Abstract
Background
Traditional medical research infrastructures relying on the Centers of Excellence (CoE) model (an infrastructure or shared facility providing high standards of research excellence and resources to advance scientific knowledge) are often limited by geographic reach regarding patient accessibility, presenting challenges for study recruitment and accrual. Thus, the development of novel, patient-centered (PC) strategies (e.g., the use of online technologies) to support recruitment and streamline study procedures are necessary. This research focused on an implementation evaluation of a design innovation with implementation outcomes as communicated by study staff and patients for CoE and PC approaches for a randomized controlled trial (RCT) for patients with vasculitis.
Methods
In-depth qualitative interviews were conducted with 32 individuals (17 study team members, 15 patients). Transcripts were coded using the Consolidated Framework for Implementation Research (CFIR).
Results
The following CFIR elements emerged: characteristics of the intervention, inner setting, characteristics of individuals, and process. From the staff perspective, the communication of the PC approach was a major challenge, but should have been used as an opportunity to identify one “point person” in charge of all communicative elements among the study team. Study staff from both arms were highly supportive of the PC approach and saw its promise, particularly regarding online consent procedures. Patients reported high self-efficacy in reference to the PC approach and utilization of online technologies. Local physicians were integral for making patients feel comfortable about participation in research studies.
Conclusions
The complexity of replicating the interpersonal nature of the CoE model in the virtual setting is substantial, meaning the PC approach should be viewed as a hybrid strategy that integrates online and face-to-face practices.
Trial registrations
1) Name: The Assessment of Prednisone In Remission Trial – Centers of Excellence Approach (TAPIR).
Trial registration number: ClinicalTrials.gov NCT01940094.
Date of registration: September 10, 2013.
2) Name: The Assessment of Prednisone In Remission Trial – Patient Centric Approach (TAPIR).
Trial registration number: Clinical Trials.gov NCT01933724.
Date of registration: September 2, 2013.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12874-024-02352-w.
Keywords: Clinical trial, Research subject recruitment, Social media, Direct-to-consumer, Advertising, Granulomatosis with polyangiitis
Contributions to the literature
Research has documented the variety of challenges that clinical trials have faced regarding recruitment and engagement. Rare diseases face additional obstacles when accounting for smaller populations.
One novel solution is the use of social media recruitment, coupled with a web-based platform where patients with vasculitis could participate in a clinical trial virtually (consent/enroll, report their symptoms/self-report measures, and taper their prednisone dosage) – deemed the patient-centered (PC) approach.
Our comparison of the implementation of the PC approach to the traditional Center of Excellence (CoE) approach has important implications, including different types of studies that may be best suited for virtual design.
Background
Establishing the evidence-base for treating rare diseases is a challenging but critical area of focus. A rare disease is defined as a condition with a prevalence of less than one in 2,000 (Europe) or less than 200,000 (United States) [1]. The ability to conduct trials and advance treatments for populations with rare diseases is limited by access to patients [2]. Advances in rare disease research have included consortium building, which leverages economies of scale related to linking loci of clinical expertise and patient access to support a broader research infrastructure targeting the needs of patients with rare diseases [3].
Parallel to the focal aggregation of clinical resources at academic health centers in responding to the needs of patients with rare diseases is the traditional research infrastructure of Centers of Excellence (CoE) for a given condition. CoEs, each with a concentration of patients with certain rare diseases, can work together to increase the sample size of natural history studies and clinical trials. While the CoE approach to research leverages hubs of clinical treatment and research infrastructure, CoEs are often geographically and economically isolated from the majority of affected patients, limiting access to cutting-edge care and participation in research studies [4]. Developing novel strategies to increase participation in clinical trials presents a challenging opportunity for the field of implementation science, as consistent evaluations of the performance of novel methods must be assessed in order to determine best practices for future integration in clinical trial settings [5–7].
To improve recruitment of patients, a novel approach was designed to address patient recruitment and ongoing engagement capitalizing on social medial and web-based platforms to overcome common barriers to participation in traditional clinical trials (e.g. travel distance and small patient recruitment pools). A key innovation of the approach included the ability to recruit patients and collect clinical outcomes without the need for office visits, using direct-to-consumer advertising and marketing principles similar to those utilized by the pharmaceutical industry [8, 9]. Thus, the PC approach featured a process that was primarily conducted in an online setting, with a direct-to-patient website created where participants could enroll in the study, access online informed consent, and view a personalized portal that housed all study materials.
To capture the process and product of the study efforts, the project utilized a hybrid effectiveness-implementation framework with quantitative assessments of recruitment and retention mixed with qualitative assessments of key stakeholder perspectives on the development and implementation of the designed approach. Quantitative results published in a separate study reflect the greater success of enrollment of those confirmed eligible by their physician for CoEs ((96%) when compared to the PC approach (77%), with no significant difference found regarding subject eligibility and provider acceptance for each approach [10]. While the quantitative portion of such assessment is critical, to develop a greater understanding of why the PC approach was not as successful, qualitative feedback on the drivers of implementation process and outcomes is necessary.
As patients become more active participants in their healthcare, it is important that research investigates not only the study team members’ perspectives on implementation, but also the patients’ perspectives to ensure the needs and preferences of all stakeholders are met [11]. Previous research analyzing engagement of patients with vasculitis in research confirmed that involvement in research design and development positively impacts patients collaborating on the study team and study investigators [12]. However, there remains a lack of deep understanding of the factors involved in the successful and challenging aspects of novel recruitment methods. The specific aims of this study were to describe qualitative findings from interviews with study team members and patients involved in the clinical trial to assess the drivers of success and challenges to the implementation of the PC model of patient recruitment and engagement.
Methods
Study design
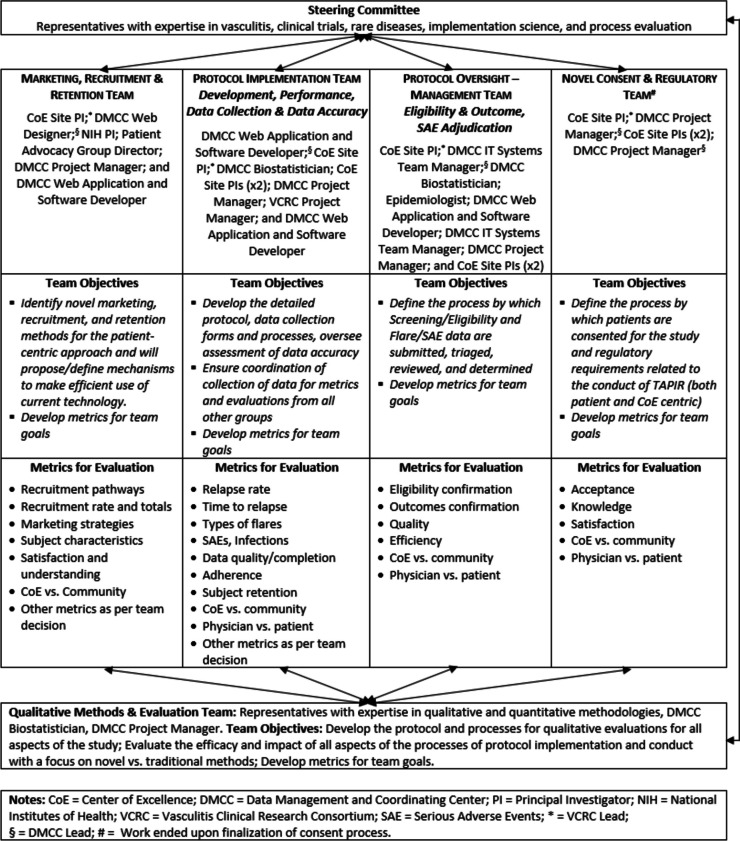
To evaluate the drivers of implimentation for this novel approach to support recruitment of participants into a randomized controlled trial (RCT) the study team designed an organizational structure as the key implementation component to develop and implement a direct-to-patient recruitment (i.e., the patient-centric, PC) arm of the trial to be compared to the traditional CoE approach. The clinical trial was designed to test the effectiveness of low-dose prednisone (i.e., 5 mg daily) as a maintenance regimen compared to no prednisone for patients with granulomatosis with polyangiitis. A notable component of the study involved the strategic inclusion of team members with expertise in vasculitis, clinical trials, and process evaluation/implementation to provide an implementation assessment that included contrasting more traditional approaches. This expertise was further organized to create distinct teams (marketing, recruitment, and retention; protocol implementation; protocol oversight/management; novel consent/regulatory), where individual skillsets were utilized across the study (see Fig. 1, Organizational Structure. Directive framework for the development and implementation of the study). Team structures also incorporated patient advocacy group representatives, clinicians, and technology support staff, who were central to the outreach approach utilized. Incorporation of collaborative teams was a central reflexivity strategy, as individuals in varying roles allowed for assumptions to be challenged and diversity of perspectives to be considered at multiple points, particularly during creation of the interview guide [12, 13]. Interviews were used to explore stakeholder perspectives regarding the process and product of the PC arm strategies and implementation in comparison to the more traditional CoE arm of the trial. The consolidated criteria for reporting qualitative research (COREQ) checklist was used throughout all phases of this research to ensure that data were explicitly and comprehensively reported [13].
Fig. 1.
Organizational Structure and directive framework for the development and implementation of the study. Notes: CoE = Center of Excellence; DMCC = Data Management and Coordinating Center; PI = Principal Investigator; NIH = National Institutes of Health; VCRC = Vasculitis Clinical Research Consortium; SAE = Serious Adverse Events; * = VCRC Lead; § = DMCC Lead; # = Work ended upon finalization of consent process
As of June 30, 2018 (the date when the PC arm was closed), a total of 61 patients in the CoE arm and all 10 patients in the PC arm completed the study by either having met a study endpoint or having completed six-months on study.
Sample
The qualitative sample for the current research included semi-structured interviews with 32 total participants. Interviews were conducted purposively across stakeholder groups with 17 study team members (10 in the CoE arm, including site physician leads and research coordinators and seven in the PC arm, representing the various committees described in Fig. 1), and interviews were conducted with 15 patients (two who dropped out of the study, 11 in the CoE arm and two in the PC arm). Two patients participated in study entry and follow-up interviews, which were included in the dataset. For demographic information on patients interviewed, see Table 1.
Table 1.
Demographics of patients interviewed
| Characteristic | N = 15 | |
|---|---|---|
| Age at Registration – Mean (STDO | 54.5 (13.8) | |
| Age at Registration – N (%) | 30–40 | 4 (26.7%) |
| 40–50 | 1 (6.7%) | |
| 50–60 | 5 (33.3%) | |
| 60–70 | 3 (20.0%) | |
| 70–78 | 2 (13.3%) | |
| Gender | Female | 7 (46.7%) |
| Male | 8 (53.3%) | |
| Race | White | 15 (100.0%) |
| Ethnicity | Not Hispanic | 11 (73.3%) |
| Unknown/Not Reported | 4 (26.7%) | |
| Study Arm | Patient Centric | 2 (13.3%) |
| Centers of Excellence | 13 (86.7%) |
Data collection
The multi-disciplinary evaluation team, including experts in program evaluation, qualitative methods, and clinical trials research, developed semi-structured interview guides for faculty and staff involved in the study infrastructure. In-depth interviews were used, as they permit the collection of rich, complex data suitable for making sense of complex processes [14, 15].
Interview guides were developed based on our team’s methodological experience and experience with the recruitment and retention of participants with rare diseases in randomized trials. Interview guides were piloted and feedback sought from collaborating faculty and staff. While tailored to elicit the study-related experiences of each stakeholder group (i.e. patients, study faculty, and staff), patient interview guides were designed to additionally explore how patients heard about the study, their drivers for participation and perceptions of risk, the influence of others on their decision to participate, reflections of their randomization arm, and their experience with the consenting process. Study team interview questions asked respondents to reflect on their role in study development and implementation, patient recruitment efforts, and the consenting process. See Additional File 1 for final interview guide. This research focused identifying implementation outcomes through evaluation of a novel approach to RCTs. Importantly, data were also collected and analyzed from the CoE arm where participants were asked to extrapolate reactions to elements of the PC arm to capture patient perspectives on PC arm elements regardless of arm assignment. This tactic supported assessing how well the design evaluation was carried out in practice as communicated by patients and team members.
Interviews were conducted by phone with all participants in 2018. Interviews were audio recorded, labeled with a confidential unique identifier, de-identified and professionally transcribed, and entered into NVivo 10.0 (QSR NVivo), a software package used to support qualitative data coding and analysis.
Framework for implementation analysis
For the initial review of early interview transcripts, the study team used a phronetic iterative approach to develop a set of codes to apply to all interview data. This process moves back and forth between existing theory, predefined questions, and emergent qualitative findings being produced by the emergent data [16, 17]. This permits for an analysis of patterns, themes, and constructs present within a given phenomenon.
To provide a structure for organizing findings, the study team augmented its iterative approach with the Consolidated Framework for Implementation Research (CFIR). The CFIR provides a useful framework for exploring the primary domains driving the development and implementation of innovative approaches to improving health and healthcare systems [18]. The CFIR elements include characteristics of the intervention, the inner setting, characteristics of individuals, and process. The described CFIR domains have been widely employed to aid researchers and practitioners in understanding the complex elements that impact the execution and long-term viability of implementation endeavors. As such, the CFIR was used to help guide the evaluation of the novel approach.
Analysis
Two coders (who served as interviewers) used a constant comparative approach when analyzing all data. In this approach, two coding schemes were applied the to the transcribed qualitative data: 1) open and axial codes that further emerged from close, line-by-line readings of the data; and 2) the a priori set of codes representing the most relevant CFIR constructs to emerge from the grounded data collection. Each code was defined and decision rules for the appropriate application of each code were developed and included in the codebook. A total of 20% of the data were coded by the two coders using the inter-rater agreement assessments available in NVivo to identify and correct areas of disagreement through group consensus to ensure coding reliability and accuracy. To enhance rigor, a third coder with expertise in health communication and recruitment methods (who did not serve as an interviewer) independently analyzed 100% of the data to confirm findings as represented with the CFIR constructs. This approach served to enhance investigator triangulation.
Results
The findings below have been categorized according to applicable constructs of the CFIR. An overview of major findings can be seen in Table 2.
Table 2.
Major Findings. Results have been organized according to the stakeholder interviewed (patient or staff) and the Consolidated Framework for Implementation Research (CFIR) [18]
| CFIR Construct | Construct Description | Applicable Principles | Staff Perspective | Patient Perspective | Best Practices & Suggestions for Successful Implementation |
|---|---|---|---|---|---|
| Characteristics of the Intervention | Multi-faceted components of the intervention (may include core or more adaptable, peripheral components) |
• Complexity • Adaptability • Trialability • Relative advantage |
• Concern that recruitment materials for the PC arm were not created with enough focus on the target audience • Online format has advantages for streamlining the consent process |
Patients report being interested in the online format for learning more about available research study opportunities, but also appreciate the advantages that face-to-face/interpersonal communication offers in the traditional CoE setting |
• For PC designs, choose study designs with clearly defined self-report measures and procedures that are easily adaptable to the online environment • Use appropriate health literacy levels in study recruitment, consent, and enrollment/procedure materials |
| Inner Setting | Structural and cultural contexts through which the implementation process must occur (e.g., communications, culture, readiness for change) |
• Available resources • Culture and climate • Readiness for implementation |
• Insufficient resource regarding marketing expertise for design of study materials • Greater communication needed at multiple touchpoints to implement editing of study materials/study process |
Not applicable |
• Hire an advertising/marketing professional • Identify one “point person” on staff to communicate all recommended study changes (with firm deadlines) to study team members • Implement a shared mechanism for editing study materials in real time to be sure patient questions/concern are being adequately addressed |
| Characteristics of Individuals | How individual choices, mindsets, and personalities impact the implementation process |
• Knowledge and beliefs about the intervention self-efficacy • Personal attributes |
• Concerns that patients will be “left behind” if they are not comfortable using online technologies | • Patients report feeling confident in their ability to learn about new studies in the online environment |
• Reiterate to staff members that the implementation of new technology in the research process is a balance – it is not an “all or nothing” approach (a mixture of CoE and PC principles will require greater flexibility on their part) • Develop hard copies of educational materials for distribution in various settings to ensure that those not online will not be “forgotten” |
| Process | Series of subprocesses (linear or non-linear) that occur at multiple levels to form the overall implementation |
• Reflecting and evaluating • Key stakeholders • Engaging • Opinion leaders |
• Face-to-face communication is still important • The online consent process is a major step in the right direction • A hybrid approach (CoE and PC elements) would work best |
• Role of the local physician is paramount – patients see this individual as an opinion leader and influential in their decision to enroll in a study |
• Engage existing online networks (e.g. Facebook groups) to leverage their followers to increase study awareness and establish greater trust • Design online/web-based elements using theory-based, PC approaches • Incorporate physicians into the design phases of online materials given their role as opinion leaders for patients |
Characteristics of the Intervention: complexity, adaptability, trialability, and relative advantage
For recruitment purposes, individual Facebook, Twitter, Google + , and YouTube accounts were created to disseminate advertisements for the study to various populations (focused by age, location, etc.). The primary element driving the novel method (i.e. the PC approach) for clinical trial innovation was the utilization of a website designed for patient recruitment, engagement, and consent.
While the use of social media and internet technologies was a central aspect of recruitment for the PC arm, all study staff noted considerable complexity in matching engagement strategies with social media platforms patients were already using. Web-based recruitment strategies supporting outreach across multiple stakeholder attributes (e.g., age and disease specifics [10]) required presenting complex information related to study intention and process. Ensuring that all recruitment materials were “user-friendly” and comprehendible for patients was a focus for the PC arm strategy. To accomplish this, designing a clear, linear patient consent process became a focal point of the PC arm’s recruitment approach. From the PC arm staff perspective, approximately half of respondents indicated that their enthusiasm for the use of a web-based platform for patient engagement throughout the study was tempered by the recognition that the intervention that was designed as a technology-based outreach would struggle to incorporate patients with technology access issues.
“…I think you need to know your audience…and then you need to know what technology is at hand and what’s available to that audience…I think especially with the fact that we only have ten centers, they’re in densely populated places, but getting the people that live out in the middle of nowhere…I think in one way we are getting them. But on the other hand, there are some patients regardless of what means you use (even if it’s the means they already use, they’re just totally disinterested still.” (PC Staff A)
Participants reported that ensuring that a trial chosen for this method is conducive to web-based technologies becomes important when considering the assessment of clinical information. Thus, the adaptability of this novel method was possible because the study had well-defined self-report measures and procedures for patients that supported a virtual engagement.
“…so this had to be data that we could collect directly from patients. And I think that we designed a trial and chose end points that are very likely to be accurate when it’s reported by patients. …so I think that’s the key issue. You can only do a study like this if it’s amenable to accurate data collection directly from patients.” (CoE Staff A)
PC staff often reflected on the tough-to-answer questions that their teams had to consider concerning the trialability (i.e., how easily potential adopters can explore your innovation) of this approach, such as how patients could best be guided through the novel approach process, as the PC arm staff often felt they had “one shot” to present the web-based information to patients, as opposed to the CoE arm which benefits from a consistent dialogue available during standard recruitment approaches and explanations.
Many PC arm staff reported patient understanding and the nuances of the language used to recruit patients as being central to the trialability of this process as well, often leading to discussions regarding the importance of patient-centered language being further developed for such web-based approaches. Soliciting patient perspective and feedback was mentioned as an important step toward ensuring that appropriate literacy levels were in place. For example, a group of patients from the Vasculitis Foundation, the major patient advocacy group for vasculitis, participated in user acceptance testing, through which they provided feedback on the study description, consent forms, and registration process. This information was used in subsequent design phases to help create a series of FAQs for the study website where patients could seek further clarification.
Representation from patient partners, patient advocacy group representatives, and clinicians within the team was a central consideration in developing an approach to outreach that would connect to patients not channeled to recruitment through traditional CoE approaches. The involvement of the Vasculitis Foundation provided a communication venue for patients using the Foundation as an information source, as well as linkage for non-academic feedback on implementation approaches and tools.
The patients interviewed from the PC arm reported the use of web-based tools as providing a relative advantage to the traditional CoE model. These patients reported having previous engagement with online resources and social media. This appeared to be a common venue for capturing the attention of participants for providing study-related information and supporting the enrollment process. As one PC arm patient reported:
“I belong to the Facebook, Wegener’s granuloma vasculitis Facebook chat page, for everybody who has it, or somebody they know who has it, and they’ve joined. Then we compare things that happened to us versus what we take and how we’re treated. And Then, of course, seeing that link, that’s how I found it, and I decided to try it.” (PC Patient A).
Yet, most patients interviewed in the CoE arm described in-person conversations with their physicians or study coordinators as being integral in their joining the study. Thus, the relative advantage – i.e., the advantage of implementing the intervention versus an alternative solution – for the perspective of CoE staff was based on all interactions being face-to-face conversations with patients as opposed to focusing on the PC arm’s online approach. CoE arm patients often cited physicians as trusted sources of information about the study, providing details about the process and eligibility that influenced how comfortable patients reported feeling about enrolling.
“Well, the doctor and his assistant in this case, [NAME]… they were very, very knowledgeable and they kept assuring me. They said, do this only if you want to be involved, but they thoroughly went over the agenda for the study and the purpose.” (CoE Patient A)
Inner setting: available resources, culture and climate, and readiness for implementation
All PC arm staff discussed how the development of a novel approach required broadening the study team to include expanded skillsets involving information technologies and mass communications in addition to the expected expertise involved in standard implementation and evaluation of clinical trials. This often presented a challenge given that the available resource of experience did not always necessarily reflect the identified specialty areas, particularly in the areas of advertising and marketing.
“You have to be able to present something [online marketing] that looks real, that looks…like a real study. I mean, this is different than any other study most physicians probably have seen having a patient bring them study materials in. So how do you make it look real, look valid, but also make it very simple that there’s not a lot of – they don’t have to do a lot of research to understand the study.” (CoE Staff B)
Obtaining feedback from patients as to why they did or did not sign up for the study was cited as an important step that must be incorporated. One PC staff member raised an important point regarding resources, commenting that they felt resources for recruitment online (time and money) were sufficient, but that proper implementation procedures related to recruitment and retention reviewed during study team meetings were crucial for addressing patient concerns and questions. Thus, adequate resources tied in with the culture and climate regarding availability of feedback and communication among staff members.
“…we launched the study on February 17th and I had social media, a document with social media messages and for the website and things like that for our newsletter, but I’ve gotten no further direction on that. And I don’t know if there’s supposed to be more…I’m just wondering if there’s a phase 2 of that. And I emailed [NAME] about it a couple of weeks ago but I haven’t heard from her. So I – I just need to follow-up with her and say, you know, is there a phase 2 of the materials. Do we need to change the message, is there round 1, round 2, round 3 of messaging…if people have signed up or have not signed up for one reason or another, if they’ve given us, can we adjust our message to address their problems – their questions.” (PC Staff B)
The culture and climate of the PC arm was described by study team respondents as one that had an overall readiness for implementation among the study team, with respondents expressing their commitment to the novel approach. Yet, these respondents reiterated the importance of all team members communicating effectively with one another, ideally with one individual appointed as being responsible for the chain of communication. PC arm staff often reported that the “point person” for all communication needs to be responsible for garnering feedback from staff members, but that deadlines for this feedback need to be firm. Waiting on feedback from others was sometimes cited as a source of frustration within PC arm communications.
“…if you had the teams that, or committees that, stuck to the deadlines and then had one person to sign off on everything, I think that would have increased the concept to implementation time by at least six months.” (PC Staff A).
Characteristics of Individuals: Knowledge and beliefs about the intervention, self-efficacy, and personal attributes
Though committed to the development and testing of novel approaches to clinical trials, half of total staff respondents focused on the dominance of the CoE model, relating to the knowledge and beliefs surrounding the implementation process. However, all PC and CoE staff respondents were also aware that finding an appropriate balance of novel methods in conjunction with the traditional CoE model showed great promise.
“…this is something that wouldn’t have been possible, even ten years ago. So it provides an opportunity to do certain types of research in a way that we’ve never done before. But again, there are certain studies you will never be able to do through this pathway. So the types, it’s not going to replace as far as that type of study, that still needs to be done on a [trial] basis, either in practices or in academic centers, where you have people receiving a medication and having a face-to-face consenting and data collection aspect. But it may provide a way to do certain types of studies that are very feasible and hopefully very time-efficient and cost-efficient and allows us to research even better.” (CoE Staff C)
Nearly one third of all study team respondents voiced concern about the perceived self-efficacy of their patients to complete study-related tasks, particularly the older patient population associated with granulomatosis with polyangiitis, being “left behind” due to gaps in technology use. One study team respondent noted that the nature of the PC arm being technology-based would inherently limit participation to those with the access and capacity to navigate the technology.
While study team members often commented on their concern of using technology to recruit study participants, some PC arm patients expressed high self-efficacy and willingness to learn more about a study via social media or the internet, highlighting a discrepancy regarding perceived versus actual experiences. PC arm patients also cited the PC approach as one that still felt personalized, making it a benefit to study participation. However, positive feelings continued to be commonly discussed by CoE patients in association with their experiences of face-to-face communication with study members.
“I mean the emails were very personable and once I accepted it online, I’m always being contacted through an email and being checked up on and things like that so that’s always reassuring. It’s kind of like you’re not just a number kind of thing.” (PC Patient B)
“Again, my kudos to my specialist and his staff, who have walked me through the process, the administrative side of this process more than the doctor has. They’ve been very honest, very open, they’ve, even to the point where if I had a question that they did not know the answer to, they were honest enough to say, I do not know the answer to that, and then tried to, you know, find an answer for me.” (CoE Patient B).
The study team identified barriers associated with the absence of local (non-CoE) physician recruitment of participants in the PC arm. Traditional CoE roles were somewhat inverted in the PC arm where interested patients would provide study information to their local rheumatologist to consider the appropriateness of the study for the enrolling patient and to provide testing results to assure eligibility. The protocol was designed to not require the local treating physician to be involved in the study team or IRB process. One staff participant noted that “because patients have so many questions that require the physician’s input to answer them, that this can only be done through face to face visits with the experts.” Several study team participants attributed the low participant follow-through (from registration to consent to randomization) to the absence of the involvement of their local treating physician.
Staff members discussed the nature of the outreach process. As a patient would engage in the study, they then needed to approach their treating physician to validate their eligibility. Several staff assumed the local treating physician would not be immediately supportive, that they might feel “threatened,” unsupported, or vulnerable to legal ramifications in the event of poor study outcomes. One staff member explained, “We’ve already convinced the patient if they’ve downloaded it. We’ve got to convince the doctor now.” Direct-to-patient recruitment did not reduce the important role a patient’s treating physician played in their study experience.
“And then, again, of course, to make sure I had the approval and the participation with my doctor here, too. That’s because if he didn’t, then I wouldn’t have enrolled, because he’d have to work with me on this.” (PC Patient A)
Local treating physicians were framed as critical in a shared decision-making process with patients. Although this study was designed to alleviate the burden put on the local treating physician, patients consistently referenced how important their local treating physician was in determining their eligibility and approval for the study.
Process: Reflecting and evaluating, key stakeholders, engaging, and opinion leaders
A particular innovation for this clinical trial included a process for online consenting. Respondents were provided study-related information about potential risks and benefits of participation via the web-based platform. When reflecting and evaluating on this study experience, study team respondents varied in their level of commitment to the novel approach, with some feeling that the face-to-face discussions were central to the standard consenting process, and few seeing little difference in terms of the information provided face-to-face versus online.
Some staff reflected on the importance of team members evaluating how they communicate with patients. Some commented on the responsibility of team members to inform participants that while web-based approaches may be novel, participants would still “be in good hands” with knowledgeable, experienced individuals that know what they are doing. Given that staff reflected on the challenges associated with engaging with patients via web-based technologies, it became all the more important that such interfaces were designed with the patient perspective in mind.
“…and so it was important for us to make sure that all of the essential components to that informed consent were written in a way that was concise and that was thorough so everything was there, but that really could keep the – I guess keep the attention of someone who was just clicking through and finding this and reading through it – that they weren’t gonna have to scroll through ten pages to get to the end and agree to Participate because we felt that we would lose patients if that was the case…” (PC Staff C).
Time was discussed as a primary factor impacting individual PC arm staff members’ ability to successfully complete tasks associated with the online recruitment and consent phases. Associated tasks included communicating their interest in study participation with the study team, working with their treating physician to validate their study eligibility, and participating in the consent process for being randomized into the trial. The constant, iterative communication among team members was integral for a smoother rollout of the novel approach, yet most study staff respondents reflected on the process using “all or nothing” language, reflecting on whether things “worked” or “did not work,” rather than emphasizing how lessons learned could be applied toward future efforts. However, many staff reiterated that one of the greatest impacts of this approach was having various staff members re-think the way they previously designed studies for the traditional CoE route. Being able to have a more holistic view of the process permitted increased understanding of various perspectives integral to the overall research process. This mindset relied upon having a broad understanding of the way that clinical trials are typically deployed, and taking a step back to evaluate the ways in which traditional processes can be adjusted to improve overall workflow.
In cases where patients heard about the study from their healthcare providers, it was clear that treating physicians held great amounts of authority in helping a patient decide whether to enroll. Respondents expressed feeling more interested in participating when their treating physicians took the time to describe how research helps impact broader patient populations. Thus, patients framed their local treating physician as informal opinion leaders.
“…but he [my doctor] was the one that came to me when I was at an appointment with him and said, “…let me tell you about this, would you be interested?” …and he said “if you participate in this study, I mean, it will help other people and it will also help me further my understanding of whether or not to keep people on a certain dosage or to taper you off completely.” So I was very happy to do it because I want to help people in the future and I want to help myself in the future, too.” (PC Patient C)
Discussion
Using a lens of implementation evaluation, this manuscript explored the development and evaluation of a novel method of recruitment and engagement in a clinical trial that leveraged technology and web-based platforms to support virtual recruitment, engagement, retention, and assessment to increase access for patients beyond the traditional approach to trials based at CoEs. The primary findings include a rich description of the challenges faced in designing and managing the study team as necessary when orchestrating such a complicated endeavor, as well as study team and patient-level perspectives on the components driving implementation. While technology continues to enhance the ability to model and study rare diseases, the use of web-based and social media enhanced recruitment and engagement strategies proved quite challenging for the model developed within the context of the population sampled. Just as with email blasts being sent to contact registries, social media approaches appeared to also have limited reach in the study. Study team members noted the low number of social media followers, little traffic on pages, and few comments on Facebook posts.
Low participation rates in clinical trials are due in part to the time and costs associated with frequent in-person visit to clinical sites [19]. The burden is heightened when considering rare diseases where patients are may be geographically and economically isolated from the few centers of excellence provided cutting-edge care for the patient’s condition [20]. Technology has the potential to provide wide-based information sharing through social media and other web-based technologies increasing awareness of clinical trial availability vastly beyond the locus of centers of excellence and support not only recruitment and enrollment/consent, but also care provision and testing within their local care networks.
As true for any trial design, while focused on developing a novel approach to recruitment, engagement, and data collection, it was critical to develop processes and workflows that were highly feasible and linked to patient preferences. There are many variables to consider when distilling the conclusions of this study. In assessing the described implementation methodology, it is difficult to separate the capacity and challenges within the inner setting driving study team function with the nuances of patient engagement with the studied clinical trial innovations. In reflecting on the implementation broadly, the data illustrate key components and considerations related to study team factors not often explored in traditional implementation studies laid out across the CFIR domains of characteristics of the intervention, inner setting, characteristics of individuals, and process.
While this manuscript considered the implementation of a novel approach for clinical trial recruitment for a specific rare disease, its findings are useful for a range of conditions or disease areas interested in increasing recruitment and accrual rates. It is clear that more research, and expanded timelines for implementation when compared to a traditional CoE approach, are necessary for such novel methods to be effective. An important consideration for future research will be the incorporation of a more holistic approach to reach patients of specialized populations. Utilizing web-based approaches may not be sufficient, but when combined with other approaches such as phone-based texting and app platforms, could prove to be quite useful for reaching a broader number of potential participants. Thus, rather than envisioning this novel method for implementation as a replacement for the traditional CoE model, future research should explore hybrid approaches whereby patients may be recruited, screened, and consented online, but are then provided with the face-to-face treatment and interaction at physical clinical sites. As one participant explained:
“I mean, I don’t know if I could choose one arm over another. I mean, I definitely think there is value to both. And I think there’s a lot of potential in the patient-centric, the internet arm of the study, just because we are becoming a very computer-dependent society and more people are doing things that way. So, you know, there’s no reason why we shouldn’t have a presence there; otherwise, I mean, we’re kind of gonna be – I don’t know. I think we need to modernize as much as anything else, just because that’s the way the world is starting to work now. But due to the nature of the fact that we are talking about clinical care, medical care, people’s health. I mean, there are also – it can’t be completely computerized. There has to be a human element present all the time, especially since, I mean, we’re talking about someone’s health. So, I mean, I think there’s room – I don’t know that there necessarily means both approaches will keep existing separately, or whether they’ll kind of combine somehow. But, I mean, yeah, it’ll be interesting to see. I think they both have their value, and maybe they might kind of morph into one hybrid or completely new thing. I don’t know that, at this point, I could say that one is better over the other.” (PC Staff D).
Our goal was to fill an important gap in the literature (to date, we are not able to find a comparison study of this nature), with existing research on individual approaches to PC versus CoE approaches for RCTs supporting our findings. One systematic review of 30 PC approaches to RCTs emphasized the importance of health professionals improving their qualitative communication skills in order to facilitate the decision-making process for patients and team members, consistent with our findings where patients often sought out feedback from their physician or support staff [21]. While this study was designed to not rely on the treating physician, it is important that all study staff be trained in communicative skills and all study elements to be best equipped to engage patients. In the context of web-based approaches used for PC innovations for RCTs, one study that utilized an online platform for chronic myeloid leukemia patients to test patient-centered innovation capabilities found patient medication adherence and physician guideline adherence to be challenging [22]. While the web-based platform resulted in increased patient empowerment and guideline adherence, the study showcased how such innovations seek to serve as a value-added feature to more traditional models of patient care, consistent with our findings [22].
Limitations
In interpreting the results of this research, some limitations should be considered. The qualitative data presented should be used for hypothesis generation. First, not every study team member was interviewed and low numbers of patients in the PC arm participating in interviews may limit the generalizability of our findings. It is possible that additional feedback would further advance understanding of the drivers of implementation for this study design. Due to small sample sizes, issues of generalizability or transferability should be considered with possible over-representation of the perspectives of patients choosing to participate in the study. It should be noted, however, that saturation was reached for all constructs. Finally, member checking was not performed for this research, which could increase validity in allowing participants the opportunity to confirm findings.
Conclusions
Future efforts should explore how existing online patient networks may be utilized to reach more patients via social media. For example, one patient commented on their interest in this study being piqued more because of it being shared within a vasculitis support group on Facebook. Thus, source credibility for web-based messages should be a consideration when aiming to develop novel approaches to clinical trial recruitment via the Internet. This argument extends beyond social media in that mutually beneficial relationships with relevant patient advocacy groups should be established and maintained to leverage various communication channels available for disseminating study calls.
Existing research has provided suggestions for ways to enhance and refine novel approaches to trial activities, for patients and providers alike. Understandably, patients are a stakeholder holder group needing to be educated on at-home RCT tasks, and communication with study staff and providers for success of such endeavors are key. Research has recommended that provider and study staff communicative training may be beneficial to facilitate increased qualitative skills in PC approaches, with previous results highlighting increased shared decision making amongst patients and team members alike [21, 23, 24]. This is consistent with our results, which showcase a need for more clear, increased communication amongst all stakeholders.
It is important to note the rise of decentralized clinical trials (DCTs). While this research utilized the RCT model in order to compare PC and CoE approaches for implementation, the DCT relies on a patient-centric approach with an emphasis on new technologies, advanced analytics, and innovative procedures to maintain communication with study participants in their home environments [24, 25]. The Drug Information Association Innovative Design Working Group provided a comprehensive list of considerations for the planning and conduct of DCTs (and PC approaches) [24]. The Group’s recommendation to operationalize novel roles for study staff as technological needs are added and adapted is particularly relevant to our findings. Additional guidelines include the incorporation of plans for patient monitoring and safety and the use of statistical analysis plans that meet the needs of a study [24].
Also consistent with our study’s structure, previous research has recommended for the creation and involvement of a variety of stakeholders for the development of communication and logistics in PC approaches (see Fig. 1) [24]. Additionally, it is important to acknowledge that technological advances come with challenges for patients. One of our participants addressed issues of patient access and familiarity with technology as areas of concern. Study budgets should be reflected to provide equipment and resources to encourage participation for a variety of scenarios. Yet, if remote participation does not include access to reasonably reliable web-based platforms and Internet, the option should not be utilized. Regarding navigability of web-based content, studies should be built with patient experience in mind. Providing clear audio-visual instructions related to online content, 24/7 support systems for when technical difficulties arise, and chatbots pre-loaded with answers to frequently asked questions related to the study are all ways to increase patient engagement [26]. As web-based platforms allow for increased patient engagement, it stands that more burden is being transferred to participants as they carry out research procedures and input data. Therefore, the importance of the informed consent process is critical, highlighting another opportunity for communication between patients and providers to be clear and consistent.
This research demonstrated both the ability to recruit using direct-to-patient techniques for a clinical trial via social media and web-based approaches, and the challenges to these strategies. Future research needs to explore further the ways that virtual platforms can increase access to clinical trials for patients experiencing rare conditions. As technologies continue to expand, it is important that research practices evolve in an effort to address recruitment challenges. Beyond social media, augmented and virtual realities in particular present future research trends that should be explored for rare disease research recruitment, considering each technology’s ability to facilitate screening, eligibility, record management, and remote data collection [27]. The realities of rare conditions drive nuances specific to their study, including the role of local providers as they relate to patient access and engagement in clinical trials. The same realities resulting in barriers to care (e.g., geographic isolation from CoEs) demand increased attention to the opportunities that social media and virtual platforms provide in improving access to information and further tethering of remote communities to the resources available within CoEs.
Supplementary Information
Additional file 1. Interview Guides. Interview guides for: patient participants (PC and CoE arms); NMCT research staff, CoE PIs, study coordinators; physicians (PC and CoE arm); opt-out interviews (CoE and PC arms).
Acknowledgements
The authors gratefully acknowledge the contribution to this study of the many patients who helped review aspects of the trial communications and processes and the patients who participated in the interviews associated with this project. A full list of Vasculitis Clinical Research Consortium members, whom we would like to acknowledge, has been provided.
Vasculitis Clinical Research Consortium
Renée Borchin5, Cristina Burroughs5, Jeffrey Krischer, PhD5, Julie Martin, RN, MEd5, Leah Madden6, Carol McAlear, MA6, Brian Rice6, Antoine Sreih, MD6, Jacquelyn Thomas, MPH6,Joyce Kullman7,Simon Carette, MD8, Julia Farquharson8, Samyukta Jagadeesh8, Christian Pagnoux, MD, MSc, MPH8, Nader Khalidi, MD9, Sandra Messier9, Diana Robins9 Martha Finco, MA10, Jennifer Godina10, Jessica Gonzalez10, Curry Koening, MS, MD10, Julieanne Nielsen10Sonya Crook, RN11, Kathleen Gartner11, Elizabeth Kisela11, Carol Langford, MHS, MD11, Lori Strozniak11,Paul Monach, MD, PhD12, Laurie Hope, RN13, Dawn McBride, RN13, Larry Moreland, MD13, Cynthia Beinhorn14, Yeoniee Kim14, Kathleen Mieras14, Ulrich Specks, MD14, Steven Ytterberg, MD14, Peter Merkel, MPH, MD15
Authors’ contributions
Peter A Merkel, Jeffrey Krischer, and Peter Cronholm conceptualized the study. Peter Cronholm and Janelle Applequist wrote the main manuscript text and prepared figures and supplementary tables. Jeffrey Krischer, Ebony Fontenot, Trocon Davis, Cristina Burroughs, Carol A McAlear, Renée Borchin, Joyce Kullman, Simon Carette, Nader Khalidi, Curry Koening, Carol A Langford, Paul Monach, Larry Moreland, Christian Pagnoux, Ulrich Specks, Antoine G Sreih, Steven R. Ytterberg and Peter A Merkel reviewed the manuscript and provided substantive edits.
Funding
This project was supported by the National Heart, Lung, and Blood Institute (R01HL115041). The Vasculitis Clinical Research Consortium (VCRC) has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54 AR057319), the National Center for Research Resources (U54 RR019497), the Office of Rare Diseases Research (ORDR), and the National Center for Advancing Translational Science (NCATS). The VCRC was part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of ORDR and NCATS. The RDCRN Data Coordinating Center (U01TR001263) was also supported by ORDR, NCATS. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
In accordance with the Declaration of Helsinki, the study was reviewed and approved by the Institutional Review Boards (IRB) at the University of South Florida (protocol number: Pro0001324) and the University of Pennsylvania (protocol number: 818769). Informed consent was obtained from staff member and patients prior to starting interviews at the time of data collection.
Consent for publication
In cases where interview quotes have been reported in this study, pseudonyms have been used to protect participant identity. Thus, consent for publication of data is not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter F. Cronholm, Email: peter.cronholm@pennmedicine.upenn.edu
Vasculitis Clinical Research Consortium:
Jeffrey Krischer, Cristina Burroughs, Carol A. McAlear, Renée Borchin, Joyce Kullman, Simon Carette, Nader Khalidi, Curry Koening, Carol A. Langford, Paul Monach, Larry Moreland, Christian Pagnoux, Ulrich Specks, Antoine G. Sreih, Steven R. Ytterberg, Peter A. Merkel, Julie Martin, Leah Madden, Brian Rice, Jacquelyn Thomas, Julia Farquharson, Samyukta Jagadeesh, Sandra Messier, Diana Robins, Martha Finco, Jennifer Godina, Jessica Gonzalez, Julieanne Nielsen, Sonya Crook, Kathleen Gartner, Elizabeth Kisela, Lori Strozniak, Laurie Hope, Dawn McBride, Cynthia Beinhorn, Yeoniee Kim, and Kathleen Mieras
References
- 1.Rare Disease Day: What is a Rare Disease? https://www.rarediseaseday.org/article/what-is-a-rare-disease. 2018. Accessed 21 Feb 2022.
- 2.Garrino L, Picco E, Finiguerra I, Rossi D, Simone P, Roccatello R. Living with and treating rare diseases: experiences of patients and professional health care providers. Qual Health Res. 2015;25:6. 10.1177/1049732315570116. [DOI] [PubMed] [Google Scholar]
- 3.Austin CP, Cutillo CM, Lau LPL, Jonker AH, Rath A, Julkowska D, et al. Future of rare diseases research 2017–2027: an IRDiRC perspective. Clin Transl Sci. 2018;11:1. 10.1111/cts.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray S. Reducing barriers to participation in clinical trials for rare diseases. ACRP. 2021;35:6. https://acrpnet.org/2021/08/16/reducing-barriers-to-participation-in-clinical-trials-for-rare-diseases/. 2021. Accessed 2022 May 10.
- 5.Paul J, Seib R, Prescott T. The internet and clinical trials: background, online resources, examples and issues. J Med Internet Res. 2005. 10.2196/jmir.7.1.e5. [DOI] [PMC free article] [PubMed]
- 6.Santoro E, Nicolis E, Franzosi MG, Tognoni G. Internet for clinical trials: past, present, and future. Control Clin Trials. 1999;20:2. 10.1016/S0197-2456(98)00060-9.S0197245698000609. [DOI] [PubMed] [Google Scholar]
- 7.Applequist J, Burroughs C, Ramirez A, Merkel P, Rothenberg ME, Trapnell B, et al. A novel approach to conducting clinical trials in the community setting: utilizing patient-driven platforms and social media to drive web-based patient recruitment. BMC Med Res Methodol. 2020;20:58. 10.1186/s12874-020-00926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Applequist J. Broadcast pharmaceutical advertising in the United States: primetime pill pushers. Lanham: Lexington; 2016. [Google Scholar]
- 9.Applequist J, Ball JG. An updated analysis of direct-to-consumer television prescription advertisements for prescription drugs. Ann Fam Med. 2018;16(3). 10.1370/afm.2220. [DOI] [PMC free article] [PubMed]
- 10.Krischer J, Cronholm PF, Burroughs C, McAlear CA, Borchin R, Easley E, et al. Experience with direct-to-patient recruitment for enrollment into a clinical trial in a rare disease: a web-based study. J Med Internet Res. 2017. 10.2196/jmir.6798. [DOI] [PMC free article] [PubMed]
- 11.Hareendran A, Gnanasakthy A, Winnette R, Revicki D. Capturing patients’ perspectives of treatment in clinical trials/drug development. Contemp Clin Trials. 2012;33. 10.1016/j.cct.2011.09.015. [DOI] [PubMed]
- 12.Olmos-Vega FM, Stalmeijer, RE, Varpio L, Kahlke R. A practice guide to reflexivity in qualitative research: AMEE guide no. 149. Med Teach. 2023;45(3). 10.1080/0142159X.2022.2057287. [DOI] [PubMed]
- 13.Linabary JR, Corple DJ, Cooky C. Of wine and whiteboards: Enacting feminist reflexivity in collaborative research. Qual Res. 2021;21(5). 10.1177/1468794120946988.
- 14.Young K, Kaminstein D, Olivos A, Burroughs C, Castillo-Lee C, Kullman J, et al. Patient involvement in medical research: what patients and physicians learn from each other. Orphanet J. Rare Dis. 2019;14(21). 10.1186/s13023-018-0969-1 [DOI] [PMC free article] [PubMed]
- 15.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:6. 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 16.Creswell JW. Qualitative inquiry and research design: choosing among five traditions. Thousand Oaks: Sage; 1998. [Google Scholar]
- 17.Lindlof TR, Taylor BC. Qualitative communication research methods. 2nd ed. Thousand Oaks: Sage; 2002. [Google Scholar]
- 18.Tracy SJ. A phronetic iterative approach to data analysis in qualitative research. J Qual Res. 2018;19:2. 10.22284/qr.2018.19.2.61. [Google Scholar]
- 19.Tracy SJ. Qualitative research methods: collecting evidence, crafting analysis, communicating impact. Hoboken: Wiley Blackwell; 2020.
- 20.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richesson RL, Lee HS, Cuthbertson D, Young K, Krischer JP. An automated communication system in a contact registry for persons with rare diseases: scalable tools for identifying and recruiting clinical research participants. Contemp Clin Trials. 2009;30:1. 10.1016/j.cct.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rees CA, Pica N, Monuteaux MC, Bourgeois FT. Noncompletion and nonpublication of trials studying rare diseases: a cross-sectional analysis. PloS Med. 2019;16(11). 10.1371/journal.pmed.1002966. [DOI] [PMC free article] [PubMed]
- 23.McMillan SS, Kendall E, Sav A, King MA, Whitty JA, Kelly F, et al. Patient-centered approaches to health care: a systematic review of randomize controlled trials. Med Care Res Rev. 2013;70(6). 10.1177/1077558713496318. [DOI] [PubMed]
- 24.Ector GICG, Westerweel PE, Hermens RPMG, Braspenning KAE, Heeren BCM, Vinck OMP, et al. The development of a web-based, patient-centered intervention for patients with chronic myeloid leukemia (CMyLifeCmyLife): design thinking development approach. J Med Internet Res. 2020;22(5): e15895. 10.2196/15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan S, Yoder LH. A concept analysis of person-centered care. J Holist Nurs. 2012;30(1). 10.1177/0898010111412189. [DOI] [PubMed]
- 26.Ghadessi M, Di J, Wang C, Toyoizumi K, Shao N, Mei C, et al. Decentralized clinical trials and rare diseases: a Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective. Orphanet J Rare Dis. 2023 18(79). 10.1186/s13023-023-02693-7. [DOI] [PMC free article] [PubMed]
- 27.Van Norman GA. Decentralized clinical trials: the future of medical product development?. JACC Basic to Transcl Sci. 2021;6(4). 10.1016/j.jacbts.2021.01.011. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Interview Guides. Interview guides for: patient participants (PC and CoE arms); NMCT research staff, CoE PIs, study coordinators; physicians (PC and CoE arm); opt-out interviews (CoE and PC arms).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.