Abstract
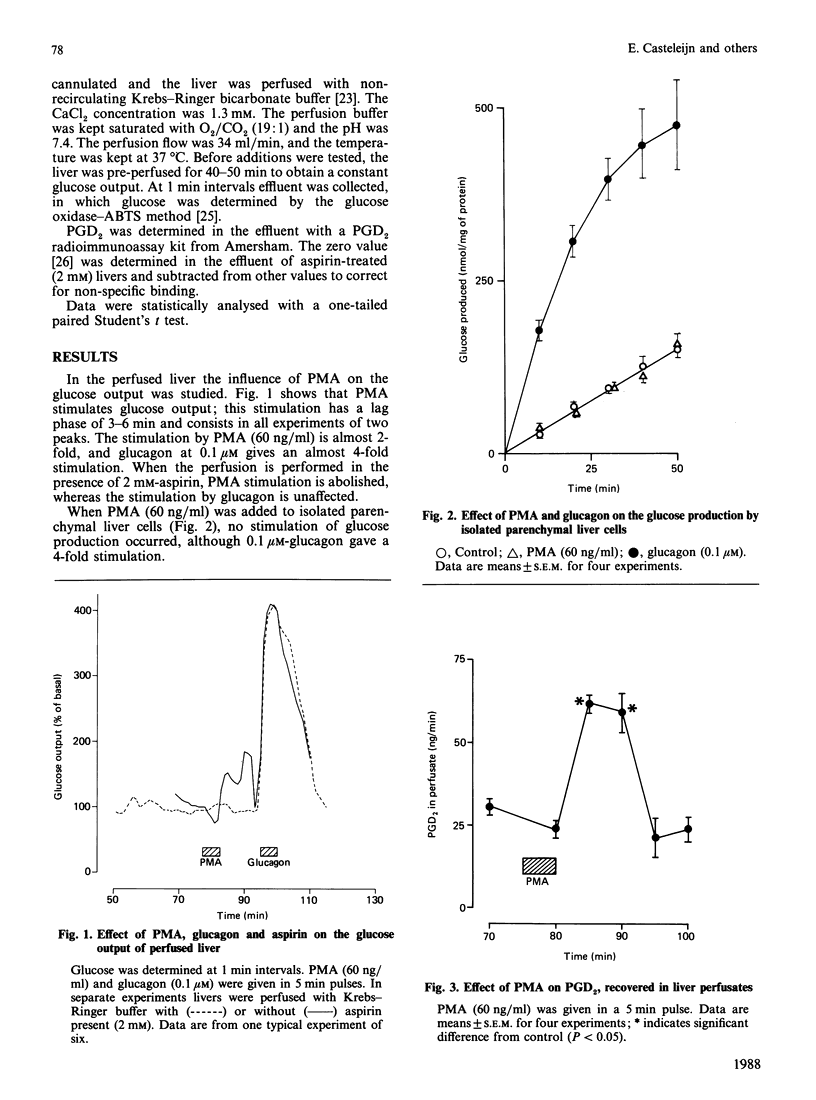
The tumour-promoting phorbol ester, phorbol 12-myristate 13-acetate (PMA), when added to the perfused liver, stimulates glycogenolysis 2-fold. This stimulation is not seen when aspirin is present in the perfusion medium. In isolated parenchymal liver cells. PMA is not able to stimulate glycogenolysis, suggesting that its effect on glycogenolysis might be indirect and depends on the presence of the non-parenchymal liver cell types. To test the possible operation of an indirect mechanism, we measured the amount of prostaglandin (PG) D2 in liver perfusates. After addition of PMA, the amount of PGD2 is doubled, in parallel with the increase in glycogenolysis. Glycogenolysis in both isolated parenchymal liver cells and perfused liver could be stimulated by the addition of PGD2. Our data indicate that stimulation of glycogenolysis in the liver by PMA may be mediated by non-parenchymal liver cells, which produce PGD2 in response to PMA. Subsequently PGD2 activates glycogenolysis in the parenchymal liver cells. The intercellular communication inside the liver in response to PMA adds a new mechanism to the complex regulation of glucose homoeostasis by the liver.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmelin M., Decker K. Synthesis of prostanoids and cyclic nucleotides by phagocytosing rat Kupffer cells. Eur J Biochem. 1984 Jul 16;142(2):219–225. doi: 10.1111/j.1432-1033.1984.tb08274.x. [DOI] [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonta I. L., Bult H., Vincent J. E., Zijlstra F. J. Acute anti-inflammatory effects of aspirin and dexamethasone in rats deprived of endogenous prostaglandin precursors. J Pharm Pharmacol. 1977 Jan;29(1):1–7. doi: 10.1111/j.2042-7158.1977.tb11227.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Briseno D. L., Hanahan D. J., Olson M. S. Glycogenolytic and haemodynamic responses to heat-aggregated immunoglobulin G and prostaglandin E2 in the perfused rat liver. Biochem J. 1987 Apr 15;243(2):493–498. doi: 10.1042/bj2430493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Hanahan D. J., Olson M. S. Platelet-activating factor-mediated vasoconstriction and glycogenolysis in the perfused rat liver. J Biol Chem. 1986 Jan 15;261(2):644–649. [PubMed] [Google Scholar]
- Buxton D. B., Shukla S. D., Hanahan D. J., Olson M. S. Stimulation of hepatic glycogenolysis by acetylglyceryl ether phosphorylcholine. J Biol Chem. 1984 Feb 10;259(3):1468–1471. [PubMed] [Google Scholar]
- Casteleijn E., van Rooij H. C., van Berkel T. J., Koster J. F. Mechanism of glucagon stimulation of fructose-1,6-bisphosphatase in rat hepatocytes. Involvement of a low-Mr activator. FEBS Lett. 1986 Jun 9;201(2):193–197. doi: 10.1016/0014-5793(86)80607-x. [DOI] [PubMed] [Google Scholar]
- Corvera S., García-Sáinz J. A. Phorbol esters inhibit alpha 1 adrenergic stimulation of glycogenolysis in isolated rat hepatocytes. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1128–1133. doi: 10.1016/0006-291x(84)90892-1. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic effects of catecholamines on liver metabolism. J Cyclic Nucleotide Res. 1979;5(4):277–287. [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena: role of calcium ions in actions of catecholamines in liver and other tissues. Am J Physiol. 1980 Jan;238(1):E3–12. doi: 10.1152/ajpendo.1980.238.1.E3. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Li S. Y., Litosch I., Wallace M. Synergistic activation of rat hepatocyte glycogen phosphorylase by A23187 and phorbol ester. Biochem Biophys Res Commun. 1984 Feb 29;119(1):88–94. doi: 10.1016/0006-291x(84)91622-x. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Shukla S. D., Debuysere M. S., Hanahan D. J., Olson M. S. The effect of acetylglyceryl ether phosphorylcholine on glycogenolysis and phosphatidylinositol 4,5-bisphosphate metabolism in rat hepatocytes. J Biol Chem. 1984 Jul 25;259(14):8685–8688. [PubMed] [Google Scholar]
- García-Sáinz J. A., Hernández-Sotomayor S. M. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoyl-phorbol-13-acetate (TPA) via cyclooxygenase products. Biochem Biophys Res Commun. 1985 Oct 15;132(1):204–209. doi: 10.1016/0006-291x(85)91008-3. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J Biol Chem. 1984 Mar 10;259(5):3283–3292. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kimura S., Nagasaki K., Adachi I., Yamaguchi K., Fujiki H., Abe K. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoylphorbol-13-acetate (TPA) via a calcium requiring process. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1057–1064. doi: 10.1016/0006-291x(84)91198-7. [DOI] [PubMed] [Google Scholar]
- Mendlovic F., Corvera S., García-Sáinz J. A. Possible involvement of cyclooxygenase products in the actions of platelet-activating factor and of lipoxygenase products in the vascular effects of epinephrine in perfused rat liver. Biochem Biophys Res Commun. 1984 Sep 17;123(2):507–514. doi: 10.1016/0006-291x(84)90259-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Patel T. B. Stimulation of hepatic glycogenolysis by phorbol 12-myristate 13-acetate. Biochem J. 1987 Jan 15;241(2):549–554. doi: 10.1042/bj2410549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuche H. J., Kaever V., Resch K. A possible role of protein kinase C in regulating prostaglandin synthesis of mouse peritoneal macrophages. Biochem Biophys Res Commun. 1986 Sep 14;139(2):604–611. doi: 10.1016/s0006-291x(86)80033-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Buxton D. B., Olson M. S., Hanahan D. J. Acetylglyceryl ether phosphorylcholine. A potent activator of hepatic phosphoinositide metabolism and glycogenolysis. J Biol Chem. 1983 Sep 10;258(17):10212–10214. [PubMed] [Google Scholar]
- van de Werve G., Proietto J., Jeanrenaud B. Control of glycogen phosphorylase interconversion by phorbol esters, diacylglycerols, Ca2+ and hormones in isolated rat hepatocytes. Biochem J. 1985 Nov 1;231(3):511–516. doi: 10.1042/bj2310511. [DOI] [PMC free article] [PubMed] [Google Scholar]


