Abstract
Context.
Patients with gastric cancer experience health-related quality of life (HRQOL) decline during adjuvant chemotherapy following gastrectomy.
Objectives.
This pilot study aimed to evaluate the preliminary effect and feasibility of electro-acupuncture (EA) for HRQOL and symptom burden in these patients.
Methods.
In this open-label, multicenter, parallel controlled trial, gastric cancer patients who planned to receive adjuvant chemotherapy were randomly assigned to receive high-dose EA (seven times each chemotherapy cycle for three cycles), low-dose EA (three times each chemotherapy cycle), or usual care only. The acupoints prescription consisted of bilateral ST36, PC6, SP4, and DU20, EX-HN3, and selected Back-shu points. Patients completed the Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) weekly, and the Edmonton Symptom Assessment System (ESAS). The primary outcome was the difference among the groups on the gastric cancer subscale (GaCS) of the FACT-Ga.
Results.
Of the 66 randomized patients, 58 were analyzed according to intention-to-treat principle, and 45 were in the per-protocol set (PPS). The average scores in PPS of GaCS were 52.12±9.71, 51.85±12.36, and 45.37±8.61 in high-dose EA, low-dose EA, and control groups, respectively. EA was significantly associated with improved average GaCS scores when compared with control group (51.98±10.91 vs. 45.37±8.61, P = 0.039). EA treatment also produced ESAS relief at the end of intervention (14.36 ± 12.28 vs. 23.91 ± 15.52, P = 0.027). Participants in EA groups had fewer grade ≥3 leukopenia (0% vs. 15.79%, P = 0.031) and neutropenia (2.56% vs. 26.31%, P = 0.012).
Conclusion.
EA showed promising effects in improving HRQOL, controlling symptom burden, and reducing toxicity during adjuvant chemotherapy in gastric cancer patients. Future adequately powered trials are feasible and needed to confirm the specific effect of EA.
Keywords: Acupuncture, health-related quality of life, symptom burden, gastric cancer, adjuvant chemotherapy
Background
Gastric cancer is the fifth most frequently diagnosed cancer and the fourth leading cause of cancer-related death worldwide.1 Gastrectomy followed by adjuvant chemotherapy remains the preferred strategy for treatment of local gastric cancer, resulting in improved long-term survival rates. However, the sequelae of gastrectomy and the toxicities of adjuvant chemotherapy lead to a significant decline in health-related quality of life (HRQOL). This is of particular concern during the first three months after gastrectomy when the most common symptoms are nausea, early satiety, reflux, and pain.2 These symptoms can even lead to discontinuation of chemotherapy. Thus, identifying interventions to control symptom burden and improve HRQOL during adjuvant chemotherapy has important clinical significance.
Acupuncture, a component of Traditional Chinese Medicine (TCM), is a popular integrative medicine intervention in oncology settings.3,4 Acupuncture has been found to reduce various symptoms and treatment-related toxicities in cancer patients,4 including pain,5,6 fatigue,7 nausea and/or vomiting,8 and peripheral neuropathy,9 which are also common in postoperative gastric cancer patients. Therefore, we hypothesized that acupuncture may also help to control symptom burden and improve HRQOL of gastric cancer patients undergoing adjuvant chemotherapy. However, efficacy of acupuncture for these patients has never been reported.
The objectives of this pilot study included: 1) To estimate the effect size of electro-acupuncture (EA) on HRQOL and symptom burden in gastric cancer patients undergoing adjuvant chemotherapy. In order to improve the generalizability of our results, EA, with unified electrical stimulation parameters, rather than manual acupuncture, was used in this study. 2) To refine an optimal EA frequency for future trials, since evidences for optimal acupuncture dosage in oncology settings are still lack. Therefore, two EA groups, high-dose and low dose, were set in this study. 3) To assess the feasibility and refine the logistics in planning for a larger clinical trial, EA and questionnaire completion rates in this study were also evaluated.
Methods
Study Participants
We conducted a multicenter, three-arm (high-dose EA, low-dose EA, and control), parallel randomized clinical trial from January 2019 through February 2020 at seven hospitals in China. This study was approved by the ethics committees of each hospital before participant enrollment at each site, including Guangdong Provincial Hospital of Traditional Chinese Medicine (BF2018–118), the First Affiliated Hospital of Sun Yat-sen University (2019−091), Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (2018NL-172), the Sixth Affiliated Hospital of Sun Yat-sen University (2020ZSLYEC-029), Guangdong Provincial People’s Hospital (GDREC2019339H), Affiliated Cancer Hospital & Institute of Guangzhou Medical University (2019–1), and the First Affiliated Hospital of Guangzhou Medical University (2019−43). All participants provided informed consent before randomization. This study has been registered in ClinicalTrials (NCT03753399), and was first released on November 27, 2018. We conducted and reported this study according to the Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist with extension for pilot trials10 and the STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) 2010 extension11 (Table S1).
Study inclusion criteria included patients: 1) who were pathologically diagnosed with stage Ⅱ/Ⅲ gastric or esophagogastric junction cancer after R0 resection and D2 lymph node dissection; 2) without tumor recurrence confirmed by image examination; 3) without chemotherapy after resection and planning to accept at least 3 cycles of adjuvant chemotherapy; 4) 18−75 years old; 5) with ECOG score≤2; 6) with normal organs function, defined as absolute neutrophil count ≥1.5 × 109/L, platelet ≥100 × 109/ L,hemoglobin ≥90 g/L, serum creatinine ≤1.5 mg/dl (133μmol/L), or creatinine clearance rate ≥60ml/min, total bilirubin ≤1.5 × upper limit of normal value (ULN), alanine transaminase ≤2.5 × ULN, and aspartate transaminase ≤2.5 × ULN; and 7) who can understand the study well and complete the study questionnaires. Exclusion criteria included patients: 1) unable to finish the baseline assessment, 2) with needle phobia; 3) currently diagnosed with psychiatric disorders (e.g., severe depression, obsessive-compulsive disorder, or schizophrenia); 4) with a history of autoimmune diseases, hematological diseases or organ transplantation, or long term use of hormones or immunosuppressors; 5) implanted with heart pacemaker; 6) with neoadjuvant radiotherapy before surgery; 7) with plans of adjuvant radiotherapy during the next three cycles of chemotherapy; 8) with current active infection; 9) who had acupuncture treatment within the previous six weeks; and 10) who were pregnant or breast-feeding.
Randomization
Participants were randomly assigned to a high-dose EA group, a low-dose EA group, or a control group (1:1:1) using the central randomization system, allowing for full allocation concealment, provided by the Institute of Clinical Pharmacology of Xiyuan Hospital, China Academy of Chinese Medical Sciences. Random assignment was stratified by resection extent (total/proximal or distal gastrectomy) and neoadjuvant chemotherapy status (yes or no).
Interventions
All participants were treated with adjuvant chemotherapy with the CapeOx or SOX regimen. The CapeOx regimen consisted of 130 mg/m2 of oxaliplatin intravenously on the first day and 1000mg/m2 of capecitabine twice daily for 14 consecutive days, every 21 days. The SOX regimen consisted of 130 mg/m2 of oxaliplatin intravenously on the first day and S-1 twice daily for 14 consecutive days, every 21 days. The dose of S-1 was also calculated according to body surface area (BSA): BSA <1.25m2, 80 mg/day; 1.25m2 ≤BSA <1.5m2, 100 mg/day; BSA >1.5m2, 120 mg/day.
Participants in the high-dose group received EA treatment seven times during each chemotherapy cycle (three times in the first week, twice per week in the next two weeks), for a total of 21 sessions during the first three chemotherapy cycles. Those in the low-dose group received EA treatment three times during each chemotherapy cycle (once per week), for a total of nine sessions during the first three chemotherapy cycles. The acupoints prescription consisted of standard points and selected points. Standard points included bilateral ST36 (Zusanli), bilateral PC6 (Neiguan), bilateral SP4 (Gongsun), DU20 (Baihui), and EX-HN3 (Yintang). Selected points were Back-shu points, in the urinary bladder meridian, chosen according to TCM differentiation (see Supplementary method). The acupoints prescription was designed according to TCM theory and our clinical experience, focusing on tonifying Qi,12 considering gastrointestinal symptoms as most common in gastric cancer patients during chemotherapy after gastrectomy.2
Licensed acupuncturists with at least 2 years of experience, from 11 acupuncture sites, performed the EA interventions. Before treating study participants with EA, all of the acupuncturists received training on the specific protocol and completed study checklists, and XSC checked documentation and provided feedback to ensure treatment fidelity. Acupuncturists inserted and manipulated the needles (25 mm or 40 mm and 0.25 mm gauge; Hanyi Medical Instrument Co., Ltd, Beijing) until patients reported De Qi, a sensation of soreness, warmth, tingling, or heaviness. For EA, participants were in sitting, prone, or in a lateral position. The acupuncture needles were inserted perpendicular, to a depth of approximately 20–30 mm for ST36, 10−20 mm for SP4 and PC6 from the skin surface. For DU20, EX-HN3, and Back-shu points, we inserted acupuncture needles 10−15 mm deep at an angle of 30 degrees to the skin. A bilateral 2-Hz current was connected to the ST36 and PC6 points, using an electrical stimulator (G6805–1 EA apparatus; Xinsheng Industrial Co., Ltd, Qingdao, China). The needles were retained for 20 minutes.
Outcome Measures
We used the Chinese version of Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) questionnaire (https://www.facit.org/) to assess HRQOL,13 which has been validated in Chinese population with excellent reliability, construct validity, and sensitivity to distinguish changes in responsible to different clinical characteristics and interventions.14 The FACT-Ga consists of five subscales, including physical well-being (PWB, seven items), social/family well-being (SWB, seven items), emotional well-being (EWB, six items), functional well-being (FWB, seven items), and the gastric cancer subscale (GaCS, 19 items). A higher score indicates better HRQOL. Patients completed the FACT-Ga before chemotherapy and then once a week during the study duration (three weeks/cycle × three cycles of chemotherapy, ten times in total) using a patient diary. The GaCS subscale was the main indicator of HRQOL in this pilot study since it is specific to gastric cancer.
We used the Chinese version of Edmonton Symptom Assessment System (ESAS) to assess the symptom burden.15 In the Chinese version of ESAS, 11 symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite loss, nonwellbeing, itching, breath shortness, and other problems, were scored using a numeric rating scale (NRS), ranging from 0 to 10. Higher scores indicate greater symptom severity. Since the itching symptom was specific for patients with jaundice, which is not common in our participants, and are not included in other versions of ESAS,16 we didn’t score the itching symptom; therefore, only 10 symptoms were recorded, and the total ESAS score ranges from 0 to 100 in this study.
The primary outcome was the average of the GaCS scores during the three cycles of chemotherapy. We chose this as the primary outcome because the effect of EA on HRQOL should persist throughout the entire phase of the intervention rather than at any specific time point.17,18 Secondary outcomes included the average scores of trial outcome index variables (TOI = PWB + FWB + GaCS), FACT-Ga total scores (PWB + SWB + EWB + FWB + GaCS), and ESAS total scores. Research staff, not blinded to treatment groups, monitored adverse events (AEs) according to National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTC AE, V4.0) before each cycle of chemotherapy.
Statistical Analysis
According to the statistics analysis plan for this study, primary outcomes should be compared according to the intention-to-treat (ITT) principle. However, due to the COVID-19 pandemic, 13.79% of participants did not complete the treatments per protocol. Therefore, we modified our analyses to compare the outcomes in per-protocol set (PPS) population; we also compared the differences in the ITT population as sensitivity analysis. Missing data were imputed using the worst observation carried forward (WOCF) method. We used student’s t test, analysis of variance (ANOVA) with least significant difference (LSD) for post hoc test, x2 test, or Fisher’s exact test to test the differences among groups. ANOVA for repeated measures was used to test the within-subject differences during the intervention and among each cycle of chemotherapy. Data were analyzed using SPSS (version 16.0). The Statistician was not blinded to the treatment groups.
We postulated that the GaCS average score would be 40 in the control group, with a standard deviation of 10, according to the trajectory investigation by Munene G et al.2 As a pilot study, we planned to enroll a total of 54 patients, randomizing 36 in EA groups (18 in the high-dose group and 18 in the low-dose group), and 18 in control group, allowing for an 81% power to detect a difference of 8.2 in GaCS between EA and control groups, the minimally important difference (MID) for GaCS,13 using a two side hypothesis test at a significance level of 0.05. We hoped that more than 90% of participants would complete the intervention per-protocol and answer all of the questionnaires, determining a good feasibility for a larger trial. Therefore, we assumed a 10% dropout rate, and a total of 60 subjects (20 per arm) were needed.
Results
Feasibility Outcomes
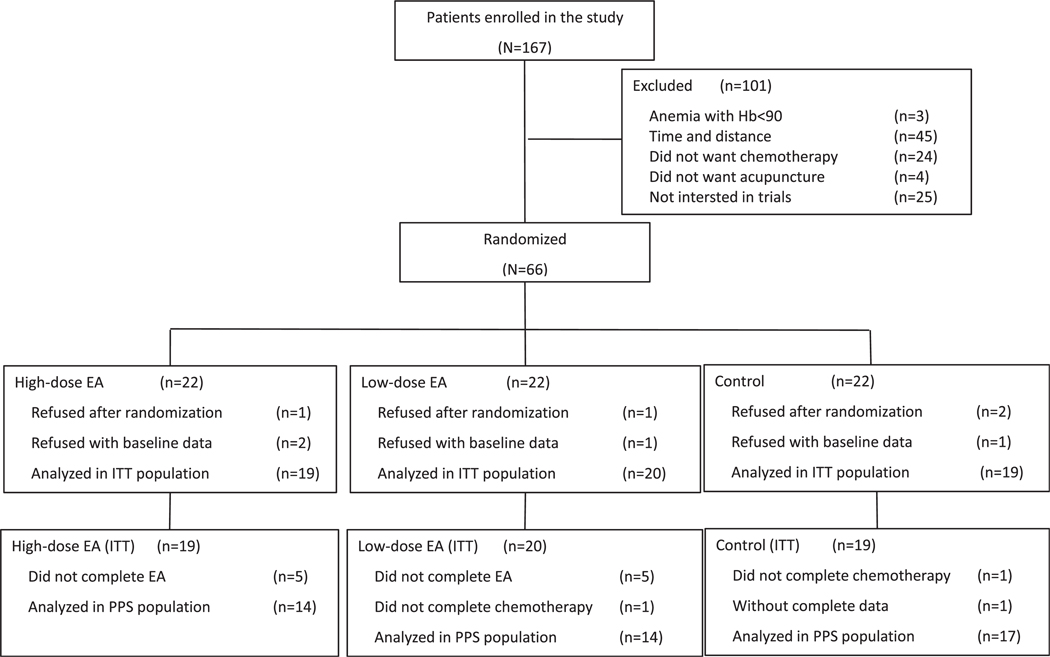
A total of 167 patients were assessed for eligibility, and 66 patients were randomized. Eight patients refused to participate after randomization or baseline data collection. Finally, a total of 58 patients, including 5 (8.62%) with missing FACT-Ga or ESAS data during the treatment, were analyzed as ITT population (Fig. 1).
Fig. 1.
Participants recruitment diagram for this study. Hb = hemoglobin; EA = electro-acupuncture; ITT = intention to treat; PPS = per-protocol set.
A total of 10 patients did not complete the acupuncture per protocol; for 6 patients, this was due to the COVID-19 pandemic. Two other patients refused the third cycle of adjuvant chemotherapy also due to the COVID-19 pandemic. In addition, one patient in the control group did not complete the FACT-Ga or ESAS data during the study. Finally, a total of 45 patients were analyzed as PPS population (Fig. 1). Regardless of the impact of COVID-19 pandemic (8/58, 13.79%), the dropout rate was 8.62% (5/58).
Patient Characteristics and Treatments
Baseline characteristics of the study population were balanced well among the groups (Table 1). The mean age was 55.95±11.23 years. Thirty-six patients (62.07%) were male, and 40 (68.97%) were diagnosed with stage Ⅲ disease. Most patients had an ECOG physical status (PS) score of 1 (86.21%, 50/58), and only one patient had a PS score of 2 (in the low-dose EA group). Thirty-two patients (55.17%) were treated with distal gastrectomy, 22 (37.93%) with total gastrectomy, and only 4 (6.90%) with proximal gastrectomy. Twenty-eight patients (48.28%) underwent laparoscopic surgery, and the others underwent open surgery. Only eight patients (13.79%) accepted neoadjuvant chemotherapy. The median time from surgery to the first cycle of adjuvant chemotherapy was 30 days (25%−75% percentile, 25−38 days). Most patients (87.93%, 51/58) were treated with the CapeOx regimen, and five (8.62%) were treated with CapeOx at first, but then changed to other oxaliplatin-containing regimens. The other two patients were treated with the SOX regimen. The median number of acupoints of each session was 12, with a range of 10−14.
Table 1.
Demographic and Clinical Characteristics of Patients in the Intention to Treat Population
| Characteristics | High-dose EA (n = 19) | Low-dose EA (n = 20) | Control Group (n = 19) |
|---|---|---|---|
| Age (year), mean ± SD | 55.00±12.41 | 58.75±8.85 | 53.95±12.19 |
| Gender | |||
| Male | 15 (78.95%) | 11 (55.00%) | 10 (52.63%) |
| Female | 4 (21.05%) | 9 (45.00%) | 9 (47.37%) |
| ECOG physical status score | |||
| 0 | 2 (10.53%) | 2 (10.00%) | 3 (15.79%) |
| (1–2)* | 17 (89.47%) | 18 (90.00%) | 16 (84.21%) |
| Stage | |||
| II | 5 (26.32%) | 6 (30.00%) | 7 (36.84%) |
| III | 14 (73.68%) | 14 (70.00%) | 12 (63.16%) |
| Resection extent | |||
| Total/proximal gastrectomy | 9 (47.37%) | 9 (45.00%) | 8 (42.11%) |
| Distal gastrectomy | 10 (52.63%) | 11 (55.00%) | 11 (57.89%) |
| Surgical approach | |||
| Laparoscope | 9 (47.37%) | 10 (50.00%) | 9 (47.37%) |
| Open surgery | 10 (52.63%) | 10 (50.00%) | 10 (52.63%) |
| Neo-adjuvant chemotherapy | |||
| Yes | 4 (21.05%) | 2 (10.00%) | 2 (10.53%) |
| No | 15 (78.95%) | 18 (90.00%) | 17 (89.47) |
| Days from surgery to adjuvant | |||
| chemotherapy, median (range) | 29 (20–58) | 30 (22–65) | 34 (19–52) |
| Number of acupoints, median (range) | 12 (10–14) | 12 (10–13) | - |
Abbreviations: EA = electro-acupuncture; SD = standard deviation; ECOG = Eastern Cooperative Oncology Group.
Only 1 patient in low-dose group was with ECOG PS=2.
EA Significantly Improved Quality of Life
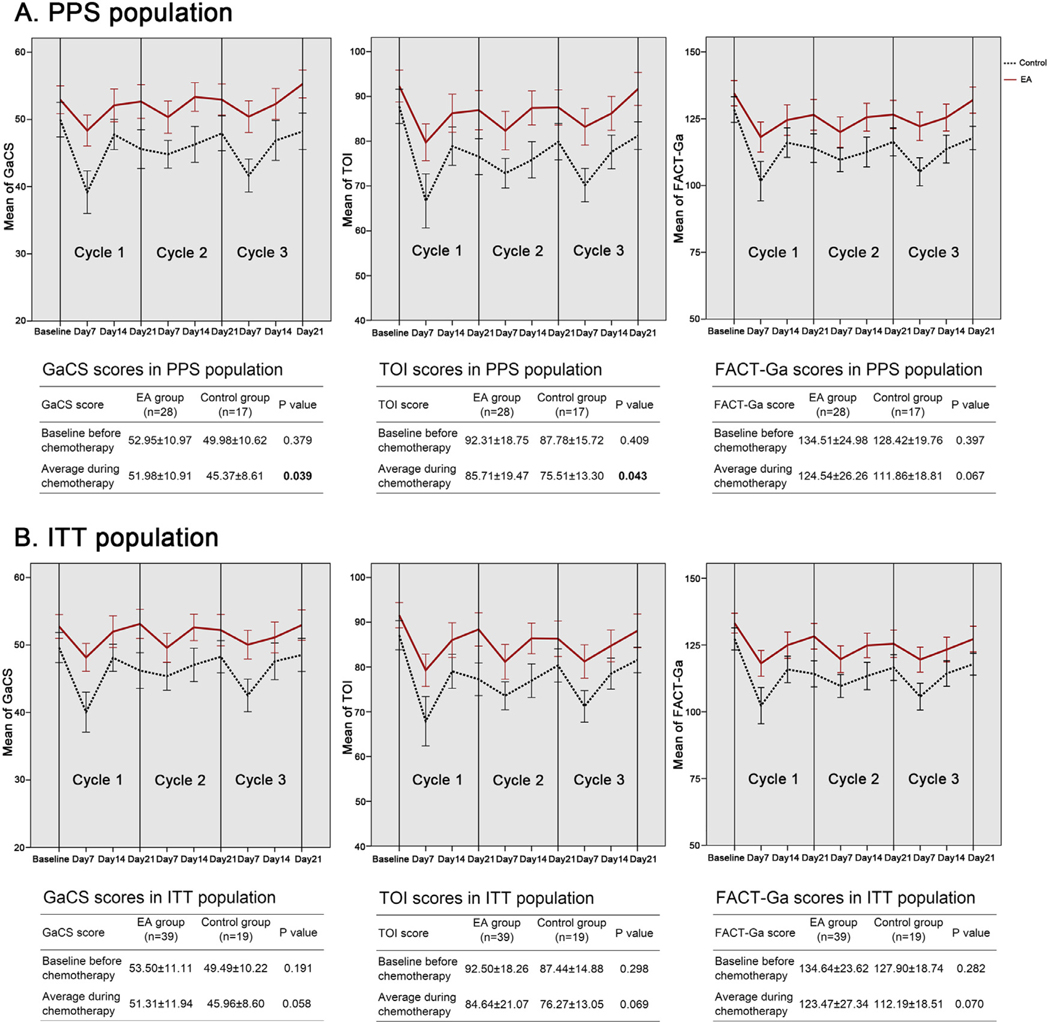
Expectedly, the GaCS (P < 0.001), TOI (P < 0.001), and FACT-Ga (P < 0.001) scores were significantly different during the intervention, with the worst scores on the day7 of each chemotherapy cycle (Fig. 2a). Differences of GaCS (P = 0.320), TOI (P = 0.403), or FACT-Ga (P = 0.525) among each cycle of chemotherapy were not significant. Average scores of GaCS (51.98 ± 10.91 vs. 45.37 ± 8.61, P = 0.039) and TOI (85.71 ± 19.47 vs. 75.51 ± 13.30, P = 0.043) during the chemotherapy for patients in EA groups were significantly higher than those in control group (Fig. 2a, Table S2). EA treatment also produced a trend of better average scores, yet not statistically significant, of FACT-Ga (P = 0.067) and other subscales (Fig. 2a, Fig. S1a). Similar trend of changes on GaCS (P = 0.058), TOI (P = 0.069), and total FACT-Ga (P = 0.070) scores between EA and control groups, although not significantly different, were also indicated in the sensitivity analysis using ITT population (Fig. 2b).
Fig. 2.
Scores of Gastric Cancer Subscale (GaCS), Trial Outcome Index (TOI), and the total Functional Assessment of Cancer Therapy-Gastric (FACT-Ga), the higher, the better, for patients in EA and control groups in per-protocol set (2a) and intention to treat (2b) populations. Data were shown as mean ± standard error (SE) in the figures and mean ± standard derivation (SD) in the tables. EA = electro-acupuncture; PPS = per-protocol set; ITT = intention to treat.
EA Helped to Reduce Symptom Burden
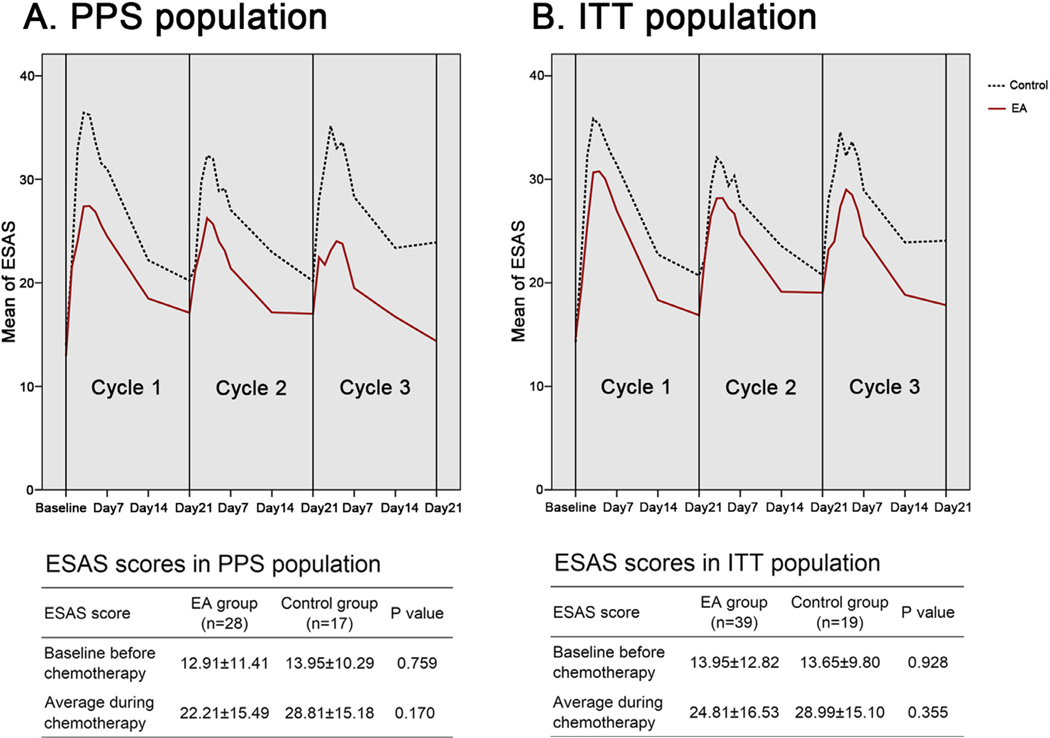
Again, the ESAS scores were significantly different during the intervention (P < 0.001), with the worst symptom burden in the first weeks after each cycle of chemotherapy (Fig. 3a). Differences of ESAS scores among each cycle of chemotherapy were not significant (P = 0.572). The average ESAS scores during intervention in the EA and control groups were not significantly different (22.21±15.49 vs. 28.81±15.18, P = 0.170). Nevertheless, the last reported ESAS scores, reported at the end of intervention, in the EA group were significantly lower than those in control group (14.36±12.28 vs. 23.91±15.52, P = 0.027, Table S2), suggesting a promising effect of EA treatment to reduce total ESAS scores (Fig. 3a). No significant differences on symptom scores recorded in ESAS questionnaire between the EA and control groups were indicated, except for the last reported feeling of nonwellbeing (2.18±1.95 vs. 3.47±2.27, P = 0.049), pain (0.82± 1.25 vs. 1.76±1.82, P = 0.045), and shortness of breath (0.82±1.28 vs. 1.88±1.69, P = 0.021, Fig. S2). Similar trend of changes on total ESAS scores between EA and control groups, although not significantly different, was also indicated in the sensitivity analysis using ITT population (Fig. 3b).
Fig. 3.
Scores of total Edmonton Symptom Assessment System (ESAS), the lower, the better, for patients in EA and control groups in per-protocol set (3a) and intention to treat (3b) populations. Data were shown as mean± derivation (SD). EA = electro-acupuncture; PPS = per-protocol set; ITT = intention to treat.
Association of EA Dose and Efficacy
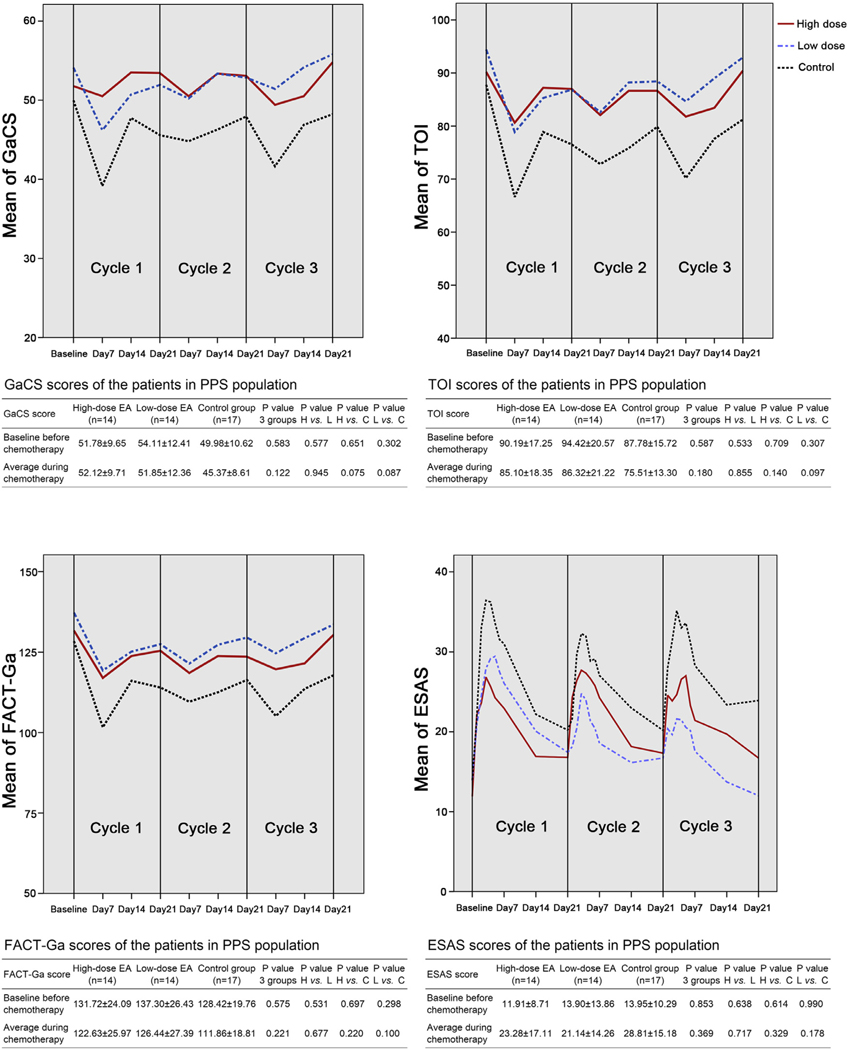
No significant differences on GaCS (p=0.945), TOI (P = 0.855), FACT-Ga (P = 0.677), ESAS (P = 0.717, Fig. 4), or other FACT-Ga subscales (Fig. S1b) between the high-dose and low-dose EA groups were indicated. Interestingly, in the symptom and psychology-associated PWB (P = 0.702), and EWB (P = 0.707) subscales, efficacy of EA was very similar between the high-dose and low-dose groups (Fig. S1b). However, in the social-associated SWB (P = 0.268) and FWB (P = 0.311) domains, high-dose EA even produced a trend of worse scores when comparing with low-dose EA, although the differences were not significant (Fig. S1b).
Fig. 4.
Scores of Gastric Cancer Subscale (GaCS), Trial Outcome Index (TOI), the total Functional Assessment of Cancer Therapy-Gastric (FACT-Ga), the higher, the better, and the total Edmonton Symptom Assessment System (ESAS), the lower, the better, for patients in high-dose EA, low-dose EA, and control groups in per-protocol set. Data were shown as mean±derivation (SD). EA = electro-acupuncture; PPS = per-protocol set; H vs. L = high-dose vs. low-dose EA; H vs. C = high-dose EA vs. control; L vs. C = low-dose EA vs. control.
Safety Data
Treatment-related AEs occurred in 94.74% (grade 3−4, 15.79%), 95.00% (grade 3−4, 15.00%), and 100.00% (grade 3−4, 31.58%) of patients in high-dose EA group, low-dose EA group, and control group, respectively (Table 2). The most common AEs were nausea (75.86%), neutropenia (60.34%), leukopenia (50.00%), peripheral sensory neuropathy (46.55%), vomiting (39.66%), anemia (39.66%), diarrhea (34.48%), and increased ALT/AST (34.48%). The most common grade 3−4 AEs was neutropenia (10.34%), vomiting (8.62%), nausea (6.90%), and leukopenia (5.17%). EA was associated with reduced grade 3−4 leukopenia (0% vs. 15.79%, P = 0.031) and neutropenia (2.56% vs. 26.31%, P = 0.012). In addition, trends of reduced grade 3−4 AEs (15.38% vs. 31.58%, P = 0.153), all grade of neutropenia (30.77% vs. 68.42%, P = 0.380) and vomiting (33.33% vs. 52.63%, P = 0.159) were indicated in EA group, although the differences were not significant.
Table 2.
Treatment-related Adverse Events of Patients in the Intention to Treat Population
| Characteristics | EA (n = 39) | Control Group (n = 19) | P | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Any Grade | Grade 3-4 | Any Grade | Grade 3-4 | Any Grade | Grade 3-4 | |
| Any adverse event | 37 (94.87%) | 6 (15.38%) | 19 (100%) | 6 (31.58%) | 1.000a | 0.153b |
| Leukopenia | 19 (48.72%) | 0 (0%) | 10 (52.63%) | 3 (15.79%) | 0.780b | 0.031 a |
| Neutropenia | 22 (30.77%) | 1 (2.56%) | 13 (68.42%) | 5 (26.31%) | 0.380b | 0.012 a |
| Thrombocytopenia | 7 (17.95%) | 0 (0%) | 2 (10.53%) | 0 (0%) | 0.703a | - |
| Anemia | 14 (35.90%) | 0 (0%) | 9 (47.37%) | 0 (0%) | 0.402b | - |
| ALT/AST increased | 11 (28.21%) | 0 (0%) | 9 (47.37%) | 0 (0%) | 0.150b | - |
| Bilirubin increased | 2 (5.13%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.000a | - |
| Creatinine increased | 1 (2.56%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.000a | - |
| Nausea | 28 (71.79%) | 3 (7.69%) | 14 (73.68%) | 1 (5.26%) | 0.880b | 1.000a |
| Vomiting | 13 (33.33%) | 3 (7.69%) | 10 (52.63%) | 2 (10.53%) | 0.159b | 1.000a |
| Diarrhea | 16 (41.03%) | 2 (5.13%) | 4 (21.05%) | 0 (0%) | 0.155a | 1.000a |
| Constipation | 2 (5.13%) | 0 (0%) | 1 (5.26%) | 0 (0%) | 1.000a | - |
| Oral mucositis | 3 (7.69%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.544a | - |
| Foot-hand syndrome | 8 (20.51%) | 0 (0%) | 3 (15.79%) | 0 (0%) | 1.000a | - |
| Peripheral sensory neuropathy | 18 (46.15%) | 0 (0%) | 9 (47.37%) | 0 (0%) | 0.931b | - |
Abbreviations: EA = electroacupuncture; ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Significant differences are shown in bold.
Fisher’s exact test.
χ2 test.
Discussion
Gastrectomy and perioperative chemotherapy has improved survival in patients with stage Ⅱ-Ⅲ gastric cancer.19,20 However, the sequelae of gastrectomy exacerbate the symptoms and decline the HRQOL, especially within the first three months.2,21,22 Furthermore, toxicity of adjuvant chemotherapy during this period aggravates the HRQOL. Unfortunately, there is a lack of effective therapies to control the symptoms and improve HRQOL when undergoing adjuvant chemotherapy. To our knowledge, this trial is the first study focusing on the use of acupuncture on symptom control and HRQOL during this specific and challenging period for patients with gastric cancer.
The best oncology clinical care should not only focus on prolonging survival, but also improving HRQOL for patients during treatment and survivorship. Increasing evidence showing the effect of acupuncture on cancer-related symptoms3,4 led us to investigate its role for patients with gastric cancer. In a clinical trial with 56 advanced gastric cancer patients, acupuncture helped to reduce gastrointestinal symptoms during chemotherapy, including nausea, vomiting, abdominal pain, and diarrhea.23 In addition, it has also been reported that acupuncture can help to prevent or reduce postoperative ileus in gastric cancer patients after gastrectomy.24–26 Consistent with these findings on specific symptoms, our data contributes promising effect of EA for improving HRQOL during the hard period of postoperative adjuvant chemotherapy. Regardless of the impact of COVID-19 pandemic, the dropout rate of less than 10% indicated good feasibility for a larger clinical trial. If the preliminary observation can be confirmed in future large clinical trials,acupuncture can potentially be integrated into oncological care delivery to improve HRQOL for patients with gastric cancer during this challenging period.
Theoretically, acupuncture dosage is as important for effectiveness as that of pharmacological agents. Yet, acupuncture frequency in oncology settings varies from daily23 to weekly,9 depending mainly on the experiences of acupuncturists rather than evidences from clinical trials, suggesting that dosage is a crucial research area for acupuncture. An individual patient meta-analysis indicates that more acupuncture sessions appeared to be associated with better outcomes in patients with chronic pain.27 However, this may not be the case in oncology settings, since our data indicated that high-dose EA (2−3 times per week) was not more efficacious than low-dose EA (once per week) in improving HRQOL. Cancer patients undergoing chemotherapy are often suffering from increased travel and financial burdens due to frequent antitumor therapies; high dose acupuncture requires more time and increases cost. This may partially explain our data that in the social-associated SWB and FWB domains (Fig. S1b), as well as depression (P = 0.337) and anxiety (P = 0.358) symptoms (not reported), high-dose EA acted worse, although not significantly, than low-dose EA. In addition, for health systems, high dose acupuncture also requires more demands on staffing and space. Therefore, once weekly acupuncture appears to be the optimal dosing for future trials, nevertheless, more confirmation is needed.
Our data suggests EA not only improved subjective HRQOL, but also reduced objective high-grade leukopenia and neutropenia. A prior exploratory meta-analysis also indicates that acupuncture use was associated with an increase in leukocytes in patients during chemotherapy or chemoradiotherapy.28 A clinically-relevant trend of higher white blood cell count for acupuncture use was also observed in a pilot randomized, shamcontrolled clinical trial in gynecologic malignancy patients undergoing chemotherapy.29 Recently, Zhang Y, et al. reported that acupuncture promoted typical Th1 cells drifting, increased IFNγ and decreased IL-4 and IL-6 levels in peripheral blood mononuclear cells and plasma in advanced stage gastric cancer patients.30 All these results, as well as our data, indicated that acupuncture may have effects on modulating the immune system in cancer patients, while basic research in animal models suggested that this may be via vagal modulation.31,32 If future clinical trial confirms that electroacupuncture can prevent grade 3 leukopnea and neutropenia, it can potentially increase the tolerability of chemotherapy thereby increase the long term survival for patients with gastric cancer.
This study has some limitations. First, we did not use sham acupuncture as a control. This trial focused on determining the preliminary effect size of electro-acupuncture for improving HRQOL for gastric cancer patients undergoing adjuvant chemotherapy. The placebo effect of acupuncture cannot be ruled out and should be further explored. Second, due to the COVID-19 pandemic and the relatively high drop-out rate, we modified our analysis approach in the PPS population, where the ITT data was used as the sensitivity analysis. Furthermore, investigators and the statistician were not blinded to treatment groups, with potential risk of bias, even though we used the WOCF method to imput missing data for the bias risk reduction. Therefore, our findings should be interpreted as preliminary rather than definitive. Lastly, this study was conducted in China and may need to be repeated in health care settings in other regions of the world to determine generalizability of findings.
Conclusions
We presented preliminary evidence that EA is associated with promising effects in improving HRQOL, controlling symptom burden, and reducing toxicity during adjuvant chemotherapy in gastric cancer patients. Future adequately powered trials are feasible and needed to confirm the specific effect of EA.
Supplementary Material
Key message.
This article describes a multi-center randomized clinical trial with 58 gastric cancer patients undergoing adjuvant chemotherapy after gastrectomy. The results indicate that electro-acupuncture was associated with improved quality of life, controlled symptom burden, and reduced toxicity of chemotherapy in these patients.
Disclosures and Acknowledgments
The work was original research that has not been published previously, except that some results were presented as a Poster (1843P) at the 2020 ESMO (European Society for Medical Oncology) Annual Meeting. Dr. Jun J. Mao has received grants from Tibet Cheezheng Tibetan Medicine Co Ltd for work performed outside of the current study. All other authors declare no conflicts of interest. We thank all the patients who participated in this study and their families. We thank Wen-wei Ou-yang from Guangdong Provinicial Hospital of Traditional Chinese Medicine for help with statistical analysis. We thank Qiaoning Yang from Xiyuan Hospital, China Academy of Chinese Medical Sciences for the help with data monitoring. We thank Jie-shan Zhang from Zengcheng Hospital of TCM, Hai-peng Li from Central Hospital of Guangdong Nongken, Zhao-hong Li from Foshan Hospital of TCM, Jun-yi Cai from the Second People’s Hospital of Guangdong Shanwei, Li-zhen Zou from Heyuan Hospital of TCM, Zi-qian Zhang and Gui-yuan Li from the First Affiliated Hospital of Guangzhou Medical University, Li-na Yang from Shenzhen Traditional Chinese Medicine Hospital, Yi-fen Wu from Dongguan People’s Hospital, and Cheng-yun Wang from Maoming Hospital of Traditional Chinese Medicine, for performing the acupuncture.
Funding
This study was funded by the National Key Research and Development Program of China (2017YFC1700603), and the Science and Technology Planning Project of Guangdong Province (2017A020213021). Dr. Mao is supported in part by a grant from the National Institutes of Health/National Cancer Institute Cancer Center (P30 CA008748) and by the Translational and Integrative Medicine Research Fund at Memorial Sloan Kettering Cancer Center.
Footnotes
Data Statement
Dr. Hai-bo Zhang has full control of all primary data. All data relevant to the study are available in the department of Scientific Research Management of Guangdong Provincial Hospital of Traditional Chinese Medicine. Data and the protocol for this study may be available upon reasonable request, after the permission from Ministry of Science and Technology of the People’s Republic of China.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jpainsymman.2021.09.009.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Munene G, Francis W, Garland SN, et al. The quality of life trajectory of resected gastric cancer. J Surg Oncol 2012;105:337–341. [DOI] [PubMed] [Google Scholar]
- 3.Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-Designated comprehensive cancer center websites. J Natl Cancer Inst Monogr 2017;2017:lgx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zia FZ, Olaku O, Bao T, et al. The national cancer institute’s conference on acupuncture for symptom management in oncology: state of the science, evidence, and research gaps. J Natl Cancer Inst Monogr 2017;2017:lgx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He YH, Guo XF, May BH, et al. Clinical evidence for association of acupuncture and acupressure with improved cancer pain a systematic review and meta-analysis. JAMA Oncol 2020;6:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao JJ, Liou KT, Baser RE, et al. Effectiveness of electroacupuncture or auricular acupuncture vs usual care for chronic musculoskeletal pain among cancer survivors: the PEACE randomized clinical trial. JAMA Oncol 2021;7:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–4476. [DOI] [PubMed] [Google Scholar]
- 8.Naeim A, Dy SM, Lorenz KA, et al. Evidence-based recommendations for cancer nausea and vomiting. J Clin Oncol 2008;26:3903–3910. [DOI] [PubMed] [Google Scholar]
- 9.Bao T, Seidman AD, Piulson L, et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer 2018;101:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 2010;7:e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang S. The meridian system and mechanism of acupuncture: a comparative review. Part 3: mechanisms of acupuncture therapies. Taiwan J Obstet Gyne 2013;52:171–184. [DOI] [PubMed] [Google Scholar]
- 13.Garland SN, Pelletier G, Lawe A, et al. Prospective evaluation of the reliability, validity, and minimally important difference of the Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) Quality-of-Life Instrument. Cancer 2011;117: 1302–1312. [DOI] [PubMed] [Google Scholar]
- 14.Zhou HJ, So JB, Yong WP, et al. Validation of the functional assessment of cancer therapy-gastric module for the Chinese population. Health Qual Life Outcomes 2012;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y, Chen H, Zheng Y, et al. Psychometric validation of the edmonton symptom assessment system in Chinese patients. J Pain Symptom Manage 2015;50:712–717.e2. [DOI] [PubMed] [Google Scholar]
- 16.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 Years Later: past, present, and future developments. J Pain Symptom Manage 2017;53:630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairclough DL. Summary measures and statistics for comparison of quality of life in a clinical trial of cancer therapy. Stat Med 1997;16:1197–1209. [DOI] [PubMed] [Google Scholar]
- 18.Sprangers MAG, Moinpour CM, Moynihan TJ, et al. Assessing meaningful change in quality of life over time: a users’ guide for clinicians. Mayo Clin Proc 2002;77:561–571. [DOI] [PubMed] [Google Scholar]
- 19.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 20.Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389–1396. [DOI] [PubMed] [Google Scholar]
- 21.Shan B, Shan L, Morris D, Golani S, Saxena A. Systematic review on quality of life outcomes after gastrectomy for gastric carcinoma. J Gastrointest Oncol 2015;6:544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu YN, Vos EL, Baser RE, et al. Longitudinal analysis of quality-of-life recovery after gastrectomy for cancer. Ann Surg Oncol 2021;28:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Fang L, Wu WY, et al. The effect of acupuncture on chemotherapy-associated gastrointestinal symptoms in gastric cancer. Curr Oncol 2017;24:E1–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung SY, Chae HD, Kang UR, Kwak MA, Kim IH. Effect of acupuncture on postoperative ileus after distal gastrectomy for gastric cancer. J Gastric Cancer 2017;17:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chae HD, Kwak MA, Kim IH. Effect of acupuncture on reducing duration of postoperative ileus after gastrectomy in patients with gastric cancer: a pilot study using sitz marker. J Altern Complement Med 2016;22:465–472. [DOI] [PubMed] [Google Scholar]
- 26.You XL, Wang YJ, Wu J, et al. Zusanli (ST36) Acupoint injection with neostigmine for paralytic postoperative ileus following radical gastrectomy for gastric cancer: a randomized clinical trial. J Cancer 2018;9:2266–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacPherson H, Maschino AC, Lewith G, et al. Characteristics of acupuncture treatment associated with outcome: an individual patient meta-analysis of 17,922 patients with chronic pain in randomised controlled trials. PLOS One 2013;8:e77438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu W, Hu D, Dean-Clower E, et al. Acupuncture for chemotherapy-induced leukopenia: exploratory meta-analysis of randomized controlled trials. J Soc Integr Oncol 2007;5:1–10. [DOI] [PubMed] [Google Scholar]
- 29.Lu WD, Matulonis UA, Doherty-Gilman A, et al. Acupuncture for chemotherapy-induced neutropenia in patients with gynecologic malignancies: a pilot randomized, shamcontrolled clinical trial. J Altern Complem Med 2009;15:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YJ, Min Q, Huang Y, et al. Efficacy of acupuncture and moxibustion as a subsequent treatment after second-line chemotherapy in advanced gastric cancer. Evid Based Complement Alternat Med 2020:8274021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres-Rosas R, Yehia G, Pena G, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014;20:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Xu CC, Lin SQ, et al. Synergistic immunoreaction of acupuncture-like dissolving microneedles containing thymopentin at acupoints in immune-suppressed rats. Acta Pharm Sin B 2018;8:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.