Abstract
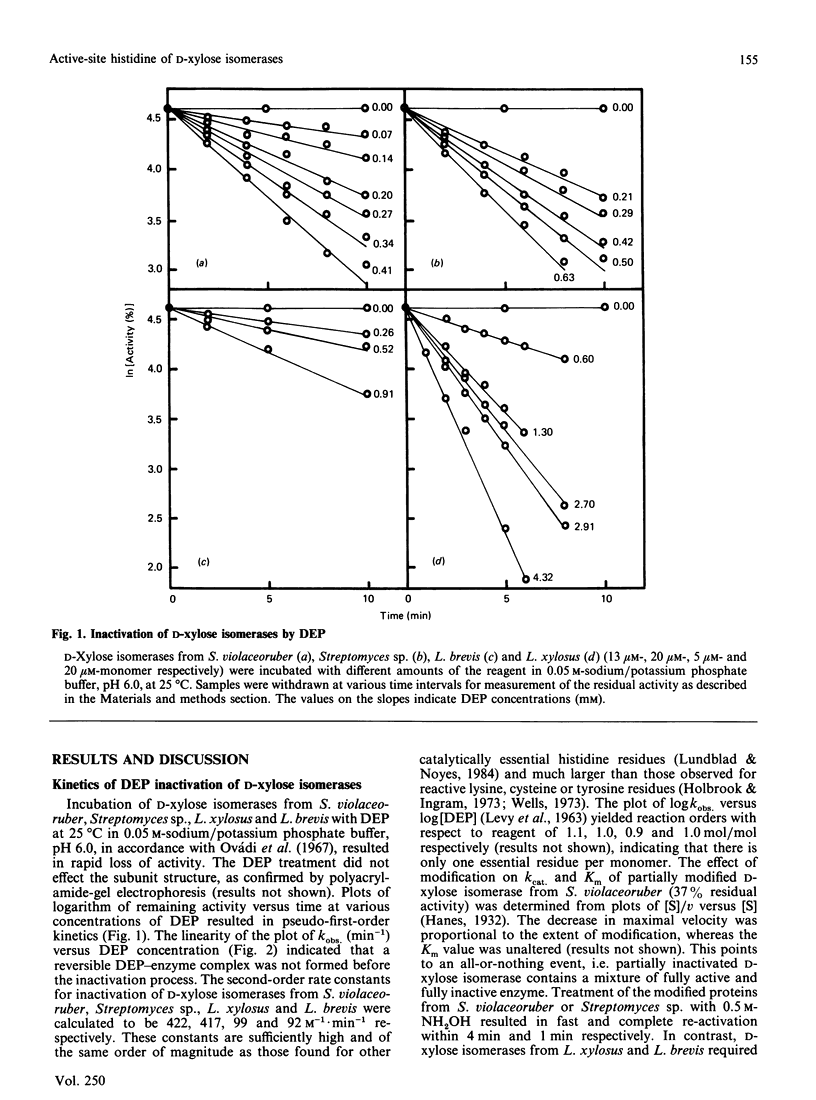
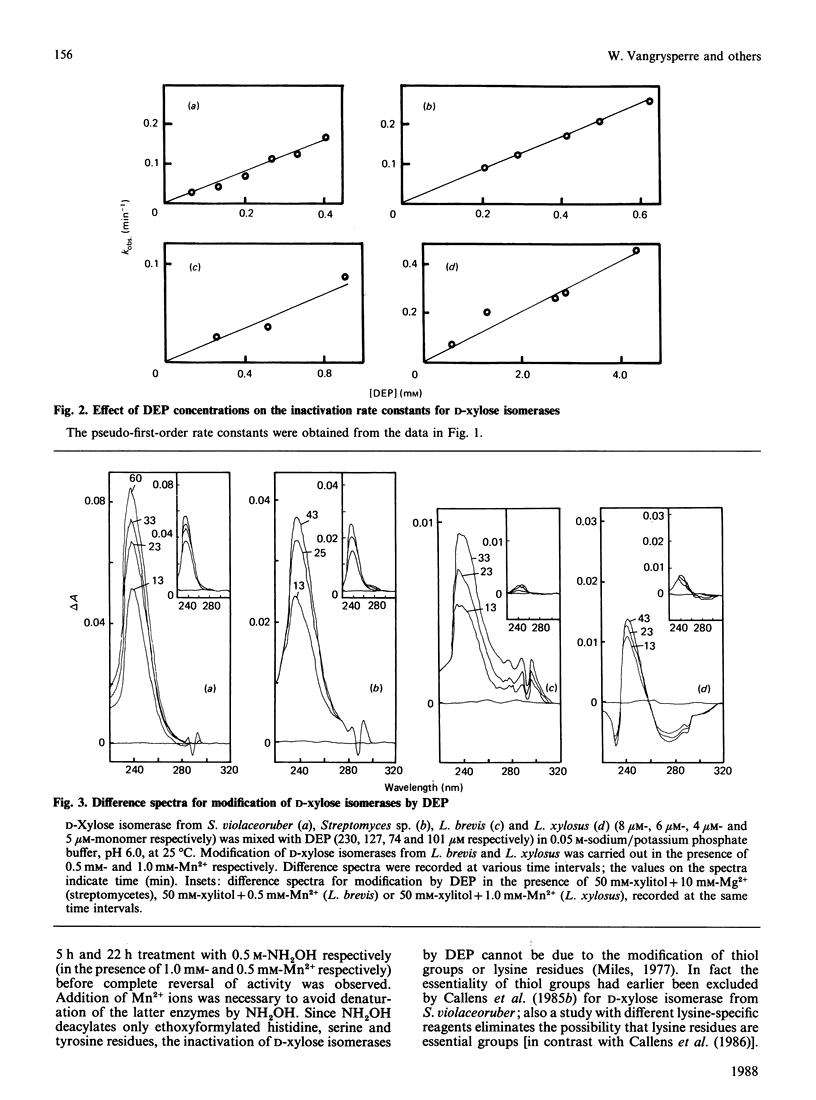
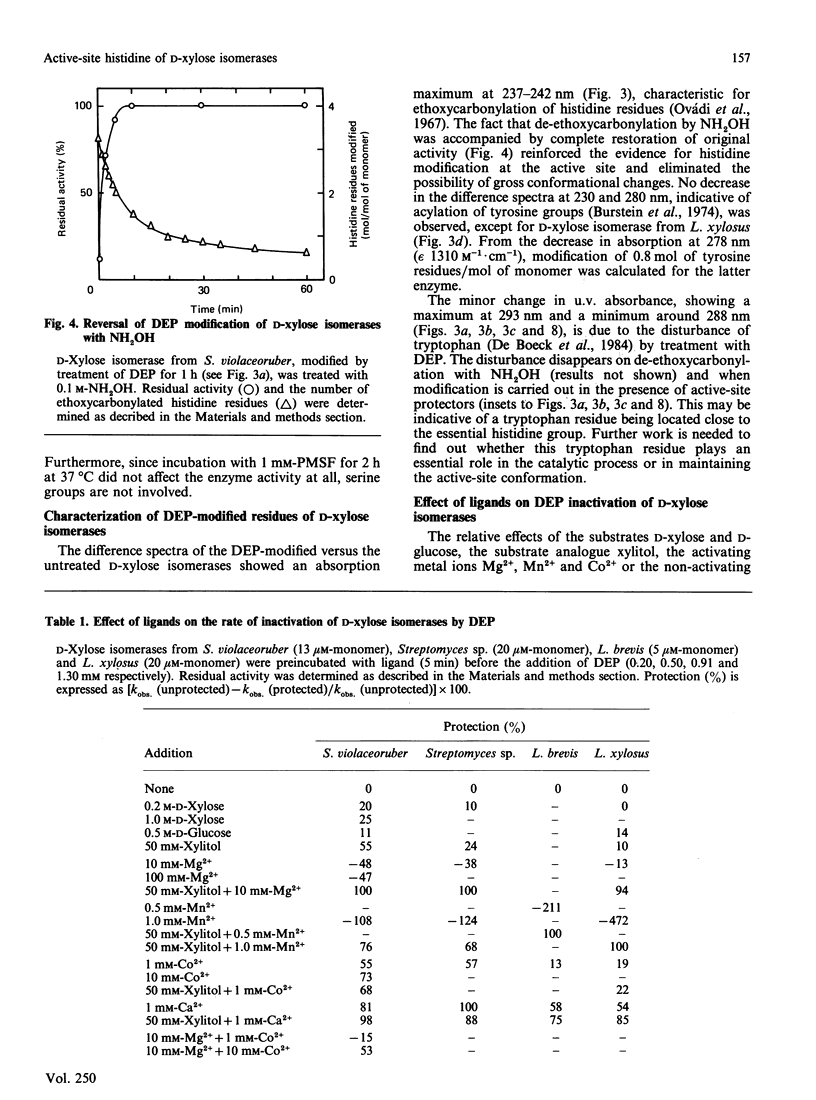
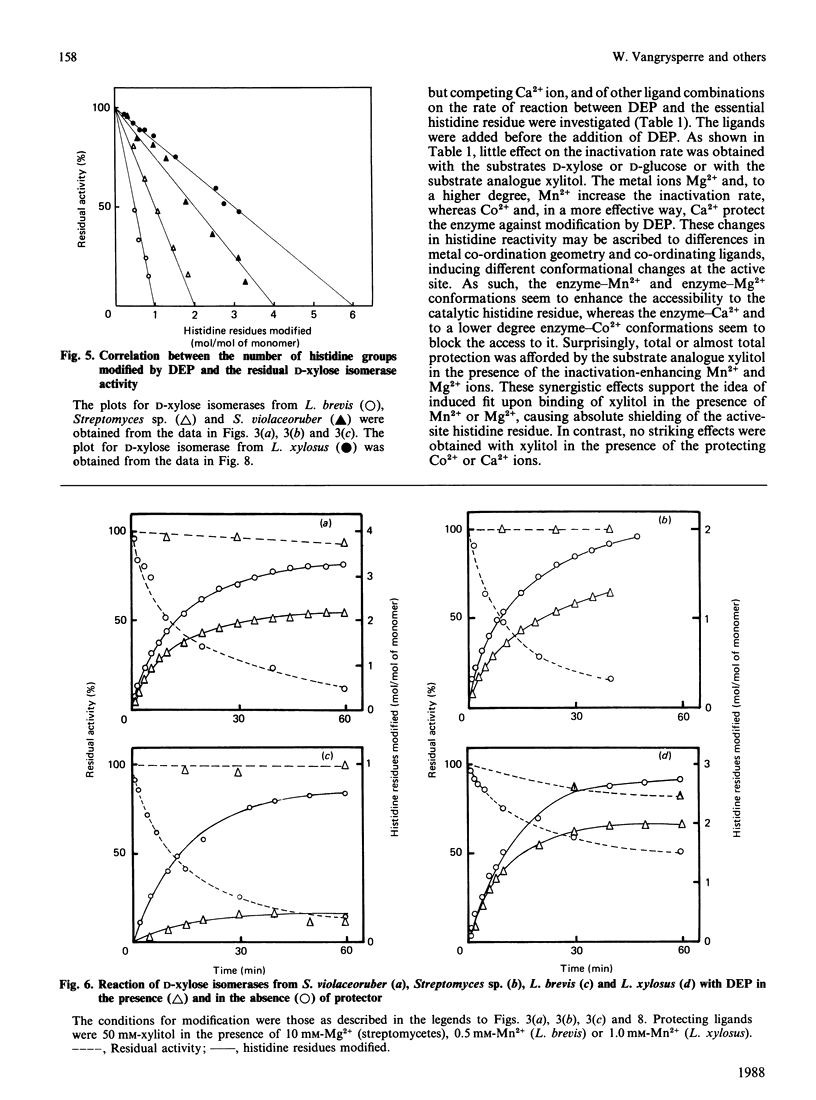
Diethyl pyrocarbonate inactivated D-xylose isomerases from Streptomyces violaceoruber, Streptomyces sp., Lactobacillus xylosus and Lactobacillus brevis with second-order rate constants of 422, 417, 99 and 92 M-1.min-1 respectively (at pH 6.0 and 25 degrees C). Activity was completely restored by the addition of neutral hydroxylamine, and total protection was afforded by the substrate analogue xylitol in the presence of either Mg2+ or Mn2+ according to the genus studied. The difference spectra of the modified enzymes revealed an absorption maximum at 237-242 nm, characteristic for N-ethoxycarbonylhistidine. In addition, the spectrum of ethoxycarbonylated D-xylose isomerase from L. xylosus showed absorption minima at both 280 and 230 nm, indicative for modification of tyrosine residues. Nitration with tetranitromethane followed by diethyl pyrocarbonate treatment eliminated the possibility that modification of tyrosine residues was responsible for inactivation, and resulted in modification of one non-essential tyrosine residue and six histidine residues. Inactivation of the other D-xylose isomerases with diethyl pyrocarbonate required the modification of one (L. brevis), two (Streptomyces sp.) and four (S. violaceoruber) histidine residues per monomer. Spectral analysis and maintenance of total enzyme activities further indicated that either xylitol Mg2+ (streptomycetes) or xylitol Mn2+ (lactobacilli) prevented the modification of one crucial histidine residue. The overall results thus provide evidence that a single active-site histidine residue is involved in the catalytic reaction mechanism of D-xylose isomerases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein Y., Walsh K. A., Neurath H. Evidence of an essential histidine residue in thermolysin. Biochemistry. 1974 Jan 1;13(1):205–210. doi: 10.1021/bi00698a030. [DOI] [PubMed] [Google Scholar]
- De Boeck H., Lis H., van Tilbeurgh H., Sharon N., Loontiens F. G. Binding of simple carbohydrates and some of their chromophoric derivatives to soybean agglutinin as followed by titrimetric procedures and stopped flow kinetics. J Biol Chem. 1984 Jun 10;259(11):7067–7074. [PubMed] [Google Scholar]
- Dyson J. E., Noltmann E. A. The effect of pH and temperature on the kinetic parameters of phosphoglucose isomerase. Participation of histidine and lysine in a proposed dual function mechanism. J Biol Chem. 1968 Apr 10;243(7):1401–1414. [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Horiuchi S., Morino Y. Inactivation of gamma-glutamyl transpeptidase by phenylmethanesulfonyl fluoride, a specific inactivator of serine enzymes. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1183–1188. doi: 10.1016/0006-291x(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Kumar S. Functional deacylases of pigeon liver fatty acid synthetase complex. J Biol Chem. 1975 Jul 10;250(13):5150–5158. [PubMed] [Google Scholar]
- LEVY H. M., LEBER P. D., RYAN E. M. INACTIVATION OF MYOSIN BY 2,4-DINITROPHENOL AND PROTECTION BY ADENOSINE TRIPHOSPHATE AND OTHER PHOSPHATE COMPOUNDS. J Biol Chem. 1963 Nov;238:3654–3659. [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- O'Connell E. L., Rose I. A. Affinity labeling of phosphoglucose isomerase by 1,2-anhydrohexitol-6-phosphates. J Biol Chem. 1973 Mar 25;248(6):2225–2231. [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. Intramolecular hydrogen transfer in the phosphoglucose isomerase reaction. J Biol Chem. 1961 Dec;236:3086–3092. [PubMed] [Google Scholar]
- Sanchez S., Smiley K. L. Properties of D-xylose isomerase from Streptomyces albus. Appl Microbiol. 1975 Jun;29(6):745–750. doi: 10.1128/am.29.6.745-750.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Sarthy A., Larson A. E., Backer M. P., Crabb J. W., Lidstrom M., Hall B. D., Furlong C. E. Xylose isomerase from Escherichia coli. Characterization of the protein and the structural gene. J Biol Chem. 1984 Jun 10;259(11):6826–6832. [PubMed] [Google Scholar]
- Schray K. J., Rose I. A. Anomeric specificity and mechanism of two pentose isomerases. Biochemistry. 1971 Mar 16;10(6):1058–1062. doi: 10.1021/bi00782a019. [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Muirhead H. The active site of glucose phosphate isomerase. FEBS Lett. 1976 May 15;65(1):50–55. doi: 10.1016/0014-5793(76)80619-9. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- TSOU C. L. Relation between modification of functional groups of proteins and their biological activity. I.A graphical method for the determination of the number and type of essential groups. Sci Sin. 1962 Nov;11:1535–1558. [PubMed] [Google Scholar]
- Wells M. A. Effects of chemical modification on the activity of Crotalus adamanteus Phospholipase A 2 . Evidence for an essential amino group. Biochemistry. 1973 Mar 13;12(6):1086–1093. doi: 10.1021/bi00730a011. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]
- Wilhelm M., Hollenberg C. P. Selective cloning of Bacillus subtilis xylose isomerase and xylulokinase in Escherichia coli genes by IS5-mediated expression. EMBO J. 1984 Nov;3(11):2555–2560. doi: 10.1002/j.1460-2075.1984.tb02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worku Y., Luzio J. P., Newby A. C. Identification of histidyl and cysteinyl residues essential for catalysis by 5'-nucleotidase. FEBS Lett. 1984 Feb 27;167(2):235–240. doi: 10.1016/0014-5793(84)80133-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka K. D-xylose isomerase from Lactobacillus brevis. Methods Enzymol. 1975;41:466–471. doi: 10.1016/s0076-6879(75)41101-6. [DOI] [PubMed] [Google Scholar]


