Abstract
Importance
Neonatal anemia has a long-term effect on children's growth and development. Anemia during pregnancy is also the most widespread nutritional deficiency among pregnant women in the world; If it leads to anemia in newborns, it will affect a wide range of people and be a public health problem worthy of attention.
Objective
To study the relationship between maternal anemia during pregnancy and neonatal hemoglobin levels.
Data sources
PubMed, Web of science, Scopus, MEDLINE, Embase, ProQuest, Dissertations & Theses Global, The Cochrane Library, China Biology Medicine Database, Chinese CNKI Database, and Chinese Wanfang Database were systematically searched from inception to August 31, 2022.
Study selection
The meta-analysis included all original studies which pertain to cohort studies, case-control studies or cross-sectional studies that investigated the relationship between maternal anemia during pregnancy and neonatal hemoglobin levels.
Data extraction and synthesis
Hemoglobin level of both anemic and non-anemic pregnant mothers and their paired newborns were pooled from the selected studies. The random-effects model was used to assess the risk of getting a lower neonatal hemoglobin level between mothers with and without pregnant anemia. Data analyses were performed from September 5, 2022, to March 10, 2023.
Main outcomes and measures
Maternal anemia during pregnancy is a risk factor of lower neonatal hemoglobin levels.
Results
The initial search yielded 4267 records of which 116 articles underwent full-text evaluation, which identified 18 articles and a total of 1873 patients that were included. The findings of the meta-analysis showed a significant difference between the two groups(MD=-1.38; 95%CI:[-1.96,-0.80]. p<0.01), while the co-effect showed that the neonatal hemoglobin value of anemic mothers was 1.38g/dL lower than that of non-anemic mothers(-1.96,-0.80), suggesting a correlation between maternal anemia lower neonatal hemoglobin levels.
Conclusions and relevance
This systematic review and meta-analysis demonstrated that maternal anemia during pregnancy were associated with a lower level of newborn hemoglobin levels. This may enable a better understanding of neonatal anemia and provide guidance towards future development of nutritional supplementation during pregnancy and the prediction of postpartum outcomes.
Trial registration
PROSPERO Identifier: CRD42022352759.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06832-1.
Keywords: Maternal anemia, Infants, Nutrition, Iron deficiency
Background
Anemia is a serious condition for newborns. Despite very limited data, several studies reported neonatal anemia prevalence of more than 20% [1] . A growing body of research shows that long-term anemia in neonatal period was associated with children’s stunting and developmental delay, and even affected health, development, and social achievement of all one’s life [2].
All nutrition of the fetus comes from the mother. However, Anemia is also the most widespread nutritional deficiency among pregnant women in the world. A total of 40.05% of pregnant women globally and about 20% of pregnant women in China suffered from anemia, which has reached the severe and moderate public health significance defined by WHO respectively [3–5]. Because of the alarmingly high prevalence, any adverse effects that maternal anemia during pregnancy may have on neonatal anemia would have a great public health impact. Therefore, a full understanding of the relationship between the maternal and neonatal anemia is of great significance for identifying public health policy priorities and developing nutritional interventions.
There are few studies on the relationship between maternal anemia during pregnancy and neonatal anemia, although the earliest studies can be traced back to the 1950s. Some studies suggest that the placental mechanism of unidirectional maternal-fetal iron transport ensures adequate iron supply for the fetus even when maternal iron is deficient [6]. The conclusion was supported by several epidemiological findings [7–14]. For example, Wedderburn et al.’s cohort study in Spain found that there was no association identified between maternal anemia in pregnancy and child anemia (χ2 = 0.004, P = 0.95) [15]. However, the opposite conclusion also exists. In a case-control study conducted in Vietnam involving 1274 paired newborns and mothers, newborns with anemia at 3 months were exposed to maternal anemia compared to the those non-anemic newborns, with an odds ratio (OR) of 1.30 (95%CI:0.97,1.74) after controlling for confounding factors [16]. In conclusion, there remains a paucity of evidence on the association between maternal and neonatal anemia and their conclusions were inconsistent. To address this research gap, we did a systematic review and meta-analysis to assess the association between maternal anemia during pregnancy and neonatal anemia.
Methods
We planned our review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17–19]. Prior to commencing the review, we preregistered a protocol with PROSPERO (Reference CRD42022352759).
Search strategy
The search strategy was developed by two principal investigators, and then reviewed by the research group consisting of one professor of obstetrics and gynecology, two doctors of obstetrics and gynecology, and two doctors of public health. Eleven online databases were searched for papers published from database inception to August 31, 2022: PubMed, Web of science, Scopus, MEDLINE, Embase, ProQuest, Dissertations & Theses Global, The Cochrane Library, China Biology Medicine Database, Chinese CNKI Database, and Chinese Wanfang Database. No language restrictions were used.
We searched for observational studies reporting the association between maternal anemia during pregnancy and neonatal anemia, including cohort studies, case-control studies, and cross-sectional studies. Search terms were a combination of the following vocabularies in title or abstract fields: (1) pregnant woman: Maternal OR pregnancy OR pregnant OR gravid OR antenatal OR prenatal OR antepartum OR gestation OR mother; AND (2) newborn: neonatal OR perinatal OR infant OR newborn OR newborns OR neon OR neonate OR neonates OR neonat OR “umbilical cord” OR Placenta OR “fetal blood”; AND (3) anemia: anemia OR anaemia OR haemoglobin OR hematocrit OR haematocrit OR iron; AND (4) study design: cohort OR follow-up OR prospective OR longitudinal OR retrospective OR “incidence study” OR “follow up” OR “case control” OR “cross sectional” NOT review[Title].
Two researchers independently used the above strategy to search the literature in the target database and checked the consistency of the results. Inconsistencies in results were resolved through discussions between the two reviewers, or with the help of a third reviewer. All the retrieved literature was managed by Endnote 20.0 software (Clarivate, PA, USA), and the duplicates were removed.
Literature screening and selection criteria
Two reviewers independently screened the literature in two stages: (1) screened each title and abstract and excluded irrelevant literature, and (2) then independently screened the full text of remaining studies using eligibility criteria. When the two reviewers had different opinions on the selection of the literature, a third reviewer was invited to participate in the discussion and decision-making. The studies were finally confirmed to be relevant by consensus were included. Additional literature was further identified by checking the reference lists of included relevant studies.
Inclusion criteria were as follows: original studies, including cohort studies, case-control studies or cross-sectional studies; the participants included neonates with anemia-related index. Subjects were divided into two groups, anemic and control, according to maternal hemoglobin level, mothers with Hb < 11 g/dL were categorized in the anemic group and those with Hb > 11 g/dL were served as control group; the outcomes included neonatal anemia or hemoglobin/iron/ferritin level which were measured through vein or peripheral blood during the first 6 months of life; primary exposure included maternal anemia or hemoglobin/iron/ ferritin levels during all stages of pregnancy; results was reported as mean scores with SDs, raw proportions, unadjusted odds ratios (ORs), relative risks (RR), correlation coefficient, or regression coefficient; full-text was access in the electronic library databases, and written in English or Chinese. We excluded studies with types of editorials, commentaries, abstracts only, brief communications, and reviews. If the articles used the same database, the one that provides the most data was included. Maternal anemia is defined as a hemoglobin concentration less than 11.0 g/dL. When the concentration ranges from 7.0 to 11.0 g/dL, it is classified as mild to moderate anemia; whereas a concentration below 7.0 g/dL is indicative of severe anemia. Neonates with hemoglobin values lower than 13.5 g/dL were considered anemic. And ID is defined as maternal serum or plasma ferritin levels < 15 ug/L and less than 12 ug/L in neonates.
Data extraction
Two reviewers extracted and cross-checked data of each included literature using data extraction sheets designed by the authors, including: the author information; published year; study settings; study design; inclusion criteria, and exclusion criteria; sample size; participant characteristics; exposures; outcomes; adjusted confounding factors, and the subgroups. After completion, the accuracy of extraction was reviewed by another reviewer.
Risk of bias assessment
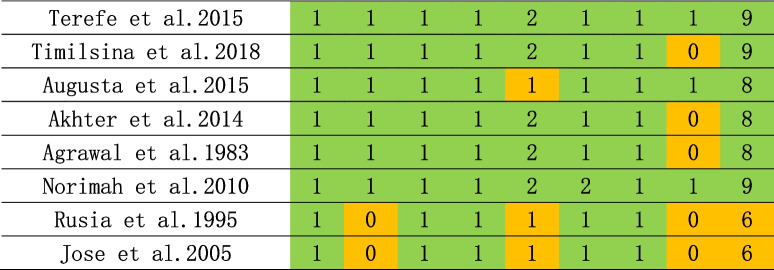
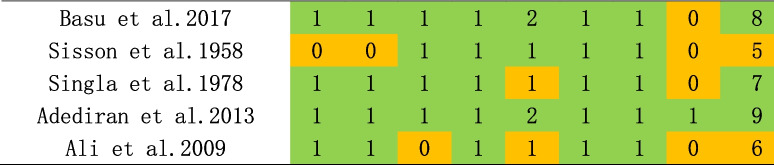
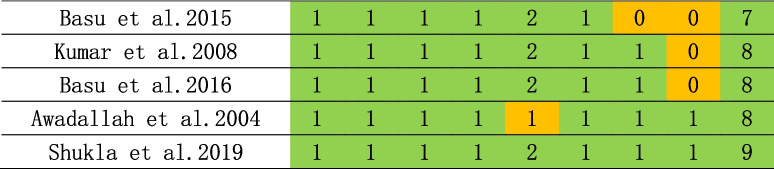
Risk of bias was assessed using the Newcastle–Ottawa Scale, a quality assessment scale recommended by the Cochrane Collaboration, for case–control and cohort studies [20]. Risk of bias of cross-sectional studies were assessed using the adapted Newcastle-Ottawa Scale. The NOS consists of eight items categorized into three dimensions: selection, comparability, and outcomes or exposures. The assessment was conducted by the three reviewers in parallel. An overall score was calculated for each study as the mean score of the reviewers, ranging from 0 to 9, with a score of less than 5 indicating a high risk of risk. Inter-rater reliability was evaluated by the kappa coefficient for each item, and the Spearman’s rank correlation coefficient for the overall score of each study. No studies were excluded based on its quality assessment.
Data analysis
We estimated pooled odds ratios (ORs) with 95% CIs for binary outcomes (anemia) and standardized mean differences (SMDs) with 95% CI for continuous outcomes (hemoglobin/iron/ferritin level). Unadjusted study outcomes were used because only few studies reported adjusted effect estimates which varied considerably with regard to the covariates included. The results of the meta-analysis will be presented as forest plots.
We used the I2statistic and Q test to indicate the proportion of total variation between study estimates due to heterogeneity. An I2value of > 50% or a Q value with p value of < 0.05 indicated significant heterogeneity. The random effect model was used if heterogeneity was significant, otherwise the fixed effect model was used. To identify and assess the sources of heterogeneity, subgroup analyses were conducted according to study design, study setting, the assessment time of neonatal anemia, and the onset time of anemia during pregnancy. Egger’s test and funnel plots were used to assess potential publication bias, and p < 0·1 was regarded as significant. Sensitivity analysis was performed by omitting each article when calculating the pooled results to assess the robustness of the conclusions of our meta-analysis.
All statistical analyses were performed with the R 3.6.3 software and “Meta” package 4.11. P value of < 0.05 indicated statistically significant.
Results
Inclusion of the study
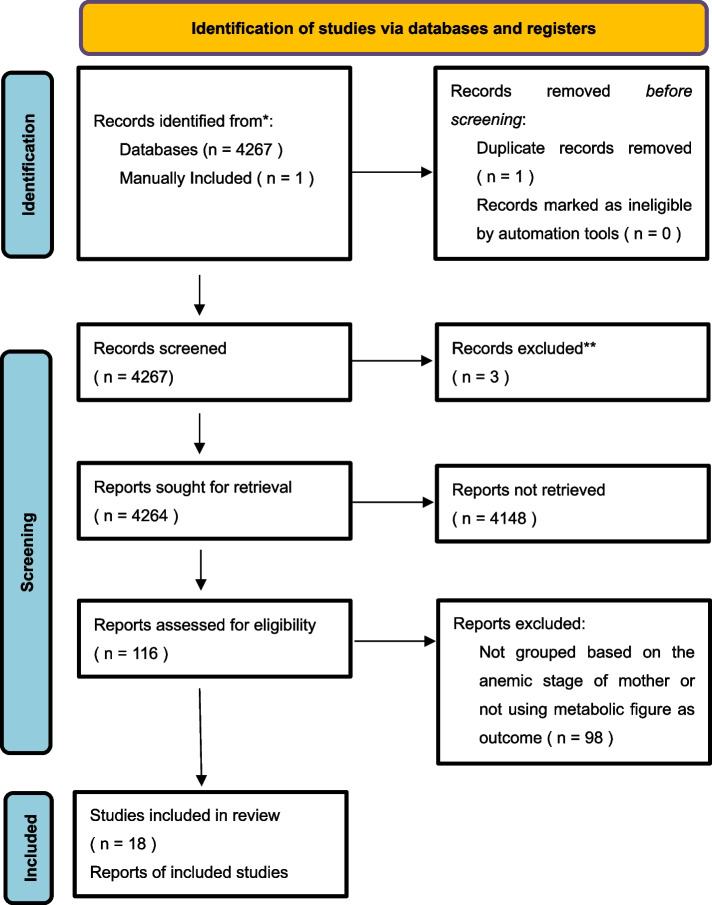
The initial search yielded 4267 records. After removal of duplicates and removal of records marked as ineligible by automation, 4267 were screened at title and abstract level, and 116 articles underwent full-text evaluation. Of those, 18 articles with a total of 1873 patients were included in review. The process of identification of studies via databases and registers were shown in Fig. 1.
Fig. 1.
Flow diagram of studies included in the systematic review and meta-analysis
Risk of bias
As shown in Tables 1, 2 and 3, NOS scale was used for the assessment of risk of bias, and most studies were considered having low bias. Four publications were deemed to be of low quality (NOS < 7) and were consequently excled. Subsequent stratified analysis were conducted, and the fact, as presented in Supplementary Table 1.
Table 1.
Table 2.
Table 3.
Characteristics of the studies
The characteristics of the studies included in the systematic review and meta-analysis are shown in Table 4. 8 cross-sectional studies, 5 case-control studies and 5 prospective-observational studies were included in the study, including 1873. The publication year of the studies varied from 1958 to 2019.
Table 4.
Analysis of the overall cohort
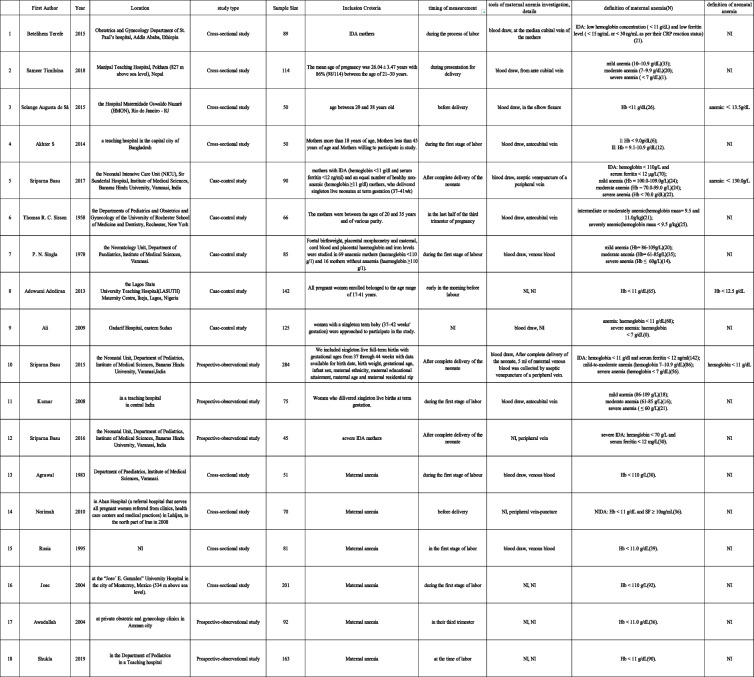
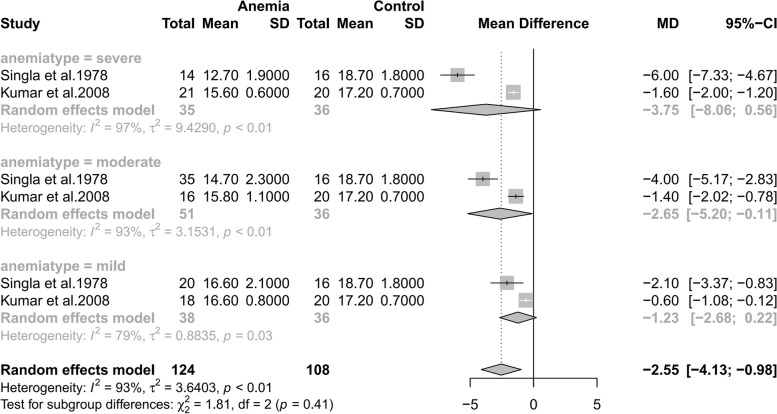
As shown in Fig. 2, eighteen studies were included in this study [21–38]. Significant heterogeneity were observed(p < 0.01,I2 = 94%). Therefore, a random effects model was adopted.
Fig. 2.
Forest plot of hazard ratios of affects of anemia during pregnancy on anemia incidence of newborns
The result showed a significant difference between the two groups(MD=−1.38; 95%CI: [−1.96,−0.80]. p < 0.01). As can be seen from the forest plot, the conclusion that maternal anemia had an impact on neonatal hemoglobin levels can be safely accepted. The co-effect showed that the neonatal hemoglobin value of anemic mothers was 1.38 g/dL lower than that of non-anemic mothers(−1.96,−0.80), suggesting a correlation between maternal anemia with lower neonatal hemoglobin levels.
Sensitivity analyses
As shown in Fig. 3, studies involved have a low sensitivity. Each study was excluded separately to see the influence on the co-effect. The result above shows that the effect of each individual data set on the sensitivity, heterogeneity and I2 of the overall figures was not statistically significant.
Fig. 3.
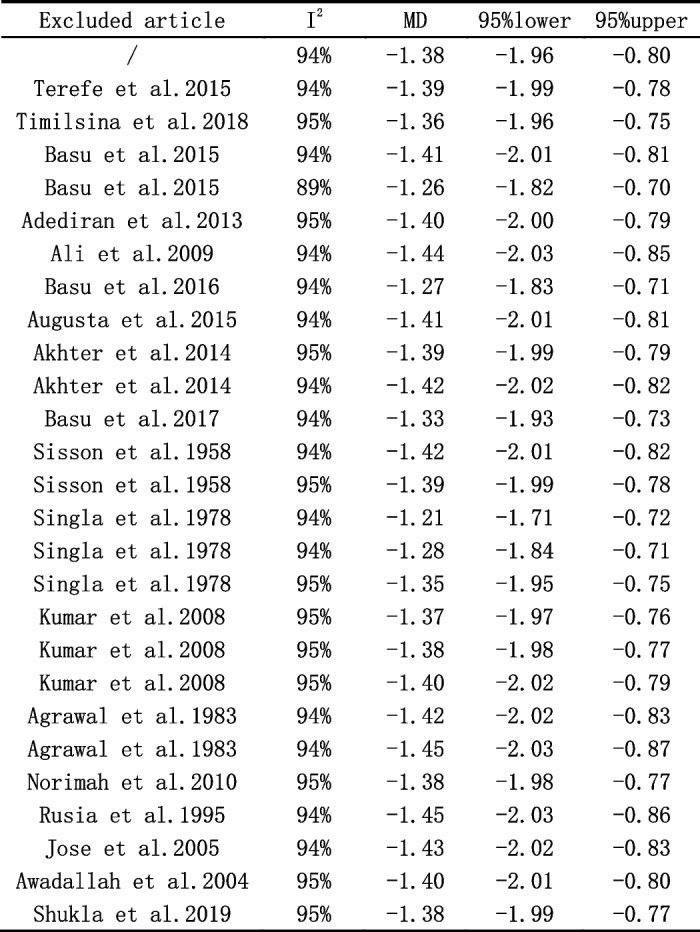
Assessment of the influence on the co-effect on each study
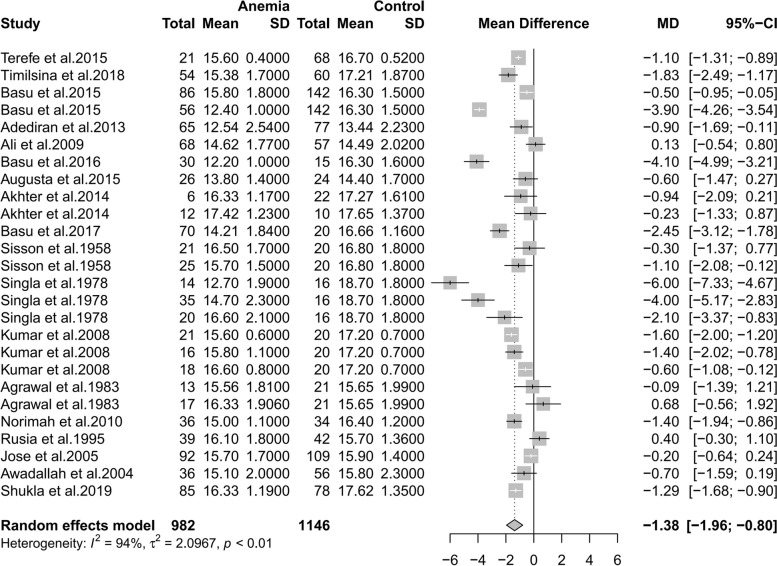
Subgroup analyses
As shown in Fig. 4, there was a correlation between maternal and infant hemoglobin whenever maternal blood samples were taken. However, maternal blood that were collected after delivery showed the most significant correlation with neonatal blood which were all taken during labor from umbilical, which indicates that collecting maternal blood after delivery may best reflect the level of neonatal hemoglobin level.
Fig. 4.
Subgroup analyses of the time of maternal blood sample collection
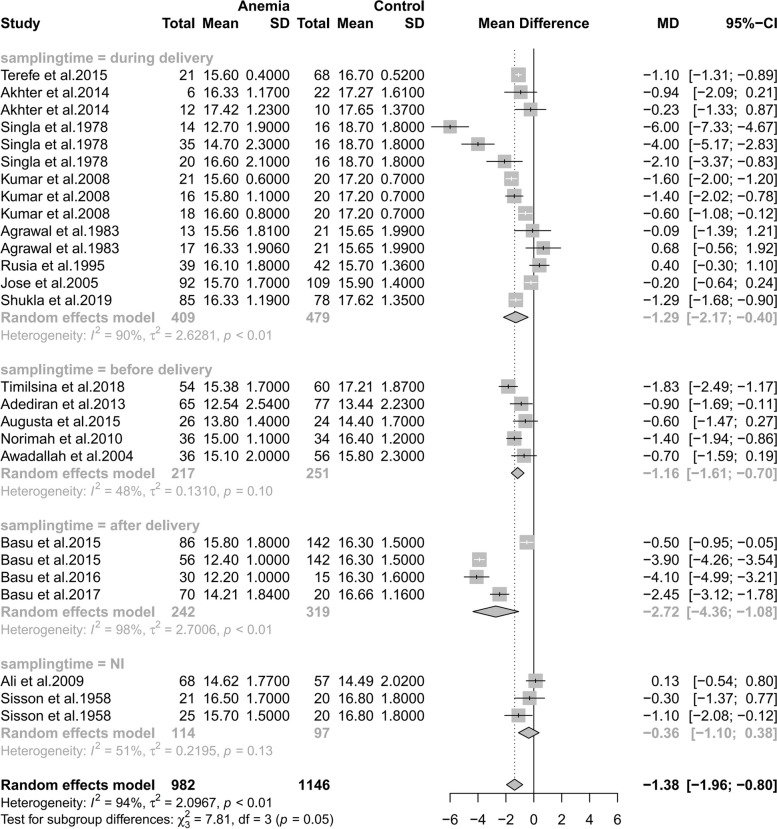
As shown in Fig. 5, there was a correlation between maternal and infant hemoglobin in both types. However, IDA mothers have a stronger correlation with neonatal hemoglobin comparing to those including all anemia types, which stressed the significance of screening for not only blood routine examination(hemoglobin concentration) but also iron index.
Fig. 5.
Subgroup analyses of anemia type
As shown in Fig. 6, maternal anemia severity display a correlation with neonatal hemoglobin level. However, number of the studies that are suitable for this subgroup analysis was small, and further research necessitates larger sample sizes and more standardized grouping criteria to provide corroborative evidence.
Fig. 6.
Subgroup analyses of maternal anemia severity
Discussion
In this systematic review and meta-analysis, we conducted a systematic review and meta-analysis of the literature on the association between maternal anemia and neonatal hemoglobin levels and explored the association between maternal anemia and neonatal hemoglobin levels, which was lacking in previous studies. Through this meta-analysis, we confirmed that maternal anemia during pregnancy increases the risk of low neonatal hemoglobin levels.
Physiologically, the fetus receives iron from the mother in the form of transferrin across the placenta-transferrin-bound iron, which is transferred directly from the maternal blood to the syncytiotrophoblast in the placental villi via transferrin receptor 1(TFR1). Upon binding to iron(Fe3+) at the apex of the syncytiotrophoblast, holo-transferrin and its bound iron are internalized and iron is released into the cytoplasm [39]. However, studies have shown that maternal iron stores gradually decrease during pregnancy, with serum ferritin levels usually reaching a nadir concentration at 35–38 weeks [40], and that may explain the reason why a high incidence of anemia in early life in infants and young children was found, due to WHO statistical projections published in the May of 2023, claiming that 40% of all children aged 6–59 months are affected with anemia [41].
However, the number of studies on neonatal anemia is relatively small, and the research on the relationship between anemia during pregnancy and anemia in neonates is even more scarce, especially the research on the association between different severity of anemia during pregnancy and anemia in neonates. It is also unclear whether anemia in infants and young children is due to maternal baseline conditions, of is caused by potential postpartum feeding problems and other issues. This may be due to the fact that the current methods of neonatal blood monitoring are subject to many ethical restrictions, and hence limited the development of these researches. According to the literature retrieval results of this study, after searching and screening for articles on PubMed and other 10 databases from the earliest time to August 31, 2022, only 18 studies were found to meet the inclusion and exclusion criteria, with 1873 subjects involved, which was a small number and again confirmed the relatively scarcity of research status. Due to the limited number, the articles included had great heterogeneity at both time and space level, and the results may have great bias, which, while inevitable, is consisten with our findings and hypotheses. In future studies, we believe that using more consistent subgroup definition criteria and sample collection methods is an optional optimization method, and further studies with large samples should be carried out to verify the relationship. In addition, we believe that the future surveillance of neonatal anemia is a matter of concern.
Our study indicates a possible association between maternal anemia and neonatal anemia, which suggests that to control maternal anemia during pregnancy can be a potential strategy to reduce the risk of neonatal anemia. However, evidence is still lack for the effectiveness of treatment towards anemia during pregnancy [42–45], and the starting time of treatment for anemia during pregnancy has not been ascertained, which needs to be verified by further experiments. In general, we should pay more attention to the management of anemia during pregnancy and the nutritional monitoring of newborns of pregnant women with anemia, and establish more sound guidelines for iron supplementation in combination with different cultural backgrounds and other factors.
In conclusion, we call for future studies with larger population sample sizes, as far as possible uniform sampling time, and as far as possible more comprehensive analysis and control of confounding factors. Despite the limitations mentioned above, our data do support the conclusion that there is an association between maternal and fetal anemia. However, this conclusion needs to be verified through more large-sample cohort studies in the future, or through innovation on the monitoring methods for neonatal anemia. And still, most importantly, management of anemia during pregnancy should be strengthened.
Supplementary Information
Acknowledgments
Clinical trial number
Not applicable.
Authors’ contributions
B.- K. Zhao and H.-F. Shi wrote the main manuscript text, M.-X. Sun, J.-X. Li and Y. Wei did the discussion part based on clinical practice, B.- K. Zhao and H.-F. Shi did the main searching and data processing part and T.-C. Wu made decisions over different opinions during research progress.
Funding
The present study was funded by the National Key Research and Development Program of China (No. 2021YFC2700700).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huifeng Shi and Yuan Wei contributed equally to this work.
Contributor Information
Huifeng Shi, Email: nsxm@pku.edu.cn.
Yuan Wei, Email: weiyuanbysy@163.com.
References
- 1.Dereje I, Etefa T, Gebremariam T, Getaye A, Tunta A, Gerbi A. Prevalence of Anemia and Associated factors among term newborns in Nekemte Specialized Hospital, Western Ethiopia. J Multidiscip Healthc. 2021;14:2607–15. 10.2147/JMDH.S326962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World health statistics 2022: monitoring health for the SDGs, sustainable development goals. Accessed 31 Aug 2024. https://www.who.int/publications/i/item/9789240051157.
- 3.Global anaemia reduction efforts among women of reproductive age: impact, achievement of targets and the way forward for optimizing efforts. Accessed 31 Aug 2024. https://www.who.int/publications/i/item/9789240012202.
- 4.Shi H, Chen L, Wang Y, et al. Severity of Anemia during pregnancy and adverse maternal and fetal outcomes. JAMA Netw Open. 2022;5(2):e2147046. 10.1001/jamanetworkopen.2021.47046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global nutrition monitoring framework: operational guidance for tracking progress in meeting targets for 2025. Accessed 31 Aug 2024. https://www.who.int/publications/i/item/9789241513609.
- 6.Barad A, Guillet R, Pressman EK, et al. Placental Iron content is lower than previously estimated and is Associated with maternal Iron status in women at Greater Risk of Gestational Iron Deficiency and Anemia. J Nutr. 2022;152(3):737–46. 10.1093/jn/nxab416. [DOI] [PubMed] [Google Scholar]
- 7.Abioye AI, Park S, Ripp K, et al. Anemia of inflammation during human pregnancy does not affect Newborn Iron Endowment. J Nutr. 2018;148(3):427–36. 10.1093/jn/nxx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balai KS, Pendse V, Gupta R, Gupta S. Effect of maternal anemia on iron status of the new born. Indian J Matern Child Health off Publ Indian Matern Child Health Assoc. 1992;3(2):54–6. [PubMed] [Google Scholar]
- 9.Chan KC, Tsun JGS, Li AM, Tam WH. Iron status of full-term infants in early infancy is not associated with maternal ferritin levels nor infant feeding practice. Br J Nutr. 2022;127(8):1198–203. 10.1017/S0007114521001975. [DOI] [PubMed] [Google Scholar]
- 10.Emamghorashi F, Heidari T. Iron status of babies born to iron-deficient anaemic mothers in an Iranian hospital. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2004;10(6):808–14. [PubMed] [Google Scholar]
- 11.Ervasti M, Sankilampi U, Heinonen S, Punnonen K. Early signs of maternal iron deficiency do not influence the iron status of the newborn, but are associated with higher infant birthweight. Acta Obstet Gynecol Scand. 2009;88(1):83–90. 10.1080/00016340802595993. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay K, Yadav RK, Kishore SS, Garewal G, Jain V, Narang A. Iron status at birth and at 4 weeks in preterm-SGA infants in comparison with preterm and term-AGA infants. J Matern-Fetal Neonatal Med off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2012;25(8):1474–8. 10.3109/14767058.2011.643328. [DOI] [PubMed] [Google Scholar]
- 13.Paiva A, Rondó deA, Pagliusi PHC, M do Latorre RA, SSR Gondim RDO, Cardoso MAA. Relationship between the iron status of pregnant women and their newborns. Rev Saude Publica. 2007;41(3):321–7. 10.1590/s0034-89102007000300001. [DOI] [PubMed] [Google Scholar]
- 14.Qaiser DH, Sandila MP, Omair A, Ghori GM. Correlation of routine haematological parameters between normal maternal blood and the cord blood of healthy newborns in selected hospitals of Karachi. J Coll Physicians Surg–Pak JCPSP. 2013;23(2):128–31. [PubMed] [Google Scholar]
- 15.Wedderburn CJ, Ringshaw JE, Donald KA, et al. Association of Maternal and child Anemia with brain structure in early life in South Africa. JAMA Netw Open. 2022;5(12):e2244772. 10.1001/jamanetworkopen.2022.44772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melissa F, Young P, Nguyen LM, Tran LQ, Khuong R, Martorell UR. Long-Term Association between Maternal Preconception Hemoglobin Concentration, Anemia, and Child Health and Development in Vietnam. J Nutr. 2023;153(5):1597–606. 10.1016/j.tjnut.2023.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg Lond Engl. 2021;88:105906. 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700–2700. 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G, Wells G, Shea B et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. In:; 2014. Accessed 31 Aug 2024. https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf.
- 21.Terefe B, Birhanu A, Nigussie P, Tsegaye A. Effect of maternal Iron Deficiency Anemia on the Iron Store of newborns in Ethiopia. Anemia. 2015;2015:808204. 10.1155/2015/808204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timilsina S, Karki S, Gautam A, Bhusal P, Paudel G, Sharma D. Correlation between maternal and umbilical cord blood in pregnant women of Pokhara Valley: a cross sectional study. BMC Pregnancy Childbirth. 2018;18(1):70. 10.1186/s12884-018-1697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Sá SA, Willner E, Duraes Pereira TA, de Souza VR, Teles Boaventura G, Blondet de Azeredo, V. ANEMIA IN PREGNANCY: IMPACT ON WEIGHT AND IN THE DEVELOPMENT OF ANEMIA IN NEWBORN. Nutr Hosp. 2015;32(5):2071–9. 10.3305/nh.2015.32.5.9186. [DOI] [PubMed] [Google Scholar]
- 24.Akhter P, Momen M, Rahman N, et al. Maternal Anemia and its correlation with Iron status of Newborn. BIRDEM Med J. 2014;4. 10.3329/birdem.v4i1.18550.
- 25.Agrawal RM, Tripathi AM, Agarwal KN. Cord blood haemoglobin, iron and ferritin status in maternal anaemia. Acta Paediatr Scand. 1983;72(4):545–8. 10.1111/j.1651-2227.1983.tb09768.x. [DOI] [PubMed] [Google Scholar]
- 26.Reihaneh H, Norimah A, Poh B, Firoozehchian F, Raheleh H. Haemoglobin and Serum Ferritin Levels in Newborn Babies Born to anaemic Iranian women: a cross-sectional study in an Iranian hospital. Pak J Nutr. 2010;9. 10.3923/pjn.2010.562.566.
- 27.Rusia U, Flowers C, Madan N, Agarwal N, Sood SK, Sikka M. Serum transferrin receptor levels in the evaluation of iron deficiency in the neonate. Acta Paediatr Jpn Overseas Ed. 1995;38(5):455–9. 10.1111/j.1442-200x.1996.tb03526.x. [DOI] [PubMed]
- 28.Jose C, Jaime-Perez JL, Herrera-Garza D, Gomez-Almaguer. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2004;36(5):598–602. 10.1016/j.arcmed.2005.03.023. [DOI] [PubMed]
- 29.Basu S, Kumar D, Anupurba S, Verma A, Kumar A. Effect of maternal iron deficiency anemia on fetal neural development. J Perinatol off J Calif Perinat Assoc. 2017;38(3):233–9. 10.1038/s41372-017-0023-5. [DOI] [PubMed]
- 30.Sisson TR, Lund CJ. The influence of maternal iron deficiency on the newborn. Am J Clin Nutr. 1958;6(4):376–85. 10.1093/ajcn/6.4.376. [DOI] [PubMed] [Google Scholar]
- 31.Singla PN, Chand S, Khanna S, Agarwal KN. Effect of maternal anaemia on the placenta and the newborn infant. Acta Paediatr Scand. 1978;67(5):645–8. 10.1111/j.1651-2227.1978.tb17816.x. [DOI] [PubMed] [Google Scholar]
- 32.Adediran A, Gbadegesin A, Adeyemo TA, et al. Cord blood haemoglobin and ferritin concentrations in newborns of anaemic and non-anaemic mothers in Lagos, Nigeria. Niger Med J J Niger Med Assoc. 2013;54(1):22–6. 10.4103/0300-1652.108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali EY, Adam GK, Ahmed S, Ali NI, Adam I. Maternal and neonatal hormonal profiles in anaemic pregnant women of eastern Sudan. J Obstet Gynaecol J Inst Obstet Gynaecol. 2009;29(4):311–4. 10.1080/01443610902878767. [DOI] [PubMed] [Google Scholar]
- 34.Basu S, Kumar N, Srivastava R, Kumar A. Effect of severe maternal Iron Deficiency Anemia on neonatal platelet indices. Indian J Pediatr. 2015;82(12):1091–6. 10.1007/s12098-015-1775-6. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Rai AK, Basu S, Dash D, Singh JS. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121(3):e673–677. 10.1542/peds.2007-1986. [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Kumar N, Srivastava R, Kumar A. Maternal and cord blood hepcidin concentrations in severe Iron Deficiency Anemia. Pediatr Neonatol. 2016;57(5):413–9. 10.1016/j.pedneo.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Awadallah S m., Abu-Elteen K h., Elkarmi A z., Qaraein S h., Salem N m., Mubarak M s. Maternal and cord blood serum levels of zinc, copper, and iron in healthy pregnant Jordanian women. J Trace Elem Exp Med. 2004;17(1):1–8. 10.1002/jtra.10032. [Google Scholar]
- 38.Shukla AK, Srivastava S, Verma G. Effect of maternal anemia on the status of iron stores in infants: a cohort study. J Fam Community Med. 2019;26(2):118–22. 10.4103/jfcm.JFCM_115_18. [DOI] [PMC free article] [PubMed]
- 39.Sangkhae V, Nemeth E. Placental iron transport: the mechanism and regulatory circuits. Free Radic Biol Med. 2019;133:254–61. 10.1016/j.freeradbiomed.2018.07.001. [DOI] [PMC free article] [PubMed]
- 40.Fisher Allison L, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017;106:S1567–74. 10.3945/ajcn.117.155812. [DOI] [PMC free article] [PubMed]
- 41.World Health Organization. Guideline: Counselling of Women to Improve Breastfeeding Practices. World Health Organization. 2018. Accessed 31 Aug 2024. https://iris.who.int/handle/10665/280133. [PubMed]
- 42.Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(8):566–76. 10.7326/M14-2932. [DOI] [PubMed] [Google Scholar]
- 43.Abioye AI, Hughes MD, Sudfeld CR, et al. The effect of iron supplementation on maternal iron deficiency anemia does not differ by baseline anemia type among Tanzanian pregnant women without severe iron deficiency anemia. Eur J Nutr. 2023;62(2):987–1001. 10.1007/s00394-022-03029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traore SS, Bo Y, Kou G, Lyu Q. Iron supplementation and deworming during pregnancy reduces the risk of anemia and stunting in infants less than 2 years of age: a study from Sub-saharan Africa. BMC Pregnancy Childbirth. 2023;23(1):63. 10.1186/s12884-023-05399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iglesias-Vázquez L, Voltas N, Hernández-Martínez C, et al. Importance of maternal Iron status on the improvement of cognitive function in children after prenatal Iron supplementation. Am J Prev Med. 2023;65(3):395–405. 10.1016/j.amepre.2023.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.