomic divergence among the groups, suggesting variations in the metabolome. Specifically, PC1 accounted for 44.7% of the overall variance, while PC2 contributed to 17.8%. Utilizing the Variable Importance in Project (VIP) scores from the OPLS-DA model’s multivariate analysis, we initially identified metabolites that showed variations across treatments. Subsequent screening of these metabolites was refined using P-values or fold changes from univariate analyses. The selection criteria for these significant differences were established as a VIP score greater than 1 in the OPLS-DA model and a P-value less than 0.05. Among the metabolites identified, five categories exhibited notable disparities: Organoheterocyclic compounds (20.9%), Lipids and lipid-like molecules (19.8%), Benzenoids (14.2%), Organic acids and derivatives (7.2%), and Phenylpropanoids (7.0%), as illustrated in Fig. 9-B. In total, 738 differential metabolites were identified between the control and drought conditions, comprising 134 down-regulated and 604 up-regulated metabolites. Comparatively, between the drought treatment and the combined drought and SLs treatment, 368 differential metabolites were identified, including 249 down-regulated and 119 up-regulated metabolites. This suggests that under drought stress, soybean leaves may accumulate certain metabolites as a defense mechanism, and the application of SLs could enhance their drought resilience.
Fig. 9.
Differential metabolites and KEGG in soybean leaves. (A) Soybean PCA figure and sample cluster heat map on day 15 of treatment, with well water (CK), drought treatment (D) and SLs treatment (D + GR) and quality control samples: PCA figure. (B) Classification statistics of all metabolites by their Chemical Taxonomy attribution information. Enrichment result factor plots in CK / D (C) and D / D + GR (D), with rich factor (number of differential genes annotated to the Pathway/total number of genes annotated to the Pathway) in the horizontal coordinates and Pathway in the vertical coordinates, the size of the dots in the plots indicates the number of differential (up-regulated or down-regulated, related to the set of genes selected during the analysis) genes annotated to the corresponding Pathway, and the darkness of the color indicates the level of significance
We further selected the top 20 KEGG pathways with the most significant enrichment for bubble map analysis (Fig. 9-C and D). These pathways are critical for various facets of plant growth and development, encompassing energy metabolism, cell membrane integrity, antioxidant mechanisms, and resilience to adverse conditions. Predominantly, in both the control and drought stress conditions, metabolites showed enrichment in pathways such as Flavone and flavonol biosynthesis, Arachidonic acid metabolism, Glycerophospholipid metabolism, Glycosylphosphatidylinositol (GPI)-anchor biosynthesis, and Cofactor biosynthesis. Moreover, in plants subjected to drought stress treated with sprayed SLs, significant enrichment was observed in Arachidonic acid metabolism, Glycerophospholipid metabolism, GPI-anchor biosynthesis, and Riboflavin metabolism. When examining the top 20 KEGG pathways, we found that Glycerophospholipid metabolism, Riboflavin metabolism, Linoleic acid metabolism, and Flavone and flavonol biosynthesis were down-regulated under drought stress (Annex 2), but up-regulated when exogenous SLs were sprayed. This suggests that SLs have a significant effect on these four metabolic pathways.
Effects of exogenous SLs on soybean endogenous hormones under drought stress
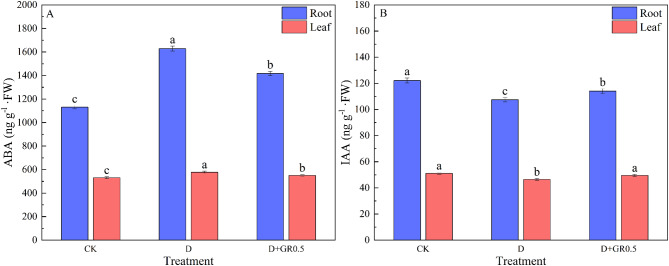
As illustrated in Fig. 10, at day 5 of drought stress, the ABA content in the roots and leaves of the drought stress treatment (D) exhibited a 43.9% and 8.8% increase in comparison to the control (CK). Conversely, the IAA content in the roots and leaves demonstrated a 12.0% and 9.1% decline, respectively. Foliar spraying with appropriate concentrations of exogenous SLs (D + GR0.5) resulted in a decrease in ABA content by 12.9% and 4.9% in roots and leaves, and an increase in IAA content by 6.1% and 6.5% in roots and leaves, respectively, in comparison with the drought stress treatment.
Fig. 10.
Effect of SLs on endogenous hormones in soybean leaves and roots. (A) ABA, (B) IAA. CK, control; D, drought stress; D + GR0.5, combined drought and 0.5 µM SLs. Vertical bars indicate the mean ± standard deviation (n = 3). Different lowercase letters indicate significant differences among treatments (P < 0.05)
Discussion
Foliar application of strigolactones (SLs) mitigate drought stress in soybean by improving chlorophyll content, osmotic regulation ability, gas exchange activity, and reactive oxygen species (ROS) in plants subjected to drought stress [17]. Drought is an abiotic stress that significantly impacts crop growth, development, and normal life processes [27, 28]. One of the earliest traits expressed by plants in response to drought is the alteration of the root phenotype [29]. Mild drought has been shown to stimulate root growth, but as drought conditions intensify and prolong, both root and leaf growth are significantly inhibited, leading to a reduction in plant height, leaf weight, and leaf area [30]. SLs, a class of plant hormones, play a role in plant responses to environmental cues, such as light and drought stress. They function as signaling molecules, coordinating plant responses to optimize growth and survival [12, 31]. Recent studies indicate that SLs are crucial in regulating root growth under drought stress [32]. SLs have been shown to modulate root system architecture by promoting the formation of longer primary roots and enhancing lateral root branching [32]. Different concentrations of SLs ranging from 0.1 to 1 µM were used in this study to affect growth at varying degrees and time intervals. Our measurements of plant and root morphology, taken on 15d and 20d, showed that drought significantly reduced the height of soybean plants, as well as their leaf area, heel volume, and root length. Spraying SLs after drought did not significantly affect plant height, but did increase root volume, root length, and leaf area to some extent. These findings are consistent with previous studies on the root system [33]. Under drought stress, plants produce higher levels of SLs, which act as signaling molecules to promote a more extended and robust root system [34]. This enhanced root system helps plants to better explore the soil for water resources. The SLs seem to have also promoted the usual development of the soybean root system under such conditions, allowing for the normal uptake and use of nutrients and the regular accumulation of dry matter.
The RWC is an important indicator that reflects the water content of the plant body and is closely related to the drought tolerance of the crop [35]. A certain amount of SLs sprayed on the leaves can increase leaf and root RWC to a certain extent. Both total aboveground RWC, root RWC, and leaf RWC decreased under drought stress, while leaf spraying with 0.5 µM of SLs significantly increased plant water content. Javadi et al. [36] reported that the RWC of the crop exhibited a gradual decline with the intensification of drought stress. Nevertheless, the results of this study revealed that an appropriate concentration of SLs could mitigate the reduction in leaf water content and wilting to some extent [16]. This improvement suggests that these phytohormones may assist plants in maintaining optimal water levels. Recent experiments have shown that SLs and ABA interact directly or indirectly, jointly regulating plant abiotic stress response [37]. Improvement in RWC of plants when treated by SLs could be explained by higher Abscisic acid content in plants. SLs were needed for efficient control of water loss by transpiration in stressed shoots [14].
Stomata are tiny pores on the leaf surface that control water loss through transpiration. SLs can regulate stomatal closure, reducing water loss and improving plant water-use efficiency during drought conditions [14]. A series of adaptive changes occur in plants under drought stress, of which the decrease in osmotic potential is an important aspect. Osmoregulation enables crops to regulate cellular osmotic pressure and maintain water balance under such conditions [38]. The present study showed that foliar spraying of SLs decreased the osmotic potential and increased the osmoregulatory capacity of the root system. In addition, under drought stress, plants can actively accumulate various organic or inorganic substances to maintain the stability of the intracellular environment. Among them, Soluble proteins serve as osmoregulatory agents that increase the water absorption or water retention capacity of cells, protecting normal metabolic processes in plants. To a certain extent, the content of ABA and soluble protein in soybean leaves may be increased under drought stress. Moreover, appropriate concentration of SLs sprayed exogenously over drought-stressed soybean seedlings increased IAA and soluble protein content (Figs. 5-D and 10). This implies that SLs are capable of effectively enhancing the osmotic substance accumulation, which helps regulate cellular osmotic potential. The ultimate result is stabilization of the intracellular environment, an important mechanism for withstanding drought stress. Previous studies have demonstrated that SLs can act as a regulator of stomatal movement, working together with ABA to regulate stomatal closure in leaves under water stress [13, 14]. ABA can induce plant root contraction to reduce water loss and also promote stomatal closure to reduce transpiration [39, 40]. These regulatory effects contribute to the water and osmotic potential of plants. Therefore, it can be inferred that there are direct or indirect interactions between SLs and ABA to regulate plant osmoregulatory capacity. Photosynthesis is crucial for plant growth, development, and energy conversion. Photosynthetic pigments are fundamental for photosynthesis in plants, influencing the capture, transfer, and conversion of light energy [41]. Drought stress hinders soybean photosynthesis, as it reduces chlorophyll content in soybean leaves, according to research. Under stress conditions, SL can increase plant stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci), thus enhancing the net photosynthetic rate (Pn) [42]. In this study, drought stress significantly hindered chlorophyll production in soybean leaves. The chlorophyll content gradually decreased with prolonged drought, and this phenomenon further impeded the normal functioning of photosynthesis. SLs effectively alleviate drought stress inhibition on soybean chlorophyll content, improve photosynthetic capacity, stomatal conductance, and increase transpiration rates. This may be due to the excess of ROS in the chloroplasts tending to balance, reducing damage to the membrane system from ROS. Then, the plant can mitigate the phenomenon of significant chlorophyll degradation due to drought stress while also sustaining the membrane structure of chloroplasts. Additionally, it can minimize water loss from the leaves, allowing stomata to remain partially open under drought conditions and enabling the plant to maximize photosynthesis. Drought stress causes disruption of reactive oxygen species metabolism in crops and excessive accumulation of reactive oxygen species in plants, leading to cell membrane lipid peroxidation and cell membrane damage, which ultimately leads to cell death [43]. In this study, we investigated the impact of exogenous application of SLs on antioxidant enzyme activities and other physiological responses in plants under drought stress. Our findings suggest that the application of SLs significantly induced enzymatic antioxidant activities in soybean plant leaves. Screening results revealed that foliar application of 0.5 µM SLs effectively enhanced antioxidant enzyme activities. Principal component analysis was performed to extract six components from the morphological and physiological indexes of drought-stressed soybean seedlings. These components mainly reflected the photosynthetic utilization capacity, cell membrane damage, and osmotic adjustment characteristics of the soybean seedlings under drought stress. Our results indicate that higher activity of intracellular antioxidant enzymes and concentration of membrane-protecting compounds can mitigate damage to seedlings under drought conditions. Additionally, our study demonstrated that drought stress significantly reduced soybean growth, photosynthesis, and chlorophyll index, but this toxicity was overcome by the foliar application of 0.5 µM SLs. Further research is needed to investigate the exact mechanism of action of SLs under drought stress in the future.
The above illustrates the effects of SLs on soybean under drought stress from morphological, photosynthetic and physiological perspectives, respectively. However, the mechanism regarding the regulation of SLs on soybean leaves is not clear. Therefore, we investigated the role of SLs in drought tolerance of soybean using transcriptomic and metabolomic approaches, which led to the identification of key metabolic pathways related to osmoregulatory capacity and antioxidant in soybean. Transcriptome sequencing analysis showed that 2798 genes were up-regulated and 4916 genes were down-regulated in soybean leaves in the normal water supply treatment compared with the drought treatment, and 357 genes were up-regulated and 603 genes were down-regulated in the drought stress treatment compared with the drought stress plus SLs treatment. The results from KEGG enrichment analysis in this study showed significant up-regulation of genes related to Alanine, aspartate and glutamate metabolism, which are amino acids involved in plant nitrogen metabolism, amino acid metabolism, regulation of energy metabolism and protein synthesis, and the up-regulation of the genes may increase the metabolism of glucose and production of ATP, affecting protein synthesis and the Nitrogen metabolism balance. The up-regulation of these genes may increase glucose metabolism and ATP production, affecting protein synthesis and nitrogen metabolism balance [44]. It has been shown that SLs and auxins interactions positively affect the accumulation of amino acids, sutherlandins and sutherlandiosides in legumes [45]. This suggests that exogenous SLs respond to adversity by modulating amino acid metabolic pathways, most notably Alanine, aspartate and glutamate metabolism in this study. In addition, our analysis by metabolome assay showed that among the top 20 KEGG pathways with the most significant enrichment, Glycerophospholipid metabolism, Riboflavin metabolism, Linoleic acid metabolism, Flavone and flavonol biosynthesis were significantly and commonly in normal water supply treatment, drought treatment, and drought plus SLs treatment. Interestingly, Arachidonic acid metabolism pathway was up/down-regulated in 3/1 genes were up/down-regulated after exogenous SLs treatment. The Glycerophospholipid metabolism pathway was up/down-regulated in 3/0 genes under drought stress, whereas 0/3 genes were up/down-regulated after exogenous SLs treatment. The Linoleic acid metabolism pathway was up/down-regulated in 1/0 genes under drought stress while 0/1 genes were up/down-regulated after treatment with exogenous SLs. The Flavone and flavonol biosynthesis pathway was up/down-regulated in 1/3 genes under drought stress while 0/1 genes were up/down-regulated after treatment with exogenous SLs (Annex 2). Arachidonic acid metabolism and Glycerophospholipid metabolism is important for the maintenance of cellular osmoregulation, which are closely related to metabolic homeostasis and osmoregulation of the cells [46]. Linoleic acid metabolism is involved in the regulation of cell membrane fluidity and permeability, which has an effect on the ionic balance and osmoregulation of cells [47]. Flavonoids in Flavone and flavonol biosynthesis may be involved in osmoregulation and have an effect on plant stress tolerance [48]. Ito et al. also pointed out that SLs and ABA participate in the regulation of flavonoid synthesis and enhance plant drought tolerance [49]. The above metabolic pathways indicate that the up-regulation of SLs regulation-related genes affects osmoregulation in a variety of ways, including regulating cell membrane permeability, modulating the balance of intra- and extracellular solute concentrations, and participating in the regulation of ion channels. Finally, the analysis of physiological indicators osmotic pressure, osmotic potential and metabolomic KEGG enrichment analysis demonstrated that SLs could respond to drought stress by up-regulating genes related to the above pathways. The present study deepens the understanding of the mechanism of SLs regulating drought resistance in soybean, and provides a useful basis for exploring the mechanism of drought resistance in soybean under drought stress and the promotion of soybean growth by SLs under drought conditions, thus providing guidance for the use of SLs in practical production to promote the improvement of soybean yield and quality, and also providing a theoretical basis for coping with drought stress brought by global warming in order to enhance the plant’s adaptability and survivability.
Conclusions
This study clearly demonstrated that drought stress had a significantly negative impact on various aspects of plant growth, such as above-ground biomass, plant relative water content, antioxidant enzyme activities, and osmoregulatory systems. Suitable concentrations of SLs partially alleviated leaf water reduction and wilting, potentially aiding plants in maintaining water balance even under conditions of increased water loss. Additionally, we revealed that the regulatory effects of different concentrations of SLs on soybean leaves and roots were time-sensitive. Consequently, it was determined that 0.5 µM SLs had the most beneficial impact on root morphogenesis, with a noticeable increase was observed 15 days after application. Transcriptome and metabolome analyses revealed 368 differentially abundant metabolites between the drought treatment group and the drought plus SLs treatment group, including 249 down-regulated and 119 up-regulated metabolites. The up-regulated genes were mainly involved in Alanine, aspartate and glutamate metabolism, which play key roles in their involvement in the utilization of nitrogen compounds, and the significantly down-regulated metabolic pathways were mainly involved in the maintenance of cellular osmoregulation and antioxidant defenses. SLs regulate the cellular osmoregulation and antioxidant defenses through the regulation of Arachidonic acid metabolism, Glycerophospholipid metabolism, Linoleic acid metabolism, Flavone and flavonol biosynthesis pathways, regulating cell membrane permeability, modulating intra- and extracellular solute concentration homeostasis, and participating in the regulation of ion channels to promote drought stress and ultimately improve drought tolerance in soybean.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank Shanghai Personalbio Technology Co., Ltd. (Shanghai, China) for help with the Eukaryotic referent transcriptome and Non-targeted metabolomics analysis.
Author contributions
L.C. and B.Q, Conceptualization; L.C. and Y.Z, methodology.; software, S.Z. and W.M.; Project administration, Z.G. and L.F.; formal analysis, S.C.; writing–original draft preparation, L.C. and B.Q.; writing–review and editing, L.C and B.Q.; visualization, W.M. and S.Z.
Funding
This work was supported by Natural Science Foundation of Heilongjiang Province (LH2022C063); Project funded by China Postdoctoral Science Foundation (2022M720695); Initiation Foundation for Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (ZRCQC202101); The “Leading the Charge with Open Competition” project by Inner Mongolia Autonomous Region (2023JBGS0006); Science and technology project of Heilongjiang Province (2021ZXJ05B02); China Agriculture Research System of MOF and MARA (CARS-04-PS19) and Heilongjiang Bayi Agricultural University Experimental demonstration base project (2022101).
Data availability
The data that supports the findings of this study, including metabolomics data, are available in the supplementary material of this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dai A, Erratum. Drought under global warming: a review. Wiley Interdisciplinary Reviews: Clim Change. 2012;3(6):617. [Google Scholar]
- 2.Wang S, Liu S, Wang J, Yokosho K, Zhou B, Yu YC, Liu Z, Frommer WB, Feng J, Chen LQ, et al. Simultaneous changes in seed size, oil content, and protein content driven by selection of SWEET homologues during soybean domestication. Natl Sci Rev. 2020;7(11):nwaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling Z, Tong L, Yang W, Yuanyu Z, Ying-Shan D. FvC5SD overexpression enhances drought tolerance in soybean by reactive oxygen species scavenging and modulating stress-responsive gene expression. Plant Cell Rep. 2019;38(9):1039–51. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Zhu Y, Li S, Zhang W, Yin C, Lin Y. Regulation of Phytohormones on the growth and development of Plant Root Hair. Front Plant Sci. 2022; 13. [DOI] [PMC free article] [PubMed]
- 5.Pravej A, Al BT, Mohammad F. Salicylic acid’s impact on growth, photosynthesis, and antioxidant enzyme activity of Triticum aestivum when exposed to salt. Molecules. 2022;28(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Babili S, Bouwmeester HJ. Strigolactones, a Novel carotenoid-derived plant hormone. Annu Rev Plant Biol. 2015;66(1):161–86. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt MD, Bhatt D. Strigolactones in Overcoming Environmental Stresses. 2020.
- 8.Golam MM, Weiqiang L, Huu NK, Masayuki F, Phan LS. Strigolactones in plant adaptation to abiotic stresses: an emerging avenue of plant research. Plant Cell & Environment; 2018. [DOI] [PubMed]
- 9.Victoria G, Soraya F, Virginie BBP, Jean-Paul PADE, Fabien P, Radoslava L, Saida M, Jean-Charles D. Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–94. [DOI] [PubMed] [Google Scholar]
- 10.Grid GCRPSC, C, RPSC, Grid et al. C RPSC, Grid. GSOL, Grid. D XGSO, Grid. C RPSC, Grid. C RPSC, Grid. C RPSC, Grid. C RPSC, Grid. A WSCU,. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008; 455(7210):195–200. [DOI] [PubMed]
- 11.Kohki A, Ken-Ichi M, Hideo H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–7. [DOI] [PubMed] [Google Scholar]
- 12.Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H. The biology of strigolactones. Trends Plant Sci. 2013;18(2):72–83. [DOI] [PubMed] [Google Scholar]
- 13.Van HC, Antonio LM, Yuriko O, Thi TU, Rie N, Yasuko W, Maho T, Motoaki S, Shinjiro Y, Van DN, et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. P Natl Acad Sci Usa. 2014;111(2):851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junwei L, Hanzi H, Marco V, Ivan V, Tatsiana C, Imran H, Andrea S, Carolien R, Claudio JBH. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta. 2015;241(6):1435–51. [DOI] [PubMed] [Google Scholar]
- 15.Min Z, Li R, Chen L, Zhang Y, Li Z, Liu M, Ju Y, Fang Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol Bioch. 2019;135:99–110. [DOI] [PubMed] [Google Scholar]
- 16.Sedaghat M, Tahmasebi-Sarvestani Z, Emam Y, Mokhtassi-Bidgoli A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol Bioch. 2017;119:59–69. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor RT, Alam P, Chen Y, Ahmad P. Strigolactones in plants: from development to abiotic stress management. J Plant Growth Regul. 2024;43(3):903–19. [Google Scholar]
- 18.Yamada Y, Umehara M. Possible roles of Strigolactones during Leaf Senescence. Plants-Basel. 2015;4(3):664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toh S, Kamiya Y, Kawakami N, Nambara E, Mccourt P, Tsuchiya Y. Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiologyplant Cell Physiol. 2011;53(1):107–17. [DOI] [PubMed] [Google Scholar]
- 20.Soma F, Mogami J, Yoshida T, Abekura M, Takahashi F, Kidokoro S, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. ABA-unresponsive SnRK2 protein kinases regulate mRNA decay under osmotic stress in plants. Nat Plants. 2017;3(1):16204. [DOI] [PubMed] [Google Scholar]
- 21.Miller DM. Studies of Root function in Zea mays. Plant Physiol. 1985;77(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wha SS, Soo KS, Soon L, Yoon KS, Soon LH, Mok PY. Enhanced resistance of transgenic sweetpotato (Ipomoea batatas Lam.) Plants to Multiple Environmental Stresses Treated with combination of water stress, high light and high temperature stresses. J Ecol Environ. 2006;29(5):479–84. [Google Scholar]
- 23.Boyer JS, James RA, Munns R, Condon T, Passioura JB. Osmotic adjustment leads to anomalously low estimates of relative water content in wheat and barley. Funct Plant Biol. 2008;35(11):1172–82. [DOI] [PubMed] [Google Scholar]
- 24.Machly AC, Chance P. Assay of catalase and peroxidases. Method Enzymol. 1955;2:764–75. [Google Scholar]
- 25.Beauchamp C. Superoxide dismutase: improved assays and an assay applicable to acrylamide gel. Anal Biochem 1971; 44. [DOI] [PubMed]
- 26.Mostafa Hassan HA, Wang, Haiping, Song J, Li X. Effects of genotypes and explants on garlic callus production and endogenous hormones. Sci Rep-Uk. 2020;10(1):4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantola S, Vibhuti, Bargali K, Bargali SS. Screening of three leguminous crops for drought stress tolerance at germination and seedling growth stage. Indian J Agr Sci. 2017; 87(4).
- 28.Ismail SSM, Cai ADF, Marian XY, Milan B, Alhaj S, Zaid HY, Hiba U, Hamada S, Muhammad A. Physiological and biochemical responses of soybean plants inoculated with arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. Bmc Plant Biol. 2021;21(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elana D, Ram LJ, Céline S, Philippe D, Pierre M. Early-stage phenotyping of Root traits provides insights into the Drought Tolerance level of soybean cultivars. Agronomy. 2021;11(1):188. [Google Scholar]
- 30.Pantalone V, EVALUATION OF ROOT TRAITS IN SOYBEAN AND APPLICABILITY TO PLANT BREEDING. Rebetzke, Gj, Burton, Jw, Carter, Te. PHENOTYPIC. Crop Sci. 1996;36(2):456–9. [Google Scholar]
- 31.Kapoor RT, Alam P, Chen Y, Ahmad P. Strigolactones in plants: from development to abiotic stress management. J Plant Growth Regul. 2023.
- 32.Cedrick M, Alan W, Sylwia S, Elisabeth S, François-Didier B, Kris G, Sofie G. The Whats, the wheres and the Hows of strigolactone action in the roots. Planta. 2016;243(6):1327–37. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, Chattopadhyay D, Gupta SK. Updating the Impact of Drought on Root Exudation: a strigolactones Perspective. J Plant Growth Regul. 2023;42(8):5131–51. [Google Scholar]
- 34.Yoram K, Pierre-Marc D, Natalie R, Einav M, Smadar W, Chaitali B, Nathalie S, Jean-Philippe C, Guillaume B, Eduard B, et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233(1):209–16. [DOI] [PubMed] [Google Scholar]
- 35.Chang L, Peng-Sen S, Shi-Rong L. A review of plant spectral reflectance response to water physiological changes. Chin J Plan Ecolo. 2016;40(01):80–91. [Google Scholar]
- 36.Javadi T, Rohollahi D, Ghaderi N, Nazari F. Mitigating the adverse effects of drought stress on the morpho-physiological traits and anti-oxidative enzyme activities of Prunus avium through β-amino butyric acid drenching. Sci Hortic-Amsterdam. 2017;218:156–63. [Google Scholar]
- 37.Xi C, Kristyna F, Harro B, Carolien RS. The role of endogenous strigolactones and their Interaction with ABA during the infection process of the parasitic weed Phelipanche ramosa in Tomato plants. Front Plant Sci. 2017;8:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhenhai C, Yanfang W, Jinjuan F, Yanye R, Cuiling G, Lijun Z, Xinglin M. Research progress in plant osmoregulation. J Maize Sci. 2007.
- 39.Zhang Q, Yuan W, Wang Q, Cao Y, Xu F, Dodd IC, Xu W. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ. 2022;45(3):871–83. [DOI] [PubMed] [Google Scholar]
- 40.Teng Z, Lyu J, Chen Y, Zhang J, Ye N. Effects of stress-induced ABA on root architecture development: positive and negative actions. Crop J. 2023;11(4):1072–9. [Google Scholar]
- 41.Hamzah KOM, SI SMA, Naser Z, Shagufta P, Abida P, Fozia A, Habib A, Baber A, AY A, et al. Foliar application of iron-lysine to boost growth attributes, photosynthetic pigments and biochemical defense system in canola (Brassica napus L.) under cadmium stress. Bmc Plant Biol. 2023;23(1):648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenglou L, Qingwang S, Hao J, Jingjing C, Xiaoliang H, Zhihai W, Zhian Z, Juan L, Yongjun Z. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci Rep-Uk. 2020;10(1):6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gad M, Nobuhiro S, Sultan C, Ron M. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–67. [DOI] [PubMed] [Google Scholar]
- 44.Norma F. The reliance of phytohormone biosynthesis on primary metabolite precursors. J Plant Physiol. 2022;268:153589. [DOI] [PubMed] [Google Scholar]
- 45.Grobbelaar MC, Makunga NP, Stander MA, Kossmann J, Hills PN. Effect of strigolactones and auxins on growth and metabolite content of Sutherlandia frutescens (L.) R. Br. Microplants in vitro. Plant Cell Tissue Organ Cult (Pctoc). 2014;117(3):401–9. [Google Scholar]
- 46.C W. Effects of dietary arachidonic acid on the growth performance, antioxidant capacity, and fatty acid metabolism of sea cucumber (Apostichopus japonicus). J Fish Sci China. 2018;25(03):555–66. [Google Scholar]
- 47.Yan G, Jia LI. Changes of fatty acid composition of membrane lipid, ethylene release and lipoxygenase activity in leaves of apricot under drought stress. J Zhejiang Agricultural University(Agric Life Sci). 2002;28(5):513–7. [Google Scholar]
- 48.Ramesh K, Sedigheh S, Nitya M, K SS, Prashanth S, Nawaz KM, Mahya B, Katsumi S, Raja RK. Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean. PLoS ONE. 2020;15(6):e233905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito S, Nozoye T, Sasaki E, Imai M, Shiwa Y, Shibata-Hatta M, Ishige T, Fukui K, Ito K, Nakanishi H, et al. Strigolactone regulates anthocyanin accumulation, acid phosphatases production and plant growth under low phosphate condition in Arabidopsis. PLoS ONE. 2017;10(3):e119724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study, including metabolomics data, are available in the supplementary material of this article.