Abstract
1. Chemiluminescence and benzoic acid hydroxylation were used to detect oxygen-centred free-radical production by 2.5 mM-H2O2 and 100 microM-Cu2+. Free radicals could not be detected by these methods when H2O2 was replaced with 10 mM-t-butyl hydroperoxide (TBH) or 10 mM-cumene hydroperoxide (CH). The inclusion of the thiol compound dithioerythritol (DTET; 100 microM) increased radical production by H2O2 and Cu2+ as judged by both assays. Mannitol scavenged radicals in the chemiluminescence system in a dose-dependent manner. 2. H2O2, TBH and CH, each with Cu2+, gave rise to substantial fragmentation of the protein bovine serum albumin (BSA). This fragmentation could be increased by the inclusion of DTET. Omission of Cu2+ or the addition of the chelator DETAPAC (diethylenetriaminepenta-acetic acid; 1 mM) lead to virtual abolition of fragmentation. Autoxidized lipid in the presence of Cu2+ caused protein fragmentation by reactions of lipid hydroperoxides. 3. Polyacrylamide-gel electrophoresis in the presence of SDS confirmed that production of fragments had occurred. 4. Susceptibility of BSA to enzymic hydrolysis by two different proteinases acting at pH 5 and pH 7.2 was increased after a limited exposure to hydroperoxides in the presence of Cu2+. 5. These results may have biological significance, particularly for proteins in lipid environments (e.g. membrane proteins and lipoproteins).
Full text
PDF

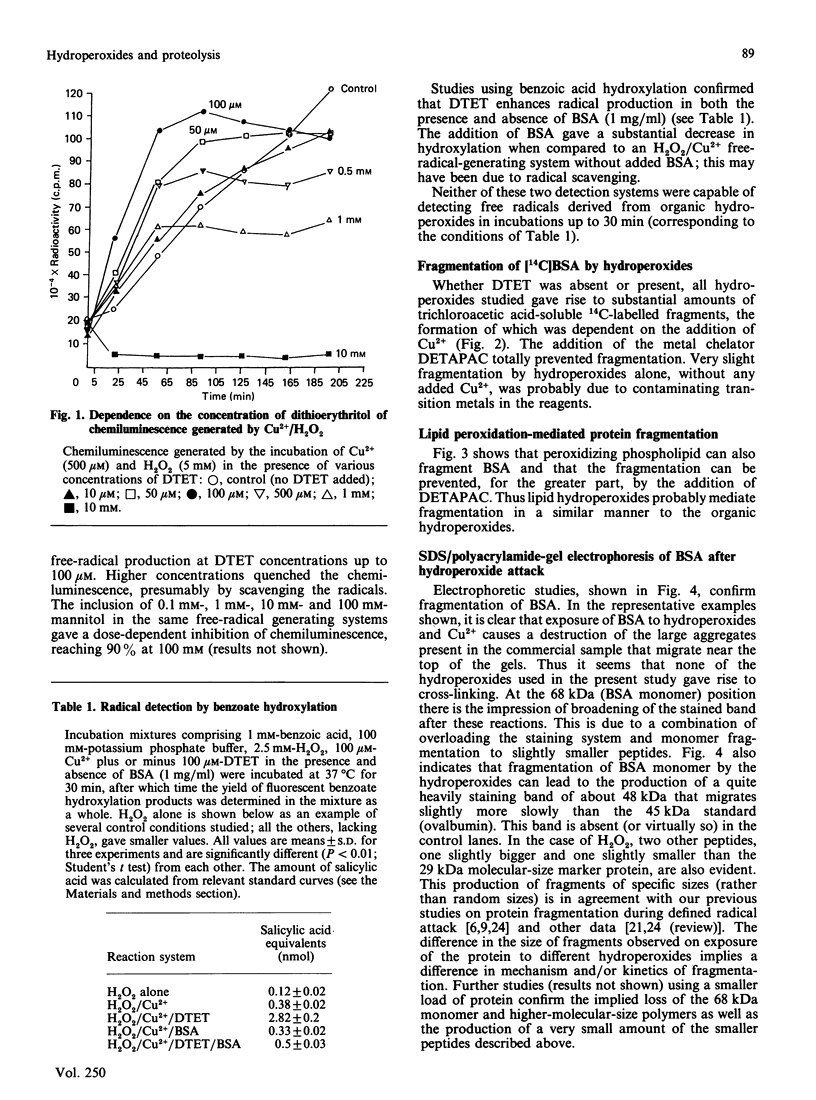
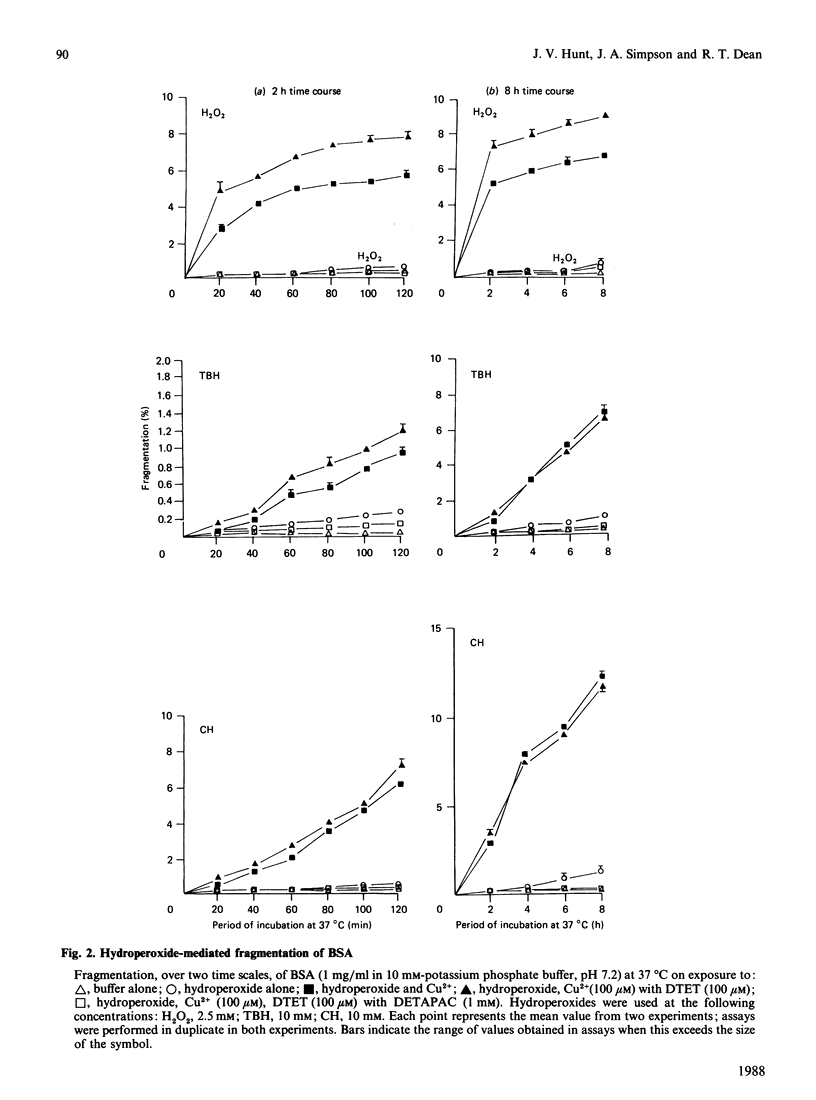

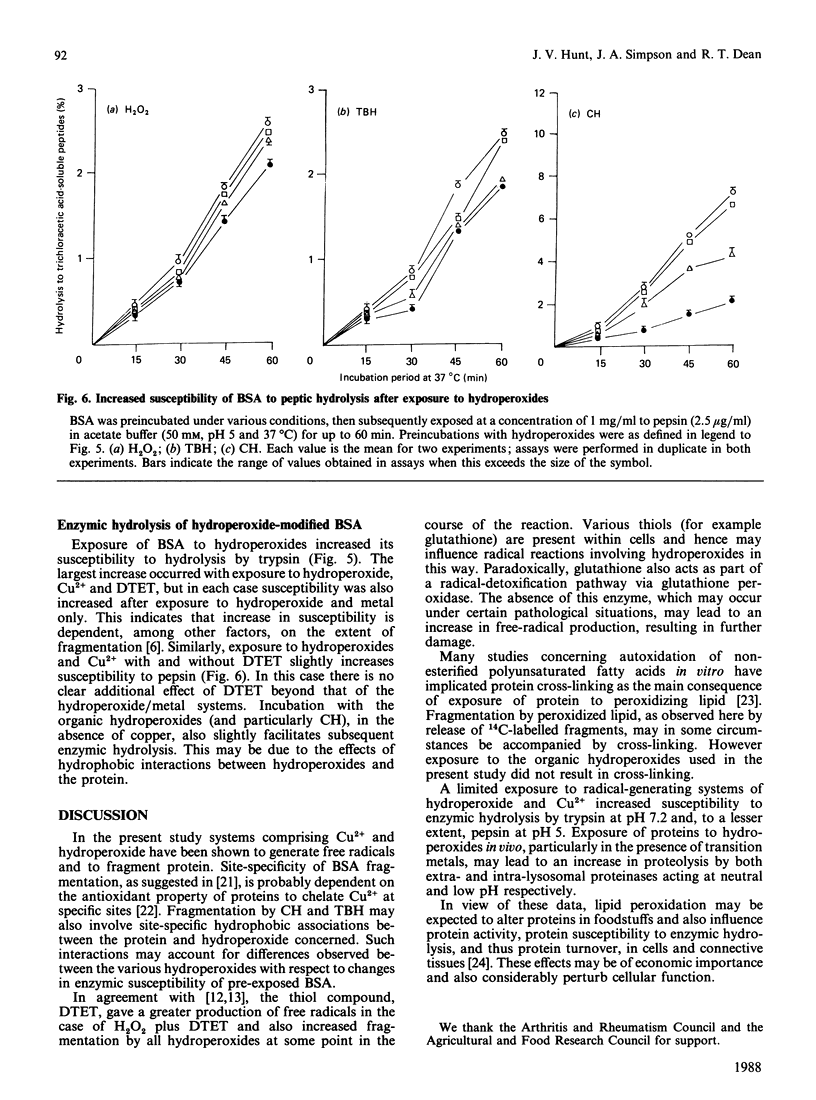

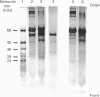
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. S., Gebicki J. M. The effect of pH on the conversion of superoxide to hydroxyl free radicals. Arch Biochem Biophys. 1984 Oct;234(1):258–264. doi: 10.1016/0003-9861(84)90348-5. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Hallett M. B., Weeks I. Chemiluminescence as an analytical tool in cell biology and medicine. Methods Biochem Anal. 1985;31:317–416. doi: 10.1002/9780470110522.ch7. [DOI] [PubMed] [Google Scholar]
- Chung M. H., Kesner L., Chan P. C. Degradation of articular cartilage by copper and hydrogen peroxide. Agents Actions. 1984 Oct;15(3-4):328–335. doi: 10.1007/BF01972367. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Hunt N. H. Free radical-induced pathology. Med Res Rev. 1985 Jul-Sep;5(3):297–332. doi: 10.1002/med.2610050303. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Pollak J. K. Endogenous free radical generation may influence proteolysis in mitochondria. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1082–1089. doi: 10.1016/0006-291x(85)90296-7. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Roberts C. R., Forni L. G. Oxygen-centred free radicals can efficiently degrade the polypeptide of proteoglycans in whole cartilage. Biosci Rep. 1984 Dec;4(12):1017–1026. doi: 10.1007/BF01116694. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Thomas S. M., Garner A. Free-radical-mediated fragmentation of monoamine oxidase in the mitochondrial membrane. Roles for lipid radicals. Biochem J. 1986 Dec 1;240(2):489–494. doi: 10.1042/bj2400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A., Jamal Z., Slater T. F. Effects of 2-mercaptopropionyl glycine on radiation-induced lipid peroxidation in liposomes and in rat liver microsomal suspensions. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Aug;50(2):323–335. doi: 10.1080/09553008614550701. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Lysosomal localization of -fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972 Aug;129(1):123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marx G., Chevion M. Site-specific modification of albumin by free radicals. Reaction with copper(II) and ascorbate. Biochem J. 1986 Jun 1;236(2):397–400. doi: 10.1042/bj2360397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Loomis W. F. Plasma membrane proteins of Dictyostelium: the spore coat proteins. Dev Biol. 1979 Aug;71(2):297–307. doi: 10.1016/0012-1606(79)90171-4. [DOI] [PubMed] [Google Scholar]
- PHELPS R. A., NEET K. E., LYNN L. T., PUTNAM F. W. The cupric ion catalysis of the cleavage of gamma-globulin and other proteins by hydrogen peroxide. J Biol Chem. 1961 Jan;236:96–105. [PubMed] [Google Scholar]
- Roubal W. T., Tappel A. L. Polymerization of proteins induced by free-radical lipid peroxidation. Arch Biochem Biophys. 1966 Jan;113(1):150–155. doi: 10.1016/0003-9861(66)90168-8. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of thiol compounds. FEBS Lett. 1982 Feb 8;138(1):33–36. doi: 10.1016/0014-5793(82)80388-8. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986 Mar 1;234(2):399–403. doi: 10.1042/bj2340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steveninck J., van der Zee J., Dubbelman T. M. Site-specific and bulk-phase generation of hydroxyl radicals in the presence of cupric ions and thiol compounds. Biochem J. 1985 Nov 15;232(1):309–311. doi: 10.1042/bj2320309. [DOI] [PMC free article] [PubMed] [Google Scholar]