Abstract
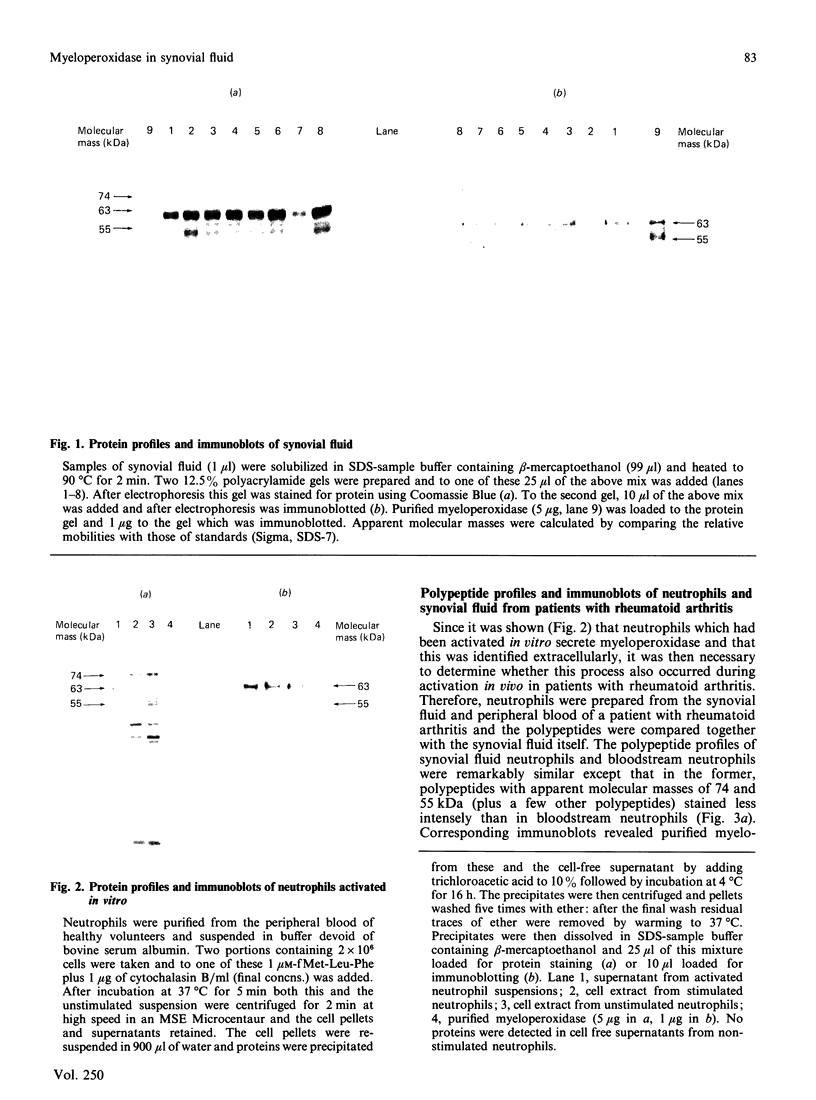
We have used rocket immunoelectrophoresis and immunoblotting to detect myeloperoxidase in synovial fluid from patients with rheumatoid arthritis. This protein was enzymatically inactive but its identity as myeloperoxidase was confirmed by comparing its subunit structure with that of the purified enzyme. When neutrophils were stimulated to secrete myeloperoxidase in vitro, a polypeptide with an apparent molecular mass of 62 kDa was detected extracellularly by immunoblotting. Neutrophils isolated from synovial fluid showed a reduced level of this 62 kDa polypeptide but it was detected extracellularly in synovial fluid by immunoblotting. Thus, we conclude that neutrophils in synovial fluid from patients with rheumatoid arthritis have been activated in vivo to secrete myeloperoxidase and propose that the products of this enzyme system can contribute to the tissue damage associated with this disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. T., Kinkade J. M., Jr Processing of a newly identified intermediate of human myeloperoxidase in isolated granules occurs at neutral pH. J Biol Chem. 1986 Jun 25;261(18):8370–8375. [PubMed] [Google Scholar]
- Andrews F. J., Morris C. J., Kondratowicz G., Blake D. R. Effect of iron chelation on inflammatory joint disease. Ann Rheum Dis. 1987 Apr;46(4):327–333. doi: 10.1136/ard.46.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Bacon P. A., Dieppe P. A., Halliwell B., Gutteridge J. M. Effect of a specific iron chelating agent on animal models of inflammation. Ann Rheum Dis. 1983 Feb;42(1):89–93. doi: 10.1136/ard.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Winyard P., Lunec J., Williams A., Good P. A., Crewes S. J., Gutteridge J. M., Rowley D., Halliwell B., Cornish A. Cerebral and ocular toxicity induced by desferrioxamine. Q J Med. 1985 Jul;56(219):345–355. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Trujillo J. M., Cook R. G., Stass S. A. Human myeloperoxidase gene: molecular cloning and expression in leukemic cells. Blood. 1986 Dec;68(6):1411–1414. [PubMed] [Google Scholar]
- Cuperus R. A., Muijsers A. O., Wever R. Antiarthritic drugs containing thiol groups scavenge hypochlorite and inhibit its formation by myeloperoxidase from human leukocytes. A therapeutic mechanism of these drugs in rheumatoid arthritis? Arthritis Rheum. 1985 Nov;28(11):1228–1233. doi: 10.1002/art.1780281106. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Formation of myeloperoxidase compound II during aerobic stimulation of rat neutrophils. Biosci Rep. 1986 Mar;6(3):275–282. doi: 10.1007/BF01115156. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Nurcombe H. L., Hart C. A. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987 Aug 1;245(3):925–928. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Swan T. F. Regulation of superoxide generation by myeloperoxidase during the respiratory burst of human neutrophils. Biochem J. 1986 Jul 15;237(2):601–604. doi: 10.1042/bj2370601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Bleomycin-detectable iron in knee-joint synovial fluid from arthritic patients and its relationship to the extracellular antioxidant activities of caeruloplasmin, transferrin and lactoferrin. Biochem J. 1987 Jul 15;245(2):415–421. doi: 10.1042/bj2450415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982 Sep 15;206(3):605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Blake D. Metal ions and oxygen radical reactions in human inflammatory joint disease. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):659–671. doi: 10.1098/rstb.1985.0171. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Nauseef W. M., Care A., Wheelock M. J., Shane S., Hudson S., Koeffler H. P., Selsted M., Miller C., Rovera G. Characterization of cDNA clones for human myeloperoxidase: predicted amino acid sequence and evidence for multiple mRNA species. Nucleic Acids Res. 1987 Mar 11;15(5):2013–2028. doi: 10.1093/nar/15.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985 Feb;65(2):484–491. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matheson N. R. The effect of antiarthritic drugs and related compounds on the human neutrophil myeloperoxidase system. Biochem Biophys Res Commun. 1982 Sep 16;108(1):259–265. doi: 10.1016/0006-291x(82)91860-5. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Miyasaki K. T., Wilson M. E., Cohen E., Jones P. C., Genco R. J. Evidence for and partial characterization of three major and three minor chromatographic forms of human neutrophil myeloperoxidase. Arch Biochem Biophys. 1986 May 1;246(2):751–764. doi: 10.1016/0003-9861(86)90332-2. [DOI] [PubMed] [Google Scholar]
- Morishita K., Kubota N., Asano S., Kaziro Y., Nagata S. Molecular cloning and characterization of cDNA for human myeloperoxidase. J Biol Chem. 1987 Mar 15;262(8):3844–3851. [PubMed] [Google Scholar]
- Nauseef W. M., Malech H. L. Analysis of the peptide subunits of human neutrophil myeloperoxidase. Blood. 1986 May;67(5):1504–1507. [PubMed] [Google Scholar]
- Nauseef W. M. Myeloperoxidase biosynthesis by a human promyelocytic leukemia cell line: insight into myeloperoxidase deficiency. Blood. 1986 Apr;67(4):865–872. [PubMed] [Google Scholar]
- Olsen R. L., Little C. Studies on the subunits of human myeloperoxidase. Biochem J. 1984 Sep 15;222(3):701–709. doi: 10.1042/bj2220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Persson A. M., Strömberg K. Biosynthesis, transport and processing of myeloperoxidase in the human leukaemic promyelocytic cell line HL-60 and normal marrow cells. Biochem J. 1984 Nov 1;223(3):911–920. doi: 10.1042/bj2230911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Polson R. J., Jawad A. S., Bomford A., Berry H., Williams R. Treatment of rheumatoid arthritis with desferrioxamine. Q J Med. 1986 Dec;61(236):1153–1158. [PubMed] [Google Scholar]
- Polson R. J., Jawed A., Bomford A., Berry H., Williams R. Treatment of rheumatoid arthritis with desferrioxamine: relation between stores of iron before treatment and side effects. Br Med J (Clin Res Ed) 1985 Aug 17;291(6493):448–448. doi: 10.1136/bmj.291.6493.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Rowley D., Gutteridge J. M., Blake D., Farr M., Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1984 Jun;66(6):691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- Weil S. C., Rosner G. L., Reid M. S., Chisholm R. L., Farber N. M., Spitznagel J. K., Swanson M. S. cDNA cloning of human myeloperoxidase: decrease in myeloperoxidase mRNA upon induction of HL-60 cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2057–2061. doi: 10.1073/pnas.84.7.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]