Abstract
Objectives
Porous, radiolucent, shape memory polymer is a new technology available in discrete peripheral vascular embolization devices. Shape memory polymers can exist in two stable shapes; crimped for catheter delivery and expanded for vessel embolization. The expanded shape memory polymer in these new devices is hemostatic, and the porous polymeric scaffold has been shown to support tissue ingrowth and eventually bioabsorbs in preclinical animal studies. This report describes clinical experience with this novel material in vascular plug devices.
Methods
a prospective, single-arm, safety study at a single center in New Zealand with longer term follow-up via retrospective imaging review. The study device was a pushable shape memory polymer vascular plug with a distal nitinol anchor coil and a proximal radiopaque marker.
Results
Ten male patients were each implanted with a single shape memory polymer vascular plug. Three inferior mesenteric arteries and an accessory renal artery were embolized during endovascular aneurysm repair. An internal iliac artery was treated prior to the open surgical repair of aorto-iliac aneurysms. An internal iliac artery and a subclavian artery were embolized to treat/prophylactically address potential endoleaks. A profunda branch was embolized prior to tumor resection, and two testicular veins were embolized to treat varicoceles. Acute technical success of target vessel embolization was achieved in all implantation cases. Patients were followed for 30 days as part of the study, and no serious adverse events with a relationship to the study device occurred. No recurrent clinical symptoms attributable to treated vessel embolization or recanalization were documented. There was no evidence of recanalization on retrospective review of follow-up imaging through a mean of 22.2 months (range, <1–44 months) post-procedure.
Conclusions
Shape memory polymer vascular embolization devices were safe and effective over the follow-up period of this small safety study. Further experience and longer term follow-up will assess further applicability.
Keywords: Shape memory polymer, peripheral embolization, endovascular aneurysm repair, endoleak, gonadal vein, vascular plug
Introduction
Shape memory polymer is a new technology in the field of peripheral vascular embolization. Shape memory polymer, available in a family of devices, is stable in a crimped shape that is compatible with catheter delivery. When a shape memory polymer device is deployed into the warm and aqueous environment of a vessel, the polymer self-expands to its maximum volume in the form of a porous and hemostatic scaffold that conforms to its surrounding anatomy. The porous shape memory polymer has been shown to support tissue ingrowth in preclinical animal studies. 1 A shape memory polymer plug has a low radial force when expanded in a vessel. The polymer pores are approximately 1000–2000 microns, which is larger than those reported for GELFOAM (30–700 microns), for reference. 2 The material is also radiolucent and bioabsorbs over time. Examples of the use of shape memory polymer-based devices in peripheral vascular embolization and aortic applications are emerging,3–5 and this report describes our early clinical experience of a device with this shape memory polymer material.
Methods
Study design and ethical approval
A prospective, open-label, single-arm, safety study of the IMPEDE Embolization Plug (Shape Memory Medical, Santa Clara, California, USA) was performed at a single center in New Zealand from August 2017 through December 2019. Local approval for human investigation (reference 17/NTA/83) and written patient consent were obtained (including for the study device, which was pre-market at the time). The study was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR): 12617000906358. The study included follow-up through 30 days. Longer term results based on standard-of-care follow-up imaging were collected with an ethics committee consent waiver for retrospective review and publication in place.
Study device
The IMPEDE Embolization Plug (included in Figure 1) is a pushable embolization device comprising an ultra-low-density polyurethane shape memory polymer plug, 6 a distal nitinol and platinum/iridium helical anchor coil, and a proximal platinum/iridium radiopaque marker. 7 The device is available in 3 sizes with expanded shape memory polymer plug dimensions of 6 mm diameter x 10 mm length, 8 mm diameter × 10 mm length, and 12 mm diameter × 15 mm length. The expanded shape memory polymer volume of the 12-mm diameter device is ∼ 1.25 mL. The diameters of the crimped form (for storage and catheter delivery) of devices are 0.032″, 0.046″, and 0.065″, respectively. The instructions for use contain all device dimensions and delivery catheter inner diameter requirements. 8 The device is supplied loaded in an introducer, and no preparation of the device is necessary prior to insertion into the delivery catheter. The instructions for use of the study device indicate the polymer starts to bioabsorb after 30 days, based on preclinical animal studies. 8 The instructions for use state a maximum storage temperature of 40°C (105 F), and the packaging has a temperature indicator label. 8 The device has a one-minute working time, defined as the time between entry into the delivery catheter and deployment into the target vessel.
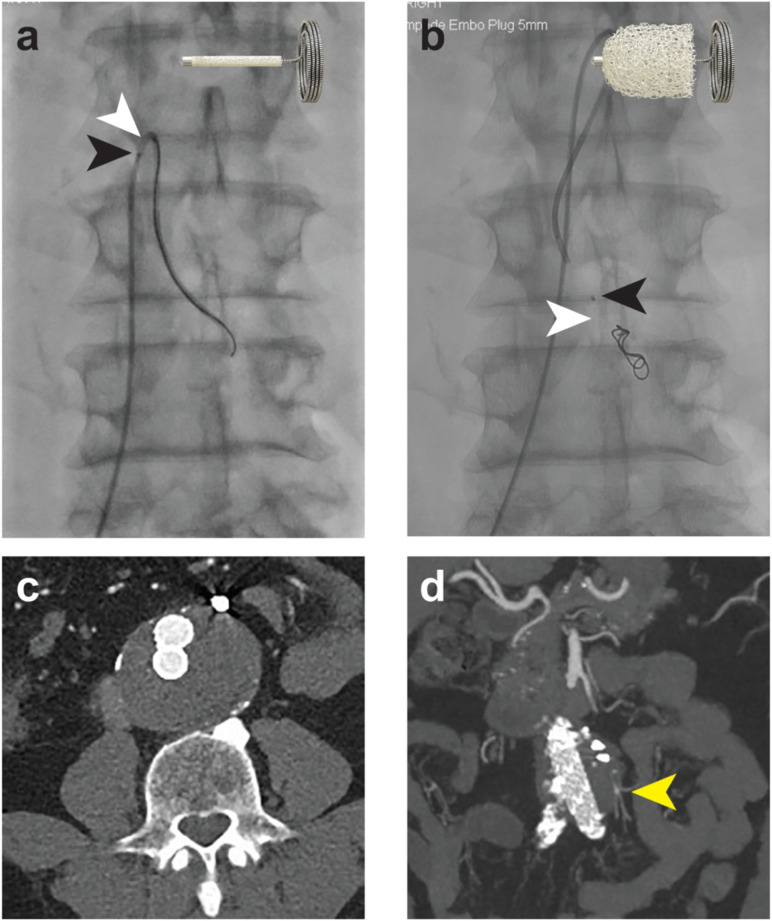
Figure 1.
Inferior mesenteric artery embolization prior to EVAR. (a) Delivery of the device in its crimped shape, where the anchor coil and proximal marker (black arrow) are clearly visible and the shape memory polymer is radiolucent (white arrow). Cartoon illustrates the position of the crimped shape memory polymer between the proximal marker and the anchor coil. (b) Post-deployment of the device into the artery, illustrating the position of the radiolucent shape memory polymer (white arrow) and proximal marker (black arrow). Cartoon illustrates the expanded shape memory polymer. (c) and (d) show 2-year axial and coronal CT imaging illustrating sustained embolized vessel occlusion. The distal branches remain patent, perfused by collaterals (yellow arrow).
Eligibility criteria
As an early device safety study of the shape memory polymer material, the study was designed to determine the safety and performance of the device in candidates for arterial or venous embolization of the peripheral vasculature. To be included, patients aged 18–75 years had a target artery or vein diameter of 2–9 mm requiring embolization. Exclusion criteria were primarily designed to exclude particularly challenging anatomy or allergies that may present unnecessary procedural or implantation risk. A full list of eligibility criteria is provided in the supplemental material.
Study endpoints
The primary endpoint of this safety study was related serious adverse event rate through 30 days, and secondary efficacy endpoints were acute technical success (target vessel embolization) and absence of recurrent clinical symptoms/recanalization through 30 days.
Results
A total of 11 patients were enrolled in the study. The study was terminated prior to full enrollment (20 patients) because the device received CE marking and FDA 510(k) approval, and a post-market study was initiated. 3 A total of 10 male patients (mean age 59 ± 19 years) were each implanted with a single study device. One enrolled patient was not implanted with a device as outlined in more detail below. As an early safety study, cases were selected to easily accommodate the unique working time of the device, where the device must be deployed into the target vessel within 1 minute of entering the delivery catheter to avoid any friction as the shape memory polymer starts to expand in the presence of blood. Three inferior mesenteric arteries and an accessory renal artery were embolized during endovascular aneurysm repair (EVAR). An internal iliac artery was treated prior to the open surgical repair of aorto-iliac aneurysms. An internal iliac artery and a subclavian artery were embolized to treat an endoleak and prophylactically address a potential endoleak, respectively. A profunda branch was embolized prior to tumor resection and hemiarthroplasty and two testicular veins were embolized to treat varicoceles.
Endovascular and surgical aneurysm repair—concomitant vessel embolization
Three inferior mesenteric arteries 3.0–4.2 mm in diameter were each implanted with devices with shape memory polymer plugs 6 mm in diameter. The low radial force of the expanded shape memory polymer means that oversizing is acceptable. The anchor coil of the device requires 2.5–4.0 cm landing zone, depending on the size of the vessel. Figure 1 shows a representative example of a 3 mm diameter inferior mesenteric artery embolization prior to EVAR as prophylactic prevention of a potential type II endoleak. Follow-up imaging at 2 years post-procedure showed the main branch remained occluded. Other vessels embolized with 6 mm diameter devices to support AAA repair were a 3.5 mm right accessory renal artery treated prior to EVAR of a juxtarenal aneurysm and a 4 mm right internal iliac artery embolization prior to open surgical aorto-iliac aneurysm repair.
Endoleaks
A type Ib endoleak post-EVAR (inadequate right common iliac artery seal) was treated via embolization of a 7 mm internal iliac artery with a 12 mm diameter device, in combination with a limb extension into the right external iliac artery (Figure 2). Follow-up through 4 years showed sustained vessel occlusion. The ostium of a 7–8 mm left subclavian artery was embolized with a 12 mm device to preemptively address a potential type II endoleak following TEVAR to treat a symptomatic aortic arch dissection (Figure 3). Long-term follow-up at 2 years also showed sustained vessel occlusion.
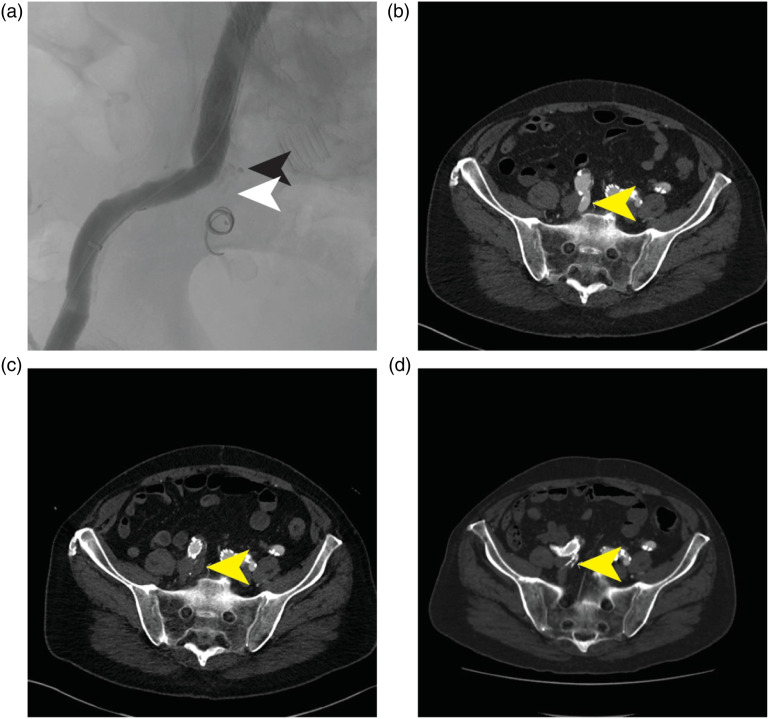
Figure 2.
Right internal iliac artery embolization to treat a type Ib endoleak post-EVAR. (a) Post-deployment of the device illustrating the position of the radiolucent shape memory polymer (white arrow) and proximal marker (black arrow). (b) Pre-procedure CT with patent internal iliac artery (yellow arrow). (c) One-month CT illustrating complete occlusion (yellow arrow). (d) Four-year CT illustrating sustained occlusion (yellow arrow).
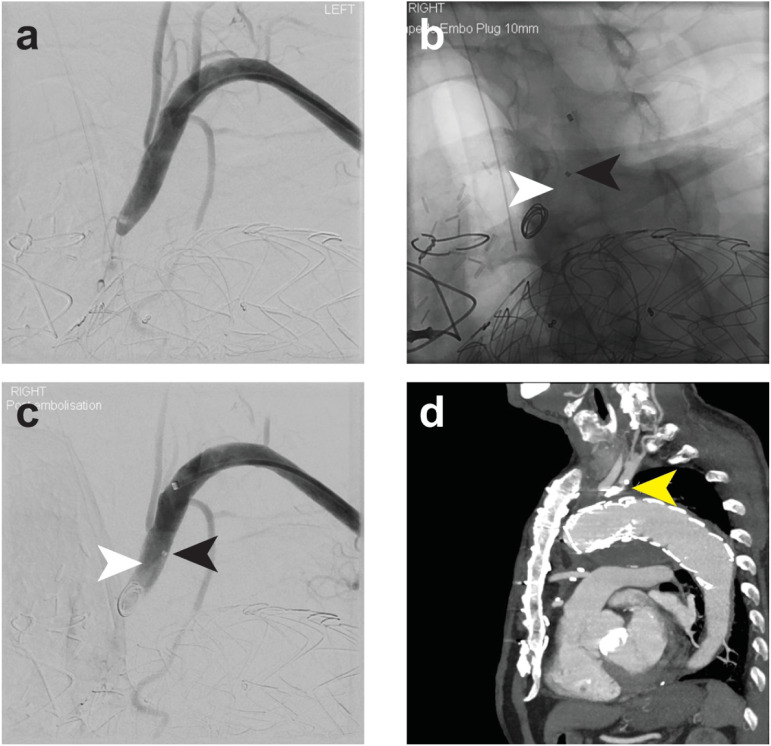
Figure 3.
Left subclavian artery embolization to prophylactically address a potential type II endoleak post-TEVAR for a symptomatic aortic arch dissection. (a) Left subclavian artery angiogram post -EVAR, illustrating stenosis at the ostium. (b and c) Post-deployment of the device illustrating the position of the radiolucent shape memory polymer (white arrow) and proximal marker (black arrow). (d) Sagittal reconstruction of 2-year CT (yellow arrow, anchor coil of the device), and sustained occlusion was observed.
Shape memory polymer plug in combination with other embolic devices
In three cases, the shape memory polymer plug was used in combination with other devices in the same vessel. Polyvinyl alcohol particles were used to embolize distally before embolization of a 2.5–4.5 mm profunda branch with a 6 mm diameter device in preparation for tumor resection in the hip. In two cases to treat varicoceles, 6–8 mm testicular veins were each implanted with a single 12 mm diameter device following the use of sodium tetradecyl sulfate foam sclerosant, which is a common approach to gonadal vein embolization. The supplemental material includes illustration of the acute technical success of a symptomatic left testicular vein embolization with a shape memory polymer device via a right internal jugular vein to left renal vein approach.
Follow-up
All patients were followed for 30 days in the prospective study. No adverse events attributable to the use of the study device occurred during that time. No patients returned to the clinic with recurrent clinical symptoms or indications of recanalization through 30 days. All patients implanted with a study device underwent follow-up imaging as part of standard of care: computed tomography (CT) (n = 7) or ultrasound (n = 3 [both varicocele cases and a right internal iliac artery embolization during EVAR]). Retrospective review of the standard-of-care follow-up imaging did not reveal any evidence of recanalization through a mean of 20.2 months (range, <1–44 months).
Discussion
Our position as a high-volume center for EVAR presented several opportunities to enroll patients needing vessel embolization during/prior to abdominal aortic aneurysm (AAA) repair and to treat endoleaks/prophylactically address potential endoleaks following EVAR and thoracic EVAR (TEVAR). On a case-by-case basis, it is our practice to preemptively embolize large diameter inferior mesenteric and accessory renal arteries arising from the aneurysm sac if we believe it may lead to type II endoleak, and the device was suited to this application. It was happenstance that all enrolled patients were men; women were not excluded from the study. As mentioned above, the working time of the shape memory polymer is a unique feature of the device that requires attention to delivery catheter selection. In our experience, a 5-6 Fr guiding catheter or sheath accommodated the device, and we avoided friction-based events. The device does not require any preparation or wetting should be avoided, if possible (as the crimped polymer will start to expand), and it is simply removed from the packaging and inserted via its introducer. The device is pushable (usually a 0.035 guidewire), and delivery is relatively straightforward using an unsheathing technique as it is important not push the shape memory polymer plug into its distal anchor coil and thereby limit the expansion of the polymer. The shape memory polymer is radiolucent, but device placement is guided by the proximal radiopaque marker and distal anchor coil. We performed device recovery in two cases. In the internal iliac artery embolization prior to surgical AAA repair, an 8 mm device was initially partially inserted but the anchor coil was not providing wall apposition, indicating that the device was too large. As the shape memory polymer portion of the plug had not exited the delivery catheter, the polymer was allowed to expand, creating friction within the catheter, and then the device and catheter were removed as one unit with light suction. A 6 mm shape memory polymer device was subsequently implanted successfully. In another case, the anchor coil did not form and therefore the device was removed with the catheter using the same recovery technique. In this case, alternate embolization devices were used to complete the case and therefore the study device was not implanted in this patient.
Integration of novel technologies into clinical practice is often a stepwise process as we initially assess safety and evaluate efficacy in different situations. While we acknowledge the anchor coil component of the shape memory polymer device in this study, the shape memory polymer material itself has numerous unique properties that differentiate it from metal-based embolization technologies; shape memory polymer devices without an anchor coil are now available. The material is radiolucent, which facilitates follow-up imaging and may be advantageous when monitoring for endoleaks post-EVAR. Shape memory polymer also has low radial force, which means the material can be used to fill vascular space without exerting substantial force on the vessel wall. In combination with the porous nature of the expanded material and the bioabsorption, it is easy to imagine prophylactic aneurysm treatment with the technology. The animal study data showing tissue ingrowth into the device are intriguing, and clinical advantages of this property may emerge in further studies on the technology.
Overall, the shape memory polymer device performed as expected and the study achieved its goal of early clinical experience.
Conclusions
Porous shape memory polymer vascular embolization devices were safe and effective over the follow-up period of this early clinical experience. Further experience and longer term follow-up will determine further applicability of the novel shape memory polymer material.
Supplemental Material
Supplemental Material for Shape memory polymer technology in peripheral vascular embolization by Andrew Holden, Andrew A Hill, and Brendan T Buckley in Vascular.
Acknowledgment
The authors thank Julie Perkins, PhD, for assistance with preparation of the manuscript, an independent consultant supported by Shape Memory Medical.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Andrew Holden is a consultant to Shape Memory Medical. All other authors have no relevant conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Shape Memory Medical.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Andrew Holden https://orcid.org/0000-0002-8170-4960
References
- 1.Jessen SL, Friedemann MC, Ginn-Hedman A, et al. Microscopic assessment of healing and effectiveness of a foam-based peripheral occlusion device. ACS Biomat Sci Eng 2020; 6: 2588–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med 2008; 19: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RA, Loftus I, Ratnam L, et al. Clinical experience with a shape memory polymer peripheral vascular embolisation plug: a case series. CVIR Endovasc 2021; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen AJS, van Schaik PM, Martens JM, et al. Embolization of the false lumen using IMPEDE-FX embolization plugs as part of treatment of an infrarenal post-dissection aneurysm: a case report. CVIR Endovasc 2020; 3: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellano D, Boghi A, Di Maggio L, et al. Shape memory polymer ovarian vein embolisation in a patient with nickel allergy. CVIR Endovasc 2021; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal P, Rodriguez JN, Small W, et al. Ultra low density and highly crosslinked biocompatible shape memory polyurethane foams. J Polym Sci B Polym Phys 2012; 50: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landsman TL, Bush RL, Glowczwski A, et al. Design and verification of a shape memory polymer peripheral occlusion device. J Mech Behav Biomed Mater 2016; 63: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Instructions for use available from the shape memory medical website, https://www.shapemem.com/ (accessed January 2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Shape memory polymer technology in peripheral vascular embolization by Andrew Holden, Andrew A Hill, and Brendan T Buckley in Vascular.